Abstract
Background:
Gut microflora influences neural development through complex mechanisms. Feeding practices, especially breastfeeding influence gut microbiome and thereby play a pivotal role in immune and neural development. Current understandings of the role of healthy distal gut microflora in the development of immune and neural systems provide insights into immunological mechanisms as one of the possible etiologies in autism spectrum disorder (ASD). Studies have shown that optimal breastfeeding is associated with lower odds of being at-risk for ASD and children with ASD are suboptimally breastfed.
Methods:
The feeding practices of children with ASD (n = 30) was compared to their typically developing siblings as matched controls (n = 30). Information regarding feeding practices was collected from mothers through a semi-structured questionnaire.
Results:
About 43.3% of children with ASD received exclusive breastfeeding, whereas 76.7% of their typically developing siblings were exclusively breastfed. Exclusive breastfeeding was associated with lower odds for ASD (odds ratio [OR] = 0.166; 95% confidence interval [CI] = 0.025–0.65), while early introduction of top feeds was associated with higher odds (OR = 6; 95% CI = 1.33–55.19). Difficulties in breastfeeding were attributed to child-related factors in 13.2% of the children.
Conclusion:
Children with ASD are suboptimally breastfed compared to their typically developing siblings. Exclusive breastfeeding may confer protection in vulnerable children. Further studies on larger prospective sample are required to establish the association.
Keywords: Autism spectrum disorder, breastfeeding, gut microbiome, immunological hypothesis
INTRODUCTION
The genetic etiology of Autism spectrum disorder (ASD) has been extensively studied and is supported by accumulating evidence.[1] The recent increasing trends in the prevalence of ASD globally has fueled a renewed interest in research into other possible etiologies.[2] Current understandings of the role of healthy distal gut microflora in the development of immune and neural systems provide insights into “Immunological mechanisms” as one of the possible etiologies in ASD.[3]
“Gut microbiome” influences neural development through complex mechanisms and is in turn influenced by the neural system. Abnormal gut flora leads to dysfunction in the “Brain-gut-microbiome axis.”[4]
Feeding practices, especially breastfeeding during the critical phase of the child's development plays a pivotal role in immune and neural development. The components of breast milk such as IgA, transforming growth factor-β, interleukin-10, erythropoietin, and lactoferrin stimulates intestinal host defenses as well as prevents inflammation.[5] The unique nutritional components of breast milk, more so of colostrum such as appropriate proportions of essential fatty acids, antioxidants, and growth factors are essential for neural and long-term cognitive development.[6] Apart from conferring protection against infections, breastfeeding also influences colonization of healthy microflora in the distal gut.[7] Gut microbiome and thereby optimal neural development is influenced by feeding practices in children.
International studies have shown that children with ASD are suboptimally breastfed.[8,9,10] Studies also have found variations in the distal gut microflora, with clostridial preponderance in children with ASD compared to typically developing peers[11] and a significant difference in children who are suboptimally breastfed or top-fed in the early years of critical brain development.[12] A previous study in the Indian settings showed that exclusive breastfeeding in children was associated with lower odds of being at-risk for ASD (odds ratio [OR] = 2.03; [95% confidence interval [CI] 0.91–4.49]).[13]
With the background of these findings, we conducted a case–control study to explore the patterns of breastfeeding in children diagnosed with ASD compared to their typically developing siblings.
MATERIALS AND METHODS
The study was conducted in the child guidance clinic of a tertiary care teaching hospital in India from May 2015 to June 2016. All consecutive children aged between 2 and 6 years, fulfilling diagnostic criteria for ASD according to DSM 5,[14] with a typically developing sibling were considered eligible for participation. Children, >6 years were excluded to minimize recall bias. The study was approved by Institute Ethics Committee. Informed consent was obtained from parents.
Thirty children with ASD and their typically developing siblings as matched controls were enrolled in the study. Siblings were chosen as controls to address potential confounding factors such as socioeconomic status, maternal education, and cultural factors that might influence feeding practices.
DSM 5 criteria[14] were employed to ascertain the diagnosis of ASD and childhood autism rating scale (CARS)[15] was used to assess the severity of autism. Mothers were interviewed regarding the feeding practices of both the children. Information regarding time of initiation of breastfeeding, whether optimal exclusive breastfeeding was done, time of introduction of top feeds, age at introduction of complementary feeds and weaning was collected through a semi-structured questionnaire. In this study, optimal breastfeeding was defined as exclusive breastfeeding up to 6 months, as per recommendations of the World Health Organization.[16] Mothers were also enquired regarding the difficulties encountered in breastfeeding, and the same was categorized into child- and mother-related factors.
Statistical analyses
Frequencies and percentages were used to represent categorical variables and means and standard deviations to represent continuous variables. The association between the feeding practices and the child being diagnosed as ASD was done using McNemar test and represented as OR with 95% CI. All tests were two-tailed and a P < 0.05 was considered statistically significant.
RESULTS
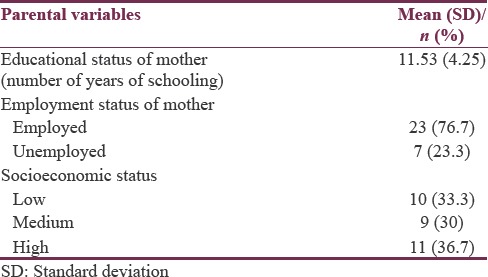
The clinical profile and sociodemographic details of participating families are depicted in Tables 1 and 2. In our study sample, mean age of children with ASD was 49.6± (21.1) months and siblings were 51.8± (20.4) months. The gender makeup was also comparable between the two groups. However, most children with ASD were first-born, and this difference in birth order was statistically significant. Average educational status of mothers was found to be 11.53± (4.2) years of schooling. Twenty-three mothers were employed, and seven were unemployed. The mean CARS score was 35.86 (±3.32).
Table 1.
Clinical profile of children with ASD and their typically developing siblings
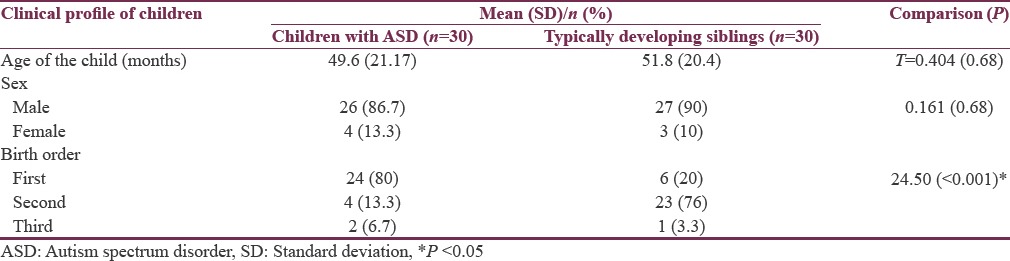
Table 2.
Parent-related factors
A significant difference in feeding practices between children with ASD and their siblings was noted. 76.7% of typically developing siblings were exclusively breastfed. Whereas, only 43.3% of children with ASD received exclusive breastfeeding up to 6 months of age and about 56.7% were introduced to top-feeds during the first 6 months of development [Table 3].
Table 3.
Comparison of feeding practices of children with autism spectrum disorder and their typically developing siblings

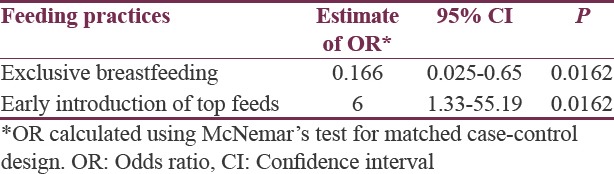
Comparing the feeding practices, children who had received exclusive breastfeeding had decreased odds of being diagnosed with ASD (OR = 0.167, 95% CI = 0.025–0.65, P = 0.0162), whereas those with early introduction of top feeds had increased odds (OR = 6.0, 95% CI = 1.33–55.19, P = 0.0162) [Table 4]. There was no association found between the severity of autism and lack of exclusive breastfeeding or early introduction of top feeds (χ2 = 0.344, P = 0.558).
Table 4.
Association of feeding practices with diagnosis of autism spectrum disorder
DISCUSSION
Recently, there is a global rise in the prevalence of ASD.[17] As genetic contribution alone does not entirely explain this alarming rise, there is a paradigm shift toward exploring environmental factors as possible etiological agents.[18] On the one hand, studies have found that children with ASD have comorbid gastrointestinal disorders more frequently compared to typically developing children and children with other neurodevelopmental disorders.[19] Studies report that food selectivity in children with ASD could be possibly to avoid gastrointestinal discomfort arising from impaired digestion as a result of altered gut flora.[20] On the other hand, extensive research has provided insights on gut microbiome, its role in neurodevelopment and the complex and atypical patterns of feeding in autism which potentially alters the gut microflora.[3] It was thus hypothesized that the brain-gut axis has a significant role in the development of ASD.
Human gut colonization by healthy microflora plays a pivotal role in immune and neural development. Gut microbiome regulates neural systems by activation of afferent circuits, mucosal immune responses and production of metabolites influencing central nervous system (CNS) and is in turn influenced by the CNS through regulation of neurotransmitters.[21] Optimal breastfeeding confers protective factors such as IgA, lactobacillus and bifidobacterium and facilitates healthy gut-micro flora symbiosis.[22] Gut colonization by healthy microflora in the critical period of development is influenced by breastfeeding and formula feeds in different directions.[23] Lack of protective factors results in an increase in harmful organisms and can lead to disruption of “Brain-Gut microbiome axis” and has been implicated as a possible etiology in neurodevelopmental disorders, especially ASD. This hypothesis is supported by animal models and is replicated in many international studies. In addition to the above-mentioned mechanisms, increased intestinal permeability or “leaky gut” has been demonstrated in children with ASD.[24] Altered microbiota and increased intestinal permeability due to activation of zonulin pathways are being implicated in the immune-mediated pathogenesis of ASD.[25]
In summary, the findings of our study are (1) children with ASD are suboptimally breastfed compared to their typically developing siblings. (2) Exclusive breastfeeding is associated with lower odds, while the early introduction of top feeds is associated with higher odds for ASD.
Our findings are consistent with existing literature. In this study, siblings were chosen as controls to address potential confounding factors that could influence feeding practices. In addition, within the family, both the children share the genetic loading and are equally vulnerable to develop ASD. Sibling study designs in ASD have been shown to uncover the role of environmental exposures in epigenetically modifying the gene expressions.[26] Furthermore, there was a significant difference in birth order observed in our study, more children with ASD being first born. This finding is in line with existing literature on birth order effect and risk of ASD.[27]
From the perspective of bonding, early initiation of breastfeeding can facilitate mother-infant bonding and attachment. Oxytocin is crucial in determining mother-infant bonding and attachment in early stage of development.[28] Role of oxytocin has been widely studied in ASD.[29]
It is important to note that only 43.3% of children with ASD received exclusive breastfeeding, whereas 76.7% of their typically developing siblings were exclusively breastfed. Children who did not receive exclusive breastfeeding had early introduction of top feeds such as infant-formulas or feeding practices as per sociocultural norms.
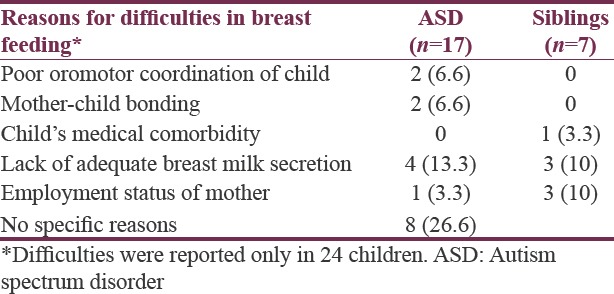
In our study, among children with ASD, child-related factors such as poor oromotor coordination, mother-child bonding that could have contributed to feeding difficulties were reported in 4 (13.2%) children. Maternal factors such as lack of adequate breast milk secretion and employment-related issues were contributory to early initiation of top feeds in 16% of children [Table 5]. There could be the possible contribution of other environmental factors apart from breastfeeding, and these have not been addressed in the current study.
Table 5.
Parental report of difficulties in breastfeeding
There is growing evidence in favor of gut microbiome and suboptimal breastfeeding practices during the critical developmental period as a possible environmental contribution, supporting the immunological theories in ASD.[30] However, this is controversial, and there is no established evidence to conclude that breastfeeding can prevent ASD. Yet, it is worth exploring the hypothesis as it could provide more insight into interventions. Currently, there are dietary interventions such as gluten-free and casein-free diet as a proposed treatment option for children with ASD, however, lack of evidence.[31] The role of microbiota-targeted therapies such as probiotics and antibiotics are also being studied.[32,33] Promoting optimal breastfeeding has already been under implementation and acceptable across settings. To overcome child-related and maternal sociooccupational factors, promoting the use of expressed breast milk (EBM) can be an effective alternative to confer the protective factors of breastfeeding in children at risk for ASD.[34] In our study, only two parents have used EBM due to feeding difficulties, yet not as exclusive feeds. This may have sociocultural relevance as the practice of EBM is less common in India.[35]
Limitations
Small sample size and possible recall bias in mothers are the limitations of our study. The role of comorbid psychiatric disorders like intellectual disability has not been accounted. Future studies with prospective design can help in establishing a cause-effect relationship. Studies in future, looking into the finer details of top feed practices can provide further insights into the association.
CONCLUSION
Children with ASD are suboptimally breastfed compared to their typically developing siblings. Breastfeeding may confer protection, and early introduction of top feeds is likely to increase the odds of being at risk for Autism in vulnerable children. Encouraging optimal breastfeeding and promoting EBM feeds can be effective and feasible interventions targeting possible environmental and immunological mechanisms underlying ASD. Further studies on the larger prospective sample are required to establish the association.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Vikas Menon and Dr. Pooja Patnaik Kuppili for their inputs on statistical methods.
REFERENCES
- 1.Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–86. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- 2.Wing L, Potter D. The epidemiology of autistic spectrum disorders: Is the prevalence rising? Ment Retard Dev Disabil Res Rev. 2002;8:151–61. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- 3.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: A new frontier in autism research. Curr Psychiatry Rep. 2013;15:337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz Heijtz R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–7. doi: 10.1016/j.siny.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156:S3–7. doi: 10.1016/j.jpeds.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 7.Bar S, Milanaik R, Adesman A. Long-term neurodevelopmental benefits of breastfeeding. Curr Opin Pediatr. 2016;28:559–66. doi: 10.1097/MOP.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 8.Al-Farsi YM, Al-Sharbati MM, Waly MI, Al-Farsi OA, Al-Shafaee MA, Al-Khaduri MM, et al. Effect of suboptimal breast-feeding on occurrence of autism: A case-control study. Nutrition. 2012;28:e27–32. doi: 10.1016/j.nut.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M, et al. Breastfeeding, infant formula supplementation, and autistic disorder: The results of a parent survey. Int Breastfeed J. 2006;1:16. doi: 10.1186/1746-4358-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanoue Y, Oda S. Weaning time of children with infantile autism. J Autism Dev Disord. 1989;19:425–34. doi: 10.1007/BF02212940. [DOI] [PubMed] [Google Scholar]
- 11.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16:444–53. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54:987–91. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- 13.Ravi S, Chandrasekaran V, Kattimani S, Subramanian M. Maternal and birth risk factors for children screening positive for autism spectrum disorders on M-CHAT-R. Asian J Psychiatr. 2016;22:17–21. doi: 10.1016/j.ajp.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 14.5th ed. Washington, DC: American Psychiatric Publishing; 2013. Association American Psychiatric. Diagnostic and Statistical Manual of Mental Disorders; p. 991. [Google Scholar]
- 15.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 16.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews. 2012;(8) doi: 10.1002/14651858.CD003517.pub2. Art. No.: CD003517. DOI: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5:160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld CS. Microbiome disturbances and autism spectrum disorders. Drug Metab Dispos. 2015;43:1557–71. doi: 10.1124/dmd.115.063826. [DOI] [PubMed] [Google Scholar]
- 19.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44:1117–27. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S, et al. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol Psychiatry. 2016;21:738–48. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinkiewicz G, Nordström EA. 353 occurrence of Lactobacillus reuteri, lactobacilli and bifidobacteria in human breast milk. Pediatr Res. 2005;58:415. [Google Scholar]
- 23.Le Huërou-Luron I, Blat S, Boudry G. Breast- v.formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23:23–36. doi: 10.1017/S0954422410000065. [DOI] [PubMed] [Google Scholar]
- 24.Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, et al. Increased serum zonulin levels as an intestinal permeability marker in autistic subjects. J Pediatr. 2017;188:240–4. doi: 10.1016/j.jpeds.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Fasano A, Hill I. Serum zonulin, gut permeability, and the pathogenesis of autism spectrum disorders: Cause, effect, or an epiphenomenon? J Pediatr. 2017;188:15–7. doi: 10.1016/j.jpeds.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Wade M, Prime H, Madigan S. Using sibling designs to understand neurodevelopmental disorders: From genes and environments to prevention programming. Biomed Res Int 2015. 2015 doi: 10.1155/2015/672784. 672784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durkin MS, Maenner MJ, Newschaffer CJ, Lee LC, Cunniff CM, Daniels JL, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168:1268–76. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galbally M, Lewis AJ, Ijzendoorn Mv, Permezel M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harv Rev Psychiatry. 2011;19:1–4. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- 29.Green JJ, Hollander E. Autism and oxytocin: New developments in translational approaches to therapeutics. Neurotherapeutics. 2010;7:250–57. doi: 10.1016/j.nurt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berding K, Donovan SM. Microbiome and nutrition in autism spectrum disorder: Current knowledge and research needs. Nutr Rev. 2016;74:723–36. doi: 10.1093/nutrit/nuw048. [DOI] [PubMed] [Google Scholar]
- 31.Millward C, Ferriter M, Calver SJ, Connell-Jones GG. Gluten- and casein-free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews. 2008;(2) doi: 10.1002/14651858.CD003498.pub3. Art. No.: CD003498. DOI:10.1002/14651858.CD003498.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract. 2011;2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert JA, Krajmalnik-Brown R, Porazinska DL, Weiss SJ, Knight R. Toward effective probiotics for autism and other neurodevelopmental disorders. Cell. 2013;155:1446–8. doi: 10.1016/j.cell.2013.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaherman VJ, Lee HC. “Breastfeeding” by feeding expressed mother's milk. Pediatr Clin North Am. 2013;60:227–46. doi: 10.1016/j.pcl.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekambaram M, Bhat V, Ahamed MAP. Knowledge, attitiude and practice of breastfeeding among postnatal mothers. Curr Pediatr. 2010;14:119–124. [Google Scholar]


