Abstract
Objective
Studies suggest a greater risk of Parkinson disease (PD) after traumatic brain injury (TBI), but it is possible that the risk of TBI is greater in the prodromal period of PD. We aimed to examine the time-to-TBI in PD patients in their prodromal period compared to population-based controls.
Methods
We identified 89,790 incident PD cases and 118,095 comparable controls > 65 years of age in 2009 using Medicare claims data. Using data from the preceding five years, we compared time-to-TBI in PD patients in their prodromal period to controls. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for TBI in a Cox regression, while adjusting for age, sex, race/ethnicity, modified Charlson comorbidity index, smoking, and alcohol use.
Results
Risk of TBI was greater in PD patients in their prodromal period across all age and sex groups, with HRs consistently increasing with proximity to PD diagnosis. HRs ranged from 1.64 (95% CI 1.52, 1.77) five years prior to diagnosis to 3.93 (95% CI 3.74, 4.13) in the year prior. The interaction between PD, TBI, and time was primarily observed for TBI attributed to falls. Motor dysfunction and cognitive impairment, suggested by corresponding ICD-9 codes, partially mediated the PD-TBI association.
Interpretation
There is a strong association between PD and a recent TBI in the prodromal period of PD. This association strengthens as PD diagnosis approaches and may be a result of undetected non-motor and motor symptoms, but confirmation will be required.
INTRODUCTION
Falls are the leading cause of traumatic brain injury (TBI) in older adults1, and the number of individuals suffering a TBI as a result of a fall is expected to rise as the population ages2. The annual incidence of TBI-related hospitalization is 79/100,000 amongst all age groups compared to 264/100,000 in those aged 75 and older3. Furthermore, adults 75 years and older have the highest rate of TBI-related hospitalization associated with falls compared to any other age group3. Older adults with Parkinson disease (PD) are at greater risk of falls compared to the general older adult population4. In a study of community dwelling individuals with early-stage PD, 48% reported at least one fall in a six month period5. During the prodromal PD period, individuals who will eventually be diagnosed with PD based on the classic motor signs may be at an increased risk of falls6. Because some falls may result in TBI7, it is not clear whether the known association between PD and TBI6,8–14 is causal. Although the possible mechanisms for falls during the prodromal period have not been elucidated, there is evidence to suggest that non-motor and motor symptoms are occurring in the years prior to PD diagnosis15,16.
The objective of the study was to determine whether the risk of TBI was greater for PD cases in their prodromal period than controls in a comparable time period and subsequently to determine whether this relative risk grew stronger as time approached PD diagnosis. We hypothesized that the risk of TBI would be greater in PD patients during their prodromal period as compared to general population controls and that this relative risk would increase over the five years leading up to PD diagnosis. We anticipated that there would be a greater risk of TBI closest to the PD diagnosis date compared to controls due to progressive non-motor and motor symptoms occurring prior to PD diagnosis. Furthermore, we hypothesized that this association would be most evident for TBIs that occurred because of falls.
SUBJECTS/MATERIALS AND METHODS
Study Design and Sample
The study was approved by the Institutional Review Board at Washington University School of Medicine and by the Centers for Medicare and Medicaid Services. For this population-based study, Medicare base files and comprehensive (inpatient, outpatient, physician/supplier Part B, durable medical equipment, and home health care) claims data from 2004–2009 were used as detailed previously17. Briefly, to be eligible for this study, individuals had to be age 66–90 years old, enrolled in Medicare Part A and/or B, and living in the United States (U.S.) in 2009. Individuals younger than 66 years and 11 months were excluded in order to allow for at least two full years of claims data. Within these study eligibility criteria we identified incident PD cases and controls, as detailed below. The 2004–2009 time period for which we had longitudinal data for the beneficiaries in this case-control study was during the five year period prior to the PD diagnosis (or control reference) date. During this five year period, termed the prodromal PD period, progressive neuropathology of PD has already developed and symptoms related to the underlying pathology of the disease are occurring.
Those individuals who met the above eligibility criteria and who had an International Classification of Diseases (ICD-9) diagnosis code of 332.0 or 332 in 2009, with no prior occurrence of these codes, were identified as incident PD cases, hereafter “PD patients in the prodromal period.” We excluded those with possible atypical parkinsonism or Lewy body dementia using ICD-9 codes 333.0 and 331.82. Controls were randomly selected from all remaining Medicare beneficiaries who met the same study eligibility criteria. Controls were not matched to PD patients on demographic factors so that we could include these demographic factors in risk factor analyses17, and this allowed the baseline hazard rate function for TBI to be representative of the study population. PD patients and controls who had a personal history of TBI using the ICD-9 diagnosis V code (V15.52) or other TBI code prior to the beginning of the study period (817 cases, 497 controls) were excluded to ensure an event was correctly identified as the first TBI.
Outcomes
TBI up until the PD diagnosis or control reference date was the primary outcome of interest. We identified TBI using the Centers for Disease Control and Prevention (CDC) criteria using the following ICD-9 diagnosis codes, as in other studies: 800.0–801.9; 803.0–804.9; 850.0–854.1; and 959.0110,18. Severity of TBI was categorized based on the CDC: mild (includes concussion) versus moderate/severe19–21. We also considered mechanism of trauma for the TBI. Trauma mechanism was defined using the external cause of injury group code (ICD-9 diagnosis E-codes) and divided into the following categories based on the CDC22: falls, motor vehicle accidents, and other mechanisms. We linked an E-code to a TBI event if it occurred within seven days of the TBI. We were able to ascertain the mechanism of injury using E-codes for 52% of PD patients in their prodromal period and 49% of controls who had a TBI.
Covariates
The Medicare files included sex, race/ethnicity, birth date (used to calculate age), ICD-9 diagnosis and procedure codes, as well as other types of procedure codes, date of Medicare enrollment, and number of months of health maintenance organization (HMO) coverage in each year. There were 26,468 unique diagnosis and procedure codes represented in our sample. We used these codes to calculate a modified version of the Charlson comorbidity index (CCI), a validated indicator of mortality that classifies and weighs various disease subgroups (e.g. cancer, renal disease, stroke, liver disease)23–25. The categories were weighted using the Deyo24 and Quan25 methods to utilize ICD-9 codes and were combined to create a single index at the patient’s year of entry25 in survival analysis, which we detail below. We also used the diagnosis and procedure codes to calculate variables for tobacco smoking and alcohol use; both are associated with PD and TBI26,27. There were no missing covariate data except for race/ethnicity in 0.1% of both PD patients and controls. This missing group was categorized as “unknown” race/ethnicity in the final model.
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 or Stata MP 14.2. We used Cox regression models to examine the risk of TBI for PD patients in their prodromal period compared to controls. The five years prior to PD diagnosis in 2009 was considered the prodromal period for PD patients. We used the five years prior to a randomly selected reference date in 2009 as the comparable period among controls. For this survival analysis, the event time, time-to-TBI, was defined as the time from study start to first TBI diagnosis code. The study start time was date of entry. For most individuals this began in 2004, that is, at the beginning of this five year period. However, the models accounted for left truncation of the data by using the date of entry in Medicare. This allowed individuals who became age-eligible for Medicare after the study start time (5.6% of PD patients in their prodromal period, 14.8% of controls) to be included in the survival analysis. For these individuals, the beginning of their Medicare eligibility truncates their study time and assumes no TBI occurred between the study start time and their entry. We examined this assumption in a sensitivity analysis. For all models, individuals were censored at the earlier of the following dates: PD diagnosis date/random control reference date or December 31st of the year prior to starting HMO coverage. Individuals were censored prior to starting any HMO coverage because not all claims would be processed through the Centers for Medicare and Medicaid Services. We verified proportional hazard assumptions for all covariates using residuals or the interaction of each covariate with time. PD case status, that is, being a PD patient in the prodromal period, was the only variable that did not meet the proportional hazards assumption for the Cox regression model, consistent with our hypothesis that risk of TBI would increase as PD diagnosis date approached. Therefore, we handled prodromal PD status by using a time-dependent coefficient in the Cox regression models. Specifically, we compared PD patients in their prodromal period to controls at fixed year periods prior to PD diagnosis/reference (1, 2, 3, 4, or 5 years prior).
To compare base hazard rates of TBI in PD patients in the prodromal period versus controls we derived hazard rate functions by calculating the discrete change in the Nelson-Aalen cumulative hazard estimates between consecutive time points while smoothing using the Epanechnikov Kernel. We stratified these hazard rate functions by sex and age (66–74, 75–84, and 85–90 years). We then fit a Cox regression model to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for TBI among PD patients in the prodromal period vs. controls in a comparable reference period, while a priori adjusting for age, sex, and race/ethnicity. We verified that there was no material effect of adjusting for the following additional variables that are related to PD, TBI, or both: modified CCI at entry year, tobacco smoking, and high alcohol usage. We then compared the log hazard ratio between each year and the prior year. We also examined the unadjusted hazard rate function for TBI by mechanism of injury.
We performed two sensitivity analyses for the above Cox models. First, we verified that results were not altered by excluding the individuals who had delayed entry because their inclusion required us to assume that they had not experienced a TBI between study start time and their entry. Second, we excluded the three months prior to PD diagnosis/reference date because prior studies of PD suggested that traumas in this time period may have facilitated PD ascertainment,6,14 and we also repeated this excluding a full year.
Finally, similar to a prior study focused on PD and TBI,14 we conducted logistic regression to examine the association between PD and TBI at different time periods prior to PD diagnosis. PD case status was our outcome and TBI the independent variable in each model. For consistency with our survival analysis, we used the same time year windows prior to PD diagnosis/control reference as before (1, 2, 3, 4 or 5 years prior, with one model per time window). We adjusted for the same covariates as described above when estimating the PD-TBI odds ratios (ORs) and 95% CIs as an estimate of relative risk.
The use of less computationally intensive logistic regression models allowed us to conduct additional analyses to determine whether any of the individual 26,468 diagnosis and procedure codes altered the PD-TBI-time interaction or confounded and/or mediated the PD-TBI association. We used logistic regression models, including all time periods without the main effect for time, to identify codes that altered the PD-TBI or PD-TBI-time OR by ≥ 5% (excluding codes synonymous with TBI and general medical care such as basic life support). Subsequently, we explored the effect of these variables as potential mediators. We used the “sgmediation28” and “ldecomp29” commands available for Stata and calculated mediation percentages for the overall PD-TBI OR and for individual time windows. We enforced that the mediator codes occurred prior to TBI unless noted. Modeling each of the time windows allowed the PD-TBI OR to vary with time, consistent with our primary survival analysis. Additionally, we calculated the mediation proportion of physical codes while excluding individuals who had any of the cognitive/psychiatric codes and vice versa.
RESULTS
PD patients (N = 89,790) were older, were more likely to be male, were less likely to have ever smoked, and, at entry year, had more comorbidities as measured by the modified CCI as compared to controls (N = 118,095) (Table 1). The PD diagnosis date (first PD code) was identified most commonly from the physician/supplier Medicare Part B file (85% of PD patients). In the five years preceding this date (or reference date for controls), there were a total of 24,421 TBIs with 18.63% of prodromal PD patients and 6.52% of controls experiencing a TBI. Approximately 84% of TBIs in both PD patients in their prodromal period and controls were classified as mild/concussive in severity. For those TBIs with an associated E-code for mechanism of injury, a majority were due to falls (82.0% of PD patients, 74.4% of controls). The next most common cause was motor vehicle accidents (3.8% of prodromal PD patients, 8.0% of controls) (Table 1).
Table 1.
Characteristics of Prodromal Parkinson Disease Cases and Controls, Overall and Among Those with Traumatic Brain Injury
| Characteristics | PD n = 89,790 (%) |
Controls n = 118,095 (%) |
TBI & PD n = 16,725 (%) |
TBI & Controls n = 7,696 (%) |
|---|---|---|---|---|
|
| ||||
| Age, years, Mean (SD) | 78.8 (6.1) | 76.0 (6.2) | 80.2 (5.8) | 79.2 (6.4) |
|
| ||||
| 66–74 | 27.6 | 47.2 | 19.5 | 27.3 |
| 75–84 | 51.7 | 40.7 | 52.6 | 47.3 |
| 85–90 | 20.7 | 12.1 | 27.8 | 25.4 |
|
| ||||
| Females | 50.2 | 57.0 | 56.8 | 66.3 |
|
| ||||
| Race/Ethnicity | ||||
| White | 88.8 | 86.4 | 90.1 | 89.2 |
| Black | 6.0 | 7.5 | 5.2 | 6.1 |
| Pacific Islander/Other | 1.0 | 1.5 | 0.8 | 0.8 |
| Asian | 1.7 | 2.2 | 1.6 | 1.7 |
| Hispanic | 2.1 | 1.9 | 1.9 | 1.7 |
| Native American | 0.3 | 0.4 | 0.4 | 0.4 |
| Unknown | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||
| TBI Severity | ||||
| Mild/Concussive | 83.7 | 83.6 | ||
| Moderate/Severe | 16.4 | 16.4 | ||
|
| ||||
| Mechanism of Injurya | ||||
| Falls | 82.0 | 74.4 | ||
| MVA | 3.8 | 8.0 | ||
| Struck by/Against | 2.3 | 3.3 | ||
| Other | 11.8 | 14.3 | ||
|
| ||||
| Modified CCI, Mean (SD)b | 1.67 (2.00) | 1.16 (1.71) | 2.06 (2.16) | 1.87 (2.09) |
|
| ||||
| 1 | 22.3 | 20.4 | 22.2 | 21.9 |
|
| ||||
| 2 | 15.6 | 12.5 | 17.4 | 16.5 |
|
| ||||
| 3 or more | 25.7 | 16.4 | 32.5 | 29.3 |
|
| ||||
| Smokingc | 42.9 | 55.4 | 27.9 | 33.7 |
|
| ||||
| Alcohold (heavy use) | 1.05 | 0.61 | 1.73 | 1.34 |
Abbreviations: PD = Parkinson disease, TBI = traumatic brain injury, MVA = motor vehicle accident, SD = standard deviation.
Mechanism of injury was defined using ICD-9 diagnosis E-codes: 52% of prodromal PD cases and 49% of controls had a TBI with an associated E-code.
≥ Median predicted probability of ever vs. never smoking divided by the number of diagnosis codes (or one for 292 cases and 6,227 controls without any diagnosis codes). Predicted probability was based on sex, race/ethnicity, birth cohort, and 661 diagnosis and procedure codes including codes specific to tobacco (ICD-9 V15.82, ICD-9 305.1, CPT 99406, CPT 99407), codes for heavy alcohol use (see below), codes for cancers with high attributable risk due to smoking (lung and larynx), and codes related to other conditions that were associated with smoking in the 2009 Behavioral Risk Factor Surveillance System (BRFSS) data40 among Americans age 66–90.
Heavy alcohol use defined using ICD-9 diagnosis codes (291.1, 291.2, 291.3, 291.4, 291.5, 291.81, 291.82, 291.89, 291.9, 303.00, 303.01, 303.02, 303.03, 303.90, 303.91, 303.92, 303.93, 305.00, 305.01, 305.02, 305.03, 357.5, 425.5, 571.0, 571.1, 571.2, 571.3, 790.3, V11.3, V79.1, E860.0), ICD-9 procedure codes (94.46, 94.53, 94.61, 94.62, 94.63, 94.67, 94.68, 94.69), and HCPCS/CPT code (G0443).
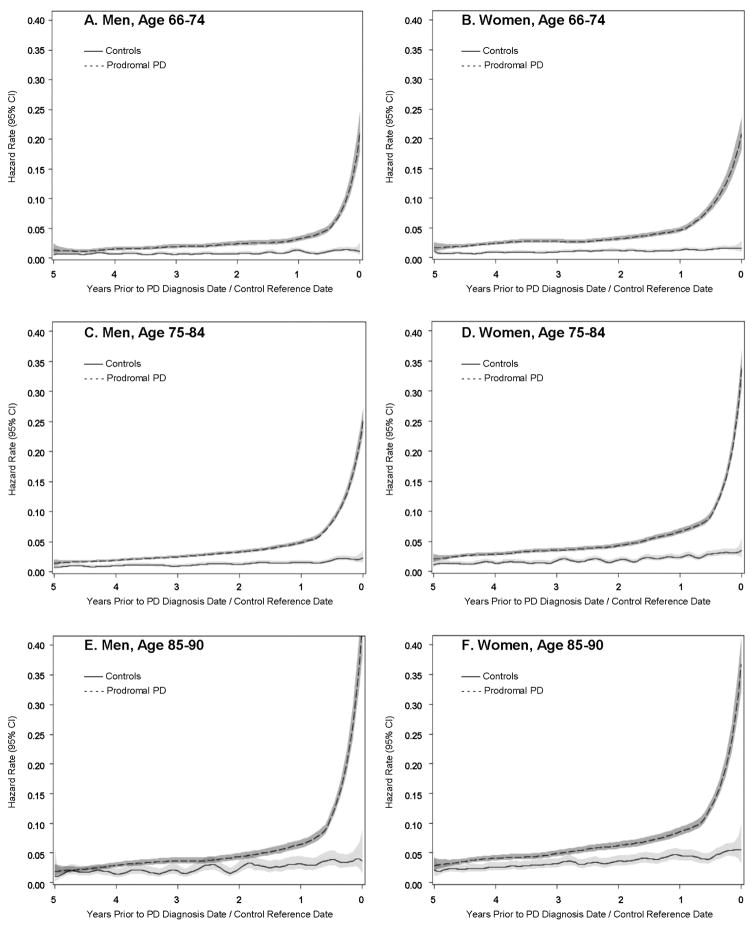
In the unadjusted models of the hazard rate function, PD patients in their prodromal period were more likely to have a TBI compared to controls in the comparable period, regardless of age and sex, throughout the entire five years prior to the diagnosis/reference date (Figure 1). The Cox regression model adjusted for age, sex, and race/ethnicity confirmed that risk of TBI was greater in PD patients than controls in all time periods (Table 2). Results were similar in the fully adjusted model that also included modified CCI, smoking, and alcohol usage. HRs consistently increased as time approached PD diagnosis/reference, with the largest HR in the year immediately prior to PD diagnosis/reference. The respective differences in the log HRs between consecutive years was significant, except for 3 years prior vs. 4 years prior (all other p ≤ 0.003). Similarly, there was a significant difference in the log HR between the year prior to PD diagnosis/reference and each prior year (all p < 0.0001).
Figure 1. Traumatic Brain Injury Hazard Rate Function by PD Case Status Stratified by Sex and Age.
Hazard rate for TBI in unadjusted models smoothed using the Epanechnikov Kernel with gray regions representing the 95% confidence intervals. Prodromal PD case status was associated with an increased risk of TBI compared to controls for all age and sex strata. The hazard rate grew larger with proximity to PD diagnosis/control reference date for prodromal PD cases compared to controls.
Table 2.
Hazard Ratios for Traumatic Brain Injury for Prodromal Parkinson Disease Cases Compared to Controls
| Basic Modela,b | Fully Adjusted Model a,c,g | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Years prior to PD diagnosis | ||||||
| 0 to < 1 year prior | 4.22* | 4.02 – 4.43 | < 0.0001 | 3.93* | 3.74 – 4.13 | < 0.0001 |
| 1 to < 2 years prior | 2.47* | 2.33 – 2.62 | < 0.0001 | 2.30* | 2.17 – 2.44 | < 0.0001 |
| 2 to < 3 years prior | 2.17 | 2.03 – 2.31 | < 0.0001 | 2.01 | 1.89 – 2.15 | < 0.0001 |
| 3 to < 4 years prior | 2.12* | 1.98 – 2.28 | < 0.0001 | 1.98* | 1.85 – 2.12 | < 0.0001 |
| 4 to < 5 years prior | 1.76 | 1.63 – 1.89 | < 0.0001 | 1.64 | 1.52 – 1.77 | < 0.0001 |
|
| ||||||
| Age, per Year | 1.056 | 1.054 – 1.058 | < 0.0001 | 1.056 | 1.054 – 1.058 | < 0.0001 |
|
| ||||||
| Female | 1.35 | 1.31 – 1.38 | < 0.0001 | 1.53 | 1.48 – 1.57 | < 0.0001 |
|
| ||||||
| Race/Ethnicity | ||||||
| White | 1.00 | (Reference) | 1.00 | (Reference) | ||
| Black | 0.89 | 0.84 – 0.94 | < 0.0001 | 0.86 | 0.81 – 0.91 | < 0.0001 |
| Pacific Islander/Other | 0.81 | 0.70 – 0.93 | 0.003 | 0.87 | 0.75 – 0.99 | 0.04 |
| Asian | 0.85 | 0.77 – 0.94 | 0.001 | 0.88 | 0.79 – 0.97 | 0.01 |
| Hispanic | 0.92 | 0.84 – 1.01 | 0.089 | 0.93 | 0.84 – 1.02 | 0.10 |
| Native American | 1.15 | 0.94 – 1.41 | 0.177 | 1.07 | 0.87 – 1.31 | 0.53 |
| Unknown | 1.18 | 0.80 – 1.75 | 0.410 | 1.16 | 0.78 – 1.72 | 0.46 |
|
| ||||||
| Modified CCId | ||||||
| 0 | 1.00 | (Reference) | ||||
| 1 | 1.28 | 1.23 – 1.32 | < 0.0001 | |||
| 2 | 1.45 | 1.40 – 1.51 | < 0.0001 | |||
| 3 or more | 1.77 | 1.71 – 1.83 | < 0.0001 | |||
|
| ||||||
| Smokinge,f | 1.60 | 1.53 – 1.67 | < 0.0001 | |||
|
| ||||||
| Alcohol (heavy use)f | 1.89 | 1.71 – 2.09 | < 0.0001 | |||
Based on 88,973 PD cases and 117,598 controls from a case-control study; excludes 817 cases and 497 controls with prior head injury. Abbreviations: HR = hazard ratio, CI = 95% confidence intervals.
Adjusted for age, sex, and race/ethnicity.
Adjusted for age, sex, race/ethnicity, modified CCI as of baseline, smoking, and alcohol use.
Modified CCI (truncated Charlson comorbidity index as described by Deyo24 and Quan25 methods) at year of study entry for each participant.
Predicted probability of ever vs. never smoking.
See Table 1 footnotes for diagnosis and procedure codes used to identify ever smoking, and heavy alcohol use.
The difference in log hazard ratios between the year prior to diagnosis and the average of all previous years was 0.6958 (CI 0.6350, 0.7565, p-value < .0001). Additionally, we compared the difference in log hazard ratios between consecutive years as follows: 1 year prior vs. 2 years prior (0.5426, CI 0.4644, 0.6208, p-value < .0001), 2 years prior vs. 3 years prior (0.1358, CI 0.0472, 0.2244, p-value 0.0027), 3 years prior vs. 4 years prior (0.0156, CI -0.0803, 0.1115, p-value 0.7496), and 4 years prior vs. 5 years prior (0.1741, CI 0.0712, 0.2770, p-value 0.0009). All were significant except for 3 years prior vs. 4 years prior.
We indicated with an asterisk(*) if there was a statistically significant increase from the previous year.
In sensitivity analyses excluding the three months prior to PD diagnosis or control reference date, the time dependency of the PD case status coefficient remained significant (p < 0.0001) even though the TBI HR in the final three month period was particularly large (6.78, 95% CI 6.21, 7.40, p < 0.0001, not shown in tables). When excluding the final three month period, the TBI HR in the year prior to PD diagnosis was attenuated but remained larger than each of the four preceding years (HR = 2.82, 95% CI 2.65, 2.99, p < 0.0001, not shown in tables). This attenuation made the relationship between the TBI HRs and time-to-PD diagnosis more linear. The PD case status coefficient’s interaction with time (time dependency) also remained significant when excluding the entire year prior to PD diagnosis or control reference date (p < 0.0001, not shown in tables). Sensitivity analyses showed the effect of left truncation did not materially alter results (not shown).
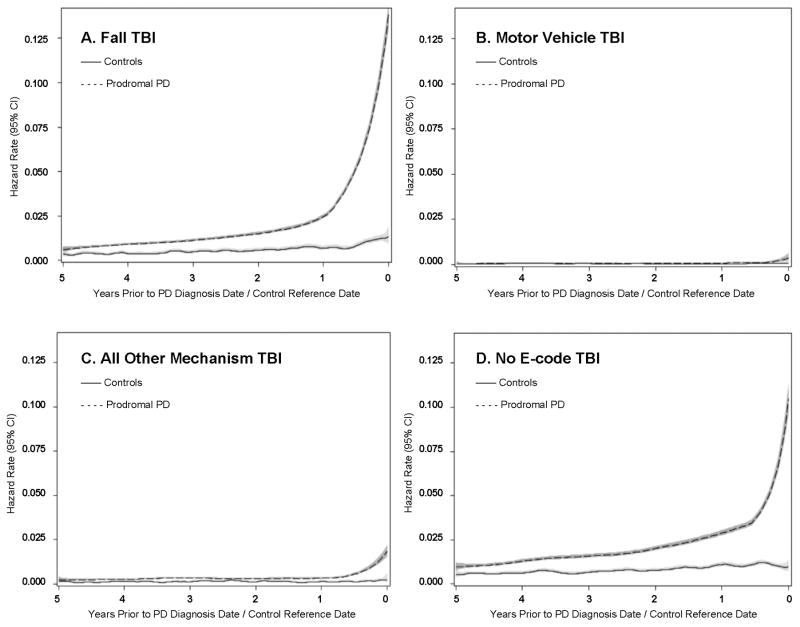
The time dependency of the PD case status coefficient was most evident for TBIs secondary to a fall (Figure 2a). In contrast, there was no clear difference in time-to-TBI due to motor vehicle accidents or other specified mechanisms between PD patients in their prodromal period and controls, other than in the final months prior to diagnosis/reference (Figure 2b and 2c). Results for TBI patients with an unknown mechanism of injury were intermediate between falls and other causes of TBI (Figure 2d). We confirmed this general pattern for each of the mechanisms when simultaneously stratifying by sex and age (results not shown).
Figure 2. Traumatic Brain Injury Hazard Rate Function by PD Case Status and Mechanism of Injury.
Hazard rate for TBI due to mechanism of injury in unadjusted models smoothed using the Epanechnikov Kernel with gray regions representing the 95% confidence intervals. The PD-TBI-time interaction was most apparent for TBIs from falls (Figure 2A) compared to any other mechanism of injury (Figure 2B. Motor Vehicle TBI and Figure 2C. All Other Mechanism TBI). Figure 2D presents all TBI in prodromal PD cases compared to controls without a recorded mechanism of injury.
The PD-TBI ORs for the logistic regression models stratified by the period prior to PD diagnosis were consistent with the results from the Cox regression (Table 3). The relative risk of PD was greater in those who had a TBI compared to those who had not and increased as the time prior to PD diagnosis decreased. This PD-TBI-time interaction was unchanged by the addition of any individual diagnosis or procedure code.
Table 3.
Odds Ratios for Parkinson Disease (PD) in Relation to TBI, by Time Window
| Time Window, Years Prior to PD Diagnosis/Reference | PD-TBI OR (95% CI)a | Total PD Cases, N | Total Controls, N | Cases with a TBI, N (%) | Controls with a TBI, N (%) |
|---|---|---|---|---|---|
| < 5 to 4 | 1.55 (1.44 – 1.68) | 87117 | 108237 | 1742 (2.0%) | 1121 (1.0%) |
| < 4 to 3 | 1.88 (1.75 – 2.02) | 89279 | 116125 | 2244 (2.5%) | 1248 (1.1%) |
| < 3 to 2 | 1.89 (1.77 – 2.02) | 89683 | 117497 | 2623 (2.9%) | 1484 (1.3%) |
| < 2 to 1 | 2.14 (2.01 – 2.27) | 89779 | 117878 | 3344 (3.7%) | 1707 (1.4%) |
| < 1 to 0 | 3.77 (3.58 – 3.96) | 89790 | 118095 | 6772 (7.5%) | 2136 (1.8%) |
Odds ratio and 95% confidence interval, adjusted for age, sex, race/ethnicity, modified CCI as of baseline, smoking, and alcohol use as in primary models.
We identified 14 codes that confounded and/or mediated the overall PD-TBI association (Table 4). Notably, four of the codes indicated possible motor dysfunction: abnormality of gait (ICD-9 781.2), lack of coordination (ICD-9 781.3), and two codes for falls (E888.9, V15.88). Using the “sgmediation” command, which provided the most conservative estimates, the gait code alone mediated 29.6 % (95% CI 28.8, 30.5) of the PD-TBI association. The 14 identified codes also included 10 codes for psychiatric and cognitive disorders. Of these 10, the five codes included in the dementia/cognitive impairment group mediated 37.9 % (95% CI 37.0, 38.8) of the PD-TBI association. When limiting to individuals with no cognitive/psychiatric codes, the mediation proportion of physical codes was 43.1% for all years (N = 116,552, not shown in tables). When limiting to individuals with no physical codes, the mediation proportion of cognitive/psychiatric codes was 41.7% (N = 126,023, not shown in tables).
Table 4.
Percent of PD-TBI Association Mediated by Selected Diagnosis Codes, Overall and by Time Window
| Excluding Diagnosis Codes Observed after TBI | Overall (All Codes) | |||||||
|---|---|---|---|---|---|---|---|---|
| Years Prior to PD Diagnosis | ||||||||
| Potential Mediators Before Specified Time Windowa (ICD-9 Diagnosis Code) | 4 to < 5 | 3 to < 4 | 2 to < 3 | 1 to < 2 | 0.25 to < 1b | 0 to 0.25 | All years > 0.25 years b | All yearsc |
| Physical | --d | |||||||
| Abnormality of Gait (781.2) | 4.1% | 6.6% | 5.2% | 6.0% | 2.2% | 29.6% | 29.4% | |
| Lack of Coordination (781.3) | 2.0% | 2.4% | 2.3% | 2.4% | 0.0% | 17.9% | 17.3% | |
| Falls (E888.9 & V15.88) | 1.5% | 2.2% | 2.1% | 1.9% | 0.0% | 34.0% | 35.2% | |
| Cognitive/Psychiatric | ||||||||
| Dementia/Cognitive Impairment (331.0, 290.0, 331.9, 294.11, 294.10) | 6.4% | 10.1% | 8.6% | 9.7% | 5.0% | 37.9% | 35.4% | |
| Delirium (780.97, 780.09) | 1.7% | 4.9% | 4.7% | 4.5% | 2.2% | 31.5% | 32.0% | |
| Mental Disorder NEC (294.80) | 5.2% | 6.6% | 5.7% | 6.5% | 4.2% | 28.7% | 27.4% | |
| Psychosis NOS (298.9) | 2.5% | 5.0% | 4.1% | 3.7% | 0.0% | 19.8% | 17.9% | |
| Depressive Disorder NEC (311.0) | 7.7% | 9.4% | 6.7% | 6.1% | 2.7% | 21.4% | 18.5% | |
Abbreviations: PD = Parkinson disease, TBI = traumatic brain injury, ICD-9 = International Classification of Diseases Version 9 – Clinical Modification, NEC = not elsewhere classified, NOS = not otherwise specified, CI = Confidence Interval.
Sgmediation with TBI as the outcome variable, PD case status as the independent variable, the mediator(s) as specified, and the same covariates: age, sex, race/ethnicity, modified CCI as of baseline, smoking, and alcohol use.
The first 3 months (0.25 years) prior to diagnosis were excluded due to evidence of ascertainment bias.
TBI in any time period and mediators in any time period.
Not calculated due to limited time period for which codes could be observed prior to TBIs in this time period.
DISCUSSION
In this large population-based study, TBIs occurred more frequently in PD patients during their prodromal period compared to controls in a comparable time period. In particular, TBI risk increased consistently in each of the five years prior to PD diagnosis in PD patients relative to controls. Not only was this PD-TBI-time interaction highly significant, but this pattern was consistent in all sex and age groups. Our results are consistent with another large population-based study which observed a similar relationship between PD, TBI, and time-to- PD diagnosis using administrative data14. The aforementioned study and ours revealed a strong association between TBI and PD in the three months prior to PD diagnosis, regardless of underlying mechanism. The association between TBI and PD in the final three months may be due to enhanced ascertainment of PD resulting from increased use of medical care following a TBI from any cause. However, even when we excluded this three month period, or a full year, a clear interaction between PD, TBI, and time to PD diagnosis/reference remained. The association between PD, TBI, and time was primarily observed for TBI attributed to falls and less evident for other mechanisms of injury. These findings were consistent with our original hypothesis that undetected non-motor and motor symptoms may contribute to falls and hence TBI. Interestingly, the magnitude of the PD-TBI association for each of the five years was remarkably similar to risk estimates observed in a large population-based study comparing PD patients in their prodromal period to controls with regard to hip fractures6. It was unlikely that hip fractures were associated causally with PD, but rather a result of progressive symptoms that led to falls and subsequent trauma. Collectively, these results strongly suggest that the positive association between TBI and PD in the several years prior to PD diagnosis is due to progressive PD-related symptoms that may contribute to falls.
Our conclusion contrasts with another recent large population-based study that used administrative health data in older Americans10. In this seven-year study restricted to inpatient/emergency room records, the authors compared time to PD between TBI patients and non-TBI trauma patients and observed a significant PD HR of 1.44. Because of the unique approach comparing TBI and trauma patients, the study suggested a causal association between TBI and PD and not reverse causation. However, because the mean time to PD hospitalization was 3.1 to 3.3 years in the comparison groups, it is possible that some of the PD patients had been diagnosed with PD earlier in another healthcare setting, perhaps even prior to baseline. It is important to note that the majority of our PD cases were diagnosed outside of a hospital. Another large population-based study observed a mean delay of three years between initiation of anti-parkinsonian medication and hospitalization with PD14. Although our study time-frame was shorter, the comprehensive claims data, which included comprehensive outpatient data, allowed for a more accurate estimation of PD diagnosis date.
Our study suggests that a combination of non-motor and motor symptoms prior to PD diagnosis may contribute to TBIs through falls. Our mediation analysis suggested that abnormality of gait, lack of coordination, and prior history of falls were among the primary mediators of the PD-TBI association. Cognitive and psychiatric disorders, notably dementia/cognitive impairment, also contributed to this association and should be an important consideration in future studies investigating the PD-TBI association. The mediation proportions were comparable even when separating the analysis to include only physical or only cognitive/psychiatric codes. The magnitude of the contribution of both motor and non-motor symptoms of PD patients in their prodromal period was modest when we restricted to individual years but substantially larger when considering all years. Nevertheless, the higher estimates should be interpreted with caution since any of the individual 26,411 diagnosis and procedure codes could have appeared after a TBI occurred. Additionally, in the three months prior to diagnosis, these mediation proportions drop, and we suspect this is because of the influence of the ascertainment bias during this period.
The mechanisms leading to a fall in PD patients in their prodromal period should be investigated in future studies. Identifying which PD patients are at greatest risk of falls in the prodromal period and after diagnosis is important. In older adults, falls are considered a common “geriatric syndrome” that occurs with the accumulated effects of multiple impairments among multiple organ systems that may affect the response of an aging physiological system with limited reserve30. In adults aged 65 and older, TBIs were among the top three most common injuries associated with a ground level fall7, which is generally thought to be a minor mechanism of injury. In the case of PD patients in their prodromal period aged 65 and older, we hypothesize that a combination of minor disturbances, such as mild depression, mild cognitive deficits, and subtle alterations in walking biomechanics may predispose this patient population to an increased risk of falls. In the closest years to PD diagnosis, both non-motor and motor symptoms such as balance impairment may be present15,16.
An important strength of our study was the use of a large U.S. population-based sample of Medicare beneficiaries. Medicare is used by 98% of Americans ages 65 and older, making this study broadly representative of the U.S. population. As noted above, another strength of our study was the comprehensiveness of the claims data, which included five different file types in all study years (including outpatient and physician/supplier Medicare Part B claims). The majority of TBIs in our study were defined as mild/concussive, which was consistent with the current literature, with these milder injuries representing 70% to 90% of all TBIs31. This underscored the comprehensive identification of TBI in our sample.
While the very large sample size of our study, the rigorous case definitions based on comprehensive claims data, and the accurate reflection of the distribution of TBI severity compared to population estimates31 were important strengths of this study, there were several potential limitations. First, we were unable to consider TBI that occurred more than five years before PD diagnosis, yet several studies8,9,11,12 have suggested that early TBIs may be causally associated with PD. Therefore, we could not exclude the possibility that TBIs in earlier life may have contributed to the development of PD. Second, our case definitions for TBI required physician and hospital coding in the setting of clinical practice, which may have led to some misclassification of TBI. Finally, PD may have been misclassified. It is possible that certain patients may have had symptoms of PD for several years and were never diagnosed. Some patients may have been misdiagnosed with PD and instead had atypical parkinsonian disorders such as multiple system atrophy or progressive supranuclear palsy. However, we excluded cases with atypical parkinsonism ICD-9 codes, and these conditions were quite rare in comparison to PD.
Another alternative explanation for an association between TBI and PD is that the TBI itself may trigger or accelerate a neurodegenerative pathology. Mouse models of TBI suggest that aged mice were more sensitive to TBI induced a-synuclein pathology than younger mice32. There is also compelling experimental evidence of trauma induced amyloid-beta aggregation in mice33. Although the PD patients in this study were in the prodromal period and likely had extrastriatal pathology, it is possible that head trauma may have accelerated striatal protein aggregation with resultant onset of motor parkinsonism. The TBIs in our sample represented mostly mild/concussive injuries, and it is unclear if a single mild/concussive TBI would be sufficient to induce a-synuclein aggregation. Well-designed studies of elderly TBI patients with and without recent TBI including cerebrospinal fluid, imaging, and clinical assessments may help clarify these relationships.
The most important implication of this work was the finding of the marked increase in incidence of TBI even prior to PD diagnosis, emphasizing the possible importance of earlier diagnosis of PD for primary prevention of TBI. While many have advocated for levodopa sparing strategies in the past34, our study provides compelling evidence that early diagnosis and treatment of motor dysfunction may decrease the burden of TBI in these patients. A superimposed TBI may exacerbate the worsening health of patients with PD who already have a higher risk of poor health outcomes compared to the general population35–39. Even in individuals who have already sustained a TBI, prevention of a second TBI could avoid more disastrous consequences, such as a superimposed intracranial hemorrhage requiring surgical intervention and further loss of independence. Earlier diagnosis of PD by healthcare providers through identification of non-motor and motor symptoms suggestive of prodromal PD may provide a window of treatment opportunity during which disease associated morbidity in PD patients may be reduced.
Acknowledgments
This study was supported by the Rehabilitation Medicine Scientist Training Program, 5K12 HD001097-19 (ACS), NIEHS K24ES017765-07 (BAR), Michael J. Fox Foundation (BAR, SSN, MNW) and American Parkinson Disease Association (BAR, SSN, MNW).
Footnotes
Author Contributions
Study concept and design: all authors.
Data acquisition and analysis: ACS, MNW, SSN, AS
Drafting of the manuscript and figures: all authors.
Potential Conflicts of Interest
All authors have nothing to disclose.
References
- 1.Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 2017;66(9):1–16. doi: 10.15585/mmwr.ss6609a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronado VG, Thomas KE, Sattin RW, Johnson RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. 2005;20(3):215–228. doi: 10.1097/00001199-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Incidence rates of hospitalization related to traumatic brain injury--12 states, 2002. MMWR Morb Mortal Wkly Rep. 2006;55(8):201–204. [PubMed] [Google Scholar]
- 4.Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson’s disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag. 2014;4(3):203–221. doi: 10.2217/nmt.14.22. [DOI] [PubMed] [Google Scholar]
- 5.Kerr GK, Worringham CJ, Cole MH, et al. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- 6.Nystrom H, Nordstrom A, Nordstrom P. Risk of Injurious Fall and Hip Fracture up to 26 y before the Diagnosis of Parkinson Disease: Nested Case-Control Studies in a Nationwide Cohort. PLoS Med. 2016;13(2):e1001954. doi: 10.1371/journal.pmed.1001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CV, Rix K, Klein AL, et al. A Comprehensive Investigation of Comorbidities, Mechanisms, Injury Patterns, and Outcomes in Geriatric Blunt Trauma Patients. Am Surg. 2016;82(11):1055–1062. [PubMed] [Google Scholar]
- 8.Bower JH, Maraganore DM, Peterson BJ, et al. Head trauma preceding PD: a case-control study. Neurology. 2003;60(10):1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- 9.Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016;73(9):1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner RC, Burke JF, Nettiksimmons J, et al. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol. 2015;77(6):987–995. doi: 10.1002/ana.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman SM, Tanner CM, Oakes D, et al. Head injury and Parkinson’s disease risk in twins. Ann Neurol. 2006;60(1):65–72. doi: 10.1002/ana.20882. [DOI] [PubMed] [Google Scholar]
- 12.Kenborg L, Rugbjerg K, Lee PC, et al. Head injury and risk for Parkinson disease: results from a Danish case-control study. Neurology. 2015;84(11):1098–1103. doi: 10.1212/WNL.0000000000001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PC, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology. 2012;79(20):2061–2066. doi: 10.1212/WNL.0b013e3182749f28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugbjerg K, Ritz B, Korbo L, et al. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Chen H, Schwarzschild MA, et al. Perceived imbalance and risk of Parkinson’s disease. Mov Disord. 2008;23(4):613–616. doi: 10.1002/mds.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrag A, Horsfall L, Walters K, et al. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 17.Searles Nielsen S, Warden MN, Camacho-Soto A, et al. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017 doi: 10.1212/WNL.0000000000004536. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuthbert JP, Corrigan JD, Whiteneck GG, et al. Extension of the representativeness of the Traumatic Brain Injury Model Systems National Database: 2001 to 2010. J Head Trauma Rehabil. 2012;27(6):E15–27. doi: 10.1097/HTR.0b013e31826da983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carroll CP, Cochran JA, Guse CE, Wang MC. Are we underestimating the burden of traumatic brain injury? Surveillance of severe traumatic brain injury using centers for disease control International classification of disease, ninth revision, clinical modification, traumatic brain injury codes. Neurosurgery. 2012;71(6):1064–1070. doi: 10.1227/NEU.0b013e31826f7c16. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 21.Thurman DJ National Center for Injury Prevention and Control (U.S.) Guidelines for Surveillance of Central Nervous System Injury. Atlanta, GA: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Recommended framework for presenting injury mortality data. MMWR Recomm Rep. 1997;46(RR14):1–30. [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 26.Parry-Jones BL, Vaughan FL, Miles Cox W. Traumatic brain injury and substance misuse: a systematic review of prevalence and outcomes research (1994–2004) Neuropsychol Rehabil. 2006;16(5):537–560. doi: 10.1080/09602010500231875. [DOI] [PubMed] [Google Scholar]
- 27.Searles Nielsen S, Franklin GM, Longstreth WT, et al. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol. 2013;74(3):472–477. doi: 10.1002/ana.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 29.Buis ML. Direct and indirect effects in a logit model. Stata J. 2010;10(1):11–29. [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric Syndromes: Clinical, Research, and Policy Implications of a Core Geriatric Concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(Suppl):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 32.Uryu K, Giasson BI, Longhi L, et al. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol. 2003;184(1):214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- 33.Washington PM, Morffy N, Parsadanian M, et al. Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer’s disease mouse model. J Neurotrauma. 2014;31(1):125–134. doi: 10.1089/neu.2013.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PD Med Collaborative Group. Gray R, Ives N, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196–1205. doi: 10.1016/S0140-6736(14)60683-8. [DOI] [PubMed] [Google Scholar]
- 35.Driver JA, Kurth T, Buring JE, et al. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70(16 Pt 2):1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach OH, Broen MP, Weber WE. Motor outcomes during hospitalization in Parkinson’s disease patients: a prospective study. Parkinsonism Relat Disord. 2013;19(8):737–741. doi: 10.1016/j.parkreldis.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Guttman M, Slaughter PM, Theriault ME, et al. Burden of parkinsonism: a population-based study. Mov Disord. 2003;18(3):313–319. doi: 10.1002/mds.10333. [DOI] [PubMed] [Google Scholar]
- 38.Guttman M, Slaughter PM, Theriault ME, et al. Parkinsonism in Ontario: comorbidity associated with hospitalization in a large cohort. Mov Disord. 2004;19(1):49–53. doi: 10.1002/mds.10648. [DOI] [PubMed] [Google Scholar]
- 39.Hely MA, Morris JG, Traficante R, et al. The sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry. 1999;67(3):300–307. doi: 10.1136/jnnp.67.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]