Abstract
Background
There have been inconsistent reports of decreased vaccine effectiveness (VE) against influenza viruses among older adults (aged ≥65 years) compared with younger adults in the United States. A direct comparison of VE over multiple seasons is needed to assess the consistency of these observations.
Methods
We performed a pooled analysis of VE over 5 seasons among adults aged ≥18 years who were systematically enrolled in the U.S. Flu VE Network. Outpatients with medically-attended acute respiratory illness (cough with illness onset ≤7 days prior to enrollment) were tested for influenza by reverse transcription polymerase chain reaction. We compared differences in VE and vaccine failures among older adult age group (65–74, ≥75, and ≥65 years) to adults aged 18–49 years by influenza type and subtype using interaction terms to test for statistical significance and stratified by prior season vaccination status.
Results
Analysis included 20,022 adults aged ≥18 years enrolled during the 2011–12 through 2015–16 influenza seasons; 4,785 (24%) tested positive for influenza. VE among patients aged ≥65 years was not significantly lower than VE among patients aged 18–49 years against any subtype with no significant interaction of age and vaccination. VE against A(H3N2) viruses was 14% (95% confidence interval [CI]−14% to 36%) for adults ≥65 years and 21% (CI 9%-32%) for adults 18–49 years. VE against A(H1N1)pdm09 was 49% (95% CI 22%-66%) for adults ≥65 years and 48% (95% CI 41%-54%) for adults 18–49 years and against B viruses was 62% (95% CI 44%–74%) for adults ≥65 years and 55% (95% CI 45%-63%) for adults 18–49 years. There was no significant interaction of age and vaccination for separate strata of prior vaccination status.
Conclusions
Over 5 seasons, influenza vaccination provided similar levels of protection among older and younger adults, with lower levels of protection against influenza A(H3N2) in all ages.
Keywords: Influenza, human, influenza vaccines, influenza vaccine effectiveness, older adults
INTRODUCTION
Older adults (aged ≥65 years) are at high risk for complications from influenza, including hospitalization and death [1–3]. During 2010–2013, older adults comprised 54–70% of influenza-associated hospitalizations and 73–85% of influenza-associated deaths in the United States [4]. Influenza vaccination is the primary method of prevention of infection and has been recommended for all adults aged ≥65 years since the 1960s [5, 6]. Understanding factors that contribute to differences in vaccine effectiveness (VE) by age and achieving the highest possible VE is a priority, especially for this at-risk group.
The U.S. Flu VE Network publishes estimates of influenza VE each season [7–11], including VE by age group. VE has been lower in older adults compared with younger adults in some seasons [7, 8, 10], but not others [9, 12]. A prior pooled estimate of U.S. Flu VE Network data examining intra-season waning of VE did not find statistical differences in VE among adults aged <60 and ≥60 years [13]. A recent meta-analysis has shown that although the vaccine is generally effective in adults aged ≥65 years, VE was often lower as compared with younger adults, particularly in seasons when A(H3N2) was the predominant circulating virus [14].
Most of these prior studies have only assessed VE in single seasons, or in single large age groups. We hypothesized that the effectiveness of standard dose inactivated influenza vaccines would be lower among older adults compared with younger adults. We pooled 5 seasons of data from the U.S. Flu VE Network to describe and compare VE among adults aged ≥65 years with adults aged 18–49 years.
MATERIALS AND METHODS
US Flu VE Network
We used data from sites participating in the U.S. Flu VE Network Study [7–9, 11] from the 2011–12 through 2015–16 influenza seasons. The network enrolled patients aged ≥6 months who presented to outpatient providers for an acute respiratory illness (ARI) of ≤7 days duration with cough (or fever/feverishness in the 2011–12 season) during periods of local influenza circulation across five sites. Interviews were performed at enrollment to collect demographic data and self-reported current health status. Oral and nasal swab specimens were collected and tested for influenza by reverse transcription polymerase chain reaction (RT-PCR) [7]. Patients with inconclusive RT-PCR results and those with two influenza viruses detected were excluded. Patients were considered to have high-risk medical conditions [15] based on ICD-9 or ICD-10 codes corresponding to a high-risk condition in the electronic medical record in the year before enrollment. At selected sites, patients without vaccination documented in the medical record or vaccine registry were considered vaccinated if plausible date and location of vaccination were provided by self-report. Receipt of prior season vaccination was obtained only from medical records or state immunization information systems. Patients who received influenza vaccines other than standard dose inactivated vaccines were excluded. We limited this analysis to enrolled patients aged ≥18 years.
Vaccine effectiveness analysis
As reported in previous VE reports by the U.S. Flu VE Network [7–11], we assessed VE using the test-negative case-control design [16, 17], with cases defined as patients with lab-confirmed influenza and controls as patients testing negative for influenza. We calculated the odds of vaccination among influenza-positive cases compared to influenza-negative controls (OR), and VE was calculated as [1-OR] × 100%. The logistic regression model was adjusted for network site, age, sex, race/ethnicity, presence of any high-risk medical condition, self-rated general health status (excellent, very good, good, fair, poor), interval between illness onset and specimen collection (0–2 days, 3–4 days, or 5–7 days), month of illness onset, and season. Variables included in the model were decided a priori to compare with prior US Flu VE Network estimates. Within each age category, age in months was modeled using linear tail-restricted cubic spline functions with multiple knots, as previously described [8, 9].
To test the hypothesis that adults aged ≥65 years had lower VE than adults aged 18–49 years, we used an interaction term for age group and vaccination for each model. We compared VE among adults aged 50–64 years, 65–74 years, ≥75 years, as well as combined ≥65 years, with the reference group of adults aged 18–49 years. Given wide variations in VE by type and subtype [7–11] we conducted analyses by influenza type and subtype. Influenza A categories included A(H1N1)pdm09 viruses, which predominated during the 2013–14 and 2015–16 seasons, and A(H3N2) viruses which predominated during the 2011–12, 2012–13, and 2014–15 seasons. Influenza B lineages were combined into a single influenza B category due to small numbers. We performed a secondary analysis for A(H3N2) viruses removing the 2014–15 season, when the majority of circulating A(H3N2) viruses were antigenically different than the viruses in the seasonal vaccines. For comparison with previous studies, additional sensitivity analyses included use of adults aged 50–64 years as the referent group and analysis for VE against A(H3N2) viruses restricted to patients with influenza-like illness (ARI and fever) and to those with documented vaccination status only.
Given prior reports of repeated vaccination being associated with lower VE [18–20] and the fact that the older age groups have consistently higher vaccination rates [21], we tested for a significant difference in VE by age group in stratified models by current season and immediate prior season vaccination status (vaccinated in both current and prior season vs. unvaccinated in both seasons, and vaccinated in current season only vs. unvaccinated in both seasons) for influenza A(H3N2).
We also hypothesized that if VE was lower among older adults, the relative odds of vaccine failure among enrolled vaccinated patients would be higher compared with adults aged 18–49 years. To directly compare vaccinated older and younger adults, we performed a post-hoc analysis limited to individuals vaccinated in the current season. Using the same covariates, we calculated the adjusted OR of influenza among vaccinated adults aged 65–74 years, ≥75 years and ≥65 years, each compared to vaccinated adults aged 18–49 years as a referent group in separate models.
Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). We tested for significant difference in VE by age group using a p value <0.05 for the age-vaccination interaction term.
RESULTS
Patient characteristics
From the 2011–12 through 2015–16 influenza season, the U.S. Flu VE Network enrolled 20,907 outpatients aged ≥18 years. A total of 885 were excluded from the analyses because of receipt of vaccine <14 days prior to illness onset or unknown date or location of self-reported vaccination (n=385), receipt of high-dose influenza vaccine (n=296) or live attenuated influenza vaccine (n=126), inconclusive RT-PCR results (n=47), illness onset >7 days prior to enrollment (n=30), and co-detection of influenza A and B viruses (n=1). Of the remaining 20,022 patients included in the analysis, 56% were aged 18–49 years, 27% were aged 50–64 years, 11% were aged 65–74 years, and 6% were aged ≥75 years (Table 1).
Table 1.
U.S. Flu VE Network population characteristics, 2011–2016 (N=20,022)
| 18–49 years N = 11,131 |
50–64 years N = 5,489 |
65–74 years N = 2,174 |
>75 years N = 1,228 |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | Influenza positive (%) | n | Influenza positive % | n | Influenza positive % | n | Influenza positive % | |
|
| ||||||||
| Sex | ||||||||
| Female | 7131 | 1581 (22) | 3370 | 807 (24) | 1255 | 275 (22) | 743 | 163 (22) |
| Male | 3998 | 1040 (26) | 2118 | 578 (27) | 919 | 233 (25) | 485 | 106 (22) |
|
| ||||||||
| Race/ethnicity | ||||||||
| White, non-Hispanic | 8547 | 2058 (24) | 4477 | 1126 (25) | 1906 | 453 (24) | 1083 | 235 (22) |
| Black, non-Hispanic | 774 | 174 (22) | 385 | 104 (27) | 100 | 24 (24) | 51 | 12 (24) |
| Hispanic | 843 | 182 (22) | 258 | 63 (24) | 52 | 11 (21) | 41 | 6 (15) |
| Other, non-Hispanic | 933 | 201 (22) | 358 | 91 (25) | 108 | 19 (18) | 49 | 15 (31) |
|
| ||||||||
| High risk conditions* | ||||||||
| 0 | 8131 | 1995 (25) | 2740 | 736 (27) | 676 | 166 (25) | 198 | 41 (21) |
| ≥1 | 3000 | 627 (21) | 2749 | 650 (24) | 1498 | 342 (23) | 1030 | 228 (22) |
|
| ||||||||
| Reported general health statust† | ||||||||
| Excellent | 2680 | 765(29) | 1046 | 294 (28) | 386 | 103 (27) | 152 | 43 (28) |
| Very good | 4707 | 1116 (24) | 2117 | 555 (26) | 851 | 209 (25) | 416 | 89 (21) |
| Good | 2933 | 602 (21) | 1725 | 405 (23) | 695 | 142 (20) | 456 | 94 (21) |
| Fair | 708 | 123 (17) | 520 | 110 (21) | 211 | 45 (21) | 171 | 36 (21) |
| Poor | 95 | 14 (15) | 73 | 18 (25) | 28 | 9 (32) | 32 | 7 (22) |
|
| ||||||||
| Vaccinated for current season | ||||||||
| Yes | 4457 | 823(19) | 3251 | 659 (20) | 1652 | 358 (22) | 1019 | 210 (21) |
| No | 6674 | 1799 (27) | 2238 | 727 (32) | 522 | 150(29) | 209 | 59 (28) |
|
| ||||||||
| Time from symptom onset to enrollment | ||||||||
| <3 days | 3473 | 1131 (33) | 1374 | 528 (38) | 528 | 201 (38) | 284 | 94 (33) |
| 3–4 days | 4431 | 1049 (24) | 2198 | 551 (25) | 812 | 172 (21) | 479 | 109 (23) |
| 5–7 days | 3227 | 442 (14) | 1917 | 307 (16) | 834 | 135 (16) | 465 | 66 (14) |
|
| ||||||||
| Season | ||||||||
| 2011–12 | 1544 | 234 (15) | 674 | 94 (14) | 240 | 36 (15) | 150 | 18 (12) |
| 2012–13 | 2200 | 719 (33) | 1032 | 364 (35) | 413 | 137 (33) | 216 | 75 (35) |
| 2013–14 | 2139 | 539 (25) | 1089 | 267 (25) | 401 | 77(19) | 214 | 25 (12) |
| 2014–15 | 2804 | 633 (23) | 1495 | 378 (25) | 711 | 197 (28) | 427 | 133 (31) |
| 2015–16 | 2444 | 497 (20) | 1199 | 283 (24) | 409 | 61 (15) | 221 | 18 (8) |
|
| ||||||||
| Type and subtype | ||||||||
| Influenza A unsubtyped | – | 53 | – | 24 | – | 9 | – | 3 |
| A(H1N1)pdm09 | – | 848 | – | 459 | – | 100 | – | 31 |
| A(H3N2) | – | 1182 | – | 583 | – | 293 | – | 207 |
| Excluding 2014–15 | – | 660 | – | 302 | – | 125 | – | 90 |
| Influenza B | – | 539 | – | 320 | – | 106 | – | 28 |
Presence of one or more electronic medical record code for a high-risk condition in the prior year, as defined by the ACIP guidance for conditions that increase the risk of complications from influenza
n = 20,002 (11,123 for 18–49 years, 5,481 for 50–64 years, 2,127 for 65–74 years, and 1,227 for ≥75 years)
Compared with adults aged 18–49 years, adults aged ≥65 years were more likely to have at least one high risk condition (74% vs. 27%) and report fair or poor health status (22% vs. 13%). They also tended to seek care later in illness (5–7 days after symptom onset). Current season vaccination was more common in adults aged ≥65 years than aged 18–49 years (79% vs 40%). Of those who were vaccinated in the current season and had records available from the previous season (n=8,658), 71% were also vaccinated in the prior season and this was highest for older adults (86% of adults aged ≥65 years vs. 60% of adults aged 18–49 years).
Overall, 15,237 (76%) tested negative for influenza and 4,785 (24%) tested positive for influenza, including 2,265 (47%) influenza A(H3N2), 1,438 (30%) influenza A(H1N1)pdm09, 89 (2%) influenza A unsubtyped, 246 (5%) influenza B Victoria lineage, 719 (15%) influenza B Yamagata lineage, and 28 (1%) influenza B undetermined lineage. The distribution of type and subtype of influenza were similar by age categories but varied by season (Table 1).
VE by subtype
Over 5 seasons, VE against influenza A(H1N1)pdm09 was 48% for adults aged 18–49 years (CI 38%-57%), 40% (CI 4%-63%) for adults aged 65–74 years, 78% (CI 39%-92%) for adults aged ≥75 years, and 49% (CI 22%-66%) for the combined group of adults aged ≥65 years (Figure 1, Supplemental Table 1). Differences in VE between older adult age groups and adults aged 18–49 years were not significant (p values for interaction >0.05) (Figure 1).
Figure 1.
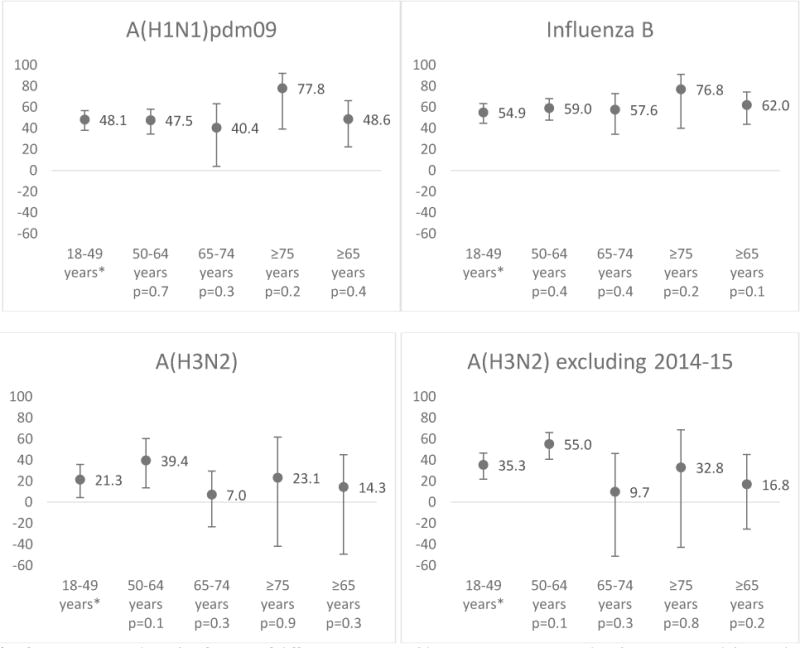
Adjusted standard dose inactivated influenza vaccine effectiveness (VE) by influenza type and subtype. VE has been adjusted for U.S. Flu VE Network site, age (by month spline), sex, race/ethnicity, high-risk health status, self-rated general health status, interval between illness onset and specimen collection, season and month of illness onset.
* Referent group and p-value for test of difference in VE in older age group compared with VE among adults aged 18–49 years using interaction terms.
† p value for the interaction term of age and vaccination (for the test of significance of the interaction term) in model using adults aged 18-49 years as the comparison group.
For influenza B viruses, VE was 55% for adults aged 18–49 years (CI 45%-63%), 58% (CI 34%-73%) for adults aged 65–74 years, 77% (40%-91%) for adults aged ≥75 years, and 62% (CI 44%-74%) for adults aged ≥65 years. Differences in VE between older adult age groups and adults aged 18–49 years were not significant (p values >0.05).
Against influenza A(H3N2) viruses, VE was 21% for adults aged 18–49 years (CI 9.4%-32%), 7.0% (CI−33% to 35%) for adults aged 65–74 years, 23% (CI −26% to 53%) for adults aged ≥75 years, and 14% (CI −14% to 36%) for adults aged ≥65 years. Differences in VE between older adult age groups and adults 18–49 years were not significant (p values >0.05). In subgroup analysis excluding the 2014–15 season, VE against A(H3N2) viruses was 35% for adults aged 18–49 years (CI 22%-46%), 10% (CI −51% to 46%) for adults 65–74 years, 33% (−43% to 68%) for adults aged ≥75 years, and 17% (CI −26% to 45%) for adults aged ≥65 years. Again, p values for the interaction terms were not significant for any of the older adult age groups.
Given lower VE against A(H3N2) compared to other subtypes, we also examined differences in VE against A(H3N2) viruses (excluding the 2014–15 season) by age group and prior vaccination status. Among patients with at least one previous year of available medical records (N=16,344), 6,163 (38%) had received influenza vaccine in both seasons and 2,495 (15%) had received vaccine in the current season only. The percentage who had received vaccine in both seasons increased with increasing age (25% of those aged 18–49 years, 44% 50–64 years, 63% 65–74 years and 75% ≥75 years). VE against A(H3N2) viruses (excluding the 2014–15 season) among those who had received vaccine in both current and prior seasons was 39% (CI 19%-54%) for adults aged 18–49 years and 1% (CI−77% to 45%) for adults aged ≥65 years (Table 2). VE against A(H3N2 viruses) (excluding the 2014–15 season) among those who had received the current season vaccine but were not vaccinated in the prior season was 42% (CI 21%-57%) for adults aged 18–49 years and 14% (CI−74% to 58%)) for adults aged ≥65 years (Table 2). P values for interaction terms were not significant (p>0.05).
Table 2.
Vaccine Effectiveness (VE) against influenza A(H3N2) viruses excluding the 2014–15 season by age group and receipt of vaccination in the year prior to enrollment among adults vaccinated during the current season.
| Current and prior season vaccination | Current season vaccination only | |||||
|---|---|---|---|---|---|---|
| n (%) | aVE* (95% CI) |
Interaction p value† |
n (%) | aVE* (95% CI) |
Interaction p value† |
|
|
18–49 years (n = 5,077) |
1,251 (25%) | 39 (19, 54) | – | 905 (18%) | 42 (21, 57) | – |
|
50–64 years (n = 2,543) |
1,130 (44%) | 60 (41, 73) | 0.36 | 423 (17%) | 56 (29, 72) | 0.55 |
|
≥65 years (n = 1,599) |
1,053 (66%) | 0.9 (−77, 45) | 0.15 | 197 (12%) | 14 (−74, 58) | 0.36 |
| 65–74 years (n = 1,011) |
615 (61%) | −22 (−159, 43) | 0.07 | 152 (15%) | 6.1 (−130, 62) | 0.30 |
| ≥75 years (n = 588) |
438 (74%) | 41 (−64, 79) | 0.64 | 45 (7.7%) | 48 (−109, 87) | 0.84 |
Adjusted for site, age (spline, month), month of enrollment, sex, race/ethnicity, presence of ≥1 high-risk condition, interval from symptom onset to enrollment, self-reported general health status, and season
p value for the interaction term of age and vaccination (for the test of significance of the interaction term) in model using adults aged 18–49 years as a comparison group
Relative odds of influenza among vaccinated persons
The relative odds of influenza among vaccinated persons varied with type and subtype, and age group (Table 3). Relative odds of influenza A(H1N1)pdm09 were lower in the oldest adults aged ≥75 years (aOR 0.4, p<0.01) and the combined age group of adults aged ≥65 years (aOR 0.7, p=0.01) compared with young adults but not for adults aged 65–74 years. Relative odds of influenza B were slightly higher in adults aged 50–64 (aOR 1.4, p=0.01) compared with adults aged 18–49 years but were not significantly different for older groups. Vaccinated older adults had greater relative odds of having influenza A(H3N2) compared with vaccinated adults aged 18–49 years (65–74 years: aOR 1.7, p<0.01, ≥75 years: aOR 2.1, p<0.01, ≥65 years: aOR 1.9, p<0.01). Results were similar in analyses excluding the 2014–15 season.
Table 3.
Relative odds of influenza among persons who received standard dose inactivated influenza vaccine during the current season by age group, compared with young adults aged 18–49 years.
| 18–49 years | 50–64 years | 65–74 years | ≥75 years | ≥65 years | |||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | aOR* (95% CI †) | n (%) | aOR* (95% CI†) | n (%) | aOR* (95% CI†) | n (%) | aOR* (95% CI†) | |
| A(H1N1)pdm09 | 212 (5.5) | 195 (7.0) | 1.4 (1.1, 1.7) | 59 (4.4) | 0.8 (0.6, 1.2) | 18 (2.2) | 0.4 (0.3, 0.7) | 77 (3.5) | 0.7 (0.5, 0.9) |
| A(H3N2) | 452 (11) | 318 (11) | 1.1 (0.9, 1.3) | 230 (15) | 1.7 (1.4, 2.1) | 171 (17) | 2.1 (1.6, 2.6) | 401 (16) | 1.9 (1.6, 2.3) |
| Excluding 2014–15 | 224 (7.7) | 142 (7.1) | 1.0 (0.8, 1.3) | 96 (10) | 1.7 (1.2, 2.3) | 75 (12) | 2.2 (1.6, 3.2) | 171 (11) | 1.9 (1.4, 2.4) |
| Influenza B | 144 (3.8) | 134 (4.9) | 1.4 (1.1, 1.8) | 63 (4.6) | 1.3 (1.0, 1.9) | 18 (2.2) | 0.6 (0.4, 1.1) | 81 (3.7) | 1.1 (0.8, 1.5) |
aOR = Adjusted relative odds ratio. Adjusted for site, age (spline, month), month of enrollment, sex, race/ethnicity, presence of ≥1 high-risk condition, interval from symptom onset to enrollment, self-reported general health status, and season
CI = 95% confidence interval
Bolded text indicates statistical significance
Sensitivity analyses of VE by age group
The interaction between age and vaccination was not significant for older adult age groups compared with adults aged 18–49 years in analysis limited to those with fever in addition to ARI, a more specific but less sensitive marker for influenza-like illness or when limited to persons with documented vaccination history only (Supplemental Table 2).
In a sensitivity analysis using adults aged 50–64 years as the referent group, interaction between age and vaccination was not significant for A(H1N1) or B viruses for any groups (Supplemental table 3). For A(H3N2), the interaction for age and vaccination was significant (overall and excluding the 2014–15 season) in adults aged 65–74 years vs. 50–64 years (p=0.04 and 0.03) and the combined age group of adults ≥65 years vs. 50–64 years(p=0.05 and 0.02), but not for adults aged ≥75 years for (p=0.52 and 0.36).
DISCUSSION
Combining 5 seasons from the U.S. Flu VE Network, we found comparable estimates in most analyses of influenza vaccine effectiveness among older and younger adult patients presenting with acute respiratory illness for outpatient care. We found no consistent pattern of lower vaccine effectiveness among any of the older adult age groupings (65–74 years, ≥75 years, or ≥65 years) compared with younger adults. For A(H1N1)pdm09 and influenza B viruses, there was no statistically significant effect modification comparing older age groups to younger adults. For A(H3N2) viruses, VE was not significant for any age group ≥65 years whereas VE was significant among adults aged 18–49 and 50–64 years. However, there were no statistically significant differences in VE between the older age groups and adults aged 18–49 years. In addition, there was no pattern of decreasing VE among the oldest adults aged ≥75 years as compared with adults aged 65–74 years.
While this analysis suggests that VE against influenza in ambulatory settings is not statistically lower in older age groups compared to adults aged 18–49 years, evidence from immunogenicity studies show decreased immune response among older adults [22–24]. This is thought to be due to immunosenescence, a gradual decline in immune function with age including decreased antibody response following immunization and greater reliance on T-cell mediated response [25]. Multiple serologic studies have found lower immune response to standard dose influenza vaccination in older adults compared with younger adults [22, 23]. In addition to this decreased initial immune response, waning of antibody response and VE over the course of a single season has been reported to be more pronounced in the elderly [26, 27], though this has not been seen consistently [13]. Despite lower antibody levels, those produced may still be enough to provide clinical protection against influenza virus infection. In addition, antibodies from prior years may provide residual protection, and there is a possibility of seroprotection despite each subsequent vaccination leading to smaller boosts of antibody levels. The effects of prior vaccination vs. immunosenescence are difficult to distinguish since older adults have the highest vaccination rates. With low numbers of unvaccinated older adults over multiple seasons, we have low power to assess for the effects of prior vaccination within this age group [28]. Adjuvanted vaccines lead to greater immune response in clinical trials and have recently been licensed in the United States for persons aged ≥65 years [22, 23]. Serologic studies have suggested higher immune response of older adults to high-dose influenza vaccination [24, 25] and one randomized controlled trial has found higher relative effectiveness of high-dose vaccine compared with standard [29]. We were not able to assess VE of high-dose influenza vaccine here, and more information on VE is needed for these newer, more immunogenic vaccines (high-dose and adjuvanted).
Our findings concur with VE estimates published for 2013–14, an A(H1N1)pdm09-predominant season [9]. Differences in the other yearly VE estimates from the U.S. Flu VE Network may be due to the use of documented vaccination only [7, 8] or to our use of a reference group, adults aged 18–49 years. Our findings also support those of a recent meta-analyses of multiple test-negative design case-control studies that found that seasonal influenza vaccination was effective against laboratory confirmed influenza in elderly adults seeking care in ambulatory settings [33] and found no difference in VE among older adults (≥60 years) and younger adults (<60 years) [12].
We set our reference group to be adults aged 18–49 years a priori with the intent to assess differences in VE among a wide age range, however, occasionally VE is lower in those aged 18–49 years compared with those aged 50–64 years [7, 8, 10]. Results in our analysis were similar using 50–64 years as a reference group, although we did detect significant effect modification of age on VE against influenza A(H3N2) viruses in adults aged 65–74 years and ≥65 years vs. 50–64 year olds.
We did not find differences in interactions between age and vaccine effect when stratifying by prior vaccination. Although influenza vaccination has been recommended for all persons aged ≥6 months since 2010, adults aged ≥65 years were one of the first groups for which annual vaccination was recommended and this group has consistently had the highest annual immunization rates in the United States [34, 35]. Data from recent observational studies suggests possible lower VE point estimates in individuals after repeated vaccination during some seasons [36, 37]. We would expect this effect to be most pronounced in the highly vaccinated population of older adults.
We hypothesized that with non-statistically significant differences in VE, we would find a similar proportion of vaccine-failures in each age group. This was the case for all influenza subtypes, except for influenza A(H3N2) viruses, where we found increased relative odds of vaccine failure among older adults compared with younger adults. Interaction terms, such as we used to calculate VE by age group, generally have lower power and an analysis of vaccine-failures by age group, such as we used to calculate the relative odds of vaccine failure, has more power to detect differences by age group. The higher relative odds of vaccine failure in older adults suggests that VE against A(H3N2) is lower even though the interaction terms were not significant in the VE models. However, these results should be interpreted with caution, as there may be differences in exposure, and therefore risk of influenza, by age group [38]. The proportion of influenza positive cases may also reflect disease severity and care-seeking behaviors that differ by age [39].
Our analysis was subject to several limitations. We relied on interaction terms of age and vaccination to assess for effect modification by age, however we may have been limited to detect a true effect due to small sample sizes in some strata and seasons. Older adults have high and consistent vaccination coverage across multiple years, which limits the size of the unvaccinated groups. Unvaccinated older adults are likely different in unmeasured ways compared with the majority who are vaccinated annually. The test-negative design has been frequently used to assess VE, but may be subject to residual confounding [16, 40] and potential biases, which may underestimate the true VE [41]. In addition, vaccination history included plausible self-report as well as documentation of vaccination, which may lead to misclassification. Vaccines may be given in nontraditional settings, making documentation more difficult to obtain. We included those with a plausible self-report of vaccination and performed a sensitivity analysis limiting to only individuals with documented vaccination history and found similar results consistent with previous studies [7]. We excluded individuals who received high-dose vaccination, as we did not have enough sample size to compare VE for high-dose to standard dose. The pooled nature of the analysis may be weighted towards seasons with higher sample size; however, the percentage of sample size comprised of each age group was relatively stable by season. The U.S. Flu VE Network only examines VE against medically-attended illness among outpatients and VE may differ when examining community illness or influenza-associated hospitalizations or among institutionalized persons or persons receiving care in subspecialty clinics. The results of this analysis may not be generalizable to those with more severe illness.
Conclusions
We found that over five influenza seasons, influenza vaccination provided similar levels of protection among older and younger adults for most seasons across influenza type and subtype. Influenza vaccination may have provided less protection against A(H3N2) among older adults compared with younger adults as evidenced by the higher relative odds of influenza among vaccinated older adults. We found no consistent pattern of lower VE with increasing age and did not detect significant modification of VE by age group. Further, we did not observe lower VE of standard dose inactivated influenza vaccine among the oldest adults (aged ≥75 years). Improving effectiveness of influenza vaccines for adults aged ≥65 years and younger age groups remains a priority.
Supplementary Material
Acknowledgments
The authors would like to thank the research staff at all study sites and individuals who participated in this study.
FUNDING
This work was supported by the Centers for Disease Control and Prevention (CDC) through cooperative agreements with the University of Michigan (U01 IP000474), Group Health Research Institute (U01 IP000466), Marshfield Clinic Research Institute (U01 IP000471), University of Pittsburgh (U01 IP000467), and Baylor Scott and White Health (U01 IP000473) and by the National Institutes of Health (NIH) (grant UL1TR001857 to the University of Pittsburgh).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
MPN has research funding from Merck & Co., Inc., RKZ has research funding from Merck & Co., Inc. and Sanofi Pasteur, Inc. EAB, EAM, HQM, and MJG report past research support from MedImmune.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. People at High Risk of Developing Flu-Related Complications. 2016 https://www.cdc.gov/flu/about/disease/high_risk.htm [accessed 27 March 2017]
- 2.Thompson W, Shay D, Weintraub E, Brammer L, Cox N, Anderson L, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H, Thompson W, Viboud C, Ringholz C, Cheng P, Steiner C, Abedi G, Anderson L, Brammer L, Shay D. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. CID. 2012;54(10):1427–36. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PloS one. 2015;10(3):e0118369. doi: 10.1371/journal.pone.0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016;65:1–54. doi: 10.15585/mmwr.rr6505a1. [DOI] [PubMed] [Google Scholar]
- 6.Bridges CB, Winquist AG, Fukuda K, Cox NJ, Singleton JA, Strikas RA. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–38. quiz CE1–7. [PubMed] [Google Scholar]
- 7.Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58:319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis. 2015;211:1529–40. doi: 10.1093/infdis/jiu647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, et al. Influenza Vaccine Effectiveness Against 2009 Pandemic Influenza A(H1N1) Virus Differed by Vaccine Type During 2013–2014 in the United States. J Infect Dis. 2016;213:1546–56. doi: 10.1093/infdis/jiv577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treanor JJ, Talbot HK, Ohmit SE, Coleman LA, Thompson MG, Cheng PY, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55:951–9. doi: 10.1093/cid/cis574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, et al. 2014–2015 Influenza Vaccine Effectiveness in the United States by Vaccine Type. Clin Infect Dis. 2016;63(12):1564–73. doi: 10.1093/cid/ciw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16:942–51. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinands JM, Fry AM, Reynolds S, Petrie J, Flannery B, Jackson ML, et al. Intraseason waning of influenza vaccine protection: Evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis. 2017;64(5):544–550. doi: 10.1093/cid/ciw816. [DOI] [PubMed] [Google Scholar]
- 14.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 15.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 16.Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–8. doi: 10.1016/j.vaccine.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31:3104–9. doi: 10.1016/j.vaccine.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Baz I, Casado I, Navascues A, Diaz-Gonzalez J, Aguinaga A, Barrado L, et al. Effect of Repeated Vaccination With the Same Vaccine Component Against 2009 Pandemic Influenza A(H1N1) Virus. J Infect Dis. 2017;215:847–55. doi: 10.1093/infdis/jix055. [DOI] [PubMed] [Google Scholar]
- 19.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. Influenza vaccine effectiveness in the community and the household. Clin Infec Dis. 2013;56:1363–9. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis. 2016;63:21–32. doi: 10.1093/cid/ciw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. FluVaxView Influenza Vaccination Coverage. 2016 https://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html [accessed 20 April, 2017]
- 22.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7:385–94. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 24.Smith NM, Bresee JS, Shay DK, Uyeki TM, Cox NJ, Strikas RA. Prevention and Control of Influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–42. [PubMed] [Google Scholar]
- 25.McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–7. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, MC IC. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine. 2017;35:212–21. doi: 10.1016/j.vaccine.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Belongia EA, Sundaram ME, McClure DL, Meece JK, Ferdinands J, VanWormer JJ. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2015;33:246–51. doi: 10.1016/j.vaccine.2014.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosterin Hopping A, McElhaney J, Fonville JM, Powers DC, Beyer WE, Smith DJ. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine. 2016;34(4):540–6. doi: 10.1016/j.vaccine.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiazGrandados C, Dunning A, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, Martin E, Gurunathan S, Nathan R, Greenber D, Tornieporth N, Decker M, Talbot H. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;37(7):635–45. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 30.De Donato S, Granoff D, Minutello M, Lecchi G, Faccini M, Agnello M, et al. Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. Vaccine. 1999;17:3094–101. doi: 10.1016/s0264-410x(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Food and Drug Administration. Fluad. 2016 https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/ucm473989.htm [accessed 12 May, 2017]
- 32.DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine. 2013;31:861–6. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Darvishian M, Bijlsa MJ, Hak E, van den Heuvel ER. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case-control studies. Lancet Infect Dis. 2014;14:1228–39. doi: 10.1016/S1473-3099(14)70960-0. [DOI] [PubMed] [Google Scholar]
- 34.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Influenza vaccination coverage estimates for persons 6 months and older by State, HHS Region, and the United States, National Immunization Survey-Flu (NIS-Flu) and Behavioral Risk Factor Surveillance System (BRFSS), 2010–11 through 2015–16 influenza seasons. 2016 http://www.cdc.gov/flu/fluvaxview/reportshtml/trends/index.html [accessed 5 December, 2016]
- 36.Ohmit SE, Petrie JG, Malosh RE, Fry AM, Thompson MG, Monto AS. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis. 2015;211:1519–28. doi: 10.1093/infdis/jiu650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59:1375–85. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucharski AJ, Kwok KO, Wei VW, Cowling BJ, Read JM, Lessler J, et al. The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population. PLoS pathogens. 2014;10:e1004206. doi: 10.1371/journal.ppat.1004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biggerstaff M, Jhung MA, Reed C, Fry AM, Balluz L, Finelli L. Influenza-like illness, the time to seek healthcare, and influenza antiviral receipt during the 2010–2011 influenza season-United States. The J Infect Dis. 2014;210:535–44. doi: 10.1093/infdis/jiu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orenstein EW, De Serres G, Haber MJ, Shay DK, Bridges CB, Gargiullo P, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–31. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 41.Ferdinands JM, Shay DK. Magnitude of potential biases in a simulated case-control study of the effectiveness of influenza vaccination. Clin Infect Dis. 2012;54:25–32. doi: 10.1093/cid/cir750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


