Abstract
We conducted a descriptive case study to examine the effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on urinary angiotensinogen excretion, which represents the function of the intrarenal renin–angiotensin system, in patients with type 2 diabetes. An SGLT2 inhibitor (canagliflozin 100 mg/day, ipragliflozin 25 mg/day, dapagliflozin 5 mg/day, luseogliflozin 2.5 mg/day or tofogliflozin 20 mg/day) was administered for 1 month (n=9). ELISA kits were used to measure both urinary intact and total angiotensinogen levels. Treatment with SGLT2 inhibitors significantly decreased hemoglobin A1c, body weight, systolic blood pressure and diastolic blood pressure (8.5±1.3 to 7.5%±1.0%, 82.5±20.2 to 80.6±20.9 kg, 143±8 to 128±14 mm Hg, 78±10 to 67±9 mm Hg, p<0.05, respectively), while urinary albumin/creatinine ratio was not significantly changed (58.6±58.9 to 29.2±60.7 mg/g, p=0.16). Both total urinary angiotensinogen/creatinine ratio and intact urinary angiotensinogen/creatinine ratio tended to decrease after administration of SGLT2 inhibitors. However, these changes were not significant (p=0.19 and p=0.08, respectively). These data suggest that treatment with SGLT2 inhibitors does not activate the intrarenal renin–angiotensin system in patients with type 2 diabetes.
Keywords: SGLT2 inhibitor, urinary angiotensinogen, type 2 diabetes, blood pressure, urinary albumin
Significance of this study.
What is already known about this subject?
Urinary angiotensinogen has been proven to be a useful biomarker for monitoring the intrarenal renin–angiotensin system (RAS).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors may induce polyuria and dehydration. Dehydration activates the internal RAS.
SGLT2 inhibitor increased the urinary angiotensinogen levels of patients with type 1 diabetes (not type 2 diabetes).
What are the new findings?
SGLT2 inhibitor did not increase total urinary angiotensinogen levels of patients with type 2 diabetes (not type 1 diabetes).
SGLT2 inhibitor did not increase intact urinary angiotensinogen levels of patients with type 2 diabetes.
There are not many reports about the change of intact urinary angiotensinogen levels of patients with type 2 diabetes.
How might these results change the focus of research or clinical practice?
SGLT2 inhibitors do not activate RAS in patients with type 2 diabetes.
Introduction
Diabetes mellitus is a common disease worldwide,1 2 and results in many complications such as blindness, renal disease and myocardial dysfunction.3 Sodium-glucose cotransporter 2 (SGLT2) inhibitors increase excretion of glucose in the urine by inhibiting urinary reabsorption of glucose,4 independent of the effect of insulin.5 6 SGLT2 inhibitors block the reabsorption of glucose by SGLT2 at the S1 portion of the proximal tubule in the kidney.7 8 The American Diabetes Association has indicated that SGLT2 inhibitors are the second-line treatment after metformin and other oral hypoglycemic agents, basal insulin and glucagon-like peptide-1 receptor agonists.9 Clinical studies have shown that in patients with type 2 diabetes mellitus, administration of SGLT2 inhibitors resulted in sustained decreases in glycoproteins, hemoglobin A1c (HbA1c), body weight (BW), blood pressure (BP)10 and urine albumin/creatinine ratio.11–13 Recently, the beneficial role of SGLT2 inhibitors on cardiovascular and renal function was reported in the EMPA-REG OUTCOME trial.14 15
However, it has also been reported that SGLT2 inhibitors have many side effects, such as an increase in urine volume and polydipsia. In particular, Haneda et al 16 reported that SGLT2 inhibitors increased hematocrit and blood urea nitrogen/creatinine (BUN) ratio. These data suggest that treatment with SGLT2 inhibitors causes dehydration if water intake is not sufficient. In addition, it is feared that dehydration activates the renin–angiotensin system (RAS) in the kidney. Indeed, Cherney et al 17 reported that urinary total angiotensinogen excretion, which represents local RAS activity in the kidney,18 19 significantly increased owing to treatment with SGLT2 inhibitors in patients with type 1 diabetes mellitus. However, the effect of SGLT2 inhibitors on urinary angiotensinogen excretion in type 2 diabetes patients has not been investigated yet.
Therefore, we conducted a descriptive case study to examine the effects of SGLT2 inhibitors on urinary angiotensinogen in patients with type 2 diabetes.
Methods
Subjects
Studies were conducted on patients with type 2 diabetes whose blood glucose was inadequately controlled with diet/exercise therapy in combination with various oral antihyperglycemic drugs. Fourteen Japanese adults with type 2 diabetes (six men and eight women) aged 60.2±15.9 (31–81) were enrolled in this study. The study ran from September 1, 2014 to September 1, 2015 and was carried out by the Hadanoeki – Minamiguchi Clinic and Kagawa University Hospital. Study subjects had not been hospitalized for metabolic complications. The subjects had HbA1c ≧6.5% and creatinine clearance ≧60 mL/min. The exclusion criteria were insulin dependence, type 1 diabetes, pregnancy, severe anemia, macroalbuminuria, severe illness (including urinary tract infection), chronic liver disease, thyroid disease, adrenal disease and malignancy. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and was approved by the ethics committee of Kagawa University (26-045). All patients gave written informed consent prior to participation.
Study design
In this study, an SGLT2 inhibitor (canagliflozin 100 mg/day, ipragliflozin 25 mg/day, dapagliflozin 5 mg/day, luseogliflozin 2.5 mg/day or tofogliflozin 20 mg/day) was administered once daily for 1 month as an add-on therapy to other oral antihyperglycemic drugs (sulfonylurea, glinide, α-glucosidase inhibitor, biguanide, thiazolidines or dipeptidylpeptide-4 inhibitor), insulin and glucagon-like peptide-1. During the observational period, medications such as antihypertensive agents were not changed. No patient was treated with any diuretics. If BP was not well controlled, the study was stopped. If any adverse events occurred, for example severe skin disorders, liver failure, cardiovascular disorder and cerebrovascular disorder, these patients were excluded. We measured urinary angiotensinogen and urinary albumin before and after administering SGLT2 inhibitors (baseline and after 1 month). HbA1c, BW, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were also measured.
Assays
We measured urinary angiotensinogen levels by using a sandwich ELISA as described.18 20 HbA1c was determined using a latex Glycohemoglobin Standardization Program. Urinary albumin was measured using an assay kit (AutoWako Microalbumin; Wako Pure Chemical Industries, Osaka, Japan) and expressed as a protein to creatinine ratio in spot urine samples. HbA1c was measured by using high-performance liquid chromatography.
Statistical analysis
Urinary angiotensinogen, urinary albumin, BW, HbA1c, SBP and DBP before and after treatment with SGLT2 inhibitors were expressed as mean±SD. Statistical analysis was performed with Student’s t-test, and statistical significance was defined as p<0.05.
Results
The total number of samples in this study was nine (four men and five women). Five patients were excluded from the study due to discontinuation of the SGLT2 inhibitor. Treatment was terminated in five patients because one developed angina pectoris while the other four patients had poor compliance. The mean age of the group was 58.3±17.7 (31–81). Mean HbA1c was 8.5%±1.3%, mean BW was 82.5±20.2 kg, mean SBP was 143±8 mm Hg and mean DBP was 78±10 mm Hg.
Table 1 shows SGLT2 inhibitors significantly decreased BW, HbA1c, alanine transaminase (ALT), γ-glutamyltranspeptidase (γGTP) and total cholesterol. However, aspartate transaminase (AST) was not significantly changed. Creatinine did not change significantly, while BUN was slightly increased after administration of SGLT2 inhibitors. There was no significant relationship between changes in plasma ALT levels and those in intact or total urinary angiotensinogen/creatinine ratio before and after administration of an SGLT2 inhibitor (r=−0.42 and 0.16, respectively). Similarly, there was no significant relationship between changes in plasma AST levels and those in intact or total urinary angiotensinogen/creatinine ratio before and after administration of an SGLT2 inhibitor (r=0.10 and 0.04, respectively).
Table 1.
Laboratory data before and after administration of an SGLT2 inhibitor
| Before | After | p | |
|---|---|---|---|
| AST (U/L) | 26±8 | 27±8 | 0.72 |
| ALT (U/L) | 38±21 | 34±20 | 0.012 |
| γGTP (U/L) | 68±54 | 42±26 | 0.013 |
| BUN (mg/dL) | 14.1±5.4 | 16.8±5.2 | 0.046 |
| Cr (mg/dL) | 0.69±0.17 | 0.74±0.18 | 0.16 |
| T.Cho (mg/dL) | 182±34 | 171±34 | 0.039 |
| HDL-C (mg/dL) | 51±17 | 50±17 | 0.37 |
| TG (mg/dL) | 171±87 | 159±97 | 0.57 |
| Ht (%) | 41±6 | 38±15 | 0.54 |
| UA (mg/dL) | 5.5±1.5 | 5.2±1.2 | 0.22 |
| HbA1c (%) | 8.5±1.3 | 7.5±1.0 | 0.0003 |
| BW (kg) | 82.5±20.2 | 80.6±20.9 | 0.013 |
Before and after administration of SGLT2 inhibitors.
ALT, alanine transaminase; AST, aspartate transaminase; BW, body weight; BUN, blood urea nitrogen/creatinine; γGTP, γ-glutamyltranspeptidase; Cr, creatinine; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein - cholesterol; Ht, hematocrit; SGLT2, sodium-glucose co-transporter 2; T.Cho, total cholesterol; TG, triglyceride; UA, uric acid.
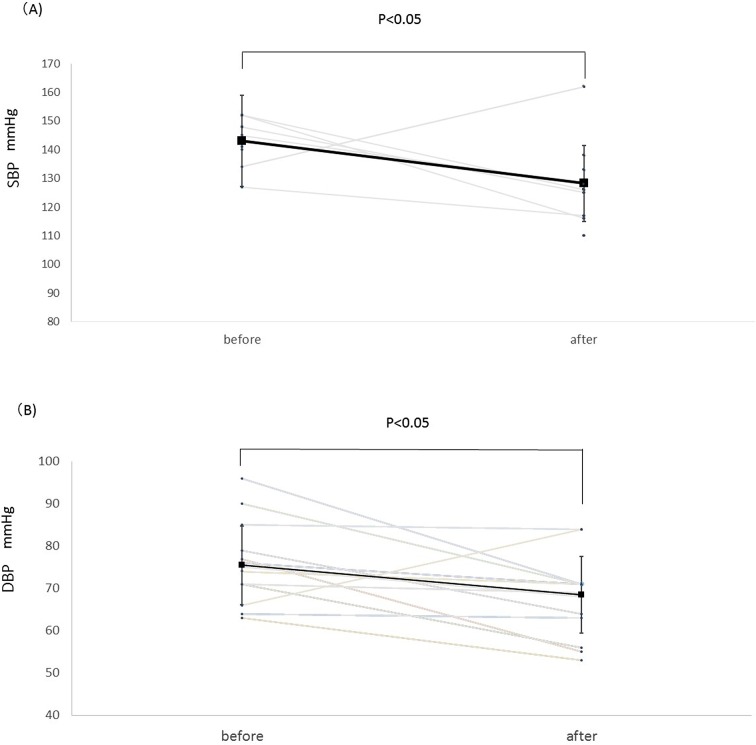
Figure 1A shows the trend in SBP. Treatment with SGLT2 inhibitors significantly decreased SBP from 143±8 mm Hg to 128±14 mm Hg (p<0.05).
Figure 1.
Change in SBP (A) and DBP (B) before and after administration of an SGLT2 inhibitor. Square points are shown as mean. SGLT2 inhibitors tended to decrease SBP and DBP, so these changes are statistically significant (p<0.05) before versus after treatment with an SGLT2 inhibitor. DBP, diastolic blood pressure; SBP, systolic blood pressure; SGLT2, sodium-glucose co-transporter 2.
Similarly, SGLT2 inhibitors significantly decreased DBP from 78±10 mm Hg to 67±9 mm Hg (p<0.05, figure 1B).
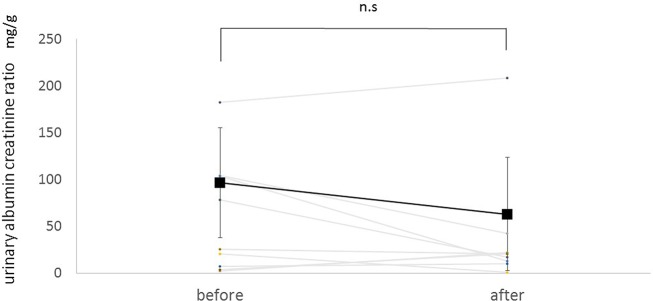
Figure 2 shows the trend in urinary albumin/creatinine ratio. Mean ratio at baseline was 58.6±58.9 mg/g. Treatment with SGLT2 inhibitors tended to decrease urinary albumin/creatinine ratio by 29.4 mg/g. However, these changes were not statistically significant (p=0.16).
Figure 2.
Change in urinary albumin/creatinine ratio before and after administration of an SGLT2 inhibitor. Square points are shown as mean. SGLT2 inhibitors tended to decrease urinary albumin/creatinine ratio, but these changes are not statistically significant (p=0.16). n.s., not significant; SGLT2, sodium-glucose co-transporter 2.
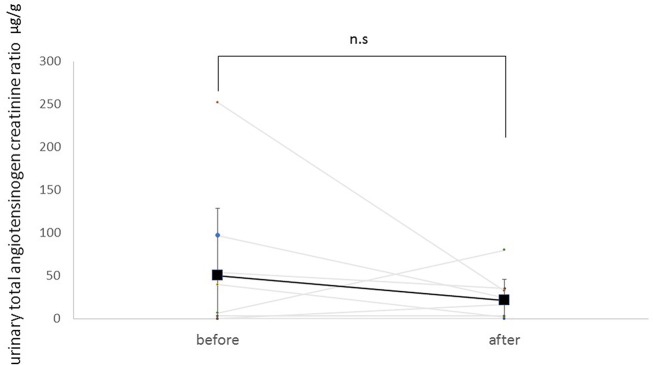
Figure 3 shows the trend in total urinary angiotensinogen/creatinine ratio. Mean ratio at baseline was 50.4±78.1 µg/g. Treatment with SGLT2 inhibitors tended to decrease total urinary angiotensinogen/creatinine ratio by 28.8 µg/g. However, these changes were not statistically significant (p=0.19).
Figure 3.
Change of urinary total angiotensinogen/creatinine ratio before and after administration of an SGLT2 inhibitor. Square points are shown as mean. SGLT2 inhibitors tended to decrease total urinary angiotensinogen/creatinine ratio, but these changes are not statistically significant (p=0.19). n.s., not significant; SGLT2, sodium-glucose co-transporter 2.
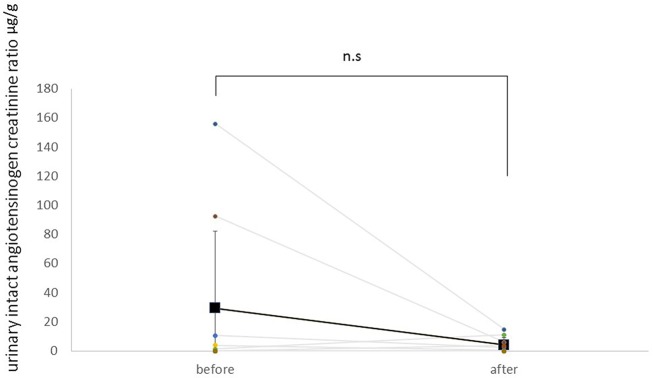
Figure 4 shows the trend in intact urinary angiotensinogen/creatinine ratio. Mean ratio at baseline was 29.4±53.0 µg/g. Treatment with SGLT2 inhibitors tended to decrease intact urinary angiotensinogen/creatinine ratio by 25.1 µg/g. However, these changes were not statistically significant (p=0.09).
Figure 4.
Change of urinary intact angiotensinogen/creatinine ratio before and after administration of an SGLT2 inhibitor. Square points are shown as mean. SGLT2 inhibitors tended to decrease intact urinary angiotensinogen/creatinine ratio, but these changes are not statistically significant (p=0.09). n.s., not significant; SGLT2, sodium-glucose co-transporter 2.
Discussion
Recent studies have shown that SGLT2 inhibitors increase plasma renin and total urinary angiotensinogen/creatinine ratio in patients with type 1 diabetes.17 21 These results suggest that treatment with an SGLT2 inhibitor stimulates RAS activity in the kidney. However, several preclinical studies have indicated that intrarenal RAS activity is much higher in type 2 diabetes than in type 1 diabetes.18 22 23 Therefore, we proposed a descriptive case study to examine the effect of SGLT2 inhibitors on urinary angiotensinogen excretion in patients with type 2 diabetes. We found that the baseline urinary total angiotensinogen/creatinine ratio in patients with type 2 diabetes was approximately 50 µg/g, which is much higher than in patients with type 1 diabetes,24 25 suggesting that intrarenal RAS is activated by augmentation of angiotensinogen. Our data also showed that urinary total angiotensinogen/creatinine ratio tended to be decreased by the administration of SGLT2 inhibitors, although the difference of total urinary angiotensinogen/creatinine ratio was not statistically significant. Collectively, these data support the hypothesis that SGLT2 inhibitors do not further activate intrarenal RAS in patients with type 2 diabetes whose basal RAS activation is already activated.
It is important to emphasize that urinary intact angiotensinogen was also measured in the present study. To the best of our knowledge, this is the first study involving the direct measurement of urinary intact angiotensinogen by ELISA in the human population. Intact urinary angiotensinogen is specific for angiotensinogen while total urinary angiotensinogen is associated with both intact angiotensinogen and des-angiotensin I angiotensinogen.26 Intact urinary angiotensinogen/creatinine ratio after administration of SGLT2 inhibitors was lower than the baseline urinary angiotensinogen/creatinine ratio, although this difference was not significant (p=0.09). Thus, the finding that administration of SGLT2 inhibitors did not change either total or intact angiotensinogen/creatinine ratio in the urine supports the hypothesis that intrarenal RAS is not activated by treatment with SGLT2 inhibitors in patients with type 2 diabetes.
In the present study, both total and intact angiotensinogen levels in the urine were measured. Since intact angiotensinogen does not contain cleaved fragments unlike total angiotensinogen,26 actual functional angiotensinogen concentration can be determined. The present study measured, for the first time, urinary intact angiotensinogen in humans. Our data showed that changes in urinary intact angiotensinogen excretion were similar to those in urinary total angiotensinogen in patients with type 2 diabetes. Future studies will be needed to determine the specific changes in urinary intact angiotensinogen.
In the present descriptive study, we examined the effects of several types of SGLT2 inhibitors on urinary angiotensinogen excretion. Clinical studies have shown that these SGLT2 inhibitors showed a similar reduction in HB1Ac.16 27–31 These data suggest that urinary excretion rates of glucose are also similar among all SGLT2 inhibitors; therefore, we speculate that renal effects of each SGLT2 inhibitors are also similar. Obviously, future studies are needed to determine whether all SGLT2 inhibitors similarly affect urinary angiotensinogen. In this study, some patients were treated with thiazolidines and other antihyperglycemic agents, in addition to an SGLT2 inhibitor. We did not observe any side effect such as edema and hypoglycemia in these patients before and after starting an SGLT2 inhibitor. Thus, it seems likely that the influence of these antihyperglycemic drugs on RAS is considered to be minimal. Plasma renin activity and aldosterone levels are useful markers to monitor systemic activities of the renin–angiotensin–aldosterone system.32 Further studies are needed to examine the effects of long treatment with an SGLT2 inhibitor on plasma renin activity and aldosterone levels in patients with type 2 diabetes. Further studies will also needed to compare the different effects of SGLT2 inhibitor on intrarenal RAS activity between types 1 and 2 diabetes with same protocols.
DeFronzo et al 22 showed that patients with macroalbuminuria demonstrated decreased urinary albumin levels, to levels consistent with microalbuminuria, following treatment with SGLT2 inhibitors. Similarly, treatment with SGLT2 inhibitors decreased urinary albumin excretion in patients with type 2 diabetes who showed both microalbuminuria and macroalbuminuria.11 In the present descriptive case study, SGLT2 inhibitors tended to decrease urinary albumin/creatinine ratio, but these changes were not statistically significant (p=0.16). A failure to observe a significant reduction in albuminuria may be due to small sample size and/or short observation period. In this study, almost all patients with diabetes showed normoalbuminuria and the urinary albumin/creatinine ratio was not changed. These data are in agreement with those in the subanalysis of the EMPA-REG OUTCOME study, showing that SGLT2 inhibitors did not prevent the occurrence of microalbuminuria.15 Since we measured urinary albumin in spot urine samples, further studies with pooled urine samples should be performed in larger sample size with a longer observation period.
In agreement with other clinical studies,11 23 the present study showed that SGLT2 inhibitors reduce both BP and HbA1c in patients with type 2 diabetes. We also observed that treatment with SGLT2 inhibitors significantly decreased both ALT and γGTP. Ohki et al 33 reported a significant reduction in serum ALT levels following treatment with SGLT2 inhibitors in Japanese, type 2 diabetic patients with non-alcoholic fatty liver disease. Interestingly, they also showed that SGLT2 inhibitors improved the index of Fibrosis-4, which is a marker for predicting the fibrous change of fatty liver.34 In this study, treatment with an SGLT2 inhibitor significantly decreased plasma ALT and AST levels; however, these changes were not significantly correlated with those in urinary intact or total angiotensinogen/creatinine ratio.
In conclusion, the present descriptive case study has indicated that treatment with SGLT2 inhibitors does not activate intrarenal RAS in patients with type 2 diabetes. Further clinical studies using larger sample sizes and longer observation periods are required to corroborate this conclusion.
Footnotes
Contributors: TF, KM and AN designed this study. TY, TF and MM performed the studies. TY and AN wrote the paper. HI and KM revised the manuscript. TY, HK, HI and KM performed sample measurements and analyses.
Competing interests: None declared.
Patient consent: We have obtained a consent form with permission of Kagawa University’s ethics committee.
Ethics approval: Kagawa University, Japan.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–31. 10.2337/diacare.21.9.1414 [DOI] [PubMed] [Google Scholar]
- 2. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:2568–9. 10.2337/diacare.27.10.2568 [DOI] [PubMed] [Google Scholar]
- 3. Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci 2005;109:143–59. 10.1042/CS20050025 [DOI] [PubMed] [Google Scholar]
- 4. Kim Y, Babu AR. Clinical potential of sodium-glucose cotransporter 2 inhibitors in the management of type 2 diabetes. Diabetes Metab Syndr Obes 2012;5:313–27. 10.2147/DMSO.S22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallon V, Sharma K. Sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Curr Opin Nephrol Hypertens 2010;19:425–31. 10.1097/MNH.0b013e32833bec06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in Type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes 2014;38:356–63. 10.1016/j.jcjd.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 7. Turk E, Martín MG, Wright EM. Structure of the human na+/glucose cotransporter gene SGLT1. J Biol Chem 1994;269:15204–9. [PubMed] [Google Scholar]
- 8. Kanai Y, Lee WS, You G, et al. The human kidney low affinity na+/glucose cotransporter SGLT2. delineation of the Major renal reabsorptive mechanism for D-glucose. J Clin Invest 1994;93:397–404. 10.1172/JCI116972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care 2016;39(Suppl 1):S4–5. 10.2337/dc16-S003 [DOI] [PubMed] [Google Scholar]
- 10. Rosenwasser RF, Sultan S, Sutton D, et al. SGLT-2 inhibitors and their potential in the treatment of diabetes. Diabetes Metab Syndr Obes 2013;6:453–67. 10.2147/DMSO.S34416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 2016;59:1860–70. 10.1007/s00125-016-4008-2 [DOI] [PubMed] [Google Scholar]
- 12. Heerspink HJ, Johnsson E, Gause-Nilsson I, et al. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab 2016;18:590–7. 10.1111/dom.12654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohan DE, Fioretto P, Tang W, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962–71. 10.1038/ki.2013.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 15. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in Type 2 diabetes. N Engl J Med 2016;375:323–34. 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 16. Haneda M, Seino Y, Inagaki N, et al. Influence of renal function on the 52-Week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in japanese patients with type 2 diabetes mellitus. Clin Ther 2016;38:66–88. 10.1016/j.clinthera.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 17. Cherney DZ, Perkins BA, Soleymanlou N, et al. Sodium glucose cotransport-2 inhibition and intrarenal RAS activity in people with type 1 diabetes. Kidney Int 2014;86:1057–8. 10.1038/ki.2014.246 [DOI] [PubMed] [Google Scholar]
- 18. Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007;59:251–87. 10.1124/pr.59.3.3 [DOI] [PubMed] [Google Scholar]
- 19. Kamiyama M, Zsombok A, Kobori H. Urinary angiotensinogen as a novel early biomarker of intrarenal renin-angiotensin system activation in experimental type 1 diabetes. J Pharmacol Sci 2012;119:314–23. 10.1254/jphs.12076FP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Konishi Y, Nishiyama A, Morikawa T, et al. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension 2011;58:205–11. 10.1161/HYPERTENSIONAHA.110.166843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–97. 10.1161/CIRCULATIONAHA.113.005081 [DOI] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384–93. 10.2337/dc14-2364 [DOI] [PubMed] [Google Scholar]
- 23. Inagaki N, Goda M, Yokota S, et al. Effects of baseline blood pressure and low-density lipoprotein cholesterol on safety and efficacy of canagliflozin in Japanese patients with Type 2 diabetes mellitus. Adv Ther 2015;32:1085–103. 10.1007/s12325-015-0255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cherney DZ, Xiao F, Zimpelmann J, et al. Urinary ACE2 in healthy adults and patients with uncomplicated type 1 diabetes. Can J Physiol Pharmacol 2014;92:703–6. 10.1139/cjpp-2014-0065 [DOI] [PubMed] [Google Scholar]
- 25. Saito T, Urushihara M, Kotani Y, et al. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci 2009;338:478–80. 10.1097/MAJ.0b013e3181b90c25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satou R, Kobori H, Katsurada A, et al. Quantification of intact plasma AGT consisting of oxidized and reduced conformations using a modified ELISA. Am J Physiol Renal Physiol 2016;311:F1211–6. 10.1152/ajprenal.00320.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–33. 10.1016/S0140-6736(10)60407-2 [DOI] [PubMed] [Google Scholar]
- 28. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2014;2:369–84. 10.1016/S2213-8587(13)70208-0 [DOI] [PubMed] [Google Scholar]
- 29. Bode B, Stenlöf K, Sullivan D, et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract 2013;41:72–84. 10.3810/hp.2013.04.1020 [DOI] [PubMed] [Google Scholar]
- 30. Fonseca VA, Ferrannini E, Wilding JP, et al. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications 2013;27:268–73. 10.1016/j.jdiacomp.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 31. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 2014;13:65 10.1186/1475-2840-13-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol 2007;293:F956–60. 10.1152/ajprenal.00090.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohki T, Isogawa A, Toda N, et al. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line treatment for Non-Alcoholic Fatty liver disease patients with type 2 Diabetes Mellitus who do not respond to Incretin-Based therapies including Glucagon-like Peptide-1 analogs and Dipeptidyl Peptidase-4 inhibitors. Clin Drug Investig 2016;36:313–9. 10.1007/s40261-016-0383-1 [DOI] [PubMed] [Google Scholar]
- 34. Okamura Y, Ashida R, Yamamoto Y, et al. FIB-4 Index is a predictor of Background liver fibrosis and Long-Term Outcomes after curative resection of hepatocellular carcinoma. Ann Surg Oncol 2016;23(Suppl 4):467–74. 10.1245/s10434-016-5377-y [DOI] [PubMed] [Google Scholar]