Abstract
In the present work, we propose the synthesis of a new family of sugar derived 1,4:3,6-dianhydrohexitol based AA/AB-type monomers. Unprecedented diacids based on Isomannide and Isoidide were elaborated with high yields and showed interestingly high melting point ranges (240–375 °C). Optimization of reaction conditions (temperature, time of reaction, and reactant ratios) has been investigated to synthesize the key intermediate of a set of AB monomers with acid, ester, and acid chloride functionalities. Isosorbide based ether benzoic acid AB monomer was polymerized and characterized by NMR and DSC techniques. The results show a semicrystalline behavior of the obtained polymer thanks to the controlled stereoregular arrangement of the AB starting monomer.
Keywords: 1,4:3,6-Dianhydrohexitols; Isosorbide; biosourced; AB monomers; semicrystalline; poly(ether)esters
1. Introduction
Pushed by concerns about the availability of petrochemical raw materials and the necessity for a sustainable society, bio-based materials became viable alternatives to existing petrochemicals. The most abundant carbohydrates attract widespread attention in both academic and industrial communities for the production of chemicals, fuels, and energy. However, a major disadvantage of using carbohydrates directly in industrial processes is their limited thermal stability due to the presence of different reactive functional groups.
The development of new monomers for the synthesis of original materials is predominantly motivated by the need to overcome the main shortcomings of composites. Therefore, to obtain mono and bi-functional monomers the depolymerization of carbohydrates is a practical strategy,[1,2] providing building blocks such as furan-25-dicarboxylic acid,[3–5] succinic acid, [6] and 1,4:3,6-dianhydrohexitols (DAH).[7,8] One of the most explored fields concerning the application of DAH is polymers, mostly referring to step‐growth polymers. A first review was published by Braun and Bergman in 1992, in which they discussed the early developments of DAH‐based polyesters, polycarbonates and polyurethanes. Later on, Kricheldorf presented another progress report [9] mainly focusing on DAH‐derived polyesters with special optical and liquid crystalline properties.[10,11] Recently, an excellent review was published by Fenouillot [7] which covers not only the most important and latest results on this subject but also the commercial applicability as well as the industrial potential of DAH‐derived monomers. However, some limitations due to a lack of stability and/or reactivity gave rise to several attempts in order to significantly broaden their application fields.[12] Modified DAH, displaying diamine,[13,14] amines,[15] acids,[14] or extended primary alcohols [16] found application in polyamides,[13] polyesters,[8,12,17] poly(ether)ketones [18] and poly(ether)sulfones.[19]
Among our recent efforts in this field we reported the synthesis of three diamines based on Isosorbide (1), Isomannide (2), and Isoidide (3) which were suitably used for the synthesis of polyamides,[20] and poly(ether)ureas.[21] Poly(ether)esteramides, as for them, were obtained directly from aminoalcohols [22] based on Isosorbide and Isomannide or from novel bis amide-diols building blocks.[23]
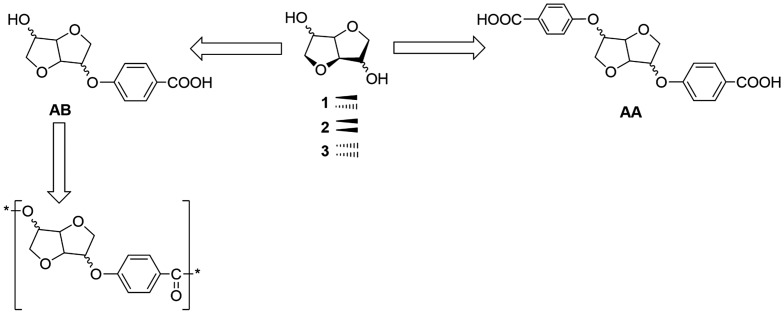
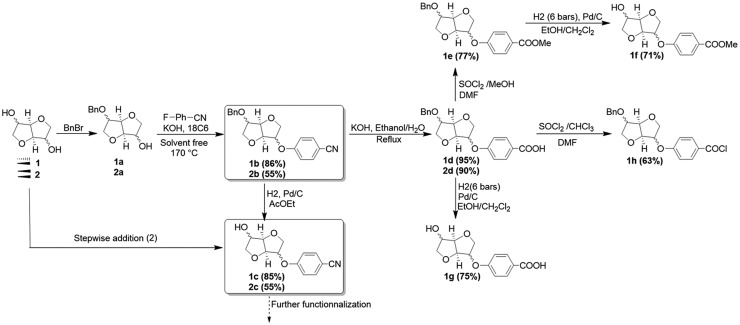
In another context, Jaffe published appealing AB type monomers containing phenyl moieties with both ester and ether linkers.[24] The DAH backbone was Isosorbide type for the former and Isoidide type for the latter. This strategy allowed them to obtain semicrystalline thermoplastics due to the controlled orientation of the hydroxyl functions for the former monomer. In this context, we propose herein a strategy to develop a new platform of DAH-based versatile monomers with a variety of functional groups. These unprecedented bio-sourced monomers exhibit AA and AB unsymmetrical functions. They could serve as monomers or precursors suitable for step‐growth (Scheme 1).
Scheme 1.
Synthetic route of novel monomers issued from dianhydrohexitols.
We present herein new monomers from the three DAH isomers starting from versatile benzonitrile compounds. Their hydrolysis led quantitatively to the corresponding diacids. In another side, dissymmetrical AB type monomers starting from protected Isosorbide or Isomannide reported for the first time are described. Their characterization and thermal properties as well as polymerization example are discussed in the present study.
2. Experimental
2.1. Materials and methods
Isosorbide was purchased from Alfa Aesar (Karleshrue, Germany), Isomannide and Isoidide were purchased from Sigma Aldrich (Milwaukee, WI, USA), previously crystallized from acetone and dried under high vacuum. 1-fluoro-4-benzonitrile (99%), 1,4,7,10,13,16-hexaoxacyclooctadecane (18-Crown-6), activated palladium supported on charcoal (10%), and thionyl chloride were purchased from Sigma-Aldrich (St Louis, MO, USA). KOH (Normapur) was purchased from Prolabo (Paris, France). Unless otherwise mentioned, all the reactants were used as received. The solvents were distilled over CaH2 and placed on molecular sieves.
1D and 2D NMR techniques were recorded at 300 and 500 MHz (Bruker WP 250). The chemical shifts are given in ppm. Differential Scanning Calorimetric data were obtained using DSC131 (SETARAM). DSC measurements were conducted with a heating and cooling rate at 10 °C/min. The first heating cycle was conducted from room temperature to 150 °C. Then samples were cooled down to room temperature. Then, a second heating scan was conducted (RT to 500 °C for the diacid) (RT to 300 °C for the AB monomers) (RT to 250 °C for the polymer). The T m and T g values were determined from the second DSC heating scan. Sample weights of about 10−15 mg were used in these experiments.
2.2. Monomers synthesis
2.2.1. Synthesis of dinitrile compounds
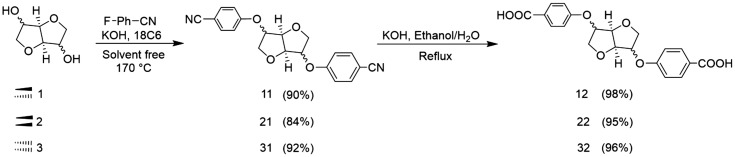
Dianhydrohexitol (10 mmol, 1.46 g), finely ground potassium hydroxide KOH (2.6 eq, 26 mmol, 1.47 g), 18-crown-6 (10%, 1 mmol, 0.265 g), and p-fluorobenzonitrile (2.5 eq, 25 mmol, 3.03 g) were placed in a cylindrical reactor equipped with a mechanical stirring. The reaction media was warmed gradually to 170 °C and stirring was maintained for4 h. The mixture was then cooled to room temperature and the crude was diluted with dichloromethane and filtered through a glass filter with celite and the residue was rinsed with dichloromethane. The solvent was then removed by rotary evaporator. The crude product was purified by flash chromatography on silica gel using a gradient eluent pentane / ethyl acetate: 70/30 (or by precipitation in cold MeOH).
2.2.1.1. 1,4:3,6-dianhydro-25-di-O-(4-cyanophenyl)-D-sorbitol (11)
Yellowish powder, 90% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.63–7.59 (dd, 4H, H9, J = 8.7 Hz, J’ = 2.2 Hz); 7.05–6.99 (m, 4H, H8); 5.05 (t, 1H, H4, J = 10 Hz, J’ = 5.2 Hz); 4.89–4.84 (m, 2H, H5 + H2); 4.64 (d, 1H, H3, J = 5.2 Hz); 4.19–4.02 (m, 4H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm) 161.06 (C7); 160.06 (C7); 134.16 (C9); 133.98 (C9); 118.93 (CN); 118.84 (CN); 115.82 (C8); 115.74 (C8); 104.91 (C10); 104.79 (C10); 8611 (C3); 8155(C2); 8150 (C5); 7751 (C4); 73.23 (C1); 71.63 (C6); IR (KBr): cm−1 2964, 2890, 2226, 1602, 1502, 1382, 1173, 1097, 817; HR-MS m/z for C20H17N2O4 [M + H]+: calcd: 349.1188, found 349.1415; T m = 130.6–137.9 °C.
2.2.1.2. 1,4:3,6-dianhydro-25-di-O-(4-cyanophenyl)-D-mannitol (21)
Brown powder, 84% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.60–7.57 (d, 4H, H9, J = 9 Hz); 7.05–7.02 (d, 4H, H8, J = 9 Hz); 4.91–4.82 (m, 2H, H5); 4.85–4.82 (m, 2H, H4); 4.13–4.01 (m, 4H, H1). 13C NMR (75 MHz, CDCl3): δ (ppm) 161.00 (C7); 133.95 (C9); 118.90 (CN); 115.69 (C8); 104.65 (C10); 80.77 (C4); 77.05 (C5); 71.45 (C6); IR (KBr): cm−1 2931, 2865, 2223, 1606, 1507, 1302, 1173, 1063, 840; HR-MS m/z for C20H17N2O4 [M + H]+: calcd 3,491,188, found 349.1186; T m = 178.9–184.4 °C.
2.2.1.3. 1,4:3,6-dianhydro-25-di-O-(4-cyanophenyl)-D-iditol (31)
Yellowish powder, 92% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.62–7.60 (d, 4H, H9, J = 8.9 Hz); 7.01–6.99 (d, 4H, H8, J = 8.8 Hz); 489 (dd, 2H, H2,J = 3.8 Hz, J = 2.1 Hz); 4.77–4.79 (m, 2H, H3); 4.25–4.07 (m, 4H, H1); 13C NMR (75 MHz, CDCl3): δ (ppm) 160.03 (C7); 134.14 (C9); 118.77 (CN); 115.79 (C8); 104.96 (C10); 85.56 (C3); 81.13 (C2); 72.16 (C1); IR (KBr): cm−1 2922, 2869, 2222, 1603, 1499, 1296, 1092, 841; HR-MS m/z for C20H17N2O4 [M + H]+: calcd 349.1188, found 349.1190; T m = 160.1–165.8 °C.
2.2.2. Synthesis of diacid compounds
The dinitrilphenyl compound (1.44 mmol, 0.5 g) was suspended in a mixture of EtOH/H2O 50/50 (20 mL) with KOH (11 eq, 15.84 mmol, 0.89 g) in a two-necked round bottom flask and stirred magnetically. The medium was warmed to 100 °C and the reaction was refluxed for 48 h. After completion (TLC control), the mixture was cooled to room temperature and a few drops of HCl (cc) were added and a precipitate began to be formed. The addition was continued to reach acidic pH (pH = 2–3). The precipitate is then filtered, washed with water and vacuum dried at 50 °C, until constant weight. The diacid was recovered as a pure white crystalline solid.
2.2.2.1. 4,4’-(((3R,3aR,6S,6aR)-hexahydrofuro[3,2-b]furan-3,6-diyl)bis(oxy))dibenzoic acid (12)
White powder, 98% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.91–7.86 (m, 4H, H9); 7.10–7.03 (m, 4H, H8); 5.05–5.01 (m, 2H, H2 + H5); 5.00 (d, 1H, H4, J = 3.5 Hz); 458 (d, 1H, H3, J = 4.4 Hz); 404–400 (m, 2H, H1 + H6); 3.92–3.89 (m, 2H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm 167.19 (COOH); 167.11 (COOH); 161.67 (C7); 160.42 (C7); 131.68 (C9); 131.43 (C9); 123.80 (C10); 123.39 (C10); 115.18 (C8); 114.96 (C8); 85.94 (C3); 8143 (C2); 80.92 (C4); 77.16 (C5); 72.78 (C1); 70.98 (C6); IR: cm−1 2941, 2665, 1676, 1596, 1240, 1170, 1083, 841; T m = 240–300 °C.
2.2.2.2. 4,4’-(((3R,3aR,6R,6aR)-hexahydrofuro[3,2-b]furan-3,6-diyl)bis(oxy))dibenzoic acid (22)
White powder, 95% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.88–7.87 (d, 4H, H9, J = 8.8 Hz); 7.13–7.11 (d, 4H, H8, J = 8.8 Hz); 5.01–4.94 (m, 2H, H5); 4.91–4.90 (m, 2H, H4); 4.03–4.00 (m, 2H, H1); 3.81–3.78 (m, 2H, H1). 13C NMR (125 MHz, CDCl3): δ (ppm) 167.00 (COOH); 161.47 (C7); 131.29 (C9); 123.25 (C10); 114.83 (C8); 80.22 (C4); 76.73 (C5); 70.65 (C6); IR: cm−1 2941, 2665, 1676, 1596, 1240, 1170, 1083, 841; T m = 300–375 °C.
2.2.2.3. 4,4’-(((3S,3aR,6S,6aR)-hexahydrofuro[3,2-b]furan-3,6-diyl)bis(oxy))dibenzoic acid (32)
White powder, 96% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.91–7.89 (m, 4H, H9); 7.13–7.11 (m, 4H, H8); 5.04 (dd, 2H, H2, J = 3.8 Hz, J = 1.6 Hz); 4.72 (s, 2H, H3); 4.13–4.10 (dd, 2H, H1, J = 10.4 Hz, J = 4.1 Hz); 3.98–3.96 (dd, 2H, H1, J = 10.4 Hz, J = 1.6 Hz); 13C NMR (125 MHz, CDCl3): δ (ppm) 166.85 (COOH); 160.21 (C7); 131.46 (C9); 123.67 (C10); 115.06 (C8); 85.24 (C3); 80.84 (C2); 71.56 (C1); IR: cm−1 2941, 2665, 1676, 1596, 1240, 1170, 1083, 841; T m = 280–315 °C.
2.2.3. Synthesis of mono-nitrile compounds
1a or 2a [22] (4.23 mmol, 1 g), finely ground potassium hydroxide KOH (1.2 eq, 5 mmol, 0.28 mg), 18-crown-6 (10%, 0.112 g),and p-fluorobenzonitrile (1.1 eq, 4.65 mmol, 0.564 g) were placed in a cylindrical reactor equipped with a mechanical stirring. The reaction media was warmed gradually to 170 °C and stirring was maintained during 4 h. The mixture was then cooled to room temperature and the crude was diluted with dichloromethane and filtered through a glass filter with celite and the residue was rinsed with dichloromethane. The solvent was then removed by rotary evaporator. The crude product was purified by flash chromatography on silica gel using a gradient eluent pentane / ethyl acetate: 60/40 (or by precipitation in cold MeOH).
2.2.3.1. 4-(((3R,3aR,6S,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzonitrile (1b)
Yellowish powder, 86% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.61–7.58 (m, 2H, H9); 7.37–7.30 (m, 5H, Hph); 7.03–7.00 (m, 2H, H8); 4.97 (t, 1H, H4, J = 5 H); 4.84–4.79 (q, 1H, H5, J = 15.6 Hz, J’ = 5.2 Hz); 4.62 (d, 1H, H3, J = 4.9 Hz); 4.58 (s, 2H, CH2); 4.14 (d, 1H, H2, J = 3.3 Hz); 4.05–3.89 (m, 4H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm) 161.25 (C7); 137.38 (Ca); 133.96 (C9); 128.49 (Cb); 127.91 (Cd); 127.69 (Cc); 119.03 (CN); 115.78 (C8); 104.60 (C10); 86.78 (C3); 82.97 (C2); 80.88 (C4); 77.86 (C5); 73.26 (C1); 71.43 (C6); 70.99 (CH2); IR (KBr): cm−1 3027, 2996, 2913, 2220, 1601, 1507, 1219, 1173, 966; HR-MS m/z for C20H20NO4 [M + H]+: calcd 338.1392, found 338.1396; T m = 84.9–86.1 °C.
2.2.3.2. 4-(((3R,3aR,6R,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzonitrile (2b)
Yellowish powder, 55% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.62–7.57 (d, 2H, J = 8.8 Hz, H9); 7.34 (s, 5H, Hph); 7.04–6.99 (d, 2H, H8, J = 8.8 Hz); 4.99–4.94 (m,1H, H4); 4.85–4.77 (q, 1H, H5, J = 10.4 Hz, J = 5.2 Hz); 4.62–4.61 (d, 1H, H3, J = 4.9 Hz); 4.58 (s, 2H, CH2); 4.13 (s, 1H, H2); 4.03–3.87 (m, 4H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm) 161.15 (C7); 137.31 (Ca); 133.86 (C9); 128.33 (Cb); 127.81 (Cd); 127.59 (Cc); 118.93 (CN); 115.70 (C8); 104.58 (C10); 86.71 (C3); 82.95 (C2); 80.78 (C4); 77.80 (C5); 73.20 (C1); 71.32 (C6); 70.94 (CH2); IR (KBr): cm−1 3100, 2986, 2226, 1615, 1572, 1382, 1173, 1020, 816; HR-MS m/z for C20H20NO4 [M + H]+: calcd 338.1392, found 338.1391; T m = 84.4–85.9 °C.
2.2.4. General debenzylation method
1b or 2b (1.81 mmol, 650 mg) was dissolved in AcOEt (25 mL) and 20% of Pd/C (0.130 g) was added. The reaction mixture was reduced under hydrogen pressure (4 bars) at 40 °C. After 36 h, the crude was filtered over thick filter paper and rinsed with acetonitrile (25 mL). After evaporation under vacuum, the product was purified by flash chromatography on silica gel using a gradient eluent pentane / ethyl acetate: 50/50.
2.2.4.1. 4-(((3R,3aR,6S,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl)oxy)benzonitrile (1c)
yellow powder, 85% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.61–7.57 (d, 2H, H9, J = 9 Hz); 7.02–6.98 (d, 2H, H8, J = 8 Hz); 4.98–4.90 (q, 1H, H4, J = 10 Hz, J = 5 Hz); 4.84–4.77 (m, 2H, H5); 4.57 (t, 1H, H3, J = 5.9 Hz); 4.35 (s, 1H, H2); 3.99–3.83 (m, 4H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm) 161.19 (C7); 134.08 (C9); 118.96 (CN); 115.82 (C8); 104.82 (C10); 81.82 (C3); 80.88 (C4); 80.79 (C5); 75.30 (C2); 71.76 (C1); 70.15 (C6); IR (KBr): cm−1 3400, 2881, 2222, 1605, 1507, 1255, 1108, 833; HR-MS m/z for C13H13NO4 [M + H]+: calcd 247.0845, found 247.0844; T m = 79.4–81.9 °C.
2.2.5. Fractionwise addition method with Isommanide
Isomannide (0.69 mmol, 0.1 g), finely ground potassium hydroxide KOH (0.6 eq, 0.414 mmol, 0.23 g), 18-crown-6 (10%, 0.018 g), p-fluorobenzonitrile (0.6 eq, 0.414 mmol, 0.05 g) were placed in a cylindrical reactor equipped with a mechanical stirring. The reaction media was warmed gradually to 150 °C and stirring was maintained during 5 h. The mixture was then cooled to room temperature. The crude was diluted with dichloromethane and filtered through a glass filter with celite and the residue was rinsed with dichloromethane. The solvent was then removed by rotary evaporator. The crude product was purified by flash chromatography on silica gel using a gradient eluent pentane / ethyl acetate: 50/50.
2.2.5.1. 4-(((3R,3aR,6R,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl)oxy)benzonitrile (2c)
White powder, 84% yield; 1H NMR (300 MHz, CDCl3): δ (ppm) 7.63–7.59 (m, 4H, H9); 7.06–7.01 (m, 4H, H8); 4.90–4.83 (m, 2H, H5 + H4); 4.55–4.53 (m, 1H, H3); 4.31–4.26 (m, 1H, H2); 4.18–3.74 (m, 4H, H1 + H6); 13C NMR (75 MHz, CDCl3): δ (ppm) 161.09 (C7); 134.02 (C9); 118.94 (CN); 115.76 (C8); 104.80 (C10); 81.78 (C3); 80.85 (C4); 80.77 (C5); 75.23 (C2); 71.74 (C1); 70.12 (C6); IR (KBr): cm−1 3500, 2913, 2220, 1601, 1507, 1456, 1220, 1044, 968; HR-MS m/z for C13H13NO4 [M + H]+: calcd 247.0845, found 247.0842; T m = 119.6–125.1 °C.
2.2.6. Synthesis of the monoacids
1b or 2b (2.96 mmol, 1 g) was suspended in a mixture of EtOH/ H2O 50/50 (20 mL) with KOH (11 eq, 32.6 mmol, 1.83 g) in a two-necked round bottom flask and stirred magnetically. The medium was warmed to 100 °C and the reaction was refluxed for 48 h. After completion (TLC control), the mixture was cooled to room temperature and a few drops of HCl (cc) were added up to precipitate formation. The addition was continued until acidic pH was reached (pH = 2–3). The precipitate was then filtered, washed thoroughly with water and vacuum dried at 50 °C until constant weight. The acid was recovered as a pure white crystalline solid.
2.2.6.1. 4-(((3R,3aR,6S,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzoic acid (1d)
White powder, 95% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.87–7.71 (m, 2H, H9); 7.34–7.28 (m, 5H, Hph); 7.15–7.05 (m, 2H, H8); 5.00–4.91 (m, 1H, H4 + H5); 4.56–4.55 (m, 1H, H3); 4.52–4.50 (m, 2H, CH2); 4.03 (s, 1H, H2); 3.95–3.67 (m, 4H, H1 + H6); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 167.00 (COOH); 161.55 (C7); 138.02 (Ca); 131.23 (C9); 128.32 (Cb); 127.72 (Cc); 127.61 (C10); 123.12 (Cd); 114.80 (C8); 85.93 (C3); 82.78 (C2); 80.27 (C4); 77.22 (C5); 72.48 (C1); 70.42 (C6); 70.99 (CH2); IR: cm−1 2950, 2555, 1674, 1596, 1244, 1170, 1083, 841; T m = 148–156 °C.
2.2.6.2. 4-(((3R,3aR,6R,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzoic acid (2d)
White powder, 90% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.87–7.83 (m, 2H, H9); 7.39–7.27 (m, 5H, Hph); 7.08–7.07 (m, 2H, H8); 4.99–4.90 (m, 2H, H4 + H5); 4.56–4.48 (m, 3H, H3 + CH2); 4.05–3.75 (m, 5H, H2 + H1 + H6); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 166.85 (COOH); 161.45 (C7); 137.95 (Ca); 131.10 (C9); 128.19 (Cb); 127.59 (Cc); 127.48 (Cd); 123.10 (C10); 114.71 (C8); 85.88 (C3); 82.76 (C2); 80.17 (C4); 77.17 (C5); 72.42 (C1); 70.29 (C6); 70.21 (CH2); IR:cm−1 2950, 2655, 1676, 1596, 1254, 1170, 1082, 850; T m = 137–148 °C.
2.2.6.3. 4-(((3R,3aR,6S,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl)oxy)benzoic acid (1g)
The general debenzylation method was applied to 1d (1 g, 2.8 mmol) with 20% of Pd/C (0.2 mg) in a mixture of CH2Cl2/EtOH (15 mL/5 mL) and was reduced under hydrogen pressure (6 bars) at 40 °C.
White powder, 75% yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm 7.82–7.80 (d, 2H, H9, J = 8.8 Hz); 7.03–7.02 (d, 2H, H8, J = 8.8 Hz); 4.96–4.93 (m, 1H, H4); 4.79–4.77 (t, 1H, H5, J = 5 Hz); 4.36–4.34 (t, 1H, H3, J = 4.9 Hz); 4.11–4.08 (m, 1H, H2); 4.07–4.04 (dd, 1H, H1 + H6, J = 9.1 Hz, J’ = 6.3 Hz); 3.80–3.77 (dd, 1H, H1 + H6, J = 9 Hz, J’ = 6.5 Hz); 3.74–3.71 (t, 1H, H1 + H6, J = 7.6 Hz, J’ = 7.6 Hz); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 167.39 (COOH); 160.23 (C7); 129.21 (C9); 126.67 (C10); 114.35 (C8); 81.78 (C3); 79.72 (C4); 77.33 (C5); 71.95 (C2); 71.56 (C1); 70.62 (C6); IR :cm−1 3429, 2937, 16,741,598, 1425, 1296, 1244, 1084, 927, 841; T m = 152–175 °C.
2.2.7. Synthesis of acid chloride
2.2.7.1. 4-(((3R,3aR,6S,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzoylchloride (1h)
1d (1.126 mmol, 0.3 g) was refluxed in a mixture of freshly distilled thionyl chloride (1.71 mmol, 0.124 mL) dry chloroform (1 mL), and a few drops of DMF until a clear solution was obtained. The reaction mixture was then concentrated for 1 h under vacuum, the residue was diluted with 5 mL of dry toluene and concentrated again. This procedure was repeated twice to remove residual thionyl chloride. Finally, the product was crystallized from its concentrated toluene solution. Brown solid, 63% yield; 1H NMR: (500 MHz, DMSO-d6): δ (ppm): 7.87–7.71 (m, 2H, H9); 7.34–7.28 (m, 5H, Hph); 7.15–7.05 (m, 2H, H8); 5.00–4.91 (m,1H, H5 + H4); 4.55–4.54 (m, 1H, H3); 4.51–4.50 (m, 2H, CH2); 4.03 (s, 1H, H2); 3.95–3.67 (m, 4H, H1 + H6); 13C NMR: (125 MHz, DMSO-d6): δ (ppm): 167.05 (COCl); 161.58 (C7); 138.03 (Ca); 134.07 (C10); 131.27 (C9); 128.34 (Cb); 127.73 (Cc); 127.62 (Cd); 114.81 (C8); 85.98 (C3); 82.84 (C2); 80.32 (C4); 77.26 (C5); 72.52 (C1); 70.46 (C6); 70.31 (CH2).
2.2.8. Synthesis of ester
2.2.8.1. Methyl4-(((3R,3aR,6S,6aR)-6-(benzyloxy)hexahydrofuro[3,2-b]furan-3-yl)oxy)benzoate (1e)
The same procedure of the acid chloride was applied in the presence of methanol (10 mL). White powder, 77% yield; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.07–7.77 (m, 2H, H9); 7.40–7.28 (m, 5H, Hph); 7.06–6.99 (m, 2H, H8); 4.98–4.95 (m, 1H, H5); 4.87–4.83 (m, 1H, H4); 4.65–4.63 (m, 1H, H3); 4.60–4.58 (m, 2H, CH2); 4.20–3.94 (m, 8H, H2 + CH3+H1 + H6); 13C NMR (125 MHz, CDCl3): δ (ppm) 171.09 (COOMe); 161.00 (C7); 137.48 (Ca); 132.12 (C10); 129.30 (C9); 128.47 (Cb); 127.87 (Cd); 127.69 (Cc); 115.00 (C8); 86.72 (C3); 83.24 (C2); 80.85 (C4); 77.83 (C5); 73.32 (C1); 71.44 (CH2); 70.72 (C6); 60.34 (CH3); T m = 125–145 °C.
2.2.8.2. Methyl 4-(((3R,3aR,6S,6aR)-6-hydroxyhexahydrofuro[3,2-b]furan-3-yl)oxy)benzoate (1f)
The general debenzylation method was applied to 1e (0.81 mmol, 0.3 g) with 20% of Pd/C (0.6 g) in a mixture of CH2Cl2/EtOH (5 mL/5 mL), and was reduced under hydrogen pressure (6 bars) at 40 °C.
White powder, 71%yield; 1H NMR (500 MHz, DMSO-d6): δ (ppm) 7.87–7.82 (m, 2H, H9); 7.15–7.03 (m, 2H, H8); 5.19–5.16 (m,1H, H5); 4.97–4.95 (m, 1H, H4); 4.9–4.87 (m, 1H, H3); 4.35 (s, 1H, H2); 4.11–3.64 (m, 7H, CH3+H1 + H6); 13C NMR: (125 MHz, DMSO-d6): δ (ppm): 170.00 (COOMe); 160.09 (C7); 131.66 (C10); 129.69 (C9); 114.86 (C8); 88.45 (C3); 79.70 (C4); 79.14 (C5); 78.88 (C2); 78.61 (C1); 75.11 (C6); 69.83 (CH3).
2.3. Polymer synthesis
The substrate 1g (1.88 mmol, 0.5 g) was charged into a cylindrical glass reactor and stirred magnetically. The reaction mixture was stirred at 150 °C for 1 h without vacuum and a homogeneous medie was obtained. The temperature was then increased gradually to 200 °C, and the polymerization was kept undergo for 2 h under vaccuum. After cooling to room temperature, the crude was dissolved in DMF (3 mL) and precipitated into cold MeOH (10 mL) giving rise to a brown solid. 1H NMR: (500 MHz, DMSO-d6): δ (ppm): 7.92–7.82 (m, 4H, H9,); 7.21–7.07 (m, 4H, H8); 5.31 (s, 1H, H3); 5.22 (s, 1H, H2’); 5.08 (m, 2H, H2 + H5); 5.08–4.94 (m, H4 ‘ + H5’); 4.91–4.89 (m, H3’’); 4.65 (s, 1H, H4); 4.35–4.34 (m, H2”); 4.12–3.95 (m, H1 + H6); 3.91–3.63 (m, H1’ + H6’ + H1’’ + H6’’); 13C NMR: (125 MHz, DMSO-d6): δ (ppm): 167.44 (C11’); 166.95 (C11’’); 164.64 (C11); 162.17 (C7); 162.05 (C7’’); 161.45 (C7’); 131.31 (C10); 131.18 (C9); 129.24 (C9’); 121.62 (C10’’); 121.45 (C10’); 115.00 (C8); 114.77 (C8’’); 114.37 (C8’); 88.61 (C3); 85.96 (C4); 80.53 (C3’’); 79.98 (C5); 78.05 (C4’); 77.40 (C5’’); 77.15 (C5’); 75.24 (C2); 74.94 (C2’); 72.63 (C1); 70.72 (C6’); 70.11 (C6); IR :cm−1 3404, 2875, 1710, 1599, 1505, 1244, 1186, 767; T m = 83–113 °C.
3. Results and discussion
The synthesis of the dinitrile monomers was optimized compared to the anterior works.[25] The previous method reported for the dinitrile compound synthesis, described the use of microwave irradiation at 170 °C during 30 min. In our context, the first step was carried out under solvent-free conditions and adapted to the classical heating method at 170 °C which renders it more accessible. The dinitrile was obtained by a SNAr reaction between the DAH and p-fluorobenzonitrile in basic medium. This method was applied in the absence of any organic solvent, using KOH as the base and 18-crown-6 as the phase transfer agent. After 4 h, the dinitrile compound was recovered by precipitation or by flash chromatography on silica gel. This method was extended to the two other dianhydrohexitols namely Isomannide 2 and Isoidide 3 (Scheme 1). After exact mass confirmation using HRMS analysis, NMR studies were carried out to confirm the structures and highlight the characteristic signal of each dianhydrohexitol type (Figures S2 and S3 in the Supporting Information).
We first focused our efforts on the hydrolysis of the dinitrile functions to the corresponding diacids (Scheme 2). Various aqueous nitrile hydrolysis procedures (both acidic and basic) gave unsatisfactory results, since none of the wide range of investigated conditions gave a full conversion of the nitrile groups. Due to the high polarity of mono nitrile-acid intermediate and the targeted diacid, effective separation of the latter from the crude reaction mixture turned out to be challenging. We then investigated the basic hydrolysis in the presence of KOH by using a mixture of EtOH/H2O. The diacid was then obtained by precipitation without any further purification and recovered in high purity after several water washings.
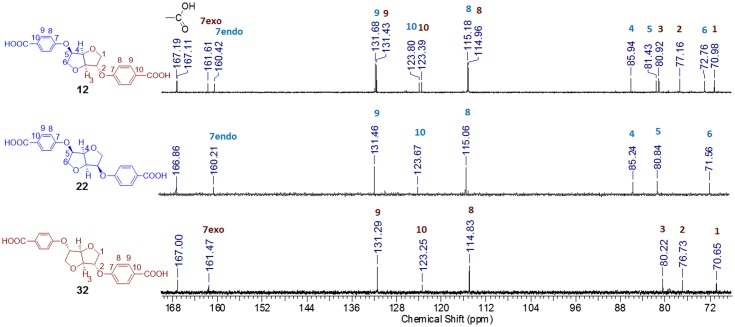
Based on our latest results we aimed to demonstrate the effect of the starting DAH stereochemistry on the products properties. The comparison of the DAH core signals (Figure 1) clearly showed that no epimerization occurred during all the process. Indeed, symmetrical Isomannide and Isoidide exhibited three types of signals, whereas Isosorbide patterns showed six. In the same trend, under the influence of the endo/exo alternation of the signals for the latter, the carbons directly attached to the hydroxyl functions split into two signals (C7: 160.4 and 161.6 ppm). This overlaid allowed us as well to attribute without any doubt.
Figure 1.
13C spectra of diacids 12, 22 and 32 in DMSO-d6.
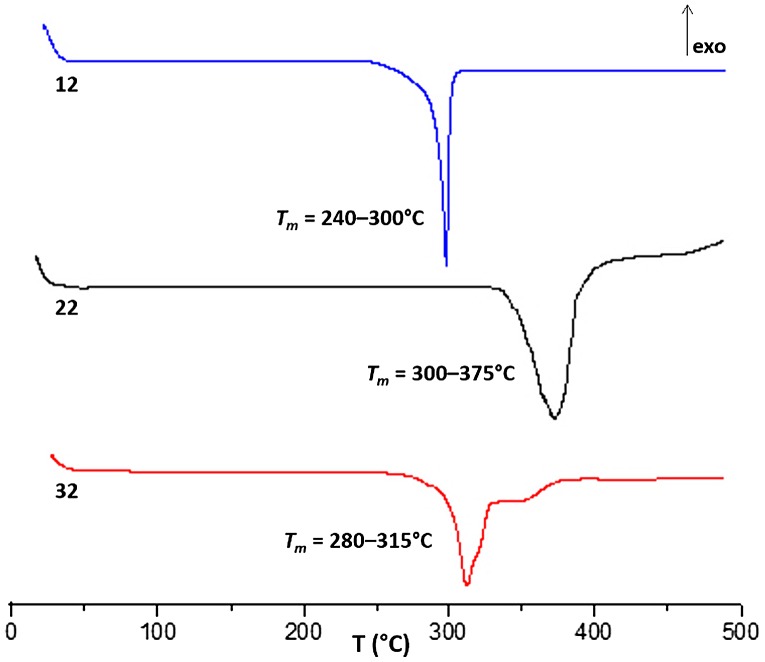
Thermal analysis of these new diacids (Figure 2) showed interestingly high melting ranges (240–375 °C) which make them being potential candidates for polymers with high thermal performance. As a trend, it seemed that stereochemistry of the starting DAH influences the melting point, the Isomannide homologue 22 presenting the highest T m range. This could probably be explained by an additional intramolecular association favored by the endo orientation of both benzoic acid moieties .
Figure 2.
DSC curves of 12, 22 and 32 measured between 17 and 500 °C at 10 °C/min heating.
Scheme 2.
Synthesis of biosourced monomers AA derived from dianhydrohexitols.
In order to extend the viability and flexibility of this approach, we decided to develop a set of self polymerizable AB type monomers. It is well established that such monomers present the benefit of avoiding side reaction as well as an accurate control of the stoichiometry. The monoarylation on Isosorbide requires the preliminary protection of one hydroxyl function due to the difference of reactivity of both OH functions. Due to the stereochemistry and configurational restrictions, the two hydroxyl functions of Isosorbide react differently.[26] The strategy was then based on a prior protection of the exo hydroxyl function with monobenzylation,[27] followed by a nucleophilic substitution on the free left hydroxyl group. The obtained monophenylnitrile intermediates 1b/2b represents a new chemical platform (Scheme 3). In fact, several pathways could be imagined starting from this intermediate. After debenzylation, it could afford the deprotected alcohol 1c or 2c. The latter would allow selective modification on the alcohol side considering the nitrile function as an acid precursor. Based on our recent works regarding selective mono arylation on symmetrical Isomannide 2,[22] a similar strategy was considered. Lowering the temperature to 150 °C and using 0.5 eq of arylating agent afforded the desired compound 2c in only one step with 84% yield (Table 1, entry 12).
Scheme 3.
Synthesis of biosourced monomers AB derived from dianhydrohexitols.
Table 1. Optimisation of reaction parameters and yields of products.
| Entry | Reagent | Quantity of reagent (g) | T (°C) | Addition mode | Reaction time (h) | Results a |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 100 | Simultaneous | 24 | 44 | |
| 2 | 0.1 | 150 | Simultaneous | 18 | 55 | |
| 3 | 0.5 | 150 | Simultaneous | 24 | 36 | |
| 4 | 1 | 170 | Simultaneous | 24 | 40 | |
| 5 | 1a | 1 | 170 | Simultaneous | 4 | 86 |
| 6 | 3 | 170 | Simultaneous | 4 | 84 | |
| 7 | 5 | 170 | Simultaneous | 4 | 81 | |
| 8 | 10 | 170 | Simultaneous | 4 | 82 | |
| 9 | 0.1 | 170 | Simultaneous | 4 | 57 | |
| 10 | 2a | 1 | 170 | Simultaneous | 4 | 55 |
| 11 | 6 | 170 | Simultaneous | 4 | 52 | |
| 12 | 2 | 0.1 | 150 | Fractionwise | 5 | 84%mono/7%di |
Yields in isolated products.
Since the protected nitrile compound 1b/2b is the key intermediate in our strategy we explored several ways to optimize the isolated yield (Scheme 3). To this end, reaction parameters such as temperature, time, and reactant ratios were examined. Monobenzylated Isosorbide and p-fluorobenzonitrile were reacted, in solvent-free conditions, in presence of KOH as a base and 18-Crown-6 as a phase-transfer agent. After precipitation in cold methanol or chromatography purification, the protected nitrile compound 1b/2b was isolated as a yellow crystalline powder with high yield (84%). A scale up to 10 g could be achieved conveniently without yield loss (Table 1, entry 8).
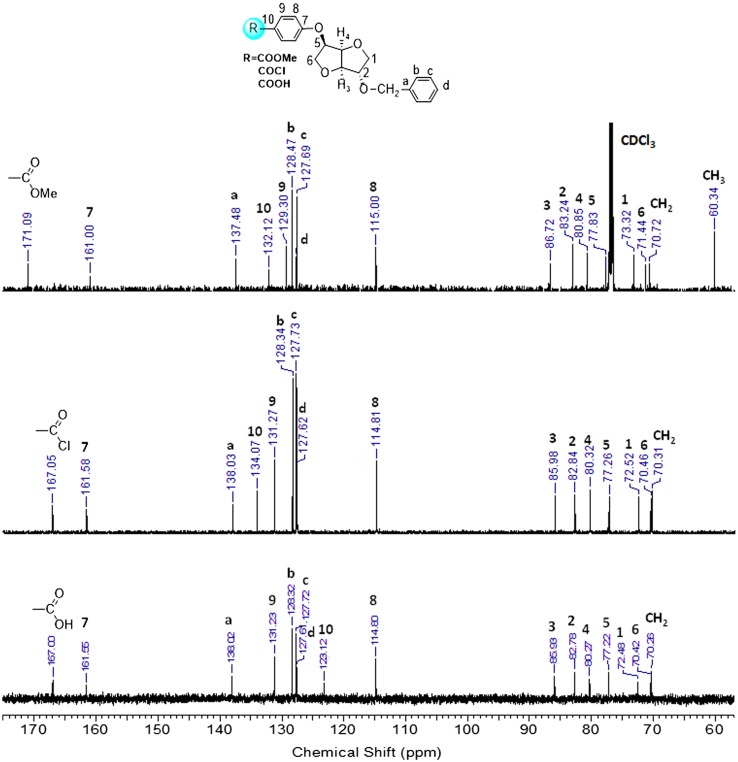
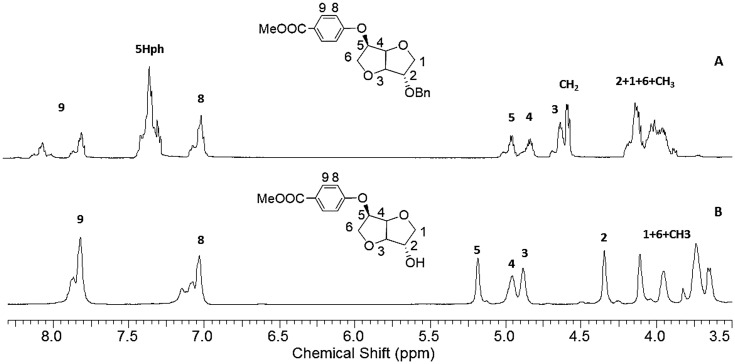
Further steps to the desired AB monomer deserved some interesting comments. Indeed, we figured out that a preliminary deprotection of the alcohol prevented the hydrolysis of the nitrile function to take place. In order to overcome this problem, we focused our efforts on working with the protected monomer 1b or 2b. The hydrolysis step was then carried out at the first place to afford 1d or 2d with high yield (90–95%). In order to render these AB monomers the most versatile possible, we explored several ways to achieve their synthesis. Starting from the protected acid reactant 1d (Figure 3(A)), a debenzylation was conducted in a mixture of EtOH and CH2Cl2 to afford the product 1g with 75% yield. On the other hand, the ester analogue was synthesized starting from 1d in one pot via joint addition of SOCl2/MeOH leading to 1e with 77% yield (Figure 3(C)). The obtained protected ester revealed more soluble. The debenzylation led to 1f with 71% yield. The disappearance of signals due to the benzyl group clearly indicated the occurrence of the reaction (Figure 4). Working in a mixture of SOCl2/CHCl3 gave rise to the protected acyl chloride 1 h with 63% (Figure 3(B)).
Figure 3.
13C spectra of 1g (A) (DMSO-d6), 1h (B) (DMSO-d6) and 1e (C) (CDCl3).
Figure 4.
1H spectra of 1e (A) in DMSO-d6and 1f (B) in CDCl3.
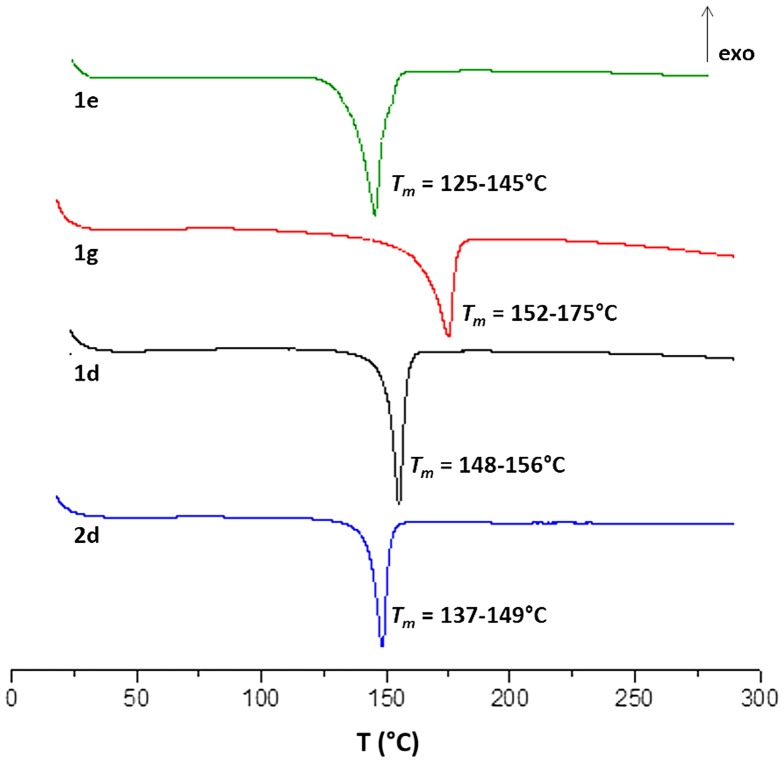
Worth noting, as mentioned above, the stereochemistry influences drastically the thermal behavior of the DAH monomers (Table 2). First, comparing Isosorbide based protected acid 1d with his homologue isoidide 2d showed a slightly higher T m. When 1d was deprotected to 1g the T m increased probably due to the additional OH bonding of the free hydroxyl (Figure 5). Second, Jaffe and al. reported a melting point for Isoidide based phenyl ester analogue of 117 °C,[24] whilst the corresponding protected Isosorbide analogue 1e melts in the range of 125–145 °C.
Figure 5.
DSC curves of 2d, 1d, 1g and 1e measured between 18 and 300 °C at 10 °C/min heating.
Having all these appealing monomers in hand, we aimed to show a proof of concept starting from the Isosorbide based acid analogue 1g considering it as the most promising monomer due to its commercial availability and straight forward synthesis. We then investigated the potential use of these monomers as building blocks in polymer materials synthesis. In addition, to the utility of AB monomers type in terms of accurate stoichiometry in our case, we expect them to give rise to a polymer with stereoregular sequences which would induces some crystallinity ordering. We carried out the polymerization of 1g in bulk. The reaction media was heated at 150 °C to ensure the melting of 1g and then increased to 200 °C to carry on the polycondensation. A precipitation of the crude material that is previously dissolved in DMF was conducted in cold MeOH leading to a solid material. FTIR and NMR analysis were conducted in order to follow the polymerization.
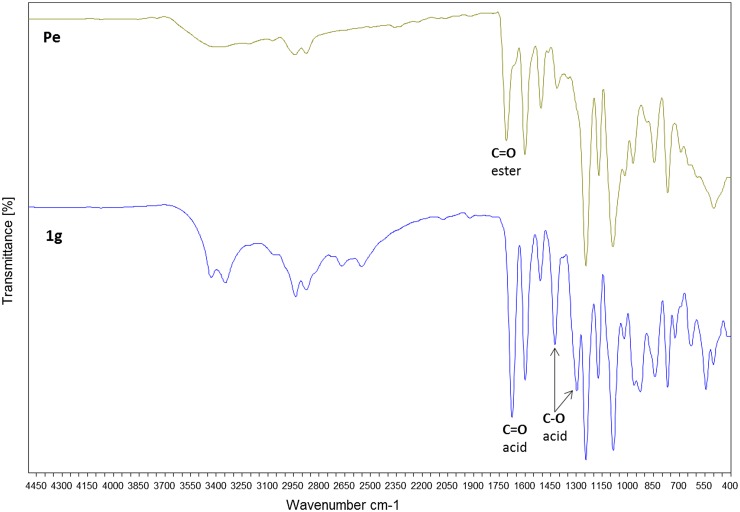
FTIR analysis (Figure 6) evidenced the successful polymerization reaction. In fact, bands at 1676, 1425 and 1296 cm−1 related to the C–O–C stretching vibration of the acid function disappeared as well as the large band at 3400 cm−1.[28,29] A new band corresponding to the neoformed ester bond was observed at 1710 cm−1.
Figure 6.
FT-IR spectra of 1g and Pe recorded at room temperature.
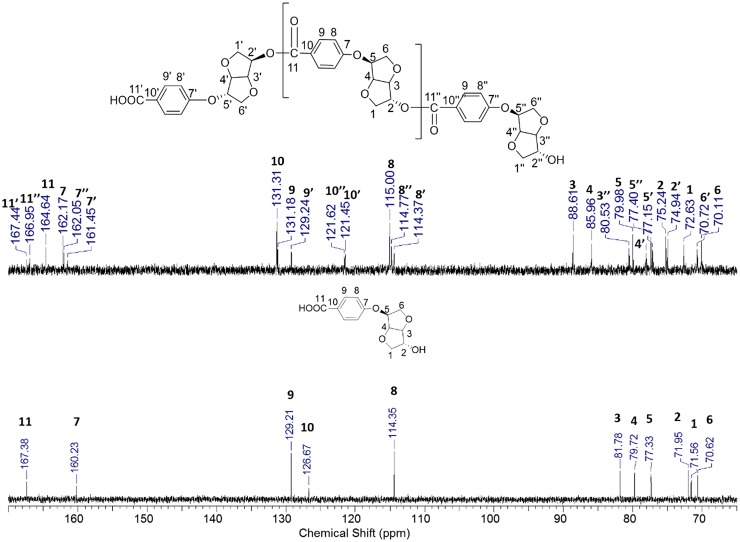
13C NMR spectra of polyester Pe overlaid with starting monomer 1g are shown in Figure 7. We observed the presence of signals with high intensity and others with low ones corresponding to the carbons from the main chain of the polymer and the end chain moieties, respectively. Looking carefully to the 13C NMR spectra superposition of monomer 1g and polymer Pe, we noticed the appearance of newly formed carbonyl signal relative to ester group C11 of the polymer backbone, besides signals C11’ and C11” corresponding to the acid and to the ester functions in the end chain respectively. Moreover, the spectrum showed novel signals owing to the main chain polymer. Several signals were greatly shifted due to the change in the chemical neighborhood. This shift was different from one carbon to another, depending on its location along the polymer chain. Especially, signals corresponding to C3 and C4 in the Isosorbide backbone were detected respectively at 88.6 and 85.7 ppm. This remarkable shift was observed in previously reported Isosorbide based polyester terephthalates.[8] C1 appeared at 72.6 ppm. C2, which is directly linked to the hydroxyl function, shifted from 71.9 to 75.2 ppm. We observed also new signals corresponding to aromatic C9, C8, and C10. The latter remarkably shifted from 126 ppm in the monomer to 131 ppm due to the neoformed ester bond.
Figure 7.
13C spectra of the monomer 1g and the polymer Pe in DMSO-d6.
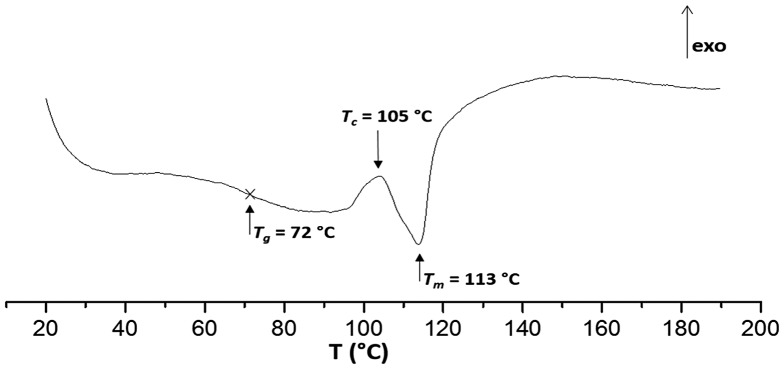
Thermal properties of the polymer Pe were next investigated by DSC analysis (Figure 8). While 1g had a melting point T m = 152–175 °C (Figure 5), we observed for Pe a melting point range of T m = 83–113 °C. As expected, this strategy gratefully afforded semicrystalline polymer starting from Isosorbide ether benzoic acid, which have never been reported. Anterior works reporting the direct use of Isosorbide with terephthaloyl chloride gave rise to random exo/endo sequences alternation rendering the obtained polymer amorphous.[30,31]
Figure 8.
DSC curves of Pe measured between 18 and 250 °C at 10 °C/min heating.
4. Conclusion
New biosourced monomers bearing nitrile, acid, ester, and acid chloride functionalities were synthesized based on Isosorbide and its isomers Isomannide and Isoidide. The aim of this work was to set up a wide range of appealing and promising precursors starting from a renewable platform for biobased AA and AB chemicals derived. The process involved a first solvent-free SNAr step on p-fluorobenzonitrile followed by an optimized quantitative hydrolysis of the nitrile function. The stereochemistry proved to be of great impact on the thermal properties of these monomers which melted in a range from 240 to 375 °C for AA type diacids and from 80 to 175 °C for AB type monomers. For the latter, a comprehensive synthesis strategy was undertaken in order to forecast the scope of obtaining a set of versatile AB type precursors bearing different functionalities. For the first time, polymer based on the novel Isosorbide ether benzoic acid has been successfully synthesized via melt polymerization. The obtained polymer revealed semicrystalline thanks to the stereoregular arrangement provided by the AB pattern .
Table 2. Thermal properties of monomers AA and AB measured by DSC.
| DAH | Product | Yield (%) | Tm (°C) |
|---|---|---|---|
| 1 | 11 | 90 | 130–137 |
| 12 | 98 | 240–300 | |
| 1b | 86 | 84–86 | |
| 1c | 85 | 79–81 | |
| 1d | 95 | 148–156 | |
| 1e | 77 | 125–145 | |
| 1f | 71 | – | |
| 1g | 75 | 152–175 | |
| 1h | 63 | – | |
| 2 | 21 | 84 | 178–184 |
| 22 | 95 | 280–315 | |
| 2b | 55 | 84–85 | |
| 2c | 84 | 119–125 | |
| 2d | 90 | 137–149 | |
| 3 | 31 | 92 | 160–165 |
| 32 | 96 | 300–375 |
Nevertheless, challenges remain to be overcome regarding the processing of such polymers and the improvement of their aspects. These new monomers showed high thermal stability which can contributes to limit sublimation and reduce the color production in melt polymerizations. Moreover, the isosorbide derived AB monomers, thanks to their controlled stoichiometry and stereochemistry, could give rise to polymers with high molecular weights and T g thus increasing thermal stability of polycondensates which is important for polyesters processing such as bottles, films, sheets, and fibers. AA type monomers which showed high thermal stability, could be successfully incorporated into commercial available polymers like PET to afford copolymers with enhanced thermal properties and an inherent biodegradability to help overcoming current environmental problems.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Ministry of Higher Education, Scientific Research, Tunisia, together with EGIDE in the framework of the CMCU project [Project PHC Utique 13G/1211].
Supplemental data
Supplemental data for this article can be accessed here http://dx.doi.org/10.1080/15685551.2016.1239175
Supplementary Material
Acknowledgments
The authors want to thank all INRAP staff for collaboration.
References
- [1]. Aden A, Bozell J, Holladay J et al.. Top value added chemicals from biomass volume I — Results of screening for potential candidates from sugars and synthesis gas. Produced by Staff at the Pacific Northwest National Laboratory (PNNL) and the National Renewable Energy Laboratory (NREL); 2004. [Google Scholar]
- [2]. Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates – the US Department of Energy’s ‘Top 10’ revisited. Green Chem. 2010;12:539-554. [Google Scholar]
- [3]. Gandini A. Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules. 2008;41:9491–9504. [Google Scholar]
- [4]. Gandini A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: a review of recent progress. Polym. Chem. 2010;1:245–251. 10.1039/B9PY00233B [DOI] [Google Scholar]
- [5]. Moreau C, Belgacem M, Gandini A. Recent catalytic advances in the chemistry of substituted furans from carbohydrates and in the ensuing polymers. Top. Catal. 2004;27:11–30. 10.1023/B:TOCA.0000013537.13540.0e [DOI] [Google Scholar]
- [6]. Bechthold I, Bretz K, Kabasci S, et al. . Succinic acid: a new platform chemical for biobased polymers from renewable resources. Chem. Eng. Technol. 2008;31:647–654. 10.1002/(ISSN)1521-4125 [DOI] [Google Scholar]
- [7]. Fenouillot F, Rousseau A, Colomines G, et al. . Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): a review. Prog. Polym. Sci. 2010;35:578–622. 10.1016/j.progpolymsci.2009.10.001 [DOI] [Google Scholar]
- [8]. Sablong R, Duchateau R, Koning CE, et al. . Incorporation of isosorbide into poly(butylene terephthalate) via solid-state polymerization. Biomacromolecules. 2008;9:3090–3097. 10.1021/bm800627d [DOI] [PubMed] [Google Scholar]
- [9]. Kricheldorf HR. “Sugar Diols” as Building Blocks of Polycondensates. J Macromol Sci Part C Polym Rev. 1997;37:599–631. [Google Scholar]
- [10]. Kricheldorf HR, Chatti S, Schwarz G, et al. . Macrocycles 27: cyclic aliphatic polyesters of isosorbide. J. Polym. Sci Part A: Polym. Chem. 2003;41:3414–3424. 10.1002/pola.v41:21 [DOI] [Google Scholar]
- [11]. Kricheldorf HR, Weidner SM. Isohexide derivatives from renewable resources as chiral building blocks. Macromol. Chem. Phys. 2013;214:726–733. 10.1002/macp.v214.6 [DOI] [Google Scholar]
- [12]. Wu J, Eduard P, Jasinska-Walc L, et al. . Fully Isohexide-Based Polyesters: Synthesis, Characterization, and Structure − Properties Relations. Macromolecules. 2013;46:384–394. [Google Scholar]
- [13]. Van Es DS, Koning CE. Fully isohexide-based polyesters: synthesis, characterization, and structure − properties relations. 2013. [Google Scholar]
- [14]. Caouthar AA, Loupy A, Bortolussi M, et al. . Synthesis and characterization of new polyamides based on diphenylaminoisosorbide. J. Polym. Sci. Part A: Polym. Chem. 2005;43:6480–6491. [Google Scholar]
- [15]. Wu J, Eduard P, Thiyagarajan S, et al. . Isohexide derivatives from renewable resources as chiral building blocks. ChemSusChem. 2011;4:599–603. 10.1002/cssc.v4.5 [DOI] [PubMed] [Google Scholar]
- [16]. Thiyagarajan S, Gootjes L, Vogelzang W, et al. . Chiral building blocks from biomass: 2,5-diamino-2,5-dideoxy-1,4-3,6-dianhydroiditol. Tetrahedron. 2011;67:383–389. 10.1016/j.tet.2010.11.031 [DOI] [Google Scholar]
- [17]. Rose M, Palkovits R. Isosorbide as a renewable platform chemical for versatile applications-quo vadis? ChemSusChem. 2012;5:167–176. 10.1002/cssc.201100580 [DOI] [PubMed] [Google Scholar]
- [18]. Wu J, Eduard P, Thiyagarajan S, et al. . Semicrystalline polyesters based on a novel renewable building block. Macromolecules. 2012;45:5069–5080. 10.1021/ma300782h [DOI] [Google Scholar]
- [19]. Bennour H, Medimagh R, Fildier A, et al. . Hyperbranched cyclic and multicyclic poly(etherketone)s by polycondensation of isosorbide and isomannide with 2,6,4’-trifluorobenzophenone and 1,3,5-tris(4-fluorobenzoyl) benzene. High Perform. Polym. 2013;26:144–155. [Google Scholar]
- [20]. Belgacem C, Medimagh R, Fildier A, et al. . Synthesis and characterization of isosorbide-based α, ω-dihydroxyethersulfone oligomers. Des. Monomers Polym. 2015;18:64–72. 10.1080/15685551.2014.947554 [DOI] [Google Scholar]
- [21]. Medimagh R, Mghirbi S, Saadaoui A, et al. . Synthesis of biosourced polyether-amides from 1,4-3, 6-dianhydrohexitols: Characterization by NMR and MALDI–ToF mass spectrometry. C. R. Chim. 2013;16:1127–1139. 10.1016/j.crci.2013.05.004 [DOI] [Google Scholar]
- [22]. Medimagh R, Smaidia MM, Bennour H, et al. . Synthesis and thermal properties evaluation of biosourced poly(ether)ureas and copoly(ether)ureas from 1,4:3,6-dianhydrohexitols. Polym. Int. 2014;64:513–520. [Google Scholar]
- [23]. Medimagh R, Saadaoui A, Mghirbi S, et al. . New biosourced alternated poly(ether)Ester-Amides (PeEA): synthesis and combined NMR/MALDI ToF MS characterization. J. Polym. Res. 2014;21:486–497. 10.1007/s10965-014-0486-4 [DOI] [Google Scholar]
- [24]. M’sahel M, Elmahdi A, Medimagh R, et al. . Synthesis and characterization of novel biosourced building blocks from isosorbide. Des. Monomers Polym. 2016;19:108–118. 10.1080/15685551.2015.1124317 [DOI] [Google Scholar]
- [25]. Feng X, East AJ, Hammond WB, et al. . Overview of advances in sugar-based polymers. Polym. Adv. Technol. 2011;22:139–150. 10.1002/pat.v22.1 [DOI] [Google Scholar]
- [26]. Caouthar A, Roger P, Tessier M, et al. . Synthesis and characterization of new polyamides derived from di(4-cyanophenyl)isosorbide. Eur. Polymer J. 2007;43:220–230. 10.1016/j.eurpolymj.2006.08.012 [DOI] [Google Scholar]
- [27]. Pfützenreuter R, Helmin M, Palkovits S, et al. . Reaction network analysis and continuous production of isosorbide tert-butyl ethers. Catal. Today. 2014;234:113–118. 10.1016/j.cattod.2014.03.007 [DOI] [Google Scholar]
- [28]. Abenhaïm D, Loupy A, Munnier L, et al. . Selective alkylations of 1,4:3,6-dianhydro-d-glucitol (isosorbide). Carbohydr. Res. 1994;261:255–266. 10.1016/0008-6215(94)84022-9 [DOI] [Google Scholar]
- [29]. Johnson SL, Rumon KA. Infrared spectra of solid 1: 1 pyridine-benzoic acid complexes; the nature of the hydrogen bond as a function of the acid-base levels in the complex. J. Phys. Chem. 1965;69:74–86. 10.1021/j100885a013 [DOI] [Google Scholar]
- [30]. Hadzi D, Sheppard N. The infra-red absorption bands associated with the COOH and COOD groups in dimeric carboxylic acids. I. The region from 1500 to 500 cm−1 . Proc. R. Soc. A: Math. Phys. Eng. Sci. 1953;216:247–266. 10.1098/rspa.1953.0020 [DOI] [Google Scholar]
- [31]. Storbeck R, Rehahn M, Ballauff M. Synthesis and properties of high-molecular-weight polyesters based on 1,4: 3,6-dianhydrohexitols and terephthalic acid. Die Makromol. Chem. 1993;194:53–64. 10.1002/macp.1993.021940104 [DOI] [Google Scholar]
- [32]. Storbeck R, Ballauff M. Synthesis and thermal analysis of copolyesters deriving from 1,4:3,6-dianhydrosorbitol, ethylene glycol, and terephthalic acid. J. Appl. Polym. Sci. 1996;59:1199–1202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.