Abstract
During development of the nervous system, molecular signals mediating cell-cell interactions play critical roles in the guidance of axonal growth and establishment of synaptic functions. The Eph family of tyrosine kinase receptors and their ephrin ligands has been shown to mediate neuronal interactions in the development of topographic axon projection maps in several brain regions, and the loss of Eph activities result in defects in select axonal pathways. However, effects of deficiencies of the Eph signals on animal behavior have not been well documented. In this study, we showed that inactivation of a ligand of the Eph receptors, ephrin-A5, resulted in defects in maternal behavior and alterations in anxiety. Female ephrin-A5-/- mice show significant defects in nest building and pup retrieval. In addition, lower levels of anxiety were observed in both male and female null mice. These changes were not due to deficiencies in estradiol, progesterone, or corticosterone levels. Our observations suggest that ephrin-A5 plays a key role in the development and/or function of neural pathways mediating mouse maternal care and anxiety.
Keywords: Eph receptor, ephrin-A5, maternal care, nest building, pup retrieval, anxiety, estrogen, progesterone
Introduction
Proper maternal behavior is essential for survival of the offspring (Numan & Insel, 2003). It is important for growth and normal development of the young and can influence their physiological, behavioral and cognitive functions in adult life (Franklin et al., 2012). Early life stress in the form of maternal neglect, separation or limited bedding/nesting, produces behavioral and emotional changes as well as long lasting alterations in cognitive functions [reviewed in (Meaney, 2001, Molet et al., 2014, Veenema, 2009)]. Adult rats that were separated from their mothers earlier in life had exaggerated inter-male aggression, increased depression-like behavior and altered levels of arginine vasopressin (AVP) and serotonin (5-HT) in the hypothalamus (Veenema et al., 2006). In addition, differences in the hypothalamic-pituitary-adrenal (HPA) response to stress were found in the offspring of low vs high licking and grooming (LG) rats (Liu et al., 1997). Adult rats that were born and raised by high–LG mothers had decreased plasma corticosterone levels in response to restraint stress compared to those raised by low–LG mothers (Liu et al., 1997). Maternal care can also affect the development of maternal behavior in the female offspring (Melo et al., 2006, Shoji & Kato, 2009). Shoji et al. (Shoji & Kato, 2009) compared the maternal behavior of the offspring of two inbred mice, CBA/Ca and BALB/c, which differ in their levels of maternal care; CBA/Ca females exhibit nursing and pup licking more frequently and retrieve their pups faster than BALB/c females. They found that low levels of maternal care earlier in life (by BALB/c dams) decreased maternal behavior in the offspring.
In rodents, a wide range of behaviors directed toward the care of the young have been reported. These include nest building, pup retrieval, aggression towards a male intruder, nursing, pup licking and grooming, and crouching over the pups to provide thermoregulation (Weber & Olsson, 2008). Interestingly, these behaviors can be seen in non-parental virgin female rodents. When initially exposed to pups virgin female rats will avoid them. Yet, after continuous daily exposure of about 7 days the female will start caring for the young. This pup-induced behavior is referred to as sensitized maternal behavior (Numan, 2014). In contrast to rats, however, mice do not require the “sensitization period” and show spontaneous maternal behavior when presented with pups (Numan, 2014). The level of maternal care is higher in postpartum female mice which are faster to retrieve the pups, spend more time crouching over them (Gandelman et al., 1970, Stolzenberg & Rissman, 2011) and are able to build a better, more complex nest than virgin female mice (Bond et al., 2002). In addition, maternal motivation is lower in virgin compared to postpartum females (Stolzenberg & Rissman, 2011).
The use of gene knockout mice has made a significant contribution to our understanding of the genetic regulation of maternal behavior. Mice carrying a null mutation for the prolactin receptor gene (Lucas et al., 1998), the dopamine β-hydroxylase gene (Thomas & Palmiter, 1997), and the forebrain Gq/11 gene (Wettschureck et al., 2004) exhibit defects in maternal behavior compared to wild-type controls. In addition, several brain regions and neural circuits have been implicated in the development of maternal behavior, specifically the medial amygdala (MeA), the ventral tegmental area (VTA) of the midbrain, the medial prefrontal cortex (mPFC) and the hypothalamus (Gammie, 2005, Numan, 2014, Pereira & Morrell, 2011). Since maternal care encompassed a repertoire of behaviors, and each involves unique motor program, different circuits likely control distinct maternal behaviors, although some overlap may exist (Gammie, 2005). The medial preoptic area (MPOA) of the hypothalamus has been shown to be essential for the onset and maintenance of many aspects of maternal behavior (Gammie, 2005, Tsuneoka et al., 2013, Wu et al., 2014). Here, hormonal activation stimulates maternal behavior, whereas interruption of neuronal activity decreases maternal responsiveness to pups [reviewed in (Numan, 2014)]. In addition, damage to the MPOA by knife cut or electrical lesions severely disrupts pup retrieval and nest building, [reviewed in (Numan & Insel, 2003)]. Furthermore, a recent study (Wu et al., 2014) found a subset of galanin-expressing neurons within the MPOA that act as a switch to turn on and off parenting behavior in mice; ablation of these neurons in lactating female mice reduced pup retrieval, whereas its activation triggered pup grooming (Wu et al., 2014). The involvement of hormones and neurotransmitters in regulating maternal care has also been shown, specifically the neuropeptides arginine vasopressin (AVP) and oxytocin (OXY) both of which are activated around parturition and in lactation (Bosch & Neumann, 2012).
The Eph families of tyrosine kinase receptors and their ephrin ligands have been implicated in the elaboration of neural circuits and functions that regulate animal behavior (Cooper et al., 2009, Hruska & Dalva, 2012, Sheleg et al., 2015, Sheleg et al., 2013, Xu & Henkemeyer, 2012). Ephrin-A5, one of the eight known ligands of the Eph receptors, is also expressed in areas of the brain that are central for normal maternal behavior such as the hypothalamus (Deschamps et al., 2010, Zarbalis & Wurst, 2000). Ephrin-A5 transcripts were detected in the preoptic area, the suprachiasmatic nucleus (SCN), the paraventicular nucleus (PVN) and the arcute nucleus (AR) (Zarbalis & Wurst, 2000). We sought to determine whether maternal behavior is affected by ephrin-A5 deletion. Specifically, we tested ephrin-A5-/- and wild-type control lactating females for their ability to build a nest, retrieve their pups and display maternal aggression toward a male intruder. In addition, different tests for anxiety were used to assess the impact of ephrin-A5 deletion on this behavior.
Materials and Methods
Animals
Ephrin-A5 −/− mice have been described previously (Frisen et al., 1998). Animals used in this study are on a mixed background (C57BL/6 and 129/SV) and were generated using heterozygous crosses. For all behavioral tests and to check for differences in hormonal levels, knockouts and wild-type controls were obtained from the same littermates. In order to measure body weight from wild-type and knockout pups that were born and reared by wild-type and knockout dams, respectively, wild-type females were bred with wild-type males and knockout females with knockout males. The resulting pups were used only for body weight measurements. Mice were maintained on a 12 h light/dark reverse cycle (lights off from 07:00 to 19:00 h), and had free access to food and water. The temperature was maintained at 25 °C. All behavioral experiments were performed during the first phase of the dark cycle under red light. To test for maternal behavior (pup retrieval, maternal aggression and nest building), pregnant females were housed separately until time of birth and the day of birth was recorded as postnatal 0 (P0). Pup body weights were measured every 4 days until P20 and again at P60. All pups were labeled for individual identification by marking their tails. Genotyping was performed by tail DNA polymerase chain reaction (PCR) as described previously (Frisen et al., 1998). Primers 1: 5’TCCAGCTGTGCAGTTCTCCAAAACA3’ and 2 5’ATTCCAGAGGGGTGACTACCACATT3’ were used for amplification of wild-type sequences (397 bp) and primers 1 and 3 5’AGCCCAGAAAGCGAAGGAGCAAAGC3’ for amplification of the null sequences (513 bp).
Nest building
The test was performed as described previously (Deacon, 2006) with some modifications. For maternal nest building, ephrin-A5-/-, heterozygous and wild-type virgin female mice (n=5 per genotype) were housed with a heterozygous male mouse and checked for the presence of a sperm plug; once a plug was observed, the date was recorded as embryonic day 0.5 (E0.5) and the male was removed from the cage. On E18.5 the pregnant mice were transferred to a new clean home cage and provided with nesting material (nestlets; Ancare; UK agent, Lillico). Nests were then observed 1, 6 and 24 hours later and assessed on a rating scale of 1-5, according to the nest sample photographs shown by Deacon (Deacon, 2006) by two observers who were blind to the genotype and time of the nest. Observer reliability was determined with Cohen’s kappa method. A score of 1 was given if there was no nest and a score of 5 represented a perfect, fully enclosed nest. In order to assess nest build by nulliparous mice, adult virgin females (n=6 per genotype; P≥60) were individually housed overnight with food, water, and new bedding. The next morning they were provided with the nesting material and the procedure was repeated as described above.
Maternal aggression and pup retrieval test
Ephrin-A5-/- (n=9), heterozygous (n=10) and wild-type (n=8) lactating female mice were exposed to wild-type intruder male in their home cage for 5 minutes on postpartum day 4 (P4) and 6 (P6). The pups were removed from the cage 2 minutes before the behavioral test, and the test was recorded for subsequent analysis. The intruder males were sexually naïve and group housed. After the maternal aggression test, the pups were randomly distributed throughout the cage and the time to retrieve the 1st and 3rd pup was recorded. Retrieval counted only when the pup was brought into the nest completely and, a score of 180 sec was assigned if the dam failed to retrieve her pups in 3 minutes.
For the cross retrieval of pups with different genotypes, a new set of lactating female mice (ephrin-A5-/-, n=10, wild-type, n=9) were used on P2. The pups were removed from their home cage 30 minutes before the test and kept in a new separate cage. Each female was then introduced to 3 pups from a different litter such that null dams tested with wild-type pups, and wild-type dams with null pups. The time to retrieve the 1st and 3rd pup was recorded for a maximum of 5 minutes.
In both tests the females were housed with a heterozygous male mouse and transferred to a new cage 2-3 days before parturition. The cage was not changed until the end of the experiments (P7 for the retrieval of own pups and P3 for the cross retrieval of pups with different genotypes).
Elevated-plus maze (EPM)
The maze was constructed of black Plexiglas with four arms in the form of a “plus”, 30 cm above the floor. Two opposing arms of the maze (65 cm long) were enclosed in 8 cm high, black Plexiglas walls, while the two remaining arms (30 cm long) were left open. Two experiments were conducted using the EPM. In experiment 1 we tested male ephrin-A5-/- (n=7) mice that were born and reared by ephrin-A5-/- mothers and wild-type (n=6) control mice that were born and reared by wild-type mothers. In experiment 2, male and female ephrin-A5-/- (n=10 per sex), heterozygous (n=11 per sex) and wild-type (n=10 per sex) littermates that were born to heterozygous mothers were tested. Mice in the second experiment were born and reared by the same mother, and thus the effect of rearing was removed. The test began when each mouse was individually placed in the center (5 cm × 5 cm) of the maze facing an open arm and the number of times each animal entered an arm (either closed or open) as well as the duration spent in each arm were recorded for 5 minutes. The number of entrances into the open arm and/or the time spent in the open arms provides indications of anxiolytic-like behaviors, and the total number of entrances (into both open and closed arms) is a measure of locomotor activity. EPM testing was carried out under dim light and an arm entry was recorded only when all four paws crossed into the arm.
Light dark box test
The test was performed as described previously (Rossi-George et al., 2004), with some modifications. Briefly, the plexiglas box (47 × 24 × 21 cm, L × W × H) was divided into two compartments, one black-walled fully opaque (14 cm long), and the other (33 cm long) lit from the compartment ceiling by a 20 W bulb. Free passage was allowed between the compartments by a small 4 × 5 cm opening. Male and female ephrin-A5-/- (n=10 per sex), heterozygous (n=10 per sex) and wild-type (n=9 male and 10 female) mice were individually placed in the dark compartment and allowed to freely explore the box for 5 minutes. All trials were videotaped for subsequent analysis. Latency to emerge from the dark compartments, light-dark transitions, time in the light compartment and a risk assessment, in which the head and fore-paws extended into the lighted area but the remainder of the body stayed in the dark compartment (Bailey & Crawley, 2009) was recorded.
Corticosterone ELISA
Blood samples were collected by tail bleeding from male and female ephrin-A5-/- (n=5 per sex) and wild-type (n=5 per sex) littermates. Briefly, the tail was dipped in warm water (37 °C) for 30 seconds after which the tip of the tail (<0.5 cm) was removed using surgical blade. Blood (20 ul) was collected into microvette tubes (Kent Scientific, Torrington CT, cat no. MCVT200-SER) and allowed to clot at room temperature for 1 hour. The blood was then centrifuged (10,000 × g) for 5 minutes at 20 °C to isolate upper layer serum and the corticosterone levels were measured by a competitive enzyme immunoassay (Arbor assay, Ann Arbor, MI, cat No. K014). Blood collections were done in <3 min so that sampling is completed before activation of the HPA axis (Vahl et al., 2005). All standards and samples were run in triplicates. In order to compare corticosterone levels under both basal and stress conditions blood was collected twice from the same mice; once in the morning (first stage of the dark cycle) and again, a week later after a 5 minute exposure to the elevated plus maze test (stressor).
Estradiol and progesterone concentration measurements
Blood samples were collected from lactating females at P4 by tail bleeding (estradiol) as described above or cardiac puncture (progesterone). The blood was spun and the serum isolated. Serum concentration of estradiol were determined using ELISA kit (Calbiotech, Spring Valley, CA, cat# ES180S-100) and progesterone concentrations were determinate with radioimmunoassay by the core laboratory at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Statistical analysis
Data were analyzed using Statview statistical software. An unpaired Student’s t-test was used for two sample comparisons (EPM, estradiol and progesterone concentration measurements) and multivariate or repeated-measures analysis of variance (ANOVA) were used to analyze the light-dark test, maternal behavior and CORT levels. The Mann Whitney U test was used to analyze nest building and observer reliability was determined with Cohen’s kappa method. Fisher’s Exact test was used for comparisons of ratios in the pup retrieval test. The results are expressed as mean + SEM and p < 0.05 was considered significant.
Results
Altered nesting behavior in primiparous (pregnant) ephrin-A5-/- mice
Primiparous ephrin-A5-/- mice showed impairment of nesting behavior compared to heterozygous and wild-type littermates (Figure 1a, right panel). There were no significant differences between heterozygous and wild-type controls at all 3 time points tested (p>0.05). However, null mice achieved significantly lower nesting scores at six hours (U=0.5, p=0.012) and twenty four hours (U=2, p=0.028) compared to wild-type controls and lower scores at twenty four hours compared to heterozygous mice (U=3, p=0.047), showing a lower quality nest. One hour after providing the nesting material wild-type mice were already starting to make a nest, with the nesting square partially torn compared to the null mice that left the nesting square mostly intact. By six hours wild-type mice built a nearly perfect nest with more than 90 percent of the nesting square torn, whereas null mice left most of the material intact. Although by twenty four hours the null mice shredded most of the nesting square, a well formed nest was usually not found in the cage (Figure 1b). There were no significant differences in nest score between heterozygous and wild-type mice in all three time points tested. However, heterozygous mice had a significantly higher nest score then null mice at twenty four hours (U=3, p=0.047), suggesting that deletion of one copy of the gene is not sufficient to decrease nesting performance in these mice. The nest scores were determined by two blind raters, and the interrater reliabilities as determined by Cohen’s Kappa method were k=1, 0.785 and 0.916 for time points 1, 6 and 24 respectively.
Figure 1. Deficits of nesting behavior in primiparous (pregnant) ephrin-A5-/- mice.
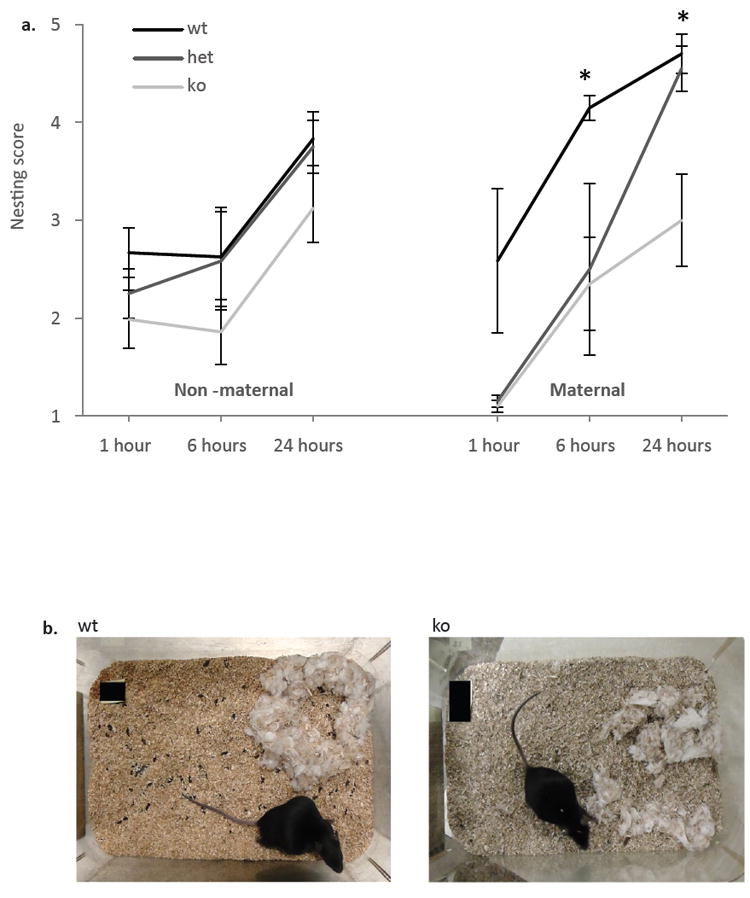
Nest building was assessed in ephrin-A5-/-, heterozygous and wild-type pregnant (n=5 per genotype) and non-pregnant females (n=6 per genotype). (a) Nest rating was lower in pregnant mutant mice compared to wild-type controls starting 6 hours after providing the nesting material (a, right panel). There were no significant differences in nest rating between the non-pregnant females (a, left panel). Data are presented as mean nest score ±SEM. (b) Representative pictures of nests built by pregnant wild-type and ephrin-A5-/- mice 24 hours after providing the nesting material. * indicates significant differences between null and wild-type mice; p<0.05.
Since non-pregnant, nulliparous mice are also able to build nests (Bond et al., 2002, Sherwin, 1997) and nesting requires normal sensorimotor behaviors (Gaskill et al., 2012), we analyzed nests built by nulliparous, virgin female mice (Figure 1a, left panel). There were no significant differences in nest score between the genotypes at all three time points suggesting that ephrin-A5 deletion does not affect the motor ability of the mice to build a non-maternal nest. The interrater reliabilities of the two blind raters for these results as determined by Cohen’s Kappa method were k=0.913, 1 and 1 for time points 1, 6 and 24 respectively. However, twenty four hours after introducing the nesting material, the nests of primiparous wild-type females rated significantly higher than those of nulliparous females (U=3, p=0.028). This difference was not observed in ephrin-A5-/- females where both primiparous and nulliparous mice built equal quality nests suggesting that the maternal contribution to nest quality is absent in the knockout mice.
No changes in maternal aggression between ephrin-A5-/- and wild-type mice
We examined maternal aggression in lactating female mice on postpartum days 4 and 6 because it has been shown that aggression is highest during the early lactation period (Svare et al., 1981). Pups were removed from the cage 2 min before the introduction of a wild-type male intruder into the home cage and the behavior of the dam was recorded for 5 minutes. A repeated measure ANOVA was used to analyze the latency to first attack as well as the number of attacks made by the dam. There were no statistically significant differences between the genotypes on either measurement (Figure 2a and b).
Figure 2. No changes in maternal aggression between ephrin-A5-/- and wild-type mice.
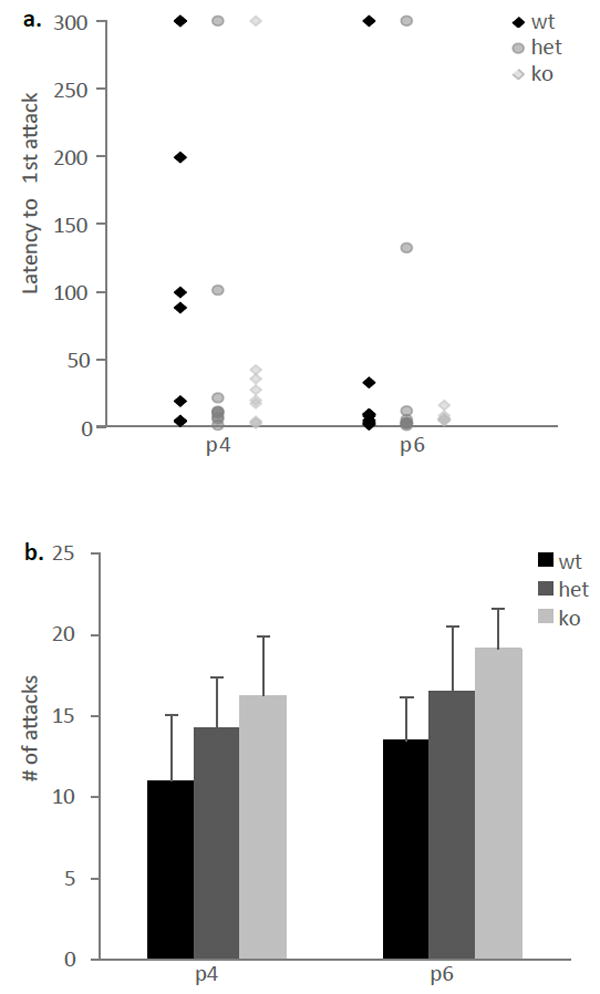
(a) The latencies to first attack by the dam on the male intruder were not significantly different between the genotypes. Data are presented as individual latencies to first attack. (b) The cumulative number of attacks made by the dam during the 5 minute aggression test was not significantly different between the genotypes. Data are presented as mean number of fights +SEM.
Reduced pup retrieval in lactating ephrin-A5-/- mice
To further evaluate the role of ephrin-A5 on maternal behavior, we examined post-partum female mice in the pup retrieval task at the end of the aggression test (Figure 3). Null mice showed an impaired performance to retrieve the pups back to their nest on both test days. A repeated measure ANOVA test showed a significant genotypic difference on P4 [F(2,23)=6.563, p=0.006] (Figure 3a) as well as P6 [F(2,23)=9.652, p=0.001] (Figure 3b). Ephrin-A5-/- female mice took longer to retrieve the pups back to the nest compared to wild-type and heterozygous littermates. With the exception of one heterozygous female which did not retrieve her pups on P4, all wild-type and heterozygous mice retrieved their pups into the nest, whereas only about 45 percent of ephrin-A5-/- female mice completed the task (Figure 3c, Fisher Exact Test p<0.05). To test whether consecutive pup exposure improved maternal behavior in null mice, the pup retrieval test was repeated two days later on P6. Repeated exposure to the pups did not improve retrieval in the null mice nor in wild-type or heterozygous females [F(2,22)=0.137, p=0.870] (Figure 3d), although a genotypic difference was still observed [F(2,22)=14.958, p<0.0001]. Finally, in order to test the possibility that the retrieval deficit was due to defective cues from the null pups such as odor and ultrasonic vocalization (USV), we measured retrieval behavior with the wild type pups. Here, a repeated measure ANOVA test also showed a significant overall genotypic difference [F(1,15)=6.539, p=0.022] where null mice took longer to retrieve the 1st (p=0.05) and 3rd (p=0.035) wild-type pup compared to wild-type dams (Figure 4). Since pup retrieval was impaired in the null mice regardless of the pups’ genotype, we concluded that the defects are in the dams and not the pups.
Figure 3. Pup retrieval is impaired in lactating ephrin-A5-/- mice.
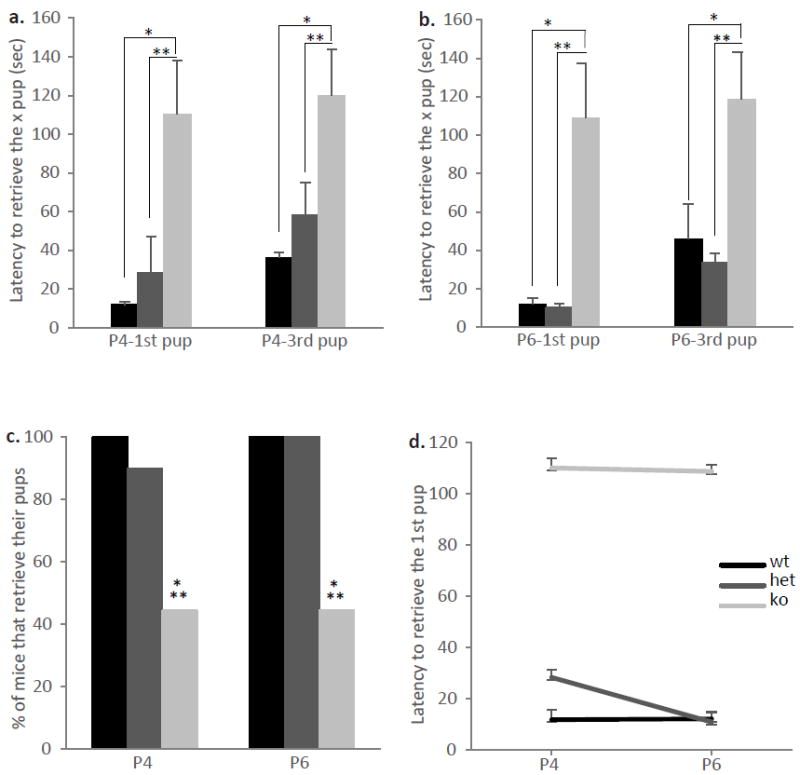
The latencies to retrieve the 1st and 3rd pup on postnatal day 4 (a) and 6 (b) were tested in ephrin-A5-/- (n=9 per test day), heterozygous (n=10) and wild-type (n=8) lactating female mice. There were significant differences between the genotypes on both test days where the mutant females took longer to retrieve their pups than both the wild-type and the heterozygous mice. Data are presented as mean latency to retrieve the x pup +SEM. (c) The percentage of females that retrieve their pups were significantly lower in ephrin-A5-/- mice compared to wild-type and heterozygous control females. (d) No differences were observed across the days tested. Data are presented as mean latency to retrieve the 1st pup ±SEM. * indicates significantly different from wild-type mice; p<0.05. ** indicates significantly different from heterozygous mice; p<0.05.
Figure 4. Retrieval of wild-type pups is impaired in lactating ephrin-A5-/- mice.
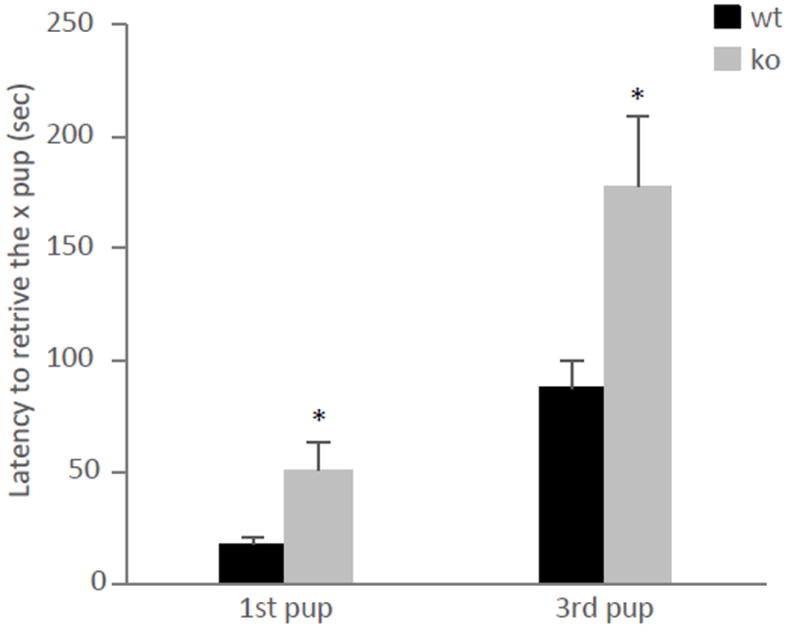
The latencies to retrieve the 1st and 3rd pup were significantly higher in ephrin-A5-/- dams (n=10) compared to wild-type (n=7) controls. Data are presented as mean latency to retrieve the x pup +SEM. * indicates significantly different from wild-type mice; p<0.05
Reduced pup survival in ephrin-A5-/- mice
We observed decreased survival rate in pups that were born and reared by ephrin-A5-/- females compared to those born and reared by wild-type controls. Within the first postpartum week, pups reared by null female had a 55 percent survival rate compared to an 80 percent survival rate for pups reared by wild-type females (Figure 5a). It is important to note that survival rate was highly correlated to the shape of the nest and to the level of pup gathering by the mother on the day of parturition; when a well formed nest was not observed in the cage, and the mother was not gathering the pups after birth, the pups would not survive well (Figure 5b).
Figure 5. Decreased survival rate of pups that were born and reared by ephrin-A5-/- dams.
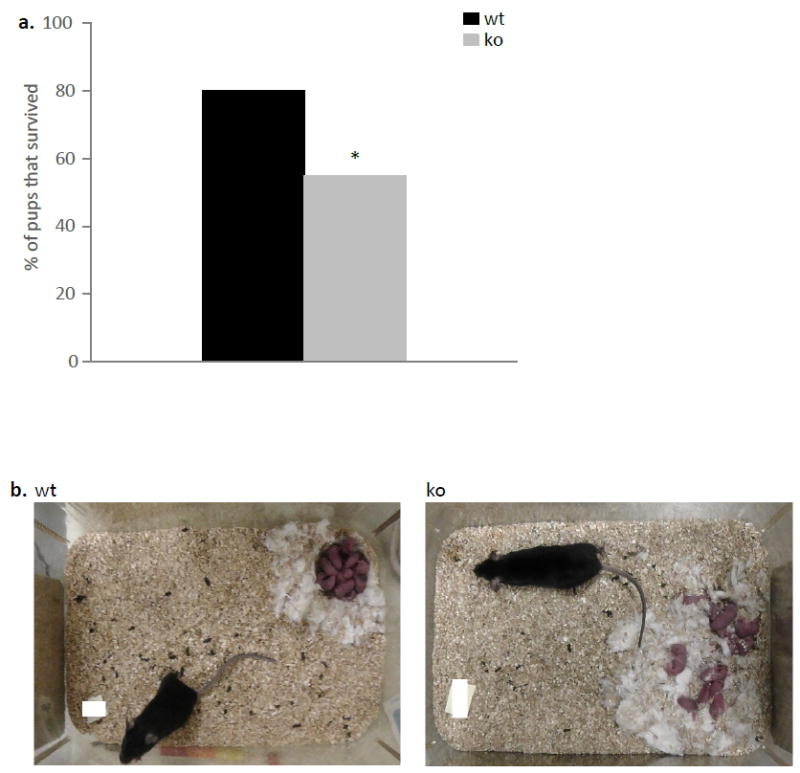
(a) Survival rate was lower for pups that were born and reared by ephrin-A5-/- mothers. Data are presented as percentage of pups that survived per genotype. (b) Representative pictures of ephrin-A5-/- and wild-type dams within the first few hours after birth.
Body weight in ephrin-A5-/- mice
Since we previously observed that ephrin-A5-/- animals had lower body weight (Sheleg et al., 2013), we sought to determine whether this reduction depends on the dam’s behavior. Body weights of heterozygous pups born and reared by ephrin-A5-/- (n=8), heterozygous (n=6), and wild-type (n=8) dams were measured every 4 days until P20 and again at P60 (Figure 6a). The rationale here is that if heterozygous pups that were reared by null dams weigh less than those reared by wild-type or heterozygous control dams, then the dam behavior probably contribute to these differences. There were no genotypic differences between the groups [F(2,61)=1.444, p=0.244] (Figure 6b). However, there was a significant effect of sex [F(1,61)=43.674, p<0.001], postnatal day [F(5,305)=11096.859, p<0.001], and postnatal day x sex interaction [F(10,305)=0.724, p=0.70]. Post hoc testing revealed that on P60 male mice weigh significantly more than female mice. The fact that heterozygous pups from all 3 dams (ephrin-A5-/-, heterozygous and wild-type) had similar body weights (Figure 6b), suggests that the growth differences between the null and wild-type mice are not due to differences in maternal behavior. Finally, we wanted to confirm our previous results (Sheleg et al., 2013) and see if the differences between null and wild-type mice are consistent with the current breeding scheme: ephrin-A5-/- and wild-type females bred with heterozygous males (See Materials and methods and Figure 6a). As expected, there was a significant effect of genotype [F(1,27)=22.719, p<0.0001] and postnatal day [F(5,135)=4666.440, p<0.0001] as well as postnatal day x genotype [F(5,135)=18.431, p<0.0001] and postnatal day x sex [F(5,135)=35.739, p<0.0001] interaction. Post hoc tests revealed that null mice weigh significantly less than wild-type mice on all the days tested (Figure 6c). In addition, a sex difference was observed on P60 with female mice weighing less than male mice. These data support our previous results, and show that null mice weigh less than wild-type mice and that the decreased level of maternal care is not likely to be responsible for this difference.
Figure 6. Body weights in Ephrin-A5 mice.
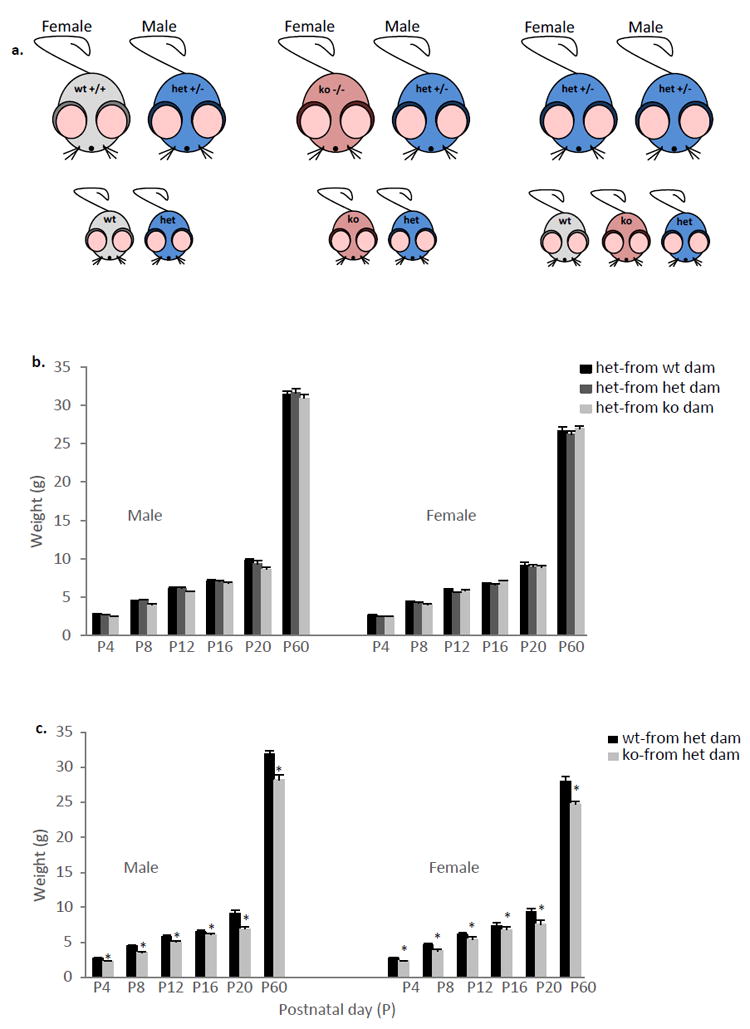
(a) Breeding scheme. (b) Body weights of heterozygous pups that were born and reared by ephrin-A5-/-, heterozygous and wild-type dams were measured. There were no significant differences between the groups. (c) Ephrin-A5-/- mice had decreased body weight compared to wild-type controls.
Serum estradiol and progesterone levels are comparable between the genotypes
Since changes in steroid hormones around parturition have been shown to play a role on maternal behavior in mammals (Knobil & Neill, 1994), we sought to determine whether serum estradiol and progesterone levels are effected by ephrin-A5 deletion. We found no differences in estradiol (Figure 7a; t=-0.490, p=0.631) and progesterone (Figure 7b; t=-1.121, p=0.5240) between the genotypes.
Figure 7. No differences in serum estradiol and progesterone levels between the genotypes.
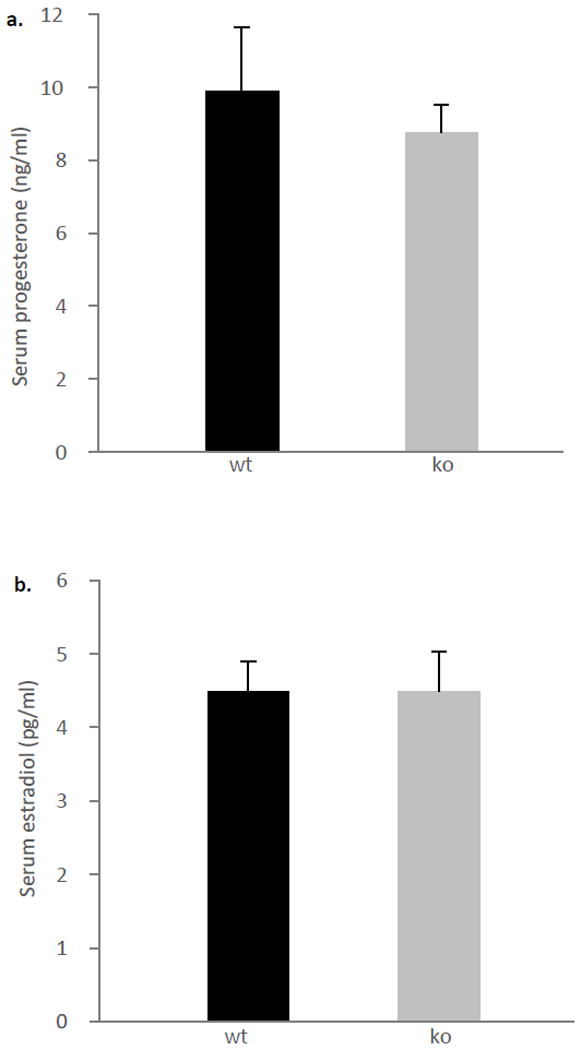
Serum progesterone (a) and estradiol (b) levels were comparable between ephrin-A5-/- (n=10 and 9 respectively) and wild-type (n=7 and 9 respectively) lactating females
Altered anxiety-like behavior in ephrin-A5-/- mice
It has been suggested that early life care influences anxiety levels in adult life (Meaney, 2001). In order to test whether the lower levels of maternal care seen in the null mice affects their offspring anxiety levels, adult ephrin-A5-/- mice that were born and raised by ephrin-A5-/- dams and wild-type mice that were born and raised by wild-type females were examined on the EPM (Figure 8). The EPM test is one of the most commonly used assays to study the psychological and neurochemical basis of anxiety behavior in rodents (Bourin et al., 2007). It takes advantage of the normal preference of mice for a dark and protected space (the closed arm) over an open and exposed area (the open arm). Student’s t-test was used to analyze the absolute number and the percentage of open arm entries, as well as the time and percentage of time spent in the open arm and the total number of entries (into both open and closed arm). Ephrin-A5-/- mice showed anxiolytic-like behaviors in the EPM test. There were genotypic differences where ephrin-A5-/- mice had a higher number of open arm entries (t=3.807, p=0.003) (Figure 8a), and higher percentage of open arm entries (t=4.068, p=0.002) (Figure 8b), as well as increased time spent in the open arm (t=4.829, p=0.0005) (Figure 8c) and higher percentage of time spent in the open arm (t=4.619, p=0.007) (Figure 8d) compared to wild-type mice. In addition, null mice had an increased number of total entries (t=4.169, p=0.002) (Figure 8e) into both arms. In order to examine whether the differences in anxiety-like behavior are due to differences in maternal care, we repeated the above study using male and female ephrin-A5-/-, heterozygous and wild-type littermate mice that were born and reared by the same heterozygous mother (Figure 9). There were again overall genotypic differences where ephrin-A5-/- mice had a higher number of open arm entries [F(2,56)=21.550, p<0.0001] (Figure 9a), and percentage of open arm entries [F(2,56)=12.657, p<0.0001] (Figure 9b) as well as increased time spent in the open arm [F(2,56)=9.365, p=0.0003] (Figure 9c) and percentage of time spent in the open arm [F(2,56)=7.330, p=0.002] (Figure 9d) compared to heterozygous and wild-type littermates. In addition, null mice had an increased number of total entries [F(2,56)=23.056, p<0.001] (Figure 9e) into both arms. There were no significant effects of sex or genotype x sex interactions in the absolute number and the percent of open arm entries as well as in the time and percent of time spent in the open arm. However a genotype x sex interaction was found in the total entries [F(2,56)=4.446, p=0.020]. Post hoc tests revealed that ephrin-A5-/- male mice had increased total entries compared to ephrin-A5-/- female mice (p=0.030). These data suggest that both male and female null mice are less anxious as revealed by increased entries and time spent in the open arm, which is usually avoided by rodents. In addition, the increase in total entries that was observed in the null mice supports our previous observation of increased locomotor activity in the null mice (Sheleg et al., 2013). Finally, since decreased anxiety was observed regardless of maternal behavior, we conclude that this change in behavior is the result of genetic factors and not exposure to early life stressors.
Figure 8. Decreased anxiety-like behavior of ephrin-A5-/- mice that were born and reared by null mice in the elevated plus maze (EPM).
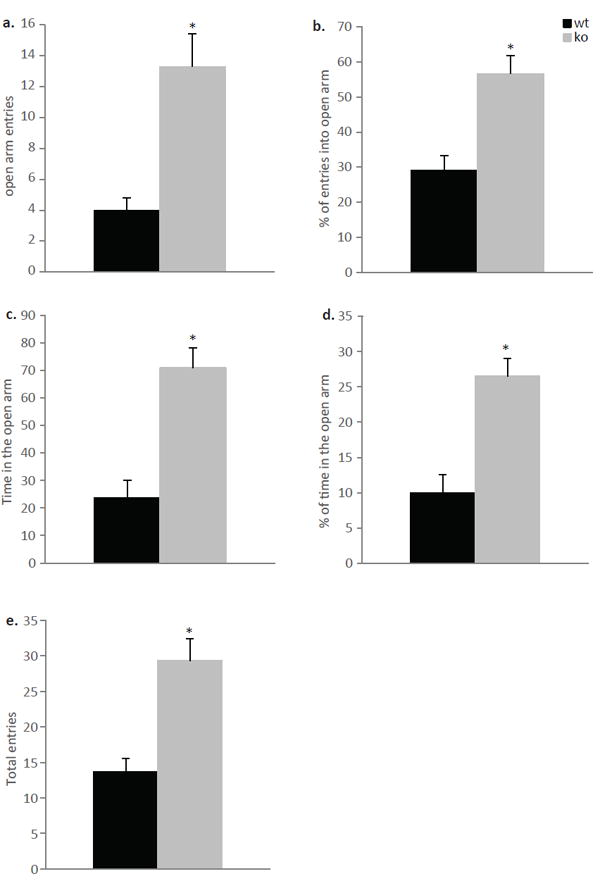
Anxiety behavior was measured on the EPM in male ephrin-A5-/- (n=7) and wild-type (n=6) mice. The number and percentage of entries into the open arm (a and b) as well as the time and percentage of time (c and d) spent in the open arm were higher in null mice compared to wild-type controls. (e) The total number of entries into both open and closed arms were significantly higher in ephrin-A5-/- mice. Data are presented as mean +SEM. * indicates significantly different from wild-type mice; p<0.05.
Figure 9. Decreased anxiety-like behavior of ephrin-A5-/- mice in the elevated plus maze (EPM).
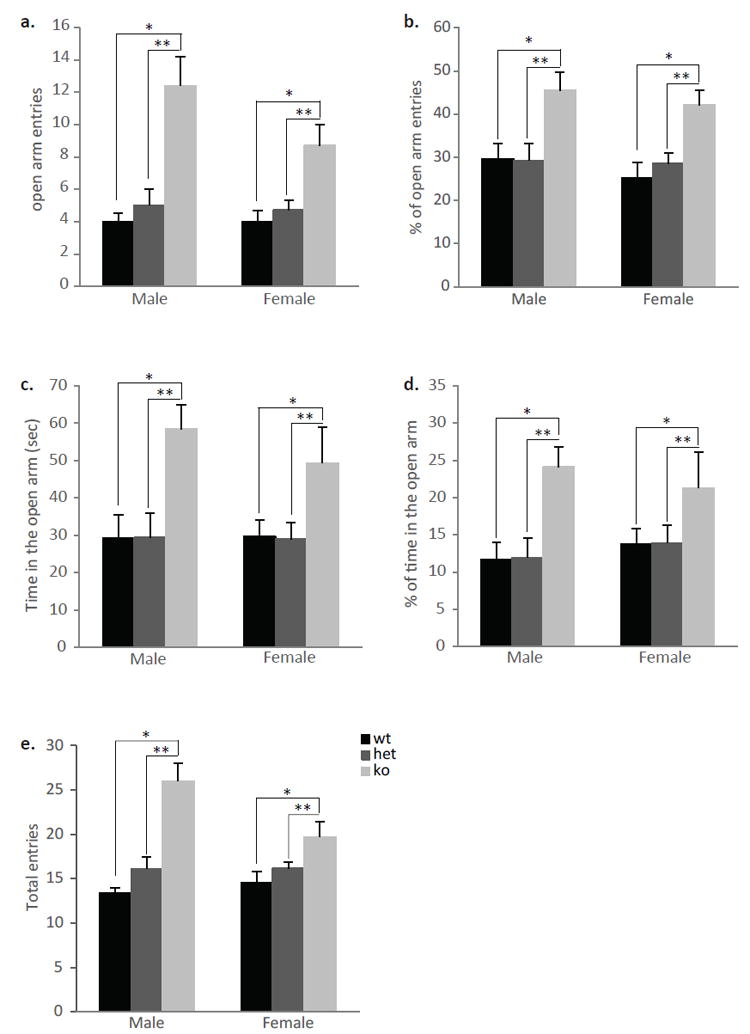
Anxiety-like behavior was measured on the EPM in male and female ephrin-A5-/- (n=10 per sex), heterozygous (n=11 per sex) and wild-type (n=10 per sex) mice. The number and percentage of entries into the open arm (a and b) as well as the time and percentage of time spent in the open arm (c and d) were higher in both male and female null mice compared to heterozygous and wild-type controls. (e) The total number of entries into both open and closed arms were significantly higher in male and female ephrin-A5-/- mice. Data are presented as mean +SEM. * indicates significantly different from wild-type mice; p<0.05. ** indicates significantly different from heterozygous mice; p<0.05.
Next we wanted to examine whether the anxiolytic-like behaviors of ephrin-A5-/- mice were specific to the EPM. To test this, we used the light –dark test which takes advantage of the fact that mice tend to avoid light and unprotected areas. Both male and female ephrin-A5-/- mice showed anxiolytic-like behavior in the tests (Figure 10). A multivariate ANOVA test showed a significant genotypic difference where ephrin-A5-/- mice spent significantly more time in the light chamber of the box [F(2,53)=11.361, p<0.0001] (Figure 10a), and had a decreased number of head pokes [F(2,53)=8.367, p=0.0007] (Figure 10b), compared to wild-type and heterozygous mice. In addition, there was a sex difference where male mice had increased head pokes compared to female mice [F(1,53)=8.367, p=0.0014]. There were no statistical differences between the genotypes in the latencies to initially enter the light or the number of transitions between the two compartments (Figure 10c and d, respectively). However, there was a significant effect of sex [F(1,53)=30.613, p<0.0001] where female mice had a consistently lower number of transitions between the two compartments across all genotypes. In addition, a genotype x sex interaction was found [F(2,53)=3.809, p=0.030], where post hoc tests revealed that ephrin-A5-/- null male initially entered the light compartment faster than ephrin-A5-/- null female (p=0.014). These results support our previous data from the EPM test and suggest that ephrin-A5 deletion decreases anxiety in mice.
Figure 10. Decreased anxiety-like behavior of ephrin-A5-/- mice in the light-dark box.
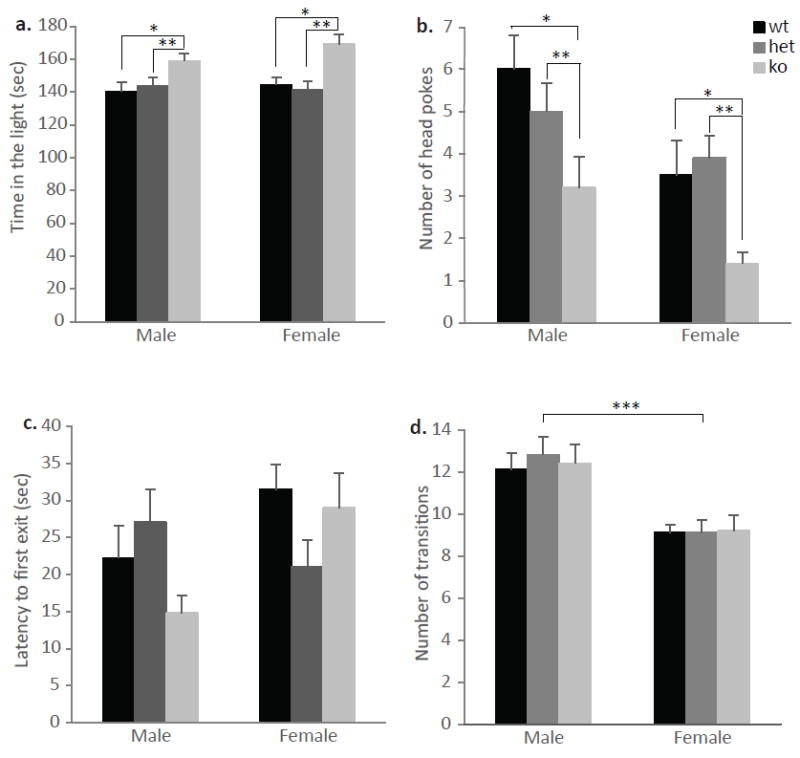
Anxiety-like behavior was measured in male and female ephrin-A5-/- (n=10 per sex), heterozygous (n=10 per sex) and wild-type (n=9 male and 10 female) mice in the light-dark box. Ephrin-A5-/- mice spend more time in the light compartment (a) and had increased number of head pokes (b) compared to heterozygous and wild-type controls. There were no genotypic differences in the latency to the first exit (c) and the number of transition between the two compartments (d). Data are presented as mean +SEM. * indicates significantly different from wild-type mice; p<0.05. ** indicates significantly different from heterozygous mice; p<0.05. *** indicates significant sex differences; p<0.05.
Corticosterone (CORT) levels are comparable between the genotypes
Activation of the hypothalamic-pituitary-adrenal (HPA) axis has been shown to play an important role in anxiety. In response to stress, corticotrophin-releasing hormone (CRH) is released from the hypothalamus, which leads to the secretion of adrenocorticotrophic (ACTH) hormone from the pituitary into the blood. ACTH in turn induces the release of glucocorticoid stress hormones from the adrenal (Miller & O’callaghan, 2002). In order to test whether the alteration in anxiety is due to changes in the activation of the HPA axis, we determined CORT concentrations, the major stress steroid, in mice. Serum CORT concentration was not significantly different between the genotypes [F(1,16)=0.963, p=0.34] nor was there a significant effect of sex [F(1,16)=0.121, p=0.73]. However, CORT levels were overall significantly higher under stress conditions compared to baseline [F(1,16)=22.379, p=0.0002] (Figure 11).
Figure 11. No differences in corticosterone (CORT) levels between ephrin-A5-/- and wild-type mice.
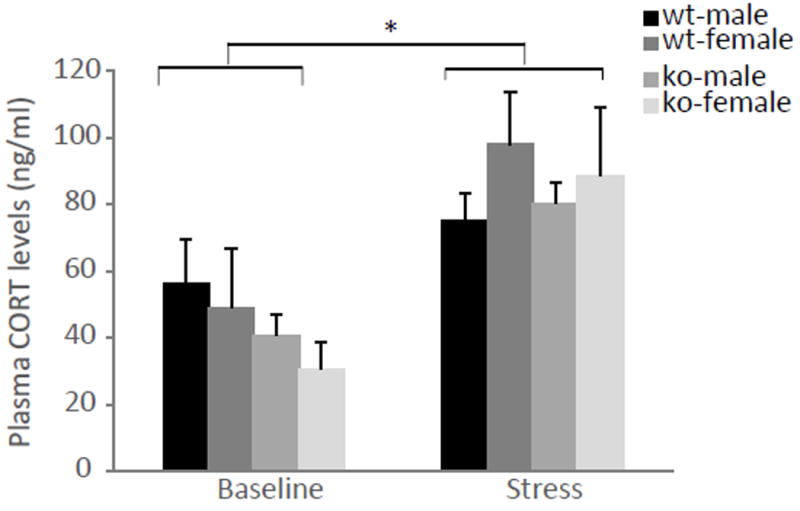
CORT levels were measured in male and female ephrin-A5-/- and wild-type mice (n=5 per sex and genotype) under baseline and mild stress conditions. There were no significantly differences between the genotypes. However, CORT levels were significantly higher under stress conditions compared to baseline. Data are presented as mean CORT levels (ng/ml) +SEM. * indicates significantly different from baseline conditions; p<0.05.
Discussion
Here, we demonstrate that genetic deletion of ephrin-A5 significantly decreased maternal behavior. Nesting behavior was reduced in lactating ephrin-A5-/- females at 6 and 24 hours after providing the nesting material. Since mice are born without the ability to regulate their body temperature, the construction of the nest is important for their survival (Weber & Olsson, 2008). It is therefore not surprising that pups born to ephrin-A5-/- females had a lower survival rate compared to those born to wild-type controls. Although maternal nest building behavior is important for the lifetime reproductive success of the mouse (Bult & Lynch, 1997) and primiparous mice tend to build the most complex quality nests (Bond et al., 2002), non-pregnant, nulliparous mouse are also capable of building a nest (Bond et al., 2002, Sherwin, 1997). This nest has been referred to as “seeping nest” and it is smaller and relatively flat compared to the maternal nest (Gandelman, 1973). Here nesting is a spontaneous behavior (Deacon, 2012), it provides shelter from predators and is essential for thermoregulation. Non-maternal nesting behavior has been shown to be affected by hippocampal damage and can be used to assess the mouse well-being (Jirkof, 2014). In addition, it requires sensorimotor behaviors such as carrying, digging, sorting and pushing (Gaskill et al., 2012). In order to examine whether the impairment in nesting seen in the primiparous ephrin-A5-/- mice was due to sensorimotor defects, we analyzed nesting in nulliparous, virgin females. We found no differences in non-maternal nesting behavior between the genotypes suggesting that the sensorimotor behaviors required for nest building are intact in the null mice. Since the difference in nesting was only observed between primiparous null and wild-type females, the defect is most likely due to responses to maternal factors such as changes in maternal motivation.
In the pup retrieval test more than 50 percent of the lactating ephrin-A5-/- females failed to retrieve their own pups back to the nest, and those that did retrieve took longer than wild-type and heterozygous littermates. It has been shown that the latency of pup retrieval decreases in virgin females with repeated exposure to pups (Stolzenberg & Rissman, 2011), as well as across the first week of postpartum in primiparous mice (Feierstein et al., 2010). In order to test whether maternal responsiveness to pups in the form of pup retrieval improves in ephrin-A5-/- females, pup retrieval was measured twice (4 and 6 days postpartum). We did not detect differences in retrieval latency across the days tested in any genotype, suggesting that repeated exposure to pups did not induce retrieval behavior in the null mice and was not sufficient to rescue the retrieval deficit. Finally, in mice, retrieval is affected by stimuli from the isolated pups. For example, USVs that are emitted from the pups are sufficient to induce retrieval behavioral (Ehret, 2005, Sewell, 1970). In this study, wild-type mothers were able to retrieve null pups, suggesting that ephrin-A5 deletion did not affect the pups’ cues toward the dams at least with respect to retrieval behavior. Moreover, wild-type pups did not elicit this behavior in the null mothers indicating that the deficit is indeed due to a maternal defect in the null mother.
The hormonal changes that accompany pregnancy and parturition have also been implicated in the regulation of maternal behavior, specifically changes in progesterone and estradiol levels (Sheehan & Numan, 2002, Terkel & Rosenblatt, 1968). For example, a state of high progesterone and low estrogen have been suggested to control maternal nest-building in primiparous mice (Lisk et al., 1969). However, we did not detect changes in hormonal levels between null and wild-type control. Differences with respect to maternal aggression were not observed between the genotypes, but since it has been shown that different neuronal circuits influence separate maternal behaviors (Gammie, 2005), it is possible that ephrin-A5 deletion affects circuits that control pup retrieval and nest building but not those that regulate maternal aggression. Consistent with this notion, lesion studies have shown that defects in maternal aggression do not affect pup retrieval in rats (Factor et al., 1993, Hansen, 1989).
Previously we reported that ephrin-A5-/- mice have decreased body weight compared to wild-type control (Sheleg et al., 2013). Here, we confirmed these results and showed that the differences in body weight were not due to the differences in maternal behavior between the genotypes since heterozygous pups that were born and raised by ephrin-A5-/- females had similar body weight to those that were born and raised by heterozygous and wild-type control females.
Ephrin-A5-/- mice had lower levels of anxiety-like behavior as revealed by increased entrance and time spent in the open arm of the EPM, as well as increased time in the light compartment of the light-dark box and decreased number of head pokes. Although it has been suggested that the quality and quantity of early life care influences anxiety (Meaney, 2001), we did not find a correlation between the two. Decreased anxiety in the EPM test was observed in the null mice regardless of the levels or quality of maternal care. Additionally, in response to mild stress (exposure to the EPM) both ephrin-A5-/- and wild-type controls showed similar activation of the HPA axis; blood CORT concentration was increased under stressful condition in both genotype with no significant differences between the genotypes. Our observations reported here suggest that genetic factors play key roles in anxiety levels of mice.
The interaction between the medial preoptic area (MPOA) and the mesolimbic system plays a pivotal role in regulating maternal behavior in rats (Numan & Stolzenberg, 2009). Numan et al. (Numan, 2014) proposed a model in which MPOA projections to the VTA induce the release of DA into the nucleus accumbens (NA). This release stops the inhibitory effect of the NA on the ventral pallidum (VP) which then becomes responsive to the stimulation from the pups. Since the mesolimbic DA system is involved in motivated behavior, it is suggested that the MPOA-VTA connection governs the appetitive aspect of maternal responses to pup stimuli which includes retrieval behavior (Numan, 2014, Stolzenberg & Numan, 2011). Previously, we have shown that ephrin-A5 and one of its receptors, EphA5, are expressed in the mesolimbic DA system and affects dopaminergic neurite growth (Cooper et al., 2009, Deschamps et al., 2009, Kimura et al., 2011). Both In vivo and in vitro data suggested that ephrin-A5 has an adhesive effect on EphA5-expressing dopaminergic neurons from the midbrain, and its absence leads to a decrease in neuronal targeting to the striatum. Thus, it is possible that the decreased maternal behavior seen in the null mice is due to alteration in the mesolimbic DA system. However, since ephrin-A5 is expressed broadly in the brain (Deschamps et al., 2009, Gao et al., 1998), and has been shown to regulate neurogenesis (Depaepe et al., 2005, Gerstmann et al., 2015, Noh & Park, 2015, Shu et al., 2016), and formation of several other neural circuits (Carvalho et al., 2006, Guellmar et al., 2009, Steinecke et al., 2014, Tadesse et al., 2013, Yates et al., 2014) the precise mechanism underlying maternal care deficiency remains to be determined by future investigations. Thus, the mechanistic connection of ephrin-A5 function to maternal behavior is at present unclear, and this gene may not regulate maternal care exclusively. In addition, other ephrins may also compensate for the loss of ephrin-A5 functions. Consistent with a potential compensatory mechanism by functionally similar ephrin genes, heterozygous ephrin-A5 mice which retained one copy of ephrin-A5 gene showed a transient defect in maternal nest making at 6 hours, but not at 24 hours (Fig. 1a) and a delay in pup retrieval at P4 but not P6 (Fig. 3). Nevertheless, the maternal care defects we have observed in the homozygous ephrin-A5-/- mice are robust and very reproducible, which support the notion that ephrin-A5 plays a role in modulating maternal behavior functions.
Acknowledgments
Research supported in part by grants to R. Zhou from NIH (1RO1EY019012 and 2PO1HD023315) and from NJ Commission on Brain Injury Research, and the Charles and Johanna Busch grant support for G. Wagner.
We thank Philip Furmanski for his insightful discussion and assistance in the assessment of the experimental results.
Footnotes
None of the authors have any disclosures or conflicts of interest to declare.
References
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [Google Scholar]
- Bond TL, Neumann PE, Mathieson WB, Brown RE. Nest building in nulligravid, primigravid and primiparous C57BL/6J and DBA/2J mice (Mus musculus) Physiology & behavior. 2002;75:551–555. doi: 10.1016/s0031-9384(02)00659-5. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Hormones and behavior. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bourin M, Petit-Demouliere B, Dhonnchadha BN, Hascoet M. Animal models of anxiety in mice. Fundamental & clinical pharmacology. 2007;21:567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- Bult A, Lynch CB. Nesting and fitness: lifetime reproductive success in house mice bidirectionally selected for thermoregulatory nest-building behavior. Behavior genetics. 1997;27:231–240. doi: 10.1023/a:1025610130282. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nature neuroscience. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Kobayashi K, Zhou R. Ephrin-A5 regulates the formation of the ascending midbrain dopaminergic pathways. Developmental neurobiology. 2009;69:36–46. doi: 10.1002/dneu.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. Journal of visualized experiments : JoVE. 2012:e2607. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Assessing nest building in mice. Nature protocols. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Deschamps C, Faideau M, Jaber M, Gaillard A, Prestoz L. Expression of ephrinA5 during development and potential involvement in the guidance of the mesostriatal pathway. Experimental neurology. 2009;219:466–480. doi: 10.1016/j.expneurol.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Deschamps C, Morel M, Janet T, Page G, Jaber M, Gaillard A, Prestoz L. EphrinA5 protein distribution in the developing mouse brain. BMC Neurosci. 2010;11:105. doi: 10.1186/1471-2202-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds -- a gate to the understanding of sound communication. Behavior genetics. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Factor EM, Mayer AD, Rosenblatt JS. Peripeduncular nucleus lesions in the rat: I. Effects on maternal aggression, lactation, and maternal behavior during pre- and postpartum periods. Behavioral neuroscience. 1993;107:166–185. doi: 10.1037//0735-7044.107.1.166. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Lazarini F, Wagner S, Gabellec MM, de Chaumont F, Olivo-Marin JC, Boussin FD, Lledo PM, Gheusi G. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Behav Neurosci. 2010;4 doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural Mechanisms of Stress Resilience and Vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O’Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20:235–243. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Gammie SC. Current models and future directions for understanding the neural circuitries of maternal behaviors in rodents. Behavioral and cognitive neuroscience reviews. 2005;4:119–135. doi: 10.1177/1534582305281086. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Induction of maternal nest building in virgin female mice by the presentation of young. Hormones and behavior. 1973;4:191–197. doi: 10.1016/0018-506x(73)90003-2. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Zarrow MX, Denenberg VH. Maternal behavior: differences between mother and virgin mice as a function of the testing procedure. Developmental psychobiology. 1970;3:207–214. doi: 10.1002/dev.420030308. [DOI] [PubMed] [Google Scholar]
- Gao PP, Yue Y, Zhang JH, Cerretti DP, Levitt P, Zhou R. Regulation of thalamic neurite outgrowth by the Eph ligand ephrin-A5: implications in the development of thalamocortical projections. Proc Natl Acad Sci U S A. 1998;95:5329–5334. doi: 10.1073/pnas.95.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PloS one. 2012;7:e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmann K, Pensold D, Symmank J, Khundadze M, Hubner CA, Bolz J, Zimmer G. Thalamic afferents influence cortical progenitors via ephrin A5-EphA4 interactions. Development. 2015;142:140–150. doi: 10.1242/dev.104927. [DOI] [PubMed] [Google Scholar]
- Guellmar A, Rudolph J, Bolz J. Structural alterations of spiny stellate cells in the somatosensory cortex in ephrin-A5-deficient mice. The Journal of comparative neurology. 2009;517:645–654. doi: 10.1002/cne.22198. [DOI] [PubMed] [Google Scholar]
- Hansen S. Medial hypothalamic involvement in maternal aggression of rats. Behavioral neuroscience. 1989;103:1035–1046. doi: 10.1037//0735-7044.103.5.1035. [DOI] [PubMed] [Google Scholar]
- Hruska M, Dalva MB. Ephrin regulation of synapse formation, function and plasticity. Molecular and cellular neurosciences. 2012;50:35–44. doi: 10.1016/j.mcn.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. Journal of neuroscience methods. 2014 doi: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Kimura K, Hikida T, Yawata S, Yamaguchi T, Nakanishi S. Pathway-specific engagement of ephrinA5-EphA4/EphA5 system of the substantia nigra pars reticulata in cocaine-induced responses. Proc Natl Acad Sci U S A. 2011;108:9981–9986. doi: 10.1073/pnas.1107592108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobil E, Neill JD. The Physiology of reproduction. Raven Press; New York: 1994. [Google Scholar]
- Lisk RD, Pretlow RA, 3rd, Friedman SM. Hormonal stimulation necessary for elicitation of maternal nest-building in the mouse (Mus musculus) Animal behaviour. 1969;17:730–737. doi: 10.1016/s0003-3472(69)80020-5. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual review of neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Developmental psychobiology. 2006;48:209–219. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Neuroendocrine aspects of the response to stress. Metabolism: clinical and experimental. 2002;51:5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- Molet J, Maras PM, Avishai-Eliner S, Baram TZ. Naturalistic rodent models of chronic early-life stress. Developmental psychobiology. 2014;56:1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Park S. Over-Expression of Ephrin-A5 in Mice Results in Decreasing the Size of Progenitor Pool through Inducing Apoptosis. Molecules and cells. 2015 doi: 10.14348/molcells.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Neurobiology of social behavior: toward understanding of the prosocial and antisocial brain. elsevier; 2014. [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer; New York: 2003. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI. Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. Journal of neuroendocrinology. 2011;23:1020–1035. doi: 10.1111/j.1365-2826.2011.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-George A, LeBlanc F, Kaneta T, Urbach D, Kusnecov AW. Effects of bacterial superantigens on behavior of mice in the elevated plus maze and light-dark box. Brain behavior and immunity. 2004;18:46–54. doi: 10.1016/s0889-1591(03)00087-4. [DOI] [PubMed] [Google Scholar]
- Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75:12–23. doi: 10.1159/000048217. [DOI] [PubMed] [Google Scholar]
- Sheleg M, Yochum CL, Richardson JR, Wagner GC, Zhou R. Ephrin-A5 regulates inter-male aggression in mice. Behavioural brain research. 2015;286:300–307. doi: 10.1016/j.bbr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheleg M, Yochum CL, Wagner GC, Zhou R, Richardson JR. Ephrin-A5 deficiency alters sensorimotor and monoaminergic development. Behavioural brain research. 2013;236:139–147. doi: 10.1016/j.bbr.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin CM. Observations on the prevalence of nest-building in non-breeding TO strain mice and their use of two nesting materials. Laboratory animals. 1997;31:125–132. doi: 10.1258/002367797780600134. [DOI] [PubMed] [Google Scholar]
- Shoji H, Kato K. Maternal care affects the development of maternal behavior in inbred mice. Developmental psychobiology. 2009;51:345–357. doi: 10.1002/dev.20375. [DOI] [PubMed] [Google Scholar]
- Shu Y, Xiao B, Wu Q, Liu T, Du Y, Tang H, Chen S, Feng L, Long L, Li Y. The Ephrin-A5/EphA4 Interaction Modulates Neurogenesis and Angiogenesis by the p-Akt and p-ERK Pathways in a Mouse Model of TLE. Molecular neurobiology. 2016;53:561–576. doi: 10.1007/s12035-014-9020-2. [DOI] [PubMed] [Google Scholar]
- Steinecke A, Gampe C, Zimmer G, Rudolph J, Bolz J. EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence. Development. 2014;141:460–471. doi: 10.1242/dev.101691. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuroscience and biobehavioral reviews. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–354. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svare B, Betteridge C, Katz D, Samuels O. Some situational and experiential determinants of maternal aggression in mice. Physiology & behavior. 1981;26:253–258. doi: 10.1016/0031-9384(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Tadesse T, Cheng Q, Xu M, Baro DJ, Young LJ, Pallas SL. Regulation of ephrin-A expression in compressed retinocollicular maps. Developmental neurobiology. 2013;73:274–296. doi: 10.1002/dneu.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Maternal behavior induced by maternal blood plasma injected into virgin rats. Journal of comparative and physiological psychology. 1968;65:479–482. doi: 10.1037/h0025817. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD. Impaired maternal behavior in mice lacking norepinephrine and epinephrine. Cell. 1997;91:583–592. doi: 10.1016/s0092-8674(00)80446-8. [DOI] [PubMed] [Google Scholar]
- Tsuneoka Y, Maruyama T, Yoshida S, Nishimori K, Kato T, Numan M, Kuroda KO. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. The Journal of comparative neurology. 2013;521:1633–1663. doi: 10.1002/cne.23251. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. American journal of physiology Endocrinology and metabolism. 2005;289:E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Frontiers in neuroendocrinology. 2009;30:497–518. doi: 10.1016/j.yfrne.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Weber EM, Olsson IAS. Maternal behaviour in Mus musculus sp.: An ethological review. Appl Anim Behav Sci. 2008;114:1–22. [Google Scholar]
- Wettschureck N, Moers A, Hamalainen T, Lemberger T, Schutz G, Offermanns S. Heterotrimeric G proteins of the Gq/11 family are crucial for the induction of maternal behavior in mice. Molecular and cellular biology. 2004;24:8048–8054. doi: 10.1128/MCB.24.18.8048-8054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin reverse signaling in axon guidance and synaptogenesis. Seminars in cell & developmental biology. 2012;23:58–64. doi: 10.1016/j.semcdb.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates NJ, Martin-Iverson MT, Rodger J. The role of ephrin-A2 and ephrin-A5 in sensorimotor control and gating. Behavioural brain research. 2014;275:225–233. doi: 10.1016/j.bbr.2014.08.061. [DOI] [PubMed] [Google Scholar]
- Zarbalis K, Wurst W. Expression domains of murine ephrin-A5 in the pituitary and hypothalamus. Mechanisms of development. 2000;93:165–168. doi: 10.1016/s0925-4773(00)00252-5. [DOI] [PubMed] [Google Scholar]


