Abstract
Background:
Nerve conduction studies are performed to diagnose the disorders of the peripheral nervous system. The reference values for nerve conduction velocity (NCV) and late responses for different nerves considerably vary in different group and type of population. Physiological factors such as age, temperature, height, and gender affect the NCV. However, there are very few studies which show the age group at which these changes become significant.
Aim and Objectives:
The aim of the study was to establish the electrophysiological data of the specific age group at which changes in NCV as well as late responses of median common peroneal nerve and also see the late response in the form of F-waves and H-reflex.
Methodology:
Study groups were divided into three categories based on the age: Group I (18–30 years) (n = 80), Group II (31–45 years) (n = 43), and Group III (46–60 years) (n = 27). Out of which, 93 patients were male and 57 were female. The NCVs were determined for median, common peroneal nerve (motor component and sensory component) along with late responses in the form of H-reflex and F-waves.
Results:
The mean and standard deviation of median, ulnar, peroneal, and tibial nerve was studied for latencies, amplitude, and velocities for both sensory and motor components. Patients with older age had longer latencies, smaller amplitudes, and slower conduction velocities compared with the younger age group. The change with age was greater in sensory nerve conduction and late responses in all the peripheral nerves.
Conclusions:
Aging has a definite correlation with the NCV and late responses of different peripheral nerves. There is a need to have reference values with relation to age.
Keywords: Gender, median, motor, nerve conduction velocity, peroneal, sensory
INTRODUCTION
Nerve conduction study (NCS) is the measure of electrical activity in a nerve. Peripheral nerves are used most commonly to measure nerve conduction velocities (NCVs).[1,2] Aging is the process that is often accompanied by changes which include slowing in muscle contractility, alteration in muscle metabolism and neuromuscular junction, and reduction in NCV. Studies have proved that the motor and sensory conduction velocities in newborn were 40%–50% of adult values, and at 3 years of age, the normal values were in the adult range for all motor and sensory NCV (SNCV).[2] Various factors influence NCS which includes age, height, gender, and body mass index.[1,2,3,4,5,6,7,8,9,10,11,12] However, few studies have been reported that show the correlation of peroneal F-wave, tibial F-wave, and H-reflex, which were performed only in one age group.[9,10,13] Thus, there is a paucity of data for changes in NCV and late response changes in different age groups.[5,6,7,8,9,10,11,12,13,14,15] Although most of the researchers agree that aging alters nerve conduction studies (NCSs), there is no specific clearly defined age group at which these changes occur.[1,2,3,4,5,6,7,8,9,10,11,12,13,14] Thus, this study aimed to analyze the effect of aging on NCV and late response studies of different age groups and to determine the group at which there are significant changes in the values and also to provide electrophysiological data for commonly tested upper and lower limb nerves in carefully screened healthy adults.
METHODOLOGY
The study was conducted in March 2014 to September 2014. The patients included in the study were 2nd-year medical and nursing students (age ≥18 years) and nonteaching staff (attenders, sweepers, clerks, and their family members of the required age group) of Meenakshi Medical College Hospital and Research Institute. Healthy individuals of different age groups (18–60 years) were selected.
Exclusion criteria
Any individual of neurological disorder or neuromuscular transmission disorder
Any individual suffering from diabetes, hypertension, renal disorder, and thyroid disorders
Any individual suffering from weakness of the upper limb and lower limb or myopathy or with a history of any neurological illness, alcoholics, smokers, obesity, and leprosy were excluded from the study. After getting ethical approval for the study, informed consent was obtained from every individual who volunteered to participate in the study. The examination was performed in a calm setting after the patient was thoroughly explained about the procedure and rest for 30 min. The considerable gap was given between examinations, so as to minimize discomforts to patient as well as to enhance their enthusiastic participation. Study groups were divided into three categories based on the age: Group I (18–30 years) (n = 80), Group II (31–45 years) (n = 43), and Group III (46–60 years) (n = 27). Out of which, 93 patients were male and 57 were female. After taking detail personal, family, and dietary history, detailed general examination and systemic examination were done. Patients were made comfortable and the procedure properly explained. Any doubts were clarified and only those who volunteered were included in the study.
Nerve conduction study
NCS was performed using the RMS EP MARK II machine. Temperature in the study room was maintained constant at 22°C–26°C. The motor NCSs were performed for median, peroneal, and tibial nerves.
Measurement of nerve conduction velocity
Data of distal motor latency (DML), motor NCV, and compound muscle action potentials (CMAPs) from the distal stimulation were analyzed for each patient. The DML is the time from the stimulus to the initial CMAP deflection of the baseline. The amplitude of CMAP was measured from the baseline to the negative peak. The onset latency is the time from the stimulus to the initial negative deflection of the baseline for a biphasic sensory nerve action potential (SNAP) or to the initial positive peak for a triphasic SNAP. For each patient, the recording data of SNAP and SNCV were included in the study. For each recording, the amplitude was measured from the baseline to the negative peak. Surface electrodes were used for the study. The recording electrodes were fixed to the patient's skin using adhesive tape; skin was prepared by scrubbing with disinfectant.
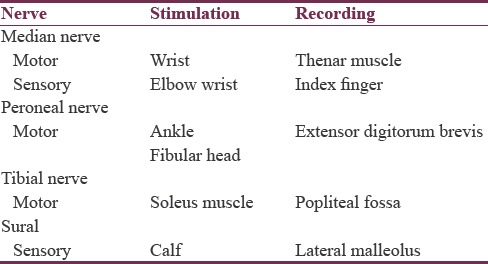
The targeted nerve was stimulated supramaximally using current with duration of 0.2 ms with the stimulator, and the action potential was picked up by the recording electrode. The length of each nerve was measured with a flexible measuring tape. Ground electrode was placed in between the stimulating and recording electrodes for safety reasons. Table 1 shows the sites for stimulation of different nerves.
Table 1.
Sites for stimulation of different nerves
Measurement of late responses
The late response study included H-reflex study and median, peroneal, and tibial F-wave studies. The stimulation and recording sites were the same as those of the motor NCS except that the cathode was placed distally. Results were based on the tracings of the supramaximal stimulations. Ten artifact-free responses were recorded. Data of minimal latency in the F-wave study were included in this study. The H-reflex study was recorded at the soleus muscle and stimulated at the popliteal fossa of the tibial nerve. The latency of the H-reflex was also included in this study. All data were entered and analyzed using SPSS software version 17 Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA). The mean values of the three age groups were compared using one-way ANOVA tests. Karl Pearson's correlation was used to show the correlationship between aging, NCVs, and late responses.
RESULTS
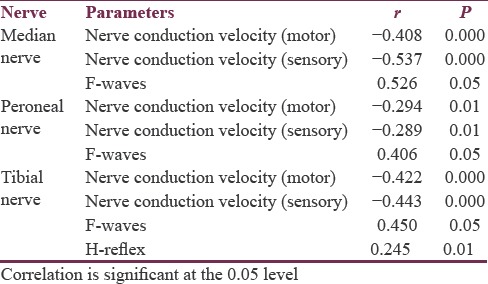
In the present study, 150 patients were recruited for different age groups (93 males and 47 females; age range: ≥18–65 years). Their body height ranged from 146.5 to 172.5 cm and the weight ranged from 40.5 to 90.8 kg. The above-mentioned data are listed in Table 2. Males had significantly higher height (P < 0.001) and weight (P < 0.001) than females. Table 3 shows CMAP for median, peroneal, and tibial nerve for different age groups. Longer latencies, smaller amplitude of MAP, and slower NCV were seen significantly with increase in age. The longer latencies of F-wave with increasing age are significant for all the nerves but are highly significant for common peroneal nerve. Table 4 shows SNAP for the peripheral nerves. Table 5 shows correlation between age and NCV (motor, sensory) of different nerves along with H-reflex and F-wave. Karl Pearson's correlation was used to assess the correlation between NCV, late responses, and age. There was a significant negative correlation between age and both median motor and sensory nerves with R value of −0.408 and −0.537. There was a significant negative correlation between age and both peroneal motor and sensory nerves with R value of −0.294 and −0.289. There was a significant negative correlation between age and both tibial motor and sensory nerves with R value of −0.422 and −0.443. Latencies of F-waves have stronger positive correlation with age for all the examined nerves (median F-waves [r = 0.526], peroneal F-waves [r = 0.406], and tibial F-waves [r = 0.450]). H-reflex (r = 0.245) showed positive correlation with aging.
Table 2.
Comparison of mean height and weight and nerve conduction velocity for male and female

Table 3.
Comparison of median nerve and common peroneal motor nerve conduction velocity between the three age groups
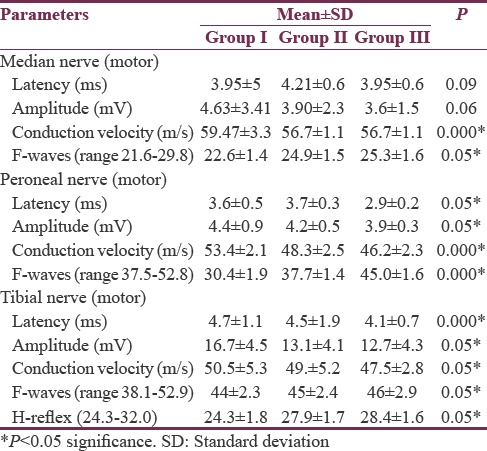
Table 4.
Comparison of sensory nerve conduction velocities for median nerve and peroneal nerve between the three age groups
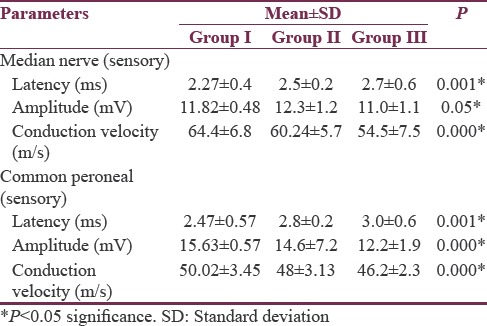
Table 5.
Effects of age and, height on nerve conduction parameters in the study subjects by correlation and multiple regression analysis
DISCUSSION
There are various factors which tend to influence NCS that includes age, height, gender, and body mass index.[1,2,3,4,5] Aging is progressive, generalized and associated with impairment of body functions resulting in loss of adaptive response to stress, and is associated with the risk of age-related diseases. Due to the different effects of nerve degeneration on aging, older patients tend to have longer distal latency, smaller CMAP and SNAP, slower NCV, and longer latency of late responses than younger patients. The reason for this is decreased nerve fibers, reduction in nerve diameter, and change in fiber membrane due to ageing.[2,12] Unlike other studies, our study aimed at finding the relation between age and the median, peroneal, and tibial NCV for both sensory and motor components along with late responses in the form of F-wave and H-reflex. Our observations showed a significant reduction in median SNCV with aging. We had grouped our patients into three groups based on age. Comparing the conduction velocities between the three age groups, the decreasing trend was well observed in the age group of ≥46 years.[5,6,7] Hence, it is evident that the values start decreasing as early as 40 years. In a study by Huang et al., effects of age, gender, height, and weight on late responses and NCS parameters showed that factors of gender, age, height, and weight influence results of late responses and NCS. Except for motor velocity, height and gender are important factors in F-wave studies while height and age are important in H-reflex study.[5] Age and gender are important factors in motor NCS. The equation on late response shows higher squared correlation coefficient than NCS. Without adjustments for these factors, the sensitivity and specificity of NCS will decrease when using the same reference data in patients with different gender, age, height, or weight. Similar findings were reported by studies[6,7,8,9] which state that conduction velocity begins to decline after 30–40 years of age, but the values normally change by < 10 m/s by the 60th years or even the 80th years.[12,13,14] The decline in nerve conduction and rise in sensory latency with increasing age may be due to loss of myelinated and unmyelinated nerve fibers in peripheral nerves with aging.[10] Senthilkumari et al. have concluded in their study that age has definite correlation with the NCS in median motor and sensory nerves. It is essential to have reference values with relation to age.[16] Age has definite effects on the duration of motor and sensory nerves; different nerves have different timing of aging. Without adjustment for age, the sensitivity and specificity of NCS will decrease when using the same reference data in patients with different age.[5,6,16] The age factor was negatively correlated to the amplitude in both motor and sensory NCSs and velocity in motor NCS.[15] The F-wave and H-reflex were positively correlated to age and height, but negatively correlated to velocity[14,15] and the same is proved in our study, we enrolled the data from persons without symptoms and signs of any neurological disorder. Awang et al. in their study revealed that there was no significant reduction in median sensory conduction speeds across different age groups, they also observed a significant reduction in median motor conduction velocity with increasing age.[8] Our study results were correlated well with the study by Tong et al. who, in their prospective cohort study, found median sensory velocities to decrease at a rate of 0.14 m/s per year of age.[10] Werner et al. in their article observed a decrease in conduction velocity at a rate of 0.41 m/s per year of age.[11] Falco et al. have shown a 10% reduction in the conduction rate at 60 years of age.[12] Interestingly, we also noted that the voltage of electrical stimulus which is needed to record a threshold action potential also increases with advancing age. We also found that, in Group III patients, an electrical stimulus of 40–50 mV was needed as compared to Group I which needed 15–25 mV. The reason for this may be decreased excitability and conduction velocity of the nerves which can be explained by the hypothesis that there is an increase in oxygen-free radicals with aging which tend to damage the enzyme systems in the mitochondria which leads to a decrease in ATP production resulting in slowing of muscle contraction, alteration in muscle metabolism, and neuromuscular junction.[13,14,15] Changes in age on NCS are greater in the nerves of lower extremities than in the median nerve in the upper limb. The results are compatible with a report on two time-point paradigms to investigate median and ulnar sensory NCS.[6] The effect of age on F-wave latency is reported to increase 0.03 ms/year in the upper and 0.1 ms/year in the lower limb.[8] In [Table 5], the results show a significant effect of age on F-wave latency in the median F-wave by increasing 0.02 ms/year in median nerve (r = 0.526), on F-wave latency in personal nerve (r = 0.406), and on F-wave latency of tibial nerve (r = 0.450). Age is an important factor in H-reflex by increasing almost by 0.04 ms/year (r = 0.245), which is in similar line of the study of Huang et al. who showed similar findings in median nerve.[5] Thus, it is essential to have reference values for the different age groups while conducting NCS.
CONCLUSIONS
Age can affect both conduction velocities as well as delayed responses in different peripheral nerves.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Robinson LR, Rubner DE, Wahl PW, Fujimoto WY, Stolov WC. Influences of height and gender on normal nerve conduction studies. Arch Phys Med Rehabil. 1993;74:1134–8. [PubMed] [Google Scholar]
- 2.Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve. 1992;15:1095–104. doi: 10.1002/mus.880151007. [DOI] [PubMed] [Google Scholar]
- 3.Hennessey WJ, Falco FJ, Goldberg G, Braddom RL. Gender and arm length: Influence on nerve conduction parameters in the upper limb. Arch Phys Med Rehabil. 1994;75:265–9. doi: 10.1016/0003-9993(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 4.Bolton CF, Carter KM. Human sensory nerve compound action potential amplitude: Variation with sex and finger circumference. J Neurol Neurosurg Psychiatry. 1980;43:925–8. doi: 10.1136/jnnp.43.10.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CR, Chang WN, Chang HW, Tsai NW, Lu CH. Effects of age, gender, height, and weight on late responses and nerve conduction study parameters. Acta Neurol Taiwan. 2009;18:242–9. [PubMed] [Google Scholar]
- 6.Bhorania S, Ichaporia RB. Effect of limb dominance on motor nerve conduction. Indian J Physiol Pharmacol. 2009;53:279–82. [PubMed] [Google Scholar]
- 7.Cai F, Zhang J. Study of nerve conduction and late responses in normal Chinese infants, children, and adults. J Child Neurol. 1997;12:13–8. doi: 10.1177/088307389701200102. [DOI] [PubMed] [Google Scholar]
- 8.Awang MS, Abdullah JM, Abdullah MR, Tharakan J, Prasad A, Husin ZA, et al. Nerve conduction study among healthy Malays. The influence of age, height and body mass index on median, ulnar, common peroneal and sural nerves. Malays J Med Sci. 2006;13:19–23. [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkwood TB. Evolutionary theory and the mechanism of aging. In: Tallis RC, Fillit HM, Brocklehurst JC, editors. Textbook of Geriatric Medicine and Gerontology. 5th ed. London: Churchill Livingston; 1998. p. 45. [Google Scholar]
- 10.Tong HC, Werner RA, Franzblau A. Effect of aging on sensory nerve conduction study parameters. Muscle Nerve. 2004;29:716–20. doi: 10.1002/mus.20026. [DOI] [PubMed] [Google Scholar]
- 11.Werner RA, Franzblau A, D’Arcy HJ, Evanoff BA, Tong HC. Differential aging of median and ulnar sensory nerve parameters. Muscle Nerve. 2012;45:60–4. doi: 10.1002/mus.22233. [DOI] [PubMed] [Google Scholar]
- 12.Falco FJ, Hennessey WJ, Braddom RL, Goldberg G. Standardized nerve conduction studies in the upper limb of the healthy elderly. Am J Phys Med Rehabil. 1992;71:263–71. doi: 10.1097/00002060-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Eman AM, Mye A, Basheer S, Waly H, Elkholy N. Egyptian demographic effect on Median nerve conduction studies. Egypt J Neurol Psychiatry Neurosurg. 2013;50:277–84. [Google Scholar]
- 14.Marbut MM, Najim RS, Mohsen MA. Determination of normal values of nerve conduction of tibial and peroneal nerves among normal healthy subjects. Tikrit Med J. 2012;18:1–8. [Google Scholar]
- 15.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 16.Senthilkumari KR, Umamaheswari, Bhaskaran M. A study on median nerve conduction velocity in different age groups. Int J Res Med Sci. 2015;3:3313–7. [Google Scholar]


