Abstract
Background:
Although the role of self-rated health (SRH) on all-cause mortality is known, we still do not know whether SRH predicts death due to specific causes (e.g., kidney disease). The current study aimed to compare Blacks and Whites on the association between SRH and mortality due to kidney diseases. A nationally representative sample of adults in the United States was used to provide generalizable results to the United States population.
Materials and Methods:
The Americans’ Changing Lives study is a nationally representative cohort, conducted from 1986–2011. The study followed 3361 Blacks (n = 1156) and Whites (n = 2205) for up to 25 years. The outcome was time to death due to kidney diseases, derived from death certificates and the National Death Index. Cox proportional hazards models were used to test whether race and baseline SRH interact on mortality due to kidney diseases.
Results:
In the pooled sample, poor SRH (odds ratio [OR] = 2.29, 95% confidence interval [CI] = 1.24–4.24) was associated with an increased risk of death due to kidney diseases over the follow-up period. Baseline SRH also showed a significant interaction with race on the outcome (OR = 0.49, 95% CI = 0.25–0.96), suggesting a stronger effect of SRH on deaths due to kidney diseases for Whites compared to Blacks. In race-specific models, poor SRH at baseline increased risk of death due to kidney diseases among Whites (OR = 2.23, 95% CI = 1.14–4.34) but not Blacks (OR = 1.14, 95% CI = 0.54–2.41).
Conclusions:
Blacks and Whites differ regarding the predictive role of baseline SRH on death due to kidney diseases over time. Factors such as SRH better predict risk of mortality for Whites than for Blacks.
Keywords: African Americans, death, ethnic groups, kidney diseases, population groups, self-rated health
Introduction
Compared to White Americans, Black Americans have lower socioeconomic status,[1] worse self-rated health (SRH),[2] and more chronic medical conditions (CMCs)[3,4] such as obesity,[5] hypertension,[6] and diabetes.[7] As part of the low social class,[8] and given that these CMCs[9] increase the risk of chronic kidney disease and its associated morbidity and mortality, Blacks are at a higher risk of kidney disease mortality in the United States.[5,10]
SRH, a single-item measure widely used in community surveys since the 1950s,[11,12] predicts subsequent risk of mortality.[13,14,15,16,17,18] Such measures are brief and efficient tools for estimating the overall health of populations.[19,20] Due to its feasibility[21] and low-cost,[22] SRH is widely applied in national surveys in many countries across the world.[22,23] In the United States, SRH has been recommended by the Institute of Medicine to monitor population health.[13,24] Not only does SRH inform about the risk of subsequent mortality,[13,14,15,16,17,18] it also predicts outcomes associated with kidney diseases.[25]
Although a large body of evidence has shown that baseline SRH strongly predicts subsequent mortality across various populations,[13,14,15,16,17,18] this predictive role may depend on race and ethnicity.[26,27,28,29,30,31,32,33,34,35,36,37] We still do not know if race also modifies the predictive role of SRH on specific causes of mortality (e.g., mortality due to kidney disease).
Despite the growing body of knowledge on race differences in the role of psychosocial factors on the health of populations,[10,38,39,40,41,42,43,44,45,46,47,48] there is still a need for studying race differences in the effect of baseline SRH on deaths due to kidney diseases in the United States. First and foremost, although individuals with poor SRH are at a higher risk of mortality compared to those with fair, good, or excellent SRH,[14,15,16,17,21,22,49] most of the existing knowledge on the SRH – mortality link is driven from all-cause rather than cause-specific mortality.[50,51] Second, there are inconsistent findings on the topic. While some studies have suggested that SRH predicts mortality regardless of race and ethnicity,[17] there is some evidence suggesting that race and ethnicity may mitigate the association.[12,29,31,52,53]
To extend the current knowledge on the associations between race and kidney disease mortality,[10] and to advance the existing knowledge on Black-White differences in health,[38] as well as racial and ethnic differences in correlates of SRH[29,48] in addition to mortality due to all-causes[45,46,47] or death due to kidney disease,[10,54] this study had three aims: (1) to test if Blacks are at a higher risk of mortality due to kidney disease compared to Whites, (2) to investigate if SRH predicts kidney disease mortality, and (3) to compare Blacks and Whites for such association. We used the Americans’ Changing Lives (ACL) data with 25 years of follow-up on a large nationally representative sample of adults in the United States; our study makes a unique contribution to the literature because capturing variance in the development of chronic renal disease requires long-term follow-up periods.[38,54,55,56] In addition, due to the fact that ACL has enrolled a nationally representative sample, our results are generalizable to the United States population.
Materials and Methods
Design and setting
The ACL is a nationally representative cohort conducted from 1986 to 2011 in the United States; detailed information on the study design is available elsewhere.[38,57,58]
Sampling and participants
The ACL enrolled a stratified multistage probability sample of adults aged 25 or above who lived in the continental United States in 1986. The study included 3617 noninstitutionalized respondents (representing 70% of sampled households and 68% of sample individuals at baseline) with an oversampling of those age 60 and older and African Americans. Wave 1 included 70% of sampled households.
Measures
Data were collected on race, demographic characteristics, socioeconomic factors, and CMCs at the baseline face-to-face interview in 1986. Mortality due to kidney diseases was collected from 1986 to 2011. Baseline SRH was the predictor of interest; mortality due to kidney diseases was the main outcome; baseline demographic characteristics, socioeconomic factors, and CMCs were covariates; and race was the focal moderator.
Race
In this study, race was defined as non-Hispanic Black or non-Hispanic White. Participant's race was defined based on self-reported race and ethnicity, collected at baseline in 1986 with several survey items. Respondents were asked an open-ended response to the question, “In addition to being American, what do you think of as your ethnic background or origins?” Respondents were then asked a multiple-choice question, “Are you White, Black, American Indian, Asian, or another race?” and allowed to answer with multiple categories. Those who responded with more than one non-White group were asked to identify which “best described” their race. The survey also assessed the state or foreign country in which the respondent, respondent's mother, and respondent's father were born, and the respondent's father's last name. Finally, participants were asked, “Are you of Spanish or Hispanic descent, that is, Mexican, Mexican American, Chicano, Puerto Rican, Cuban, or Other Spanish?” Responses from above questions were used to construct race categories of “Non-Hispanic White,” “Non-Hispanic Black,” “Non-Hispanic Native American,” “Non-Hispanic Asian,” and “Hispanic.” This analysis only included non-Hispanic White and non-Hispanic Black respondents. All Hispanic or multiethnic individuals were dropped from this analysis.
Demographic factors
Demographic indicators included age (a continuous variable as a number of years since birth) and gender (a dichotomous variable with male as the referent category).
Socioeconomic characteristics
Socioeconomic status was measured with education (<12 years of education and 12 years or more) and household income (a continuous variable as total annual income in the household).
Self-rated health
Respondents were asked to classify their SRH as excellent, very good, good, fair, or poor. Past literature has defined SRH in two distinct ways; however, we operationalized our SRH as a dichotomous measure rather than a continuous score, as the categorical measure is more robust. We collapsed this five-category scale into two categories (fair/poor vs. excellent/very good/good), a cut point that is common in the literature. This measure has shown high test–retest reliability and validity when considering its predictive power for mortality and other health outcomes.[13] Literature review shows that most studies on the SRH – mortality link have used the dichotomous SRH.[13,14,15,29,59,60]
Number of chronic medical conditions
The number of CMCs at baseline was measured using self-reported physician diagnosed CMCs.[38,39,40,41,42] All participants were asked whether a health-care provider had ever told them they had each of seven focal conditions including hypertension, diabetes, heart disease, chronic lung disease, stroke, cancer, and arthritis (any type). A sum score was calculated, potentially ranging from 0 to 7. A detailed description of the measurement of CMCs is provided elsewhere.[58] We controlled for CMCs because some (i.e., hypertension and diabetes) cause kidney disease and related death.
Mortality due to kidney diseases
The main outcome in this study was time to death due to kidney diseases since 1986 over 25 years. Information on mortality during the 25-year follow-up period was obtained via the National Death Index (NDI), death certificates, and also informants. In most cases, time and cause of death could be verified with death certificates. In a handful of cases, death could not be verified with death certificates; such cases were reviewed carefully. Actual death was certain in all cases. Only in a few cases, the date of death was ascertained from the informants or the NDI report, rather than the death certificate.[61,62] Cause of death was coded as unknown when the death certificate or NDI report was unavailable.
The International Classification of Disease (ICD)-9 and -10 codes[63,64] were used to determine the cause of mortality, whichever was current at the time the death was recorded. For ICD-9 codes, we used codes 650 (acute glomerulonephritis and nephrotic syndrome), 660 (chronic glomerulonephritis, nephritis, and nephropathy, not specified as acute or chronic, and renal sclerosis, unspecified), 670 (renal failure, disorders resulting from impaired renal function, and small kidney of unknown causes), 680 (infections of kidney), and 690 (hyperplasia of prostate). For ICD-10 codes, we used the categorization of 113 selected causes of death provided by the WHO, for which codes 97 (nephritis, nephrotic syndrome, and nephrosis), 98 (acute and rapidly progressive nephritic and nephrotic syndrome), 99 (chronic glomerulonephritis, nephritis, and nephropathy not specified as acute or chronic, and renal sclerosis unspecified), 100 (renal failure), 101 (other disorders of kidney), 102 (infections of kidney), 103 (hyperplasia of prostate), and 104 (inflammatory diseases of female pelvic organ) were used. Respondents who died due to other causes were censored at the time of death. Time of death was registered as a number of months from time of enrollment in the study to time of death, based on the month of death and the month of the baseline interview.
Statistical analysis
The ACL applied a complex sampling design (a multistage sample design involving clustering and stratification), which requires appropriate statistical techniques that account for the complex design. We applied weights based on strata, clusters, and nonresponse in all of our analyses. Subpopulation analyses for surveys were also applied.
Standard error estimation accounted for the sampling weights (due to stratification, clustering, and nonresponse) using Taylor series linearization. For multivariable analysis, Cox proportional hazards models were used to determine factors associated with time to death due to kidney diseases over the 25-year follow-up. Sample sizes reflect the unweighted sample distributions. Univariate, bivariate, and multivariable analyses were performed using Stata 13.0 (Stata Corporation, College Station, TX, USA).
Cox proportional hazards models require information on a dichotomous outcome (death due to kidney diseases) and the time when that outcome occurred (time to death due to kidney diseases). Kidney death was coded as zero if the respondent did not die or died from any other causes. Time to the kidney death event, or to censoring, was defined as the number of months from baseline to death, lost to follow-up, or the end of the year 2011. Model 1 was conducted in the pooled sample and included race, SRH, age, gender, education, income, CMC, and race by SRH interaction. Model 2 and Model 3 were conducted among Whites and Blacks, respectively. Model 2 and Model 3 included age, gender, education, income, CMC, and SRH. Hazard ratios with 95% confidence intervals (CIs) are reported. P < 0.05 was considered statistically significant. Missing data were <5%. To evaluate the proportional hazard assumptions for our Cox proportional hazards models, we used estat phtest command in the Stata for Schoenfeld residual analysis. Our models passed the tests for proportional hazards.
Results
Table 1 shows descriptive statistics for the overall sample, and then separately for Whites and Blacks. Blacks were younger and had a higher number of CMCs at baseline compared to Whites. Blacks had a lower frequency of high school graduation (all these differences by race were significant at P < 0.05) and worse SRH compared to Whites [Table 1].
Table 1.
Descriptive statistics in the pooled sample and also based on race
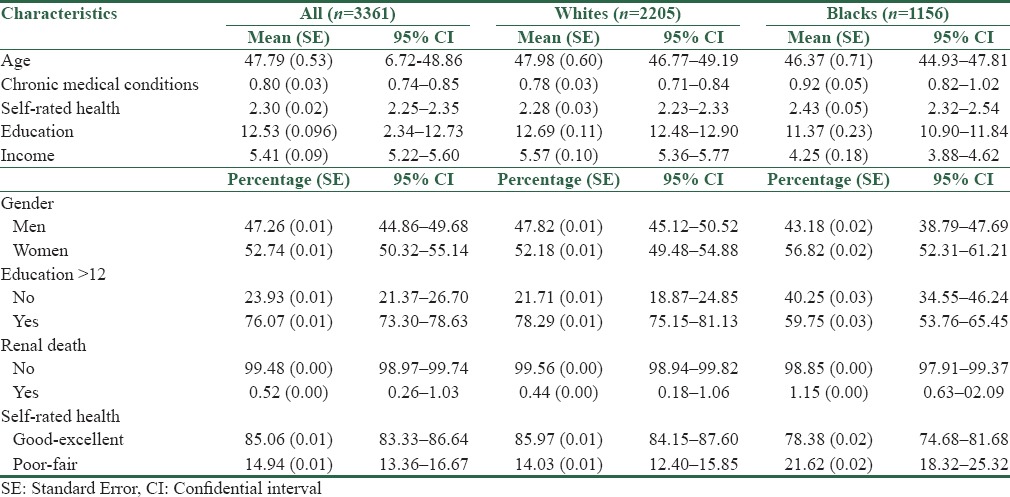
From the total 3361 Black and White individuals under follow-up, death due to kidney disease occurred in 35 individuals (1.04%). Thirteen cases of death due to kidney disease happened in Whites, whereas 22 cases of death due to kidney disease happened in Blacks. Death due to kidney disease was more common among Blacks than Whites (1.90% vs. 0.50%, P < 0.05). In an age- and gender-adjusted model, Black race was associated with 3.017 (95% CI = 1.08–8.39) higher odds of kidney disease mortality (P = 0.035).
According to Model 1 in the pooled sample, poor SRH (odds ratio [OR] = 2.29, 95% CI = 1.24–4.24) was associated with an increased risk of death due to kidney diseases over the follow-up period. Baseline SRH also showed a significant interaction with race on the outcome (OR = 0.49, 95% CI = 0.25–0.96), suggesting a stronger effect of SRH on deaths due to kidney diseases for Whites compared to Blacks [Table 2].
Table 2.
Results of Cox proportional hazards models predicting death due to renal disease in the pooled sample and also based on race
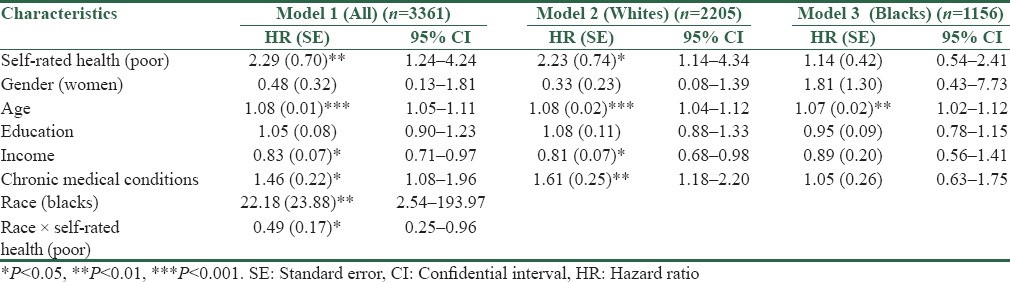
In race-specific models, SRH at baseline increased risk of death due to kidney diseases among Whites (OR = 2.23, 95% CI = 1.14–4.34) (Model 2) but not for Blacks (OR = 1.14, 95% CI = 0.54–2.41) (Model 3). Thus, our findings on race-specific models confirmed the stronger effect of SRH on deaths due to kidney diseases among Whites compared to Blacks [Table 2].
Discussion
According to our findings, compared to Whites, Blacks had worse SRH at baseline. Blacks also died sooner from kidney disease compared to Whites. We also found an interaction between race and baseline SRH on the outcome, suggesting a stronger predictive role of poor SRH on deaths due to kidney diseases for Whites compared to Blacks. Our race-specific models also confirmed our findings, as baseline SRH predicted kidney disease mortality among Whites but not Blacks. Findings are unique as we used a nationally representative sample of United States adults.
Building on the literature that suggests race may moderate the effect of SRH and other risk factors on mortality,[17,45,46,47,50] we conducted a study to investigate multiplicative effects between race, SRH, and kidney disease mortality over 25 years. We found race differences in the role of SRH on kidney disease mortality. It is yet unclear whether SRH differently predicts mortality among ethnically diverse populations.[29] The evidence is still mixed regarding group differences in the predictive role of SRH on all-cause and cause-specific mortality. While some studies suggest that race and ethnicity modify the effect of SRH on all-cause mortality,[31,52,53] other studies have failed to show such group variation on the predictive role of SRH on mortality.[17]
Our findings that SRH predicts kidney disease death among Whites is in line with previous research on the role of SRH on mortality.[13,14,15,22,49] This is also in line with the campus of researchers who believe SRH predicts risk of mortality across all racial/ethnic groups,[17] irrespective of population, geographic region, and follow-up duration.[13,14,15,17,21,22]
In the United States, race is a proxy of social class and socioeconomic status. As a result, most of the racial differences in health are due to social rather than biological factors.[65] There are studies documenting group differences in correlates of SRH including the SRH-mortality link.[17,50] A study showed that the predictive power of SRH for mortality was strongest for the lowest socioeconomic group.[30] Among Latinos in the United States, the relationship between poor SRH and mortality risk may depend on acculturation.[50] Zajacova and Dowd used data from a nationally representative sample of 9235 adults interviewed in the 2005–2008 National Health and Nutrition Examination Survey and measured SRH on two occasions, about 1 month apart. The study compared the test–retest reliability of SRH among the United States adults based on sociodemographic characteristics and showed that about 40% of the SRH responses changed over a 1 month period, and the reliability of SRH differed considerably by sociodemographic characteristics. That study found that racial/ethnic minorities and adults with less education had lower reliability of SRH reports. The authors concluded that there is a substantial amount of error in reported SRH for Blacks and other disadvantaged sociodemographic groups.[52,53] These findings are extremely important and may explain our results on the weaker predictive validity of SRH for mortality among Blacks compared to Whites.
One potential explanation for the lower predictive role of SRH on mortality risk among Blacks may be a higher tolerance of Blacks compared to Whites in reporting their health as fair/poor, in the presence of a health problem. In this view, the link between medical problems and SRH is weaker for Blacks than Whites, and Blacks who have experienced more severe health problems and other stressors may better tolerate their conditions compared to Whites. The result would be a lower agreement between health problems and SRH among Blacks than Whites. This is in line with past research[38,66,67] and the Black-White health paradox.[26,27,38,66,67]
Due to cultural issues, cognitive styles, socioeconomics, and access to health care, social groups including ethnic groups may differ in how SRH reflects “true” health status.[48,50,51] It has been previously argued that poor SRH may reflect different health needs across diverse ethnic groups.[26,27,28] Based on the literature,[30,50,52] both meanings and determinants of SRH may be specific for each population. For instance, SRH less strongly predicts health care use among Blacks than Whites.[68] The growing literature on ethnic differences in correlates of SRH may help us better understand our findings.[26,27,28,69,70] Future research is needed on population differences in the relevance of SRH and past, current, and future health.[30,50,70,71]
Our findings help us better understand the Black-White health paradox, defined as disproportionately high subjective well-being despite higher worse objective health measures, as well as stressors, CMCs, and mortality among American Blacks compared to American Whites.[38] Based on this paradox, we expect Black-White differences in the cross-sectional and longitudinal associations between subjective and objective health measures.[3,4,38,39,40,41,42,44,66,72,73,74,75,76] For example, depressive symptoms at baseline had a stronger predictive effect on the incidence of CMCs[38] or subsequent mortality due to cardiovascular conditions[77] or kidney diseases[54] among Whites than Blacks. Baseline SRH also better predicts all-cause mortality for Whites than Blacks.[29] Our finding suggests that heterogeneity in the link between subjective and objective health measures is the rule rather than the exception and is in line with the Black-White health paradox.[38,66]
Interestingly, there are previous studies that have documented better survival and lower psychological distress among Blacks with chronic kidney disease when compared to Whites, despite their higher number of medical comorbidities,[78] a pattern which aligns with the Black-White health paradox.[38,52,67] However, very little is known about potential Black-White differences in the effect of baseline depressive symptoms on subsequent risk of kidney disease or mortality from kidney causes.[43]
A recent study showed a greater risk of death due to kidney causes among Whites with higher levels of depressive symptoms, compared to that for Whites with lower levels of depressive symptoms; however, such link could not be found for Blacks.[54] Thus, not only the effect of SRH, but also depression on development, progression of chronic kidney disease, and associated death depend on race, with consistently larger effects among Whites than Blacks. These findings should be interpreted in the context of a growing literature on the race differences in the mental health-physical health link, which has provided mixed results;[39,40,41,42,43] some even contradict each other and the Black-White Health Paradox.[39,42,43,73,79]
Limitations
This study has a few limitations. First and foremost, we did not have any measure of baseline kidney disease, and only used a total number of medical conditions at baseline, which was based on self-reported data and may be subjected to recall bias.[80] Future research should specifically measure baseline kidney disease or baseline kidney function. Future research should also validate self-reported CMCs using medical record data. In addition, while SRH is subject to change over time, its change was not modeled in this study. This approach was taken because we were interested in the racial differences in the long-term effect of SRH on our outcome. Despite these limitations, the results extend our current understanding of the Black-White differences in health, the Black-White health paradox[38] in general, and racial differences in the predictive role of SRH on mortality and kidney disease[81,82,83] in particular. Three major strengths of this study include (1) a nationally representative sample, (2) a large sample size, and (3) a long-term follow-up, which are all particularly important for rare outcomes such as death due to kidney diseases.
Conclusions
The effect of baseline SRH on deaths due to kidney disease over a 25-year period in the United States can be found in Whites but not Blacks. This finding is consistent with the literature on the Black-White health paradox, which suggests a weaker effect of objective and subjective risk factors on the psychosocial well-being of Blacks compared to Whites. The links between race, ethnicity, social class, subjective aspects of health such as SRH, and objective health outcomes such as mortality are complex rather than simple. Race and ethnicity not only have main effects on health but they also change the vulnerability and resilience of populations to the effect of the very same risk and protective factors on health.
Financial support and sponsorship
The Americans’ Changing Lives (ACL) study was supported by grant #AG018418 from the National Institute on Aging (DHHS/NIH), and per the NIH Public Access Policy requires that peer-reviewed research publications generated with NIH support are made available to the public through PubMed Central. NIH is not responsible for the data collection or analyses represented in this article. The ACL study was conducted by the Institute of Social Research, University of Michigan, MI, USA.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Shervin Assari receives financial support by the Heinz C. Prechter Bipolar Research Fund, Richard Tam Fund, and also the National Institutes of Health (NIH, HD084526-01 and DK089503).
References
- 1.Karlsen S, Nazroo JY. Relation between racial discrimination, social class, and health among ethnic minority groups. Am J Public Health. 2002;92:624–31. doi: 10.2105/ajph.92.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo CC, Howell RJ, Cheng TC. Disparities in Whites’ versus Blacks’ self-rated health: Social status, health-care services, and health behaviors. J Community Health. 2013;38:727–33. doi: 10.1007/s10900-013-9671-3. [DOI] [PubMed] [Google Scholar]
- 3.Cabassa LJ, Humensky J, Druss B, Lewis-Fernández R, Gomes AP, Wang S, et al. Do race, ethnicity, and psychiatric diagnoses matter in the prevalence of multiple chronic medical conditions? Med Care. 2013;51:540–7. doi: 10.1097/MLR.0b013e31828dbb19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson-Lawrence V, Griffith DM, Watkins DC. The effects of race, ethnicity, and mood/anxiety disorders on the chronic physical health conditions of men from a national sample. Am J Mens Health. 2013;7(4 Suppl):58S–67S. doi: 10.1177/1557988313484960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CL, Szklo M, Yeh HC, Wang NY, Dray-Spira R, Thorpe R, et al. Black-white disparities in overweight and obesity trends by educational attainment in the United States, 1997-2008. J Obes 2013. 2013:140743. doi: 10.1155/2013/140743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: An overview. Cardiovasc J Afr. 2007;18:241–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97:2260–7. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007;65:809–21. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207–21. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Assari S. Distal, intermediate, and proximal mediators of racial disparities in renal disease mortality in the United States. J Nephropathol. 2016;5:51–9. doi: 10.15171/jnp.2016.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson WE, Streib GF. Situational determinants: Health and economic deprivation in retirement. J Soc Issues. 1958;14:18–45. [Google Scholar]
- 12.Schnore LF, Cowhig JD. Some correlates of reported health in metropolitan centers. Soc Probl. 1959;7:218–26. [Google Scholar]
- 13.Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 14.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. J Gen Intern Med. 2006;21:267–75. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Idler EL, Kasl SV, Lemke JH. Self-evaluated health and mortality among the elderly in New Haven, Connecticut, and Iowa and Washington counties, Iowa, 1982-1986. Am J Epidemiol. 1990;131:91–103. doi: 10.1093/oxfordjournals.aje.a115489. [DOI] [PubMed] [Google Scholar]
- 16.Singh-Manoux A, Guéguen A, Martikainen P, Ferrie J, Marmot M, Shipley M. Self-rated health and mortality: Short- and long-term associations in the Whitehall II study. Psychosom Med. 2007;69:138–43. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee DL, Liao Y, Cao G, Cooper RS. Self-reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149:41–6. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- 18.DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40:1234–46. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson I, Undén AL, Elofsson S. Self-rated health. Comparisons between three different measures. Results from a population study. Int J Epidemiol. 2001;30:326–33. doi: 10.1093/ije/30.2.326. [DOI] [PubMed] [Google Scholar]
- 20.Rohrer JE, Arif A, Denison A, Young R, Adamson S. Overall self-rated health as an outcome indicator in primary care. J Eval Clin Pract. 2007;13:882–8. doi: 10.1111/j.1365-2753.2006.00766.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawada T. Self-rated health and life prognosis. Arch Med Res. 2003;34:343–7. doi: 10.1016/S0188-4409(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 22.Sun W, Watanabe M, Tanimoto Y, Shibutani T, Kono R, Saito M, et al. Factors associated with good self-rated health of non-disabled elderly living alone in Japan: A cross-sectional study. BMC Public Health. 2007;7:297. doi: 10.1186/1471-2458-7-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q, Xie Z, Zhang T. A single-item self-rated health measure correlates with objective health status in the elderly: A survey in suburban Beijing. Front Public Health. 2014;2:27. doi: 10.3389/fpubh.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IOM. State of the USA Health Indicators: Letter Report. 2009. [Last accessed on Feb 2015]. Available from: https://www.nap.edu/catalog/12534/state-of-the-usa-health-indicators-letter-report .
- 25.Robinson-Cohen C, Hall YN, Katz R, Rivara MB, de Boer IH, Kestenbaum BR, et al. Self-rated health and adverse events in CKD. Clin J Am Soc Nephrol. 2014;9:2044–51. doi: 10.2215/CJN.03140314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim G, DeCoster J, Chiriboga DA, Jang Y, Allen RS, Parmelee P. Associations between self-rated mental health and psychiatric disorders among older adults: Do racial/ethnic differences exist? Am J Geriatr Psychiatry. 2011;19:416–22. doi: 10.1097/JGP.0b013e3181f61ede. [DOI] [PubMed] [Google Scholar]
- 27.Jang Y, Park NS, Kang SY, Chiriboga DA. Racial/ethnic differences in the association between symptoms of depression and self-rated mental health among older adults. Community Ment Health J. 2014;50:325–30. doi: 10.1007/s10597-013-9642-2. [DOI] [PubMed] [Google Scholar]
- 28.Kim G, Bryant A, Huang C, Chiriboga D, Ma GX. Mental health among Asian American adults: Association with psychiatric. Asian Am J Psychol. 2012;3:44–52. [Google Scholar]
- 29.Assari S, Lankarani MM, Burgard S. Black-white difference in long-term predictive power of self-rated health on all-cause mortality in United States. Ann Epidemiol. 2016;26:106–14. doi: 10.1016/j.annepidem.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh-Manoux A, Dugravot A, Shipley MJ, Ferrie JE, Martikainen P, Goldberg M, et al. The association between self-rated health and mortality in different socioeconomic groups in the GAZEL cohort study. Int J Epidemiol. 2007;36:1222–8. doi: 10.1093/ije/dym170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro KF, Kelley-Moore JA. Self-rated health and mortality among black and white adults: Examining the dynamic evaluation thesis. J Gerontol B Psychol Sci Soc Sci. 2001;56:S195–205. doi: 10.1093/geronb/56.4.s195. [DOI] [PubMed] [Google Scholar]
- 32.Dowd JB, Zajacova A. Does the predictive power of self-rated health for subsequent mortality risk vary by socioeconomic status in the US? Int J Epidemiol. 2007;36:1214–21. doi: 10.1093/ije/dym214. [DOI] [PubMed] [Google Scholar]
- 33.Woo H, Zajacova A. Predictive strength of self-rated health for mortality risk among older adults in the United States: Does it differ by race and ethnicity? Res Aging. 2016 doi: 10.1177/0164027516637410. pii: 0164027516637410. [DOI] [PubMed] [Google Scholar]
- 34.Finch BK, Kolody B, Vega WA. Perceived discrimination and depression among Mexican-origin adults in California. J Health Soc Behav. 2000;41:295–313. [PubMed] [Google Scholar]
- 35.Shetterly SM, Baxter J, Mason LD, Hamman RF. Self-rated health among Hispanic vs non-Hispanic white adults: The San Luis Valley Health and Aging Study. Am J Public Health. 1996;86:1798–801. doi: 10.2105/ajph.86.12.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega WA, Amaro H. Latino outlook: Good health, uncertain prognosis. Annu Rev Public Health. 1994;15:39–67. doi: 10.1146/annurev.pu.15.050194.000351. [DOI] [PubMed] [Google Scholar]
- 37.Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: A test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health. 1999;89:1543–8. doi: 10.2105/ajph.89.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assari S, Burgard S, Zivin K. Long-term reciprocal associations between depressive symptoms and number of chronic medical conditions: Longitudinal support for black-white health paradox. J Racial Ethn Health Disparities. 2015;2:589–97. doi: 10.1007/s40615-015-0116-9. [DOI] [PubMed] [Google Scholar]
- 39.Assari S. Chronic medical conditions and major depressive disorder: Differential role of positive religious coping among African Americans, Caribbean blacks and non-Hispanic whites. Int J Prev Med. 2014;5:405–13. [PMC free article] [PubMed] [Google Scholar]
- 40.Watkins DC, Assari S, Johnson-Lawrence V. Race and Ethnic Group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2:385–94. doi: 10.1007/s40615-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assari S, Lankarani MM. Chronic Medical Conditions and Negative Affect; Racial Variation in Reciprocal Associations Over Time. Front. Psychiatry. 2016;7:140. doi: 10.3389/fpsyt.2016.00140. doi: 10.3389/fpsyt.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lankarani MM, Assari S. Association between number of comorbid medical conditions and depression among individuals with diabetes; race and ethnic variations. J Diabetes Metab Disord. 2015;14:56. doi: 10.1186/s40200-015-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assari S. Chronic kidney disease, anxiety and depression among American blacks; Does ethnicity matter? Int J Travel Med Glob Health. 2014;2:133–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis TT, Guo H, Lunos S, Mendes de Leon CF, Skarupski KA, Evans DA, et al. Depressive symptoms and cardiovascular mortality in older black and white adults: Evidence for a differential association by race. Circ Cardiovasc Qual Outcomes. 2011;4:293–9. doi: 10.1161/CIRCOUTCOMES.110.957548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Assari S, Lankarani MM. Race and urbanity alter the protective effect of education but not income on mortality. Front Public Health. 2016;4:100. doi: 10.3389/fpubh.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assari S. Race, sense of control over life, and short-term risk of mortality among older adults in the United States? Arch Med Sci. 2016;8:In Press. doi: 10.5114/aoms.2016.59740. DOI: 10.5114/aoms.2016.59740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assari S, Moazen-Zadeh E, Lankarani MM, Micol-Foster V. Race, depressive symptoms, and all-cause mortality in the United States. Front Public Health. 2016;4:40. doi: 10.3389/fpubh.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assari S, Dejman M, Neighbors HW. Ethnic differences in separate and additive effects of anxiety and depression on self-rated mental health among blacks. J Racial Ethn Health Disparities. 2016;3:423–30. doi: 10.1007/s40615-015-0154-3. [DOI] [PubMed] [Google Scholar]
- 49.Nery Guimarães JM, Chor D, Werneck GL, Carvalho MS, Coeli CM, Lopes CS, et al. Association between self-rated health and mortality: 10 years follow-up to the Pró-Saúde cohort study. BMC Public Health. 2012;12:676. doi: 10.1186/1471-2458-12-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finch BK, Hummer RA, Reindl M, Vega WA. Validity of self-rated health among Latino(a) s. Am J Epidemiol. 2002;155:755–9. doi: 10.1093/aje/155.8.755. [DOI] [PubMed] [Google Scholar]
- 51.Chandola T, Jenkinson C. Validating self-rated health in different ethnic groups. Ethn Health. 2000;5:151–9. doi: 10.1080/713667451. [DOI] [PubMed] [Google Scholar]
- 52.Zajacova A, Dowd JB. Reliability of self-rated health in US adults. Am J Epidemiol. 2011;174:977–83. doi: 10.1093/aje/kwr204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SJ, Moody-Ayers SY, Landefeld CS, Walter LC, Lindquist K, Segal MR, et al. The Relationship Between Self-Rated Health and Mortality in Older Black and White Americans. J American Geriatrics Society. 2007;55:1624–9. doi: 10.1111/j.1532-5415.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 54.Assari S, Burgard S. Black-White differences in the effect of baseline depressive symptoms on deaths due to renal diseases: 25 year follow up of a nationally representative community sample. J Renal Inj Prev. 2015;4:127–34. doi: 10.12861/jrip.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 56.Landray MJ, Emberson JR, Blackwell L, Dasgupta T, Zakeri R, Morgan MD, et al. Prediction of ESRD and death among people with CKD: The Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am J Kidney Dis. 2010;56:1082–94. doi: 10.1053/j.ajkd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–34. [PubMed] [Google Scholar]
- 58.House JS, Kessler RC, Herzog AR. Age, socioeconomic status, and health. Milbank Q. 1990;68:383–411. [PubMed] [Google Scholar]
- 59.Lundberg O, Manderbacka K. Assessing reliability of a measure of self-rated health. Scand J Soc Med. 1996;24:218–24. doi: 10.1177/140349489602400314. [DOI] [PubMed] [Google Scholar]
- 60.Pérez-Zepeda MU, Belanger E, Zunzunegui MV, Phillips S, Ylli A, Guralnik J. Assessing the validity of self-rated health with the short physical performance battery: A Cross-Sectional Analysis of the International Mobility in Aging Study. PLoS One. 2016;11:e0153855. doi: 10.1371/journal.pone.0153855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Houle JN. Depressive symptoms and all-cause mortality in a nationally representative longitudinal study with time-varying covariates. Psychosom Med. 2013;75:297–304. doi: 10.1097/PSY.0b013e31828b37be. [DOI] [PubMed] [Google Scholar]
- 62.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: Results from a nationally representative prospective study of US adults. JAMA. 1998;279:1703–8. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention, National Center for Health Statistics. Instruction Manual, Part 9. ICD-10 Cause-of-Death Lists for Tabulating Mortality Statistics (Updated October 2002 to Include ICD Codes for Terrorism Deaths for Data Year 2001 and WHO Updates to ICD-10 for Data Year 2003) [Last accessed Jan 2015]. Available from: http://www.cdc.gov/nchs/data/dvs/im9_2002.pdf.pdf .
- 64.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: Preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 65.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: Sociological contributions. J Health Soc Behav. 2010;51(Suppl):S15–27. doi: 10.1177/0022146510383838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keyes CL. The Black-White paradox in health: Flourishing in the face of social inequality and discrimination. J Pers. 2009;77:1677–706. doi: 10.1111/j.1467-6494.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- 67.Barnes DM, Keyes KM, Bates LM. Racial differences in depression in the United States: How do subgroup analyses inform a paradox? Soc Psychiatry Psychiatr Epidemiol. 2013;48:1941–9. doi: 10.1007/s00127-013-0718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuvekas SH, Fleishman JA. Self-rated mental health and racial/ethnic disparities in mental health service use. Med Care. 2008;46:915–23. doi: 10.1097/MLR.0b013e31817919e5. [DOI] [PubMed] [Google Scholar]
- 69.Fleishman JA, Zuvekas SH. Global self-rated mental health: Associations with other mental health measures and with role functioning. Med Care. 2007;45:602–9. doi: 10.1097/MLR.0b013e31803bb4b0. [DOI] [PubMed] [Google Scholar]
- 70.Mawani FN, Gilmour H. Validation of self-rated mental health. Health Rep. 2010;21:61–75. [PubMed] [Google Scholar]
- 71.Levinson D, Kaplan G. What does self rated mental health represent. J Public Health Res. 2014;3:287. doi: 10.4081/jphr.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, et al. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol. 2004;43:1542–9. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 73.González HM, Tarraf W. Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. Int Psychogeriatr. 2013;25:833–41. doi: 10.1017/S1041610212002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Assari S. Race and ethnic differences in associations between cardiovascular diseases, anxiety, and depression in the United States. Int J Travel Med Glob Health. 2014;2:103–9. [PMC free article] [PubMed] [Google Scholar]
- 75.González HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. J Psychiatr Res. 2010;44:1043–51. doi: 10.1016/j.jpsychires.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams DR, González HM, Neighbors H, Nesse R, Abelson JM, Sweetman J, et al. Prevalence and distribution of major depressive disorder in African Americans, Caribbean blacks, and non-Hispanic whites: Results from the National Survey of American Life. Arch Gen Psychiatry. 2007;64:305–15. doi: 10.1001/archpsyc.64.3.305. [DOI] [PubMed] [Google Scholar]
- 77.Capistrant BD, Gilsanz P, Moon JR, Kosheleva A, Patton KK, Glymour MM. Does the association between depressive symptoms and cardiovascular mortality risk vary by race? Evidence from the Health and Retirement Study. Ethn Dis. 2013;23:155–60. [PMC free article] [PubMed] [Google Scholar]
- 78.Owen WF., Jr Racial differences in incidence, outcome, and quality of life for African-Americans on hemodialysis. Blood Purif. 1996;14:278–85. doi: 10.1159/000170274. [DOI] [PubMed] [Google Scholar]
- 79.Hankerson SH, Fenton MC, Geier TJ, Keyes KM, Weissman MM, Hasin DS. Racial differences in symptoms, comorbidity, and treatment for major depressive disorder among black and white adults. J Natl Med Assoc. 2011;103:576–84. doi: 10.1016/s0027-9684(15)30383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyer GS, Templin DW, Goring WP, Cornoni-Huntley JC, Everett DF, Lawrence RC, et al. Discrepancies between patient recall and the medical record. Potential impact on diagnosis and clinical assessment of chronic disease. Arch Intern Med. 1995;155:1868–72. [PubMed] [Google Scholar]
- 81.Kimmel PL, Cukor D, Cohen SD, Peterson RA. Depression in end-stage renal disease patients: A critical review. Adv Chronic Kidney Dis. 2007;14:328–34. doi: 10.1053/j.ackd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303:1946–53. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, et al. Association of symptoms of depression with progression of CKD. Am J Kidney Dis. 2012;60:54–61. doi: 10.1053/j.ajkd.2012.02.325. [DOI] [PubMed] [Google Scholar]


