Abstract
Background:
Dissotis rotundifolia, commonly referred to as pink lady, has several medicinal uses including peptic ulcer. This study investigated the inhibitory effects of D. rotundifolia extract on H+/K+-ATPase and also assessed its antiradical activity. In silico study of some isolated compounds of this plant was also carried out to affirm the suspected binding properties of extract to H+/K+-ATPase enzyme.
Materials and Methods:
D. rotundifolia whole plant extract was obtained after extraction process and then assessed for its ability to scavenge free radicals in four in vitro test models. Its ability to inhibit the activity of H+/K+-ATPase enzyme was also evaluated. Molecular docking was carried out on phytoconstituents, namely, vitexin, isovitexin, orientin, and isoorientin reported to be present in the whole plant extract.
Results:
Data obtained indicated that D. rotundifolia extract (DRE) exhibits strong antioxidant activity. DRE also showed inhibitory effects on H+/K+-ATPase enzyme activity. Docking studies affirmed the in vitro binding effect of the extract to H+/K+-ATPase.
Conclusion:
These findings suggest that the plant extract possess antioxidant and antipeptic ulcer activity.
Keywords: Antiradical, Dissotis rotundifolia, docking, H+/K+-ATPase
INTRODUCTION
Gastric mucosal damage is associated with the hypersecretion of gastric acid through H+/K+-ATPase action. Remarkably, all the gastric secreted biomolecules such as histamine, acetylcholine, and gastrin work through the H+/K+-ATPase pump to allow the parietal cells of gastric mucosa to produce HCl.[1] This implies that one of the major strategies to consider in the management of hyperacidity may be the use of H+/K+-ATPase inhibitors or proton pump inhibitors. Although several synthetic proton pump inhibitors (PPIs) such as omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole are available for use to manage hyperacidity in individuals with gastrointestinal disorders, most of these drugs come with several adverse effects[2,3] which are not tolerated by some individual.
Implicitly, there is the need for more research into other alternatives with less side effects, relatively less toxic, better tolerated, and globally competitive. However, plants are some of the most attractive natural sources of new drugs that have exhibited promise and also perceived to show fewer side effects.
Dissotis rotundifolia, a member of the family Melastomataceae, is used in traditional herbal medicine practice in several African countries including Ghana to manage diseases such as peptic ulcers.[4,5,6,7,8,9,10] The plant is reported to contain C-glycosylflavones, namely, isoorientin, orientin, vitexin, and isovitexin.[11] It is evident from studies on the plant that it has potential in treating a number of disorders such as rheumatism and arthritis, and it is also safe at a dose of 1000 mg/kg.[12,13,14,15,16,17,18] Based on its ethnomedicinal use in managing peptic ulcer, this study was designed to evaluate the in vitro inhibitory effect of D. rotundifolia extract (DRE) on H+/K+-ATPase enzyme and assess its binding to the enzyme through molecular docking studies of reported isolated compounds of the plant extract. This study is expected to throw light on the use of D. rotundifolia whole plant in the management of hyperacidity disorders traditionally.
MATERIALS AND METHODS
Reagents
Adenosine-5-triphosphate (ATP), Nitro blue tetrazolium (NBT), phenazonium methosulphate (PMS), naphthylenediamine dichloride, phosphoric acid, reduced glutathione, 5, 51-Dithiobis (2-nitrobenzoic acid), nicotinamide adenine dinucleotide (NADH), PMS, and Tris-HCl buffer were obtained from Sigma Aldrich, Chemical Company, USA. All other chemicals were of the highest analytical grade.
Collection of plant material
D. rotundifolia whole plant was collected and then authenticated by a curator. A voucher specimen (No. 107346) was prepared and deposited at the herbarium of the University. The whole plant was washed thoroughly, shade dried for 3 weeks, oven dried at 40°C for 3 h then pulverized into powder.
Preparation of flavonoid-rich fraction
To obtain a flavonoid-rich fraction of the plant extract, the method described by Ansah et al.[18] was employed. The filtrate obtained was concentrated and later dried at 40°C to obtain a brown coffee-colored residue, which was labeled as DRE. The crude DRE was stored at −20°C until ready for use.
Isolation of H+/K+-ATPase enzyme from sheep's stomach tissue
A stomach of freshly sacrificed sheep was obtained from a slaughter house and washed with cold distilled water and normal saline, after which it was placed in a phosphate-buffered saline and transported to the laboratory. The method described by Reyes-Chilpa et al.[19] was employed in the isolation process. The resultant parietal cells obtained was used as a source of enzyme.
Estimation of the inhibitory effect of Dissotis rotundifolia extract on H+/K+-ATPase enzyme activity
The inhibitory effect of DRE on H+/K+-ATPase enzyme was evaluated as follows: A volume of about 0.1 ml of the microsomes was mixed with 0.1 ml of double distilled water/DRE/omeprazole (20, 60, 100, 140, 200 μg/ml) and preincubated at 37°C for 1 h. After incubation, 0.2 mL of Tris-HCl (20 mM, pH 7.4); 0.2 ml of MgCl2(2 mM); and 0.2 ml of KCl (2 mM) were added to the reaction mixture. The reaction was initiated by adding 0.2 mL of ATP (2 mM) and incubated at 37°C for 30 min. The reaction was stopped by adding of 1.0 mL of ice cold 10% trichloroacetic acid followed by centrifugation at 2000 g for 10 min. The supernatant obtained was used for the determination of inorganic phosphorus generated.
The amount of inorganic phosphorus (Pi) liberated from ATP in the supernatant was determined using the method described by Shyla et al.[20] Results were expressed as mean ± standard error of the mean (SEM) on a bar graph.

Hydroxyl radical scavenging activity
The hydroxyl radical scavenging activity (HRSA) of extract and standards – citric acid and EDTA – were determined according to the method described by Klein et al.[21] The intensity of the chromophore formed was measured spectroscopically at 412 nm. Percentage HRSA was calculated as:
% HRSA = ([A0–A1]/A0) × 100,
where A0 is the absorbance of the control and A1 is the absorbance of the extract/standard.
Superoxide anion scavenging activity
The superoxide (SO) anion radical scavenging activity was measured as described by Robak and Gryglewski.[22] The absorbance was measured at 560 nm. Percentage SO anion radical scavenging ability of plant extract was calculated as:
% inhibition of superoxide radical = (Abs [control] – Abs [test])/(Abs [control]) × 100.
Where Abs is absorbance.
Nitric oxide radical scavenging activity
The nitric oxide radical scavenging activity was determined according to the method described by Green et al.[23] The absorbance was measured at 540 nm against a blank.
% NO radical scavenging activity = (Abs control–Abs sample)/(Abs control) × 100.
DPPH-radical scavenging activity
The method of Blois[24] was used to evaluate the extract scavenging ability of DPPH free radical. The absorbance was measured at 517 nm. Percentage DPPH radical scavenging activity was computed as:
% DPPH scavenging activity = (Abs control–Abs sample or standard)/(Abs control) × 100.
Anti-lipid peroxidation
A modified thiobarbituric acid-reactive species assay described by Dasgupta and De[25] was used to measure the lipid peroxide formed using egg-yolk homogenates as lipid-rich media. The absorbance was measured at 532 nm. Percentage anti-lipid peroxidation activity was calculated as: ([A0− A1/A0] × 100), where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the sample or standard. Where Ao is the absorbance value of the fully oxidized control and A1 is ([A532+ TBA]−[A532− TBA]).
Molecular docking studies
Earlier reports indicate that the whole plant D. rotundifolia contains potentially pharmacologically active C-glycosylflavones compounds, namely, isoorientin, orientin, vitexin, and isovitexin. Molecular docking was performed for these reported phytochemicals of D. rotundifolia within H+/K+-ATPase enzyme active site. Two-dimensional structures for orientin, isoorientin, vitexin, and isovitexin were downloaded from PubChem.[26]
The Protein Data Bank (PDB) file for omeprazole was acquired from DrugBank.[27,28] The protein structure file PBD ID: 2XZB PDB was downloaded from the Protein Data Bank (PDB http://www.pdb.org)[29] for use as the H+, K+-ATPase receptor.
Ligand files for orientin, isoorientin, vitexin, and isovitexin were originally downloaded in Structure Data Format. Open Babel[30] was used to convert the ligand files into PDB format. The University of California, San Francisco Chimera (Chimera) was utilized to prepare the ligand files for docking.[31] Each of the five ligands was subsequently superimposed using Chimera. A reliable control for docking was established in the overlaying of structures and the use of the same resultant grid box throughout each docking trial. The grid box was restricted to cover the binding site of 2XZB. AutoDock Vina (Vina)[32] was subsequently used to execute the docking of each ligand within 2XZB.
AutoDock Tools 1.5.6 was utilized to isolate the lowest energy pose for each docked structure.[33] Root-mean-square deviation (RMSD) was used to compare the differences in orientation within 2XZB of the docked poses of orientin, isoorientin, vitexin, and isovitexin with that of omeprazole. Due to its current therapeutic application, the orientation of omeprazole within 2XZB served as the reference pose for RMSD calculations. The Bevan Molecular Modeling Lab at Virginia Tech wrote the script used to carry out these calculations. Successful docking results were governed by an RMSD value <2.00 Å.[34]
Analysis of data
All tests were performed in triplicate, expressed as mean ± SEM and presented in tables and bar graphs where appropriate. The percentage ATPase inhibitory activity of the extract and omeprazole was also expressed in terms of 50% inhibitory concentration (IC50) value. Results were compared with the standard antiulcer drug omeprazole. Data were analyzed by Analysis of Variance followed by Dunnett's test with the level of significance set at P < 0.05 using (GraphPad Software Inc., San Diego, CA, USA).
RESULTS AND DISCUSSION
Proton-pump inhibitors work mainly by reducing gastric acid secretion during hyperacidity. Other drugs also scavenging free radicals, block histamine receptors, and elevate prostaglandin E-2 levels in the gastrointestinal mucosa. However, the use of these orthodox drugs is bedeviled with adverse effects. Plant medicine seems to be the preferred choice of treatment by rural folks due to its perceived fewer side effects. D. rotundifolia is one such plant cherished in the traditional medicine system for its numerous health benefits such as antimicrobial activity, anti-inflammatory, antihelminthics, and antipeptic ulcer effects.[35] However, the traditional use of the plant for gastropathy lacks experimental-based evidence and hence the present study. Figure 1 shows a noteworthy H+/K+-ATPase inhibition by DRE. At a maximum concentration of 200 μg/ml for DRE and omeprazole, percentage enzyme inhibition was found to be 71% and 83%, respectively [Figure 1]. DRE inhibited H+/K+-ATPase activity with an IC50 value of 34.74 μg/ml, in comparison with omeprazole with an IC50 value of 8.00 ug/ml. These results suggest that DRE has some inhibitory effects on H+/K+-ATPase though about four times lower when compared to omeprazole. Quílez et al.[36] have reported the antisecretory and H+/K+-ATPase inhibitory effects of vitexin and isovitexin isolated from the leaves of Piper carpunya plant. Implicitly, the pharmacological role of vitexin and isovitexin which has been reported to be present in DRE cannot be ignored. The presence of these flavones in the whole plant extract of D. rotundifolia may be responsible for its H+/K+-ATPase inhibitory properties.
Figure 1.
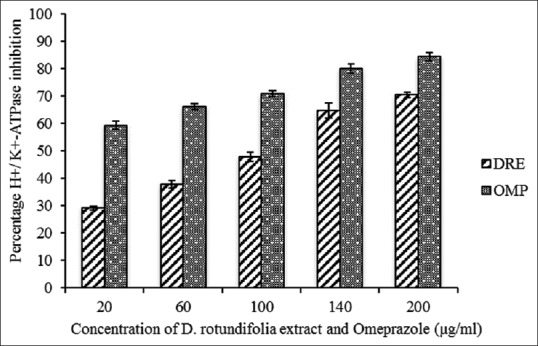
H+/K+-ATPase inhibitory activity of methanolic extract of Dissotis rotundifolia and omeprazole at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
Peptic ulcer has multietiopathogenetic factors and is generally known that one of the key underlying factors of this gastrointestinal disorder is the generation of free radicals. There is substantial evidence that oxygen-derived free radicals play an important role in the pathogenesis of injury to various tissues, including gastric tissue.[37] In addition, involvement of oxygen-derived free radicals such as the SO anion and hydroxyl radical are well established in the pathogenesis of ischemic injury of gastrointestinal mucosa.[38] Implicitly, the pivotal role of antioxidants in the prevention and healing of peptic ulcer cannot be overlooked.
In this study, DRE was investigated for its antioxidant properties to determine whether it can serve as alternative to synthetic antioxidants in reducing the free radical that mediates diseases such as peptic ulcers. Several in vitro models are used, but in this study, OH, DPPH, SO, and NO assays were used. Hydroxyl radicals are recognized as the most reactive free radical formed in biological systems. They have been implicated as highly damaging species capable of damaging biomolecules such as DNA and proteins.[39,40] In this study, the hydroxyl scavenging ability of the EDTA, citric acid, and DRE at 200 μg/mL were 93 ± 2.28, 90 ± 0.32, and 63% ± 1.28%, respectively, as shown in Figure 2. The concentrations of EDTA, citric acid, and DRE needed for 50% inhibition of OH was found to be 15.93, 14.87, and 3.50.8 μg/mL, respectively. This means IC5O for DRE is lower than citric acid and EDTA which are standards. This suggests that DRE is a more potent scavenger of hydroxyl radical than citric acid and EDTA. Implicitly, DRE could possess considerable protection against hydroxyl free radical-mediated DNA and protein damage and could therefore be considered in developing plant-based drug for oxidative stress-related diseases such as atherosclerosis and peptic ulcer.
Figure 2.
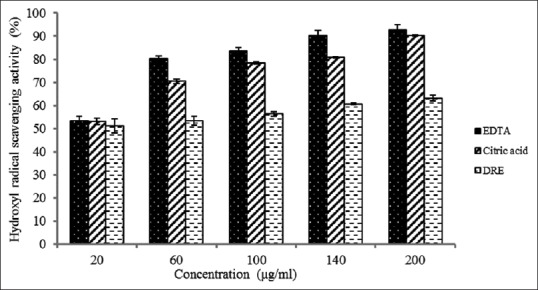
Hydroxyl radical scavenging activity of methanolic extract of Dissotis rotundifolia and standards at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
SO anion is also very harmful to cellular components. It is produced from molecular oxygen due to oxidative enzyme of body as well as through nonenzymatic reaction such as autoxidation by catecholamine.[41] SO anion is recognized to be involved in the pathogenesis of ischemic injury of gastrointestinal mucosa.[42] In this study, the SO radicals generated from dissolved oxygen by PMS-NADH coupling assay were measured by their ability to reduce NBT. At the concentration of 200 μg/mL, percentage inhibition of SO anion radical by DRE was observed to be 30.40 ± 1.28, in comparison with ascorbic acid and gallic acid with percentage inhibition found to be 49.0 ± 2.28 and 49.2 ± 0.32, respectively, at maximum concentration [Figure 3]. The IC50 values of ascorbic acid, gallic acid, and DRE were found to be 87.19, 98.90, and 93.66 μg/mL, respectively. The IC50 obtained for DRE in this study shows that it has a better potent scavenging potential for SO radicals compared to gallic acid and also gives an indication of its ability to prevent oxidative stress-related diseases that are mediated by SO oxide species.
Figure 3.
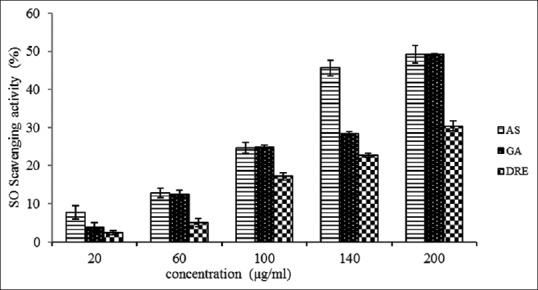
Superoxide anion radical scavenging activity of methanolic extract of Dissotis rotundifolia and standards at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
Acute exposure to NO is directly toxic to tissues and leads to vascular collapse with septic shock, whereas chronic exposure triggers various carcinomas and inflammatory conditions such as arthritis and ulcerative colitis.[43] The toxicity of nitric oxide becomes adverse when it reacts with SO radical, producing a highly reactive peroxynitrite anion (ONOO). At 200 μg/mL, the percentage inhibition of ascorbic acid, gallic acid, and DRE was found to be 81 ± 0.34, 74 ± 0.47, and 56 ± 0.64 respectively, and their respective IC50 values were 27.07, 85.71, and 32.34 μg/mL [Figure 4]. IC50 values calculated using data from Figure 4 show that DRE is a more potent scavenger of NO radical than gallic acid though not better than ascorbic acid. These results indicate that DRE has the potential of reducing and preventing oxidative stress-related diseases that are mediated by nitric oxide species.
Figure 4.
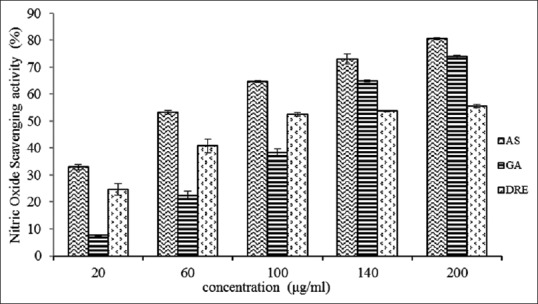
Nitric oxide radical scavenging activity of methanolic extract of Dissotis rotundifolia and standards at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
The DPPH test model involves a reaction of specific antioxidant with a stable free radical and results in a reduction in absorbance which can be measured at 517 nm. At the concentration of 200 μg/mL, the scavenging property of DRE on the DPPH radical was 83% ± 1.28%, when compared to the 98% ± 0.32% and 92% ± 2.28% for gallic and ascorbic acid, respectively [Figure 5]. The IC50 values of 12.53, 4.10, and 0.398 were recorded for DRE, ascorbic acid, and gallic acid, respectively. In the present study, DRE demonstrated significant DPPH radical-scavenging activity. Abdel-Farid et al.[44] reported a DPPH radical IC50 values of 78.18, 78.27, 76.14, respectively, for Acacia nilotica leaves, flowers, and pods, respectively. This generally depicts the fact that DPPH-scavenging ability of DRE is higher than some previously exploited natural sources of antioxidants and could be considered as an alternative to synthetic antioxidants.
Figure 5.
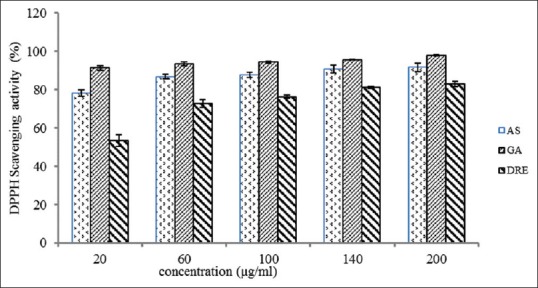
DPPH radical scavenging activity of methanolic extract of Dissotis rotundifolia and standards at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
Lipids of cell membranes are predisposed to peroxidation. In vitro anti-lipid peroxidation was determined by means of an assay system which determines the production of malondialdehyde and related compounds in egg homogenate. Malondialdehyde (MDA) is one of the major degradation products of lipid peroxidation used as a marker for oxidative stress.[45] In this experiment, at a concentration of 200 μg/mL, the inhibitory effect of DRE extract, gallic acid, and ascorbic acid in the formation of malondialdehyde was 78 ± 1.28, 73 ± 0.32, and 88% ± 2.28%, respectively [Figure 6]. The IC50 values of DRE, gallic acid, and ascorbic acid was observed to be 22.11, 50.09, and 6.44 μg/mL, respectively. DRE seem to be a more potent antioxidant in preventing lipid peroxidation than gallic acid though not better than ascorbic acid. This property of D. rotundifolia plant extracts could be attributed to the contribution of its flavonoids such as vitexin and isovitexin that have been reported to be involved in antioxidative processes.
Figure 6.
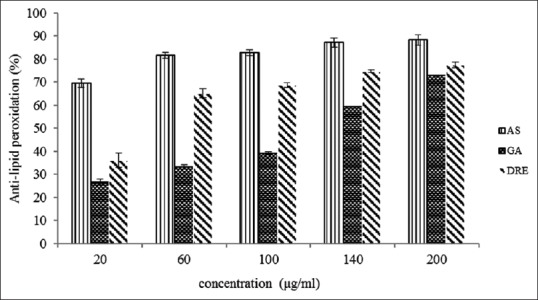
Anti-lipid peroxidation activity of methanolic extract of Dissotis rotundifolia and standards at different concentrations. Each value represents mean ± standard error of the mean (n = 3)
Molecular docking studies were performed to assess possible in vitro binding of orientin, isoorientin, vitexin, and isovitexin which are known constituents of D. rotundifolia and that of a reference drug omeprazole (5-methoxy-2 – [4-methoxy-3, 5-dimethyl-2-pyridinyl.-methyl-sulphinyl] -1H-benzimidazole) to H+/K+-ATPase enzyme. Orientin, isoorientin, isovitexin, vitexin, and omeprazole were docked into 2XZB [Figure 7]. The RMSD values indicate that isovitexin and vitexin docked more successfully than either orientin or isoorientin [Table 1]. Isovitexin and vitexin bind 2XZB in a similar fashion to the current therapeutic drug – omeprazole.
Figure 7.
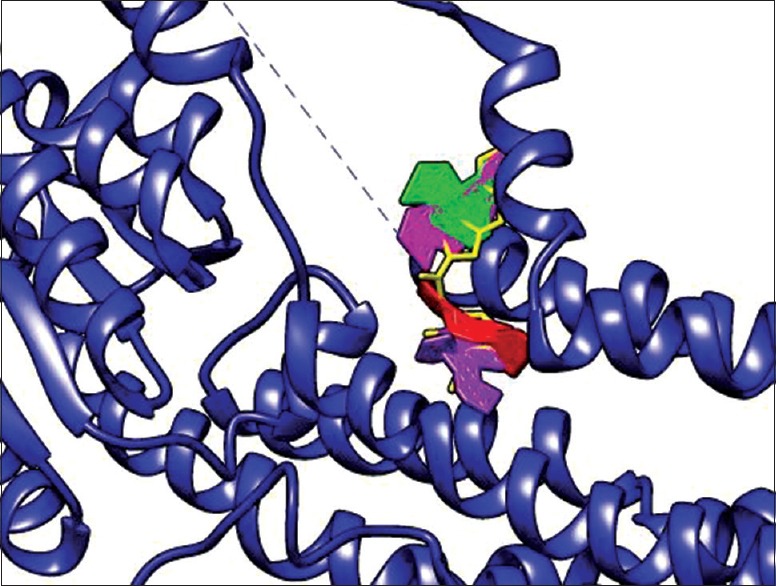
Visual representation of phytochemicals docked into 2XZB. Isovitexin (magenta), vitexin (green), orientin (purple), isoorientin (red), and omeprazole (yellow) within 2XZB receptor. Isovitexin and vitexin docked with the highest orientation similarity to omeprazole
Table 1.
Root mean square deviation and 50% inhibitory concentration values of docked phytochemicals
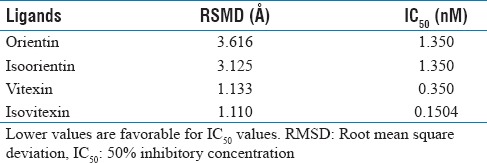
In the docking studies, the RMSD values <2.0 Š indicated successful docking. Thus, the orientation of vitexin and isovitexin within 2XZB more closely resembles the orientation of omeprazole within 2XZB than those of orientin or isoorientin. With regard to the current therapeutic utilization of omeprazole, vitexin and isovitexin show promise as a potential therapeutic.
Calculation of IC50 values affirms the inhibition potential for vitexin and isovitexin. Within the stomach, gastrin elicits parietal cell acid secretion while prostaglandins and somatostatin decrease its acid secretion. In a diseased state, gastrin binds with higher affinity than do prostaglandins or somatostatin. Selecting agonists that bind with higher affinity to H+/K+-ATPase than does gastrin, therefore, inhibits the deleterious health effects associated with gastrin binding. These nonnative agonists consequently inhibit native agonist binding. In this regard, the lower IC50 values indicate a lower concentration of an inhibitor necessary to decrease gastrin binding. Isovitexin and vitexin were notable in that they had lower IC50 values than orientin and isoorientin [Table 1]. The comparatively lower IC50 values for vitexin and isovitexin therefore suggest their therapeutic potential. Implicitly, RMSD values coupled to IC50 values therefore reinforce consideration of the therapeutic potential of vitexin and isovitexin.
CONCLUSION
Overall, the flavonoid-rich fraction of D. rotundifolia showed inhibitory effects on H+/K+-ATPase enzyme in vitro and also possesses excellent in vitro hydroxyl, SO, nitric oxide, and DPPH radical-scavenging activities and profound Fe2+-ascorbate lipid peroxidation inhibitory activity. In docking studies, it was observed that with regard to the current therapeutic utilization of omeprazole, vitexin and isovitexin known to be present in D. rotundifolia extract showed promise as a potential therapeutic agent for hyperacidity.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public. Reagents and chemicals were provided for by the Department of Biochemistry.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bamford M. H+/K+ ATPase inhibitors in the treatment of acid-related disorders. Prog Med Chem. 2009;47:75–162. doi: 10.1016/S0079-6468(08)00203-8. [DOI] [PubMed] [Google Scholar]
- 2.Waldum HL, Gustafsson B, Fossmark R, Qvigstad G. Antiulcer drugs and gastric cancer. Dig Dis Sci. 2005;50(Suppl 1):S39–44. doi: 10.1007/s10620-005-2805-4. [DOI] [PubMed] [Google Scholar]
- 3.Goel RK, Sairam K. Anti-ulcer drugs from indigenous sources with emphasis on Musa sapientum, tamrahbasma, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol. 2002;34:100–10. [Google Scholar]
- 4.Odugbemi T, editor. A Textbook of Medicinal Plants from Nigeria. Tolu Odugbemi: University of Lagos Press; 2008. pp. 134–568. [Google Scholar]
- 5.Hamisy WC, Mwaseba D, Zilihona IE, Mwihomeke ST. Status and Domestication Potential of Medicinal Plants in the Uluguru Mountain Area. A Consultancy Reported to Uluguru Mountains Conservation Project, Morogoro, Tanzania. 2000.
- 6.Jiofack T, Fokunang C, Guedje N, Kemeuze V. Ethnobotany and phytomedicine of the upper Nyong valley forest in Cameroon. Afr J Pharm Pharmacol. 2009;3:144–50. [Google Scholar]
- 7.Noumi E. Treating fibromyoma with herbal medicines in South Cameroon. Indian J Tradit Knowl. 2010;9:736–41. [Google Scholar]
- 8.Watt JM, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. London, UK: Livingstone Ltd; 1962. pp. 137–744. [Google Scholar]
- 9.Mshana NR, Abbiw DK, Addae-Mensah I, Adjanouhoun E, Ahyi MR, Ekpere JA, et al. Traditional Medicine and Pharmacopoeia, Contribution to the Revision of Ethnobotanical and Floristic Studies in Ghana. Accra: OAU STRC Technical Repearch; 2000. p. 67. [Google Scholar]
- 10.Darko IN. Ghanaian Indigenous Health Practices: The Use of Herbs (Doctoral Dissertation, University of Toronto. 2009:55. [Google Scholar]
- 11.Rath G, Touré A, Nianga M, Wolfender JL, Hostettmann K. Characterization of C-glycosylflavones from Dissotis rotundifolia by liquid chromatography - UV diode array detection - Tandem mass spectrometry. Chromatographia. 1995;41:332–42. [Google Scholar]
- 12.Soyinka JO, Oguntade TO, Onawunmi GO, Idowu TO, Ogundaini AO. Antioxidant and antimicrobial constituents of Dissotis erecta and Dissotis rotundifolia. Niger J Pharm Res. 2008;7:76–82. [Google Scholar]
- 13.Mann A, Egwim EC, Banji B, Abdukadir NU, Gbate M, Ekanem JT. Efficacy of Dissotis rotundifolia on Trypanosoma brucei brucei infection in rats. Afr J Biochem Res. 2009;3:5–8. [Google Scholar]
- 14.Abere TA, Onwukaeme DN, Eboka CJ. Pharmacognostic evaluation of the leaves of Dissotis rotundifolia Triana (Melastomataceae) Afr J Biotechnol. 2009;8:113. [Google Scholar]
- 15.Makanjuola VO, Tams GE, Ipinniwa DA. The effect of methanolic extract of Dissotis rotundifolia on cadmium induced testicular damage in Whistar rats. IOSR J Pham. 2014;4:56–65. [Google Scholar]
- 16.Offor CE. Determination of Vitamin composition of Dissotis rotundifolia leaves. Int J Curr Microbiol Appl Sci. 2015;4:211. [Google Scholar]
- 17.Aja PM, Alum EU, Ezeani NN, Ibiam UA, Egwu C. Comparative phytochemical evaluation of Dissotis rotundifolia root and leaf. Glob Vet. 2015;14:418–24. [Google Scholar]
- 18.Ansah C, Adinortey MB, Asiedu-Larbi J, Aboagye B, Asante DB, Nyarko AK. In vivo assessment of the toxic potential of Dissotis rotundifolia whole plant extract in Sprague-Dawley rats. Asian Pac J Trop Biomed. 2016;6:574–9. [Google Scholar]
- 19.Reyes-Chilpa R, Baggio CH, Alavez-Solano D, Estrada-Muñiz E, Kauffman FC, Sanchez RI, et al. Inhibition of gastric H+, K+-ATPase activity by flavonoids, coumarins and xanthones isolated from Mexican medicinal plants. J Ethnopharmacol. 2006;105:167–72. doi: 10.1016/j.jep.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Shyla B, Mahadevaiah, Nagendrappa G. A simple spectrophotometric method for the determination of phosphate in soil, detergents, water, bone and food samples through the formation of phosphomolybdate complex followed by its reduction with thiourea. Spectrochim Acta A Mol Biomol Spectrosc. 2011;78:497–502. doi: 10.1016/j.saa.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Klein SM, Cohen G, Cederbaum AI. Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry. 1981;20:6006–12. doi: 10.1021/bi00524a013. [DOI] [PubMed] [Google Scholar]
- 22.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem Pharmacol. 1988;37:837–41. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 23.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 25.Dasgupta N, De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88:219–24. [Google Scholar]
- 26.Bolton EE, Wang Y, Thiessen PA, Bryant SH. PubChem: Integrated platform of small molecules and biological activities. Annu Rep comput Chem. 2008;4:217–41. [Google Scholar]
- 27.Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank. [Last accessed on 2016 Jul 24]. Available from: http://www.drugbank.ca/drugs/DB00338 .
- 28.Abe K, Tani K, Fujiyoshi Y. Conformational rearrangement of gastric H(+), K(+)-ATPase induced by an acid suppressant. Nat Commun. 2011;2:155. doi: 10.1038/ncomms1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman HM, Battistuz T, Bhat TN, Bluhm WF, Bourne PE, Burkhardt K, et al. The protein data bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- 30.O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR, et al. Open babel: An open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF chimera – A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 32.Trott O, Olson AJ. AutoDock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and autoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis SN, Brannan L, Guri AJ, Lu P, Hontecillas R, Bassaganya-Riera J, et al. Dietary α-eleostearic acid ameliorates experimental inflammatory bowel disease in mice by activating peroxisome proliferator-activated receptor-γ. PLoS One. 2011;6:e24031. doi: 10.1371/journal.pone.0024031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baba H, Onanuga A. Preliminary phytochemical screening and antimicrobial evaluation of three medicinal plants used in nigeria. Afr J Tradit Complement Altern Med. 2011;8:387–90. doi: 10.4314/ajtcam.v8i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quílez A, Berenguer B, Gilardoni G, Souccar C, de Mendonça S, Oliveira LF, et al. Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J Ethnopharmacol. 2010;128:583–9. doi: 10.1016/j.jep.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 37.Demir S, Yilmaz M, Köseoǧlu M, Akalin N, Aslan D, Aydin A, et al. Role of free radicals in peptic ulcer and gastritis. Turk J Gastroenterol. 2003;14:39–43. [PubMed] [Google Scholar]
- 38.Tandon R, Khanna HD, Dorababu M, Goel RK. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J Physiol Pharmacol. 2004;48:115–8. [PubMed] [Google Scholar]
- 39.Hung CR, Wang PS. Gastric oxidative stress and hemorrhagic ulcer in Salmonella typhimurium-infected rats. Eur J Pharmacol. 2004;491:61–8. doi: 10.1016/j.ejphar.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Ajitha M, Rajnarayana K. Role of oxygen free radicals in human disease. Indian Drugs. 2001;38:545–54. [Google Scholar]
- 41.Naskar S, Islam A, Mazumder UK, Saha P, Haldar PK, Gupta M. In vitro and in vivo antioxidant potential of hydromethanolic extract of Phoenix dactylifera fruits. J Sci Res. 2009;2:144–57. [Google Scholar]
- 42.Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I, et al. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 2011;48:107–11. doi: 10.3164/jcbn.10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor BS, Kim YM, Wang Q, Shapiro RA, Billiar TR, Geller DA, et al. Nitric oxide down-regulates hepatocyte-inducible nitric oxide synthase gene expression. Arch Surg. 1997;132:1177–83. doi: 10.1001/archsurg.1997.01430350027005. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Farid IB, Sheded MG, Mohamed EA. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J Biol Sci. 2014;21:400–8. doi: 10.1016/j.sjbs.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]


