Abstract
Background:
Superparamagnetic iron oxide nanoparticles (SPION) have been largely considered for numerous applications in biomedicine such as magnetic resonance imaging, hyperthermia, cell tracking, anticancer treatment, and targeted delivery of drugs or genes. However, they may have side effects such body weight loss. Quercetin (QT), a strong antioxidant and free radical scavenger and a natural flavonoid, has a wide range of biological and therapeutic effects. In this study, the effect of QT on prevention of weight loss due to the using of SPION has been investigated.
Materials and Methods:
SPION and QT-SPION were administered orally at 50 and 100 mg/kg for 7 days. Then, the body weight was measured at the beginning and the end of the study.
Results:
Rats fed with 50 and 100 mg/kg SPION showed a significant weight loss, whereas those that fed with 50 mg/kg QT-SPION did not. A weight loss was observed in rats treated with 100 mg/kg of QT-SPION.
Conclusions:
The results of this study showed that quercetin could prevent weight loss due to the SPION.
Keywords: Nanoparticles, quercetin, superparamagnetic iron oxide, weight loss
Introduction
The quercetin (3,3′,4′,5,7-pentahydroxyflavone) as a flavonoid is biologically active compound that is found in eatable fruits, vegetables, and medicinal plants such as onions, cabbage, and apples in quantities of 15–30 mg kg−1 of fresh produce.[1,2,3,4] Quercetin (QT) has antitumoral,[5,6] antiallergic,[7] antioxidant effects,[8] vasorelaxation,[9] anti-ischemic,[10,11] antiviral,[12] and anti-inflammatory activity.[13,14] Furthermore, it has some other beneficial effects in metabolic disorders[15] and protection of neurons.[16,17,18,19,20]
Furthermore, QT is considered as an ergogenic supplement.[15,21,22] QT has the ability to increase aerobic exercise performance and oxidative metabolism.[22] Furthermore, adiposity reduction of QT has been studied.[23] Different studies show various effects of quercetin on weight gain. A study shows a reduction in weight gain in fat rats fed QT,[24,25] whereas another study results showed no effect of QT on weight gain in thin rats.[26,27,28]
Abovementioned beneficial effects of QT make it a potential therapeutic alternative for various disorders. However, because of the low water solubility, short biological half-life, low oral bioavailability, and poor body bioavailability of QT, it is necessary to use efficient drug delivery systems to improve QT effectiveness.[29,30,31] Therefore, a delivery system such as superparamagnetic iron oxide nanoparticles (SPION) could overcome these limitations. SPION have generated a lot of interests in biomedical applications such as magnetic resonance imaging, magnetic separation, targeted drug and gene delivery, cell tracking, tissue engineering, bioseparation, and magnetic hyperthermia.[32] However, biocompatibility and biosafety of SPION are needed to be considered. As long as we search literature, the effect of SPION on weight loss is not broadly investigated. Consequently, the aim of the current study was to evaluate the effect of SPION and QT conjugated with SPION on weight gain in rats.
Materials and Methods
All the reagents were analytical grade and used without further purification and in all experiments, deionized water was used. The chemicals used in this work were purchased from Fluka, Merck and Sigma–Aldrich chemical companies.
Synthesis of quercetin conjugated with superparamagnetic iron oxide nanoparticles
QT conjugated Fe3O4 nanoparticles (NPs) were prepared based on the existing literature with some modifications.[33] First, dextran-coated Fe3O4 NPs were synthesized through a chemical coprecipitation method. Briefly, a mixture including of FeCl3 anhydrous, FeCl2, and dextran dissolved in deionized (DI) water was put into a three-neck flask equipped with a mechanical stirrer. After the mixture was stirred and mixed completely, ammonia solution was dropped into the mixture with vigorous stirring under argon protection, until the pH of the solution reached 9. This solution was kept at 90°C for 2 h with constant stirring, and then the resultant precipitate was collected with the help of a strong external magnet. The supernatant was washed several times with DI water and ethanol and dried in oven at 70°C overnight.
QT conjugated magnetite NPs (QT-SPION) were prepared by addition of QT to dextran-coated Fe3O4 NPs. The FT-IR spectroscopy and X-ray diffraction (XRD) were recorded. The morphological features were obtained on a Hitachi S–4700 field emission-scanning electron microscope, equipped with an energy dispersive X-ray analysis detector.
Lered for various time intervals.
Animals and diets
Male Wistar rats, weighing 180–200 g, were purchased from Royan Institute (Isfahan, Iran). The animal experiments were approved by the Animal Ethics of Shiraz University and University of Isfahan. The animals were randomly divided into groups of 6 rats and were housed 3 per cage under standard conditions at a temperature of 25°C ± 2°C and relative humidity of 50%–60% and a 12-h light/dark cycle (lights on at 07:00 a.m.) with free access to tap water and diet for 1 week before the experiment. Any problem was detected during the experimental procedures.
Bodyweight measurements
Healthy male Wistar rats (n = 5/group) were given of deionized water (DI) (sham group) or treated with 50 and 100 mg/kg Fe3O4 NPs and 50 and 100 mg/kg QT-Fe3O4. A group of rats did not receive any drug or DI and served as control. Individual rat body weight was evaluated at the time of purchase, at the time of initiation of the experiment, and before sampling. Percent of the body weight changes were calculated by the following formula:
Percent of the body weight changes = % of day one body weight-day seven body weight/day one body weight
Statistical analysis
The results were reported as mean ± standard error mean for the six independent experiments. Student's t-test was used to estimate the difference between weight in day one and day seven (GraphPad Prism software version 5, San Diego, CA, USA). P < 0.05 was considered to be statistically significant.
Results
The Fourier transform-infrared spectroscopy spectrum of the synthesized QT-Fe3O4 (data not shown) showed broad absorption bands at 3386 cm−1, which were assigned to the stretching vibrations of hydroxyl groups and other characteristic bands that confirmed the successful conjugation of QT on the Fe3O4 NPs. The powder XRD pattern of the synthesized magnetic NPs was close to the pattern for crystalline magnetite Fe3O4. Field-emission scanning electron microscopy (SEM) (data not shown) results showed that the NPs are spherical and have diameters in the range of 30–50 nm. The presence of the iron and oxygen was also confirmed by the energy dispersive X-ray detector coupled to the SEM.
To investigate potential functional toxicity, the body weight of male Wistar rats was measured at the initial of experiment and before scarifying the animals. None of the rats was found dead during of the experiment. Analysis of body weight change indicated a significant reduction in body weight of rats treated with 50 mg/kg or 100 mg/kg of Fe3O4 NPs [Figure 1c and d]. The body weight loss was higher in rats treated with 100 mg/kg of Fe3O4 NPs in comparison to the rats received 50 mg/kg of Fe3O4 NPs [Figure 1c and d]. About 100 mg/kg/day QT-Fe3O4 NPs group lost weight significantly on days 7 when compared to the day one. The 50 mg/kg/day QT-Fe3O4 NPs group show an increased in their weights [Figure 1]. In 50 mg/kg/day QT-Fe3O4 NPs group, body weight neither decreased nor increased significantly.
Figure 1.
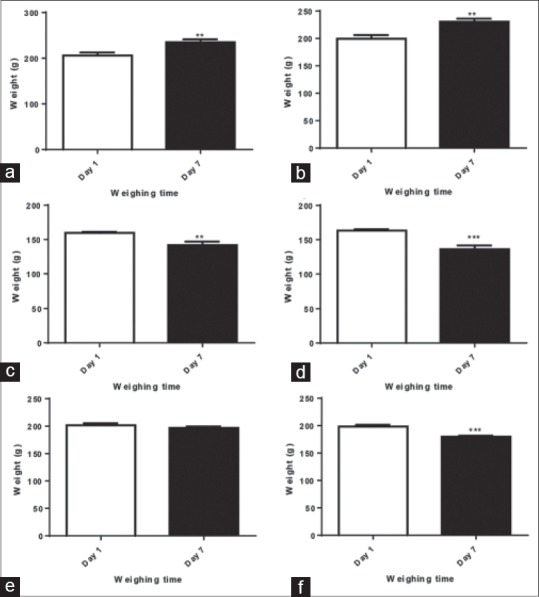
Bodyweight change of Wistar rats at day 1 and 7 of the experiment. (a) control group did not receive anything, (b) sham group received only deionized water, (c-f) rats in these groups treated with Fe3O4 at a dose of (c) 50, (d) 100 mg/kg, and quercetin conjugated Fe3O4 nanoparticles at a dose of (e) 50, (f) 100 mg/kg. All values reported are mean ± standard error mean (n = 6). (* for P < 0.05, ** for P < 0.01, and *** for P < 0.001). QT: Quercetin
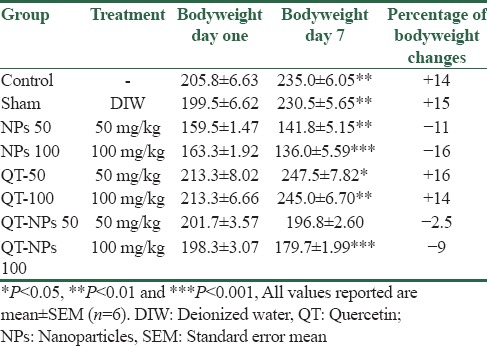
In the current study, the percentage of body weight changes were also assessed [Table 1]. The results showed a 16% weight gain and 11% weight loss. These results are in line with the abovementioned assessments.
Table 1.
Percent of body weight changes in rats treated with superparamagnetic iron oxide nanoparticles, quercetin alone and in conjugated with superparamagnetic iron oxide nanoparticles
Discussion
The results of physicochemical characterization of QT-Fe3O4 NPs are in agreement with the previously reported study.[33] The results strongly evidence that the QT was successfully conjugated to the magnetite NPs coated with dextran. An in vivo study showed that Fe3O4 NPs are relatively safe as they do not accumulate in the vital organs and are rapidly removed from the body.[34]
In the current study, rats showed body weight loss when treated with Fe3O4-NPs. All Fe3O4-NPs groups (50 and 100 mg/kg/day) showed weight loss. In a study (Hong 2008), to evaluate toxicity of Fe3O4 ferrofluid on the rats, 1.5 mL of a solution containing 4 mg Fe/mL were injected intravenous for 1 month. No rats died and no changes in body weight have been observed. The results of this study revealed that the ferrofluid has no acute fatal toxicity and can be used as a biocompatible material suitable for biomedical applications. As it can be seen the amounts of Fe NPs used is one-tenth of our study. On the other hand, QT at least at a dose of 50 mg/kg could overcome weight loss.
In another study,[35] 1 and 5 mg/kg iron oxide NP were injected to rats for 14 days. A significant weight loss was observed in rats treated with 5 mg/kg of iron oxide NPs. An explanation mechanism by iron oxide NP could be its oxidative stress.[36] Reactive oxygen species (ROS) are generated by iron oxide NPs that leads to production of the hydroxyl radicals and super hydroxide hydrogen. Consequently, this phenomenon could induce cell death (apoptosis).[37] On the other hand, iron oxide NPs could decrease superoxide dismutase and glutathione enzymes.[38] These enzymes have important roles in reducing of the ROS. QT has the antioxidant capacity, and thus, it could prevent the oxidative stress of the NPs.[39] Furthermore, it could clean up cell from ROS.[40] This capacity of QT is dose dependence.[41] This could explain why in our study rats treated with 100 mg/kg/day of QT did not show weight loss.
Conclusions
Quercetin could inhibit body weight loss due to the application of the superparamagnetic iron oxide. However, the future studies should measure food consumption and continue measurements for a longer duration. In addition, the dissolution studies of Fe3O4 NPs could identify if the Fe3O4 ion or Fe3O4-NPs contribute to the body weight responses.
Financial support and sponsorship
This work was supported by the grant from Shiraz University and help of the University of Isfahan. The authors thank Dr. Zari Pahlevanneshan of University of Isfahan for her assistance with nanoparticle preparation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kumari A, Yadav SK, Pakade YB, Singh B, Yadav SC. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf B Biointerfaces. 2010;80:184–92. doi: 10.1016/j.colsurfb.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC, Cham TM, et al. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm. 2008;346:160–8. doi: 10.1016/j.ijpharm.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Bagad M, Khan ZA. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int J Nanomedicine. 2015;10:3921–35. doi: 10.2147/IJN.S80706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty S, Stalin S, Das N, Choudhury ST, Ghosh S, Swarnakar S, et al. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials. 2012;33:2991–3001. doi: 10.1016/j.biomaterials.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Pilorget A, Berthet V, Luis J, Moghrabi A, Annabi B, Béliveau R, et al. Medulloblastoma cell invasion is inhibited by green tea (-) epigallocatechin-3-gallate. J Cell Biochem. 2003;90:745–55. doi: 10.1002/jcb.10667. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto Y, Yasuhara T, Sugimoto A, Inoue A, Hide I, Akiyama M, et al. Anti-allergic substances contained in the pollen of Cryptomeria japonica possess diverse effects on the degranulation of RBL-2H3 cells. J Pharmacol Sci. 2003;92:291–5. doi: 10.1254/jphs.92.291. [DOI] [PubMed] [Google Scholar]
- 8.Ramos FA, Takaishi Y, Shirotori M, Kawaguchi Y, Tsuchiya K, Shibata H, et al. Antibacterial and antioxidant activities of quercetin oxidation products from yellow onion (Allium cepa) skin. J Agric Food Chem. 2006;54:3551–7. doi: 10.1021/jf060251c. [DOI] [PubMed] [Google Scholar]
- 9.Novakovic A, Gojkovic-Bukarica L, Peric M, Nezic D, Djukanovic B, Markovic-Lipkovski J, et al. The mechanism of endothelium-independent relaxation induced by the wine polyphenol resveratrol in human internal mammary artery. J Pharmacol Sci. 2006;101:85–90. doi: 10.1254/jphs.fp0050863. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res. 2004;77:892–900. doi: 10.1002/jnr.20193. [DOI] [PubMed] [Google Scholar]
- 11.Duarte J, Pérez-Vizcaíno F, Zarzuelo A, Jiménez J, Tamargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- 12.Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96:229–45. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart LK, Soileau JL, Ribnicky D, Wang ZQ, Raskin I, Poulev A, et al. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 2008;57:S39–46. doi: 10.1016/j.metabol.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–7. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 16.Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf. 2009;8:397–409. doi: 10.1517/14740330903026944. [DOI] [PubMed] [Google Scholar]
- 17.Dajas F. Life or death: Neuroprotective and anticancer effects of quercetin. J Ethnopharmacol. 2012;143:383–96. doi: 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Dajas F, Andrés AC, Florencia A, Carolina E, Felicia RM. Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem. 2013;13:30–5. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- 19.Nichols M, Zhang J, Polster BM, Elustondo PA, Thirumaran A, Pavlov EV, et al. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience. 2015;308:75–94. doi: 10.1016/j.neuroscience.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Lee YJ, Bernstock JD, Nagaraja N, Ko B, Hallenbeck JM. Global SUMOylation facilitates the multimodal neuroprotection afforded by quercetin against the deleterious effects of oxygen/glucose deprivation and the restoration of oxygen/glucose. J Neurochem. 2016;138:101–16. doi: 10.1111/jnc.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep. 2009;8:206–13. doi: 10.1249/JSR.0b013e3181ae8959. [DOI] [PubMed] [Google Scholar]
- 22.Kressler J, Millard-Stafford M, Warren GL. Quercetin and endurance exercise capacity: A systematic review and meta-analysis. Med Sci Sports Exerc. 2011;43:2396–404. doi: 10.1249/MSS.0b013e31822495a7. [DOI] [PubMed] [Google Scholar]
- 23.Aguirre L, Arias N, Macarulla MT, Gracia A, Portillo MP. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011;4:189–98. [Google Scholar]
- 24.Jung CH, Cho I, Ahn J, Jeon TI, Ha TY. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. 2013;27:139–43. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- 25.Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring) 2008;16:2081–7. doi: 10.1038/oby.2008.315. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AA, Tan X, Reis JC, Badr MZ, Papasian CJ, Morrison DC, et al. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by δ-tocotrienol and quercetin. Lipids Health Dis. 2011;10:239. doi: 10.1186/1476-511X-10-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azuma K, Ippoushi K, Terao J. Evaluation of tolerable levels of dietary quercetin for exerting its antioxidative effect in high cholesterol-fed rats. Food Chem Toxicol. 2010;48:1117–22. doi: 10.1016/j.fct.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Casuso RA, Martínez-López EJ, Hita-Contreras F, Camiletti-Moirón D, Martínez-Amat A. Quercetin effects on weight gain and caloric intake in exercised rats. Biol Sport. 2014;31:63–7. doi: 10.5604/20831862.1086734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rivera F, Urbanavicius J, Gervaz E, Morquio A, Dajas F. Some aspects of the in vivo neuroprotective capacity of flavonoids: Bioavailability and structure-activity relationship. Neurotox Res. 2004;6:543–53. doi: 10.1007/BF03033450. [DOI] [PubMed] [Google Scholar]
- 30.Blasina F, Vaamonde L, Silvera F, Tedesco AC, Dajas F. Intravenous nanosomes of quercetin improve brain function and hemodynamic instability after severe hypoxia in newborn piglets. Neurochem Int. 2015;89:149–56. doi: 10.1016/j.neuint.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Galho AR, Cordeiro MF, Ribeiro SA, Marques MS, Antunes MF, Luz DC, et al. Protective role of free and quercetin-loaded nanoemulsion against damage induced by intracerebral haemorrhage in rats. Nanotechnology. 2016;27:175101. doi: 10.1088/0957-4484/27/17/175101. [DOI] [PubMed] [Google Scholar]
- 32.Barreto A, Santiago V, Mazzetto S, Denardin JC, Lavín R, Mele G, et al. Magnetic nanoparticles for a new drug delivery system to control quercetin releasing for cancer chemotherapy. J Nanopart Res. 2011;13:6545–53. [Google Scholar]
- 33.Kumar SR, Priyatharshni S, Babu VN, Mangalaraj D, Viswanathan C, Kannan S, et al. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J Colloid Interface Sci. 2014;436:234–42. doi: 10.1016/j.jcis.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 34.Boyer C, Whittaker MR, Bulmus V, Liu J, Davis TP. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010;2:23–30. [Google Scholar]
- 35.Szalay B, Tátrai E, Nyírő G, Vezér T, Dura G. Potential toxic effects of iron oxide nanoparticles in in vivo and in vitro experiments. J Appl Toxicol. 2012;32:446–53. doi: 10.1002/jat.1779. [DOI] [PubMed] [Google Scholar]
- 36.Zhu MT, Wang Y, Feng WY, Wang B, Wang M, Ouyang H, et al. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol. 2010;10:8584–90. doi: 10.1166/jnn.2010.2488. [DOI] [PubMed] [Google Scholar]
- 37.Khan MI, Mohammad A, Patil G, Naqvi SA, Chauhan LK, Ahmad I, et al. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–88. doi: 10.1016/j.biomaterials.2011.10.080. [DOI] [PubMed] [Google Scholar]
- 38.Alarifi S, Ali D, Alkahtani S, Alhader MS. Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol Trace Elem Res. 2014;159:416–24. doi: 10.1007/s12011-014-9972-0. [DOI] [PubMed] [Google Scholar]
- 39.Pool H, Quintanar D, de Dios Figueroa J, Mano CM, Bechara JE, Godínez LA, et al. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J nanomater 2012. 2012:86. [Google Scholar]
- 40.Milton Prabu S, Shagirtha K, Renugadevi J. Quercetin in combination with vitamins (C and E) improves oxidative stress and renal injury in cadmium intoxicated rats. Eur Rev Med Pharmacol Sci. 2010;14:903–14. [PubMed] [Google Scholar]
- 41.Lin HC, Cheng TH, Chen YC, Juan SH. Mechanism of heme oxygenase-1 gene induction by quercetin in rat aortic smooth muscle cells. Pharmacology. 2004;71:107–12. doi: 10.1159/000076947. [DOI] [PubMed] [Google Scholar]


