Abstract
Background and Purpose: Corneal injury can result in dysfunction of corneal nociceptive signaling and corneal sensitization. Activation of the endocannabinoid system has been reported to be analgesic and anti-inflammatory. The purpose of this research was to investigate the antinociceptive and anti-inflammatory effects of cannabinoids with reported actions at cannabinoid 1 (CB1R) and cannabinoid 2 (CB2R) receptors and/or noncannabinoid receptors in an experimental model of corneal hyperalgesia.
Methods: Corneal hyperalgesia (increased pain response) was generated using chemical cauterization of the corneal epithelium in wild-type (WT) and CB2R knockout (CB2R−/−) mice. Cauterized eyes were treated topically with the phytocannabinoids Δ8-tetrahydrocannabinol (Δ8THC) or cannabidiol (CBD), or the CBD derivative HU-308, in the presence or absence of the CB1R antagonist AM251 (2.0 mg/kg i.p.), or the 5-HT1A receptor antagonist WAY100635 (1 mg/kg i.p.). Behavioral pain responses to a topical capsaicin challenge at 6 h postinjury were quantified from video recordings. Mice were euthanized at 6 and 12 h postcorneal injury for immunohistochemical analysis to quantify corneal neutrophil infiltration.
Results: Corneal cauterization resulted in hyperalgesia to capsaicin at 6 h postinjury compared to sham control eyes. Neutrophil infiltration, indicative of inflammation, was apparent at 6 and 12 h postinjury in WT mice. Application of Δ8THC, CBD, and HU-308 reduced the pain score and neutrophil infiltration in WT mice. The antinociceptive and anti-inflammatory actions of Δ8THC, but not CBD, were blocked by the CB1R antagonist AM251, but were still apparent, for both cannabinoids, in CB2R−/− mice. However, the antinociceptive and anti-inflammatory actions of HU-308 were absent in the CB2R−/− mice. The antinociceptive and anti-inflammatory effects of CBD were blocked by the 5-HT1A antagonist WAY100635.
Conclusion: Topical cannabinoids reduce corneal hyperalgesia and inflammation. The antinociceptive and anti-inflammatory effects of Δ8THC are mediated primarily via CB1R, whereas that of the cannabinoids CBD and HU-308, involve activation of 5-HT1A receptors and CB2Rs, respectively. Cannabinoids could be a novel clinical therapy for corneal pain and inflammation resulting from ocular surface injury.
Keywords: : cannabinoids, cornea, pain, inflammation, hyperalgesia
Introduction
The cornea is a thin, transparent dome-shaped avascular tissue that is densely innervated by sensory nerve endings.1,2 Damage to these nerve endings, resulting from surgery, trauma, neurological disease, or infection, may develop into corneal neuropathic pain (CNP).2 CNP is a clinically significant problem characterized by persistent hyperalgesia, debilitating pain, photoallodynia, burning, stinging, dryness, and inflammation.3 Corneal damage can also result in an inflammatory response that involves the production of proinflammatory cytokines, neovascularization, recruitment of leukocytes, and release of neuropeptides producing inflammatory pain.4,5
Existing pharmacotherapies for ocular pain, inflammation, and CNP include topical corticosteroids, tricyclic antidepressants, GABAergic drugs, and opioids.3,6 These treatments, however, frequently fail to provide adequate pain relief and are associated with side effects.3,6 Therefore, new therapies that can alleviate pain and symptoms associated with CNP have fewer side effects and can resolve corneal inflammation are urgently required. One drug target that may have a role in the modulation of pain and inflammation is the endocannabinoid system (ECS).7,8
The ECS is an endogenous lipid signaling system that includes two G-protein-coupled receptors, cannabinoid 1 receptor (CB1R) and cannabinoid 2 receptor (CB2R), endocannabinoids, and cognate enzymes for biosynthesis and degradation of endocannabinoids.9,10 CB1Rs are widely expressed in many tissues, including in the central and peripheral nervous systems, where activation of CB1R modulates neurotransmitter release, and nerve activity.11,12 CB2R is highly expressed on immune cells and its activation is anti-inflammatory, resulting in decreased production of proinflammatory mediators and a reduction in leukocyte recruitment.13–15 Drugs that enhance activation of the ECS, including activation of both CB1R and CB2R, have shown efficacy in experimental models of pain and inflammation, including neuropathic pain.7,9,16,17
Cannabinoids have not been extensively studied in ocular surface pain and inflammation; however, CB1R is expressed in the corneal epithelium and endothelium in rodents and primates,18 and activation of CB1R has been reported to inhibit neuropeptide-induced sensitization of transient receptor potential cation channel subfamily V member 1 (TRPV1) in afferent neurons.11 Under nonpathological conditions, CB2R expression is low in the cornea and anterior ocular structures; however, increased CB2R expression in anterior ocular tissues has been suggested in experimental uveitis, where CB2R activation produces anti-inflammatory effects.19–21 Taken together, these studies suggest that cannabinoids that activate CB1R and/or CB2R may be useful for mitigating corneal pain and inflammation.
In this study, we used a mouse model of corneal hyperalgesia to investigate the antinociceptive and anti-inflammatory effects of several cannabinoids that act at CB1R and/or CB2R, or noncannabinoid receptors. These included Δ8-tetrahydrocannabinol (Δ8THC), a more stable isomer of Δ9-tetrahydrocannabinol (Δ9THC), cannabidiol (CBD), and the CBD derivative HU-308. Both Δ8THC and Δ9THC produce antinociceptive effects in pre-clinical models with similar potency via activation of CB1R.22–26 CBD lacks the behavioral effects of THC at CB1R, and may produce pharmacological actions through the activation of noncannabinoid receptors.27–29 HU-308 is a selective and highly potent agonist at CB2R,30 and has previously been shown to reduce lipopolysaccharide-induced intraocular inflammation.19
Materials and Methods
Experimental animals and corneal injury model
All animal care and experimental procedures complied with the Canadian Council for Animal Care guidelines (www.ccac.ca/) and were approved by the Dalhousie University Committee on Laboratory Animals. Male BALB/c (20–30 g; Charles River Laboratories International, Inc., Wilmington, MA) and CB2R knockout mice (CB2R−/−) were used for experiments. CB2R−/− mice were obtained by crossing male C57BL/6J CB2R−/− mice (strain B6.129P2-Cnr2tm1Dgen/J; Jackson Laboratory, Bar Harbor, ME) with inbred BALB/c female mice (Charles River) for 10 generations. Genetic loss of CB2R (Cnr2) was confirmed via polymerase chain reaction genotyping using DNA extracted from ear punches with an Accustart II Mouse Genotyping Kit (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer's instructions. Primer sequences were as follows: mouse CB2 mutant forward (moIMR0086) 5′- GGG GAT CGA TCC GTC CTG TAA GTC T-3′; mouse CB2 wild-type (WT) forward (oIMR7552) 5′- GGA GTT CAA CCC CAT GAA GGA GTA C-3′; mouse CB2 common reverse (oIMR7552) 5′- GAC TAG AGC TTT GTA GGT AGG CGG G−3′ with a single product at ∼550 bp for CB2R−/−, a single product at ∼385 bp for WT, and two products at ∼550 and 385 bp for heterozygous mice. Mice were housed in groups of 3–5, kept on a light/dark cycle (07:00–19:00/19:00–07:00), and fed ad libitum.
Corneal injury was induced using a protocol adapted from a model of corneal hyperalgesia previously described in rats by Wenk and Honda.31 Briefly, mice were anesthetized using 2–3% isoflurane gas. The center of the cornea on both eyes was cauterized with silver nitrate (MedPro®, 75% silver nitrate, 25% potassium nitrate; AMG Medical Inc., Montreal, QC, Canada) using a micro-applicator brush (Centrix, Inc., Shelton, CT). The micro-applicator brush was held in contact with the cornea for 2 sec, producing a distinct superficial white lesion of 1 mm in diameter, injuring the epithelial cell layer only. The cauterized eyes were then rinsed with saline and an ocular lubricant (Systane®; Alcon Canada, Inc., Dorval, QC, Canada) was applied to reduce corneal drying. Mice recovered fully from anesthesia within 3–5 min postcauterization. Mice were euthanized at 6 or 12 h postcauterization, and the eyes were enucleated for immunohistochemical analysis.
Assessment of behavioral pain sensitization
At 6 or 12 h postcauterization, 5 μL of 1 μM capsaicin was applied topically to the cauterized eyes to elicit a pain response.32 A sham control group was induced by touching the micro-applicator brush to the cornea for 2 sec in the absence of sliver nitrate, keeping all other parameters the same. Immediately following the application of a single dose of capsaicin, the behavioral response was video recorded for 30 sec. Videos were analyzed offline in slow motion, where the number of blinks, squints, and eye wipes to capsaicin challenge was summed to give a pain score.
Immunohistochemistry
At 12 h following corneal cauterization, eyes were enucleated and fixed in 4% PFA, followed by 30% sucrose overnight. Corneal sections (12 μm) were cut using a Leica CM1850 cryostat (Wetzlar, Germany). Sections were washed in phosphate-buffered saline (PBS) and blocked for nonspecific binding (10% normal goat serum in 0.5% Triton-X/PBS; Sigma-Aldrich, Oakville, ON, Canada) for 2 h, followed by a 48-h incubation in purified rat anti-Ly-6G antibody (1:200; Abcam, Cambridge, MA). Ly-6G is a glycosylphosphatidylinositol-anchor protein expressed predominantly on neutrophils; the anti-Ly-6G antibody allows for detection of these cells.33 Sections were then washed with PBS and incubated with a secondary antibody (1:500, goat anti-rat Alexa Fluor® 488, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Stained sections were washed in PBS and mounted on Superfrost slides (Fisher Scientific, ON, Canada) using Fluoromount (Sigma-Aldrich).
Neutrophil migration, indicative of an innate immune response, was quantified in corneal sections at 20×magnification using a Zeiss Axiovert 200 M microscope (Zeiss, Thornwood, NY). Three representative images were taken from each section of the right and left corneal peripheries and from the center of the cornea, respectively. The total number of neutrophils from these three images was counted for each section, and summed to represent the total neutrophil number for a single corneal section. A total of 5–12 sections with 120 μM intervals from each eye were analyzed and the neutrophil number was averaged.
Drugs and solutions
Δ8THC ([6aR,10aR]-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol; Cayman Chemical, Ann Arbor, MI), CBD (2-[(1R,6R)-3-Methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol; Cayman Chemical), HU-308 (4-[4-(1,1-Dimethylheptyl)-2,6-dimethoxyphenyl]-6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-methanol; Tocris Bioscience, Minneapolis, MN) were dissolved in soybean oil (Sigma-Aldrich) at different concentrations (0.2–5.0% w/v). Drugs were topically administered (5 μL) to cauterized corneas at 30, 60, and 120 min postcauterization. The CB1R antagonist AM251 (N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; Tocris Bioscience) was suspended in 10% dimethyl sulfoxide (DMSO, Sigma-Aldrich) and diluted in sterile saline. AM251 was injected at 2.0 mg/kg intraperitoneally (i.p.) fifteen min before cauterization. Capsaicin (1 μM, in 0.002% DMSO in sterile saline) was applied topically to eyes (5 μL) 6 h postinjury. The 5-HT1A receptor antagonist WAY100635 (N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate; Tocris Bioscience) was dissolved in sterile saline and injected at 1.0 mg/kg i.p., 15 min before cauterization.
Data analysis
Individual animals in each treatment group were coded and experimental data were analyzed blinded. One-way analysis of variance (ANOVA) with Dunnett's or Tukey's multiple comparison post hoc tests was used, as appropriate, to compare data between experimental groups of three or more. t-Tests were used to compare two experimental groups. The number of animals in each group was 5–12. All data reported are represented as group mean±standard deviation. Data were considered significant at p<0.05.
Results
Corneal chemical injury results in hyperalgesia and inflammation
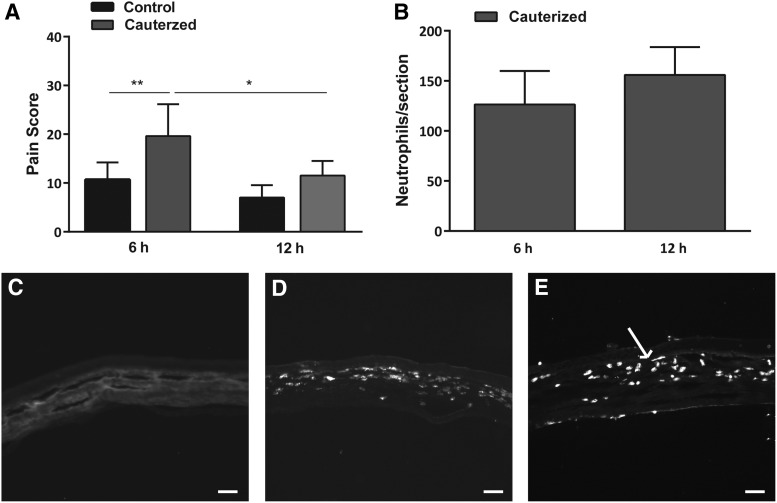
At 6 h, the pain score to 1 μM capsaicin was significantly increased in cauterized eyes (20±7, n=10) compared to sham control (11±4, n=6; p<0.01). At 12 h, no significant difference (p>0.05) was observed in pain score between sham control animals (7±3, n=5) compared to cauterized eyes (12±3, n=6). In addition, the pain score in cauterized eyes at 12 h was significantly lower than the pain score at 6 h (p<0.05; Fig. 1A).
FIG. 1.
Corneal chemical injury results in hyperalgesia and inflammation. (A) Pain responses to topical capsaicin challenge (1 μM) in noncauterized sham control eyes (n=5–6 per group) and cauterized eyes (n=6–10 per group) at 6 and 12 h postinjury. (B) Neutrophil expression in cauterized corneas at 6 and 12 h postinjury (n=5–6 per group). (C–E) Representative images of transverse sections of the central cornea from (C) sham control (noncauterized) corneas and cauterized corneas at (D) 6 h and (E) 12 h postinjury. Arrow in (E) points to one of many infiltrating neutrophils. Scale bar=50 μm. Values represent mean±SD. For statistical analysis, one-way ANOVA with Tukey's multiple comparison post hoc test was used. **p<0.01, *p<0.05. ANOVA, analysis of variance.
Immunohistochemical analysis was carried out to examine neutrophil migration, indicative of an inflammatory response, in the cornea 6 and 12 h after capsaicin challenge in either cauterized or sham eyes. Neutrophils were not observed in sham control corneas at 12 h (Fig. 1C). Figure 1B demonstrates, however, the presence of neutrophils at 6 and 12 h following cauterization (126±33, n=5, and 156±28, n=6, respectively; Fig. 1B, D, E).
Topical application of Δ8THC, CBD, and HU-308 reduces corneal pain and inflammation
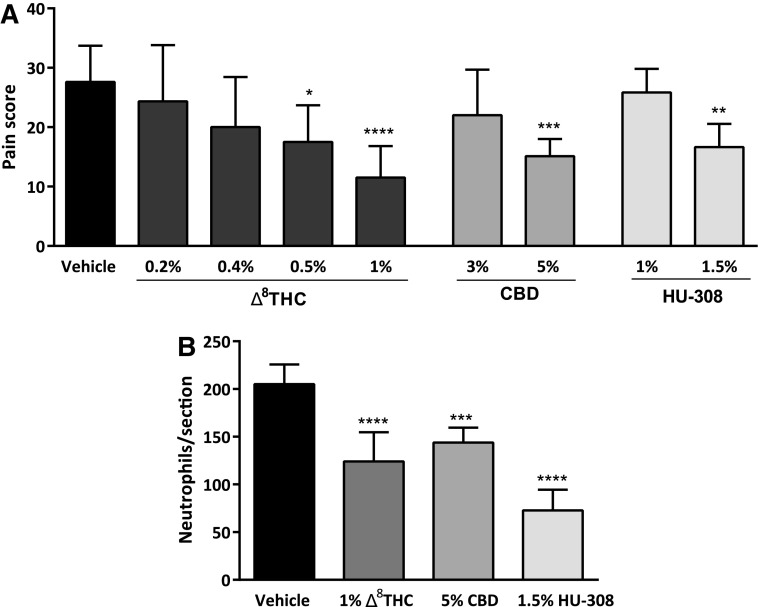
Vehicle treatment produced an average pain score of 28±6 (n=8). Different doses of topical Δ8THC, CBD, and HU-308 were examined in WT mice to establish the effective drug concentrations required to reduce corneal pain compared to the vehicle-treated group (Fig. 2A). Administration of 0.5% and 1% Δ8THC produced a significant reduction in pain scores (18±6, n=6, p<0.05; 12±5, n=12, p<0.0001, respectively). Although at lower concentrations (0.2% and 0.4%) Δ8THC did not significantly affect the pain score (n=6 in each group; p>0.05). Topical application of 5% CBD also significantly reduced the pain score (15±3, n=10, p<0.001); however, 3% CBD was not effective at reducing the pain score (n=6, p>0.05). In addition, while 1% HU-308 did not produce a significant reduction in pain score (n=6, p>0.05), administration of 1.5% HU-308 was antinociceptive (17±4, n=6, p<0.01). Therefore, given their efficacy at reducing the pain response, 1% Δ8THC, 5% CBD, and 1.5% HU-308 were used for all further experiments.
FIG. 2.
Topical administration of Δ8THC, CBD, or HU-308 reduces corneal hyperalgesia and neutrophil infiltration in WT mice after corneal cauterization. (A) Dose-response for antinociceptive effects of Δ8THC (0.2–1.0%, n=6–12 per group), CBD (3% and 5%, n=6 and10, respectively), and HU-308 (1% and 1.5%, n=6 per group) following capsaicin challenge. (B) The number of neutrophils per section in corneas from WT mice treated with 1% Δ8THC, 5% CBD, or 1.5% HU-308 at 12 h postinjury compared to vehicle-treated eyes (n=6 per group). Values represent mean±SD. For statistical analysis, one-way ANOVA with Dunnett's post hoc test (compared to vehicle) was used. ****p<0.0001, ***p<0.001, **p<0.01, *p<0.05. Δ8THC, Δ8-tetrahydrocannabinol; CBD, cannabidiol; WT, wild-type.
Neutrophil infiltration into the cornea following treatment with cannabinoids was examined. Topical administration of 1% Δ8THC, 5% CBD, or 1.5% HU-308 significantly reduced neutrophil number (124±31, 144±16, and 73±22, respectively) compared to vehicle-treated eyes (205±21; p<0.0001, p<0.001, and p<0.0001, respectively; n=6 per group).
The antinociceptive and anti-inflammatory effects of Δ8THC, but not CBD, were mediated through CB1R
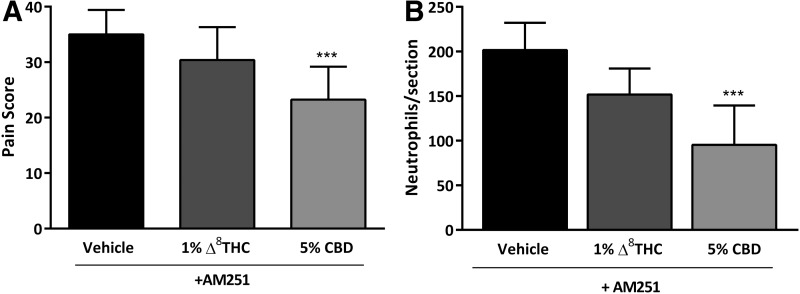
Administration of the CB1R antagonist AM251 (2.0 mg/kg, i.p.), before corneal cauterization and capsaicin stimulation, blocked the antinociceptive actions of Δ8THC (Fig. 3A; n=8, p>0.05), suggesting that Δ8THC acts via CB1R to reduce corneal pain. However, the antinociceptive actions of 5% CBD were maintained in eyes pretreated with CB1R antagonist AM251 (23±6, n=8), compared to vehicle-treated eyes plus AM251 (35±4, n=8, p<0.001; Fig. 3A).
FIG. 3.
The CB1R antagonist AM251 reduces the antinociceptive and anti-inflammatory actions of Δ8THC but not CBD. (A) Pain score measured in WT mice at 6 h postcauterization and following administration of 5 μL of topical vehicle, 1% Δ8THC, or 5% CBD (n=8 per group) in mice preadministered with AM251 (2.0 mg/kg i.p.). (B) The number of neutrophils per section at 12 h postcauterization in corneas from WT mice preadministered with AM251 (2.0 mg/kg i.p.) and treated with 5 μL of vehicle, or either 1% Δ8THC or 5% CBD (n=6 per group). Values represent mean±SD. For statistical analysis, one-way ANOVA with Dunnett's post hoc test (compared to vehicle) was used. ***p<0.001. CB1R, cannabinoid 1 receptor.
Likewise, the number of neutrophils in corneas from mice treated with AM251 and either 1% Δ8THC or vehicle was not significantly different (n=6, p>0.05). In contrast, 5% CBD treatment was still able to reduce neutrophils in corneas from mice treated with AM251 (95±44, n=6) versus vehicle-treated cauterized eyes from mice receiving AM251 (202±31, n=6, p<0.01; Fig. 3B).
The antinociceptive and anti-inflammatory effects of HU-308, but not Δ8THC or CBD, were mediated through CB2R
The involvement of CB2R in the antinocicpetive and anti-inflammatory effects of Δ8THC, CBD, and HU-308 was examined using CB2R−/− mice. Compared to WT mice receiving vehicle, the mean number of neutrophils in vehicle-treated CB2R−/− mice was significantly increased (mean difference 102±30, n=6 and 7, respectively, p<0.01); however, there was no significant difference in the pain score (n=8 in each group, p>0.05).
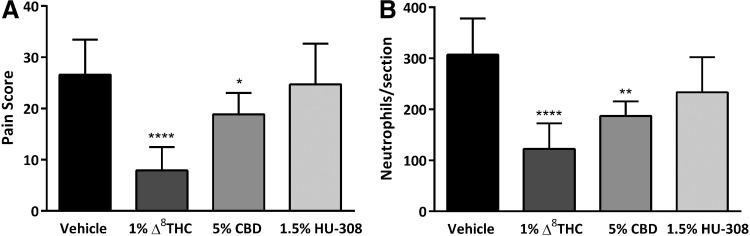
In CB2R−/− mice at 6 h postcauterization, compared to vehicle-treated eyes (27±7, n=8), application of 1% Δ8THC (8±5, n=12) or 5% CBD (19±4, n=7) significantly decreased the pain score (p<0.0001 and p<0.05, respectively). However, the antinociceptive effect of 1.5% HU-308 (n=7) was not significantly different compared to vehicle-treated animals (p>0.05; Fig. 4A), confirming the involvement of CB2R in the antinociceptive effects of HU-308, but not THC or CBD.
FIG. 4.
The corneal antinociceptive and anti-inflammatory effects of HU-308, but not Δ8THC or CBD, are mediated via CB2R. (A) Pain scores measured at 6 h postcorneal cauterization in CB2R−/− mice treated with 5 μL topical vehicle (n=8), or 1% Δ8THC (n=12), 5% CBD (n=7), or 1.5% HU-308 (n=7). (B) The number of neutrophils per section measured at 12 h postcauterization in corneas from CB2R−/− mice treated with 5 μL of vehicle (n=7), or 1% Δ8THC (n=6), 5% CBD (n=6), or 1.5% HU-308 (n=6). Values represent mean±SD. For statistical analysis, one-way ANOVA with Dunnett's post hoc test (compared to vehicle) was used. ****p<0.0001, **p<0.01, *p<0.05. CB2R, cannabinoid 2 receptor.
Consistently, at 12 h postcauterization in CB2R−/− mice, the number of neutrophils in corneas receiving either 1% Δ8THC (123±50, n=6) or 5% CBD (187±28, n=6) was significantly less than vehicle-treated corneas (307±71, n=7; p<0.0001 and p<0.01, respectively). In HU-308-treated corneas (1.5%) from CB2R−/− mice (n=6), there was no significant difference in neutrophil numbers compared to vehicle-treated eyes (p>0.05; Fig. 4B).
CBD acts at 5-HT1A receptors to reduce corneal pain and inflammation
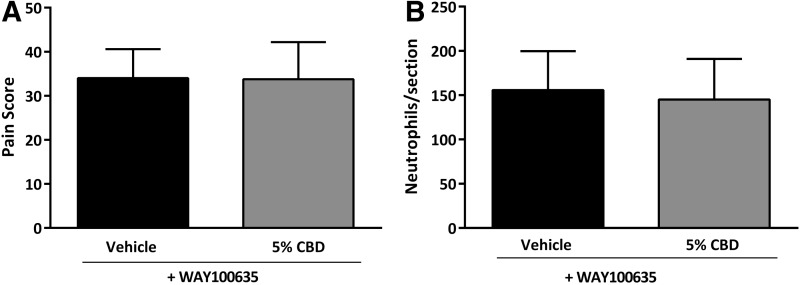
The corneal antinociceptive and anti-inflammatory effects of CBD were independent of CB1R or CB2R. Therefore, we examined an alternative non-cannabinoid receptor, 5-HT1A, which has been reported as a target for CBD in other tissues.29 Treatment of mice with the 5-HT1A receptor antagonist WAY100635 (1.0 mg/kg i.p.) was able to completely eliminate the antinociceptive actions of CBD in cauterized cornea (Fig. 5A; n=8 in each group, p>0.05). In mice treated with WAY100635, the reduction in corneal neutrophils in cauterized eyes seen with CBD treatment was also blocked (n=6 in each group, p>0.05), suggesting that 5-HT1A is the target receptor for CBD-mediated antinociceptive and anti-inflammatory actions in the cornea (Fig. 5B).
FIG. 5.
The corneal antinociceptive and anti-inflammatory effects of CBD are mediated through 5-HT1A receptor. (A) Pain score measured at 6 h postcauterization in WT mice preadministered with the 5-HT1A receptor antagonist, WAY100635 (1.0 mg/kg i.p.), and treated topically with 5 μL of either vehicle (n=8) or 5% CBD (n=8). (B) The number of neutrophils per section measured at 12 h postcauterization in corneas from WT mice treated with 5 μL of either vehicle (n=6) or 5% CBD (n=6). Values represent mean±SD. For statistical analysis, unpaired t-tests were used.
Discussion
Our results provide novel evidence that the phytocannabinoids Δ8THC and CBD and synthetic cannabinoid derivative HU-308 are antinociceptive and anti-inflammatory in an experimental model of corneal hyperalgesia. Furthermore, we demonstrate that the actions of these cannabinoids are mediated via distinct receptor targets that include CB1R and CB2R, as well as 5-HT1A receptor.
In the mammalian cornea, expression of CB1R has been reported to colocalize with TRPV1,34 the latter of which is expressed in corneal epithelium35 and endothelium,36 and sensory nerve endings of the ophthalmic branch of the trigeminal nerve innervating the cornea.37 TRPV1 is activated following damage to cornea, culminating in activation of corneal nerves and local inflammation. The release of proinflammatory cytokines and neuropeptides, including nerve growth factor and substance P, contributes to neurogenic inflammation and can lead to corneal nerve sensitization.34,38 In sensory neurons isolated from rat dorsal root ganglia, activation of CB1R by the cannabinoid agonist ACEA (arachidonoyl-2′- chloroethylamide) prevented nerve growth factor-induced sensitization of the TRPV1 receptor. This action was blocked by the CB1R antagonist AM251, suggesting that the activation of CB1R may produce analgesia by desensitization of TRPV1 receptors.39
Our results demonstrating that topical Δ8THC, acting at CB1R, reduces hyperalgesia following corneal injury are in line with these findings. In addition, we also demonstrated that Δ8THC was able to reduce the neutrophil recruitment to the cornea observed at later time points following corneal epithelial damage. The inhibition of neutrophil recruitment was blocked with the treatment of CB1R antagonist AM251, but was still present in CB2R−/− mice. This suggests that the activation of CB1R by Δ8THC is important in mitigating the innate immune response following corneal injury, which may contribute to corneal nerve sensitization.
The importance of peripheral CB1R in our study is also consistent with the actions of Δ9THC reported in other models of both acute inflammatory and neuropathic pain. For example, administration of Δ9THC (1 mg/kg i.p.) in a rat model of acute muscle pain produced antinociceptive effects, which was blocked by the CB1R antagonist AM281 and to a lesser extent by the CB2R antagonist AM630 (0.5 mg/kg i.p.).40 Furthermore, in a model of inflammatory and neuropathic pain, mice lacking CB1R in peripheral nociceptive neurons showed a reduced analgesic effect to local and systemic administration of the cannabinoid WIN55,212-2. With intrathecal application, the analgesic effect of WIN55,212-2 was absent, suggesting that peripheral CB1R in nociceptive neurons plays an important role in producing the analgesic effects of cannabinoids.41
Our data implicate 5-HT1A receptors, and not cannabinoid receptors, in both antinociceptive and anti-inflammatory actions of CBD in an experimental model of corneal injury. We showed that the actions of CBD were completely blocked by the 5-HT1A antagonist WAY100635, but were still present after CB1R block or in CB2R−/− mice. CBD has been reported in other in vitro and in vivo models to bind to 5-HT1A receptors.27,29,42 Using a heterologous cell expression system, Russo et al. reported that CBD bound to both human and rat 5-HT1A receptors with micromolar affinity, and displaced the agonist [3H]8-OH-DPAT in a concentration-dependent manner.29 In addition, CBD increased [35S]GTPγS binding, and decreased forskolin-stimulated cAMP production, which was blocked by the specific 5-HT1A antagonist NAN-190.29
In line with our findings of CBD activity at the 5-HT1A receptor, a study by Ward et al. reported that CBD administration could prevent chemotherapy-induced neuropathic pain associated with paclitaxel treatment.43 In this study, CBD was administered chronically for 14 days and prevented the onset of paclitaxel-induced mechanical and thermal sensitivity in female mice. A subsequent report showed that a subchronic dosing regimen of 2.5–10 mg/kg CBD (i.p.) was also effective in preventing paclitaxel-induced mechanical sensitivity. This effect was blocked by a 5-HT1A antagonist (WAY100635), but not a CB1R (SR141716) or CB2R antagonist (SR144528), further supporting the role of 5-HT1A in mediating the actions of CBD in preventing neuropathic pain.43
HU-308 has been reported as a selective CB2R agonist.30 In our model of corneal hyperalgesia, the antinociceptive and anti-inflammatory actions of HU-308, unlike Δ8THC and CBD, were absent in CB2R−/− mice, validating target specificity for this cannabinoid at CB2R. This is the first time a CB2R agonist has been demonstrated to reduce corneal pain, although CB2R activation has been reported to reduce ocular inflammation.20,44 In experimental uveitis, Toguri et al.19 reported that CB2R activation reduced leukocyte/endothelial adhesion in the iridial microvasculature as well as inhibited release of proinflammatory mediators, including TNFα, IL1β, Il6, CCL5, and CXCL2. Conversely, a CB2R antagonist, AM630, increased leukocyte/endothelial adhesion in experimental uveitis,19 suggesting that CB2R activity in the eye is immunosuppressive during inflammation. In a mouse model of proliferative vitreoretinopathy, CB2R−/− or pharmacological block of CB2R, also produced increased inflammation and a more severe pathology.44 Another study, in a mouse model of endotoxemia, has shown increased neutrophil recruitment to the spleen in CB2R−/− mice compared to WT control.45 In line with these results, in our experiments we observed an increase in the mean number of neutrophils in cauterized corneas in CB2R−/− mice, suggesting that loss of constitutive CB2R activity is proinflammatory in ocular tissues.20,44
Reports of the antinociceptive and antiallodynic efficacy of CB2R agonists have also been reported in other experimental models of hyperalgesia and chronic inflammatory neuropathic pain.46 While our study in cornea used a relatively acute dosing regimen, the utility of CB2R agonists used chronically was previously reported in a mouse model of paclitaxel-induced neuropathic pain.47 The authors reported that chronic CB2R activation with the CB2R-preferring agonist AM1710 was able to reverse paclitaxel-induced allodynia, an effect that was blocked in WT mice treated with the CB2R antagonist AM630, or in CB2R−/− mice. In comparison to repeated dosing with agonists such as THC that produced behavioral effects and tolerance via CB1R activation, no similar effects were observed with the CB2R-preferring agonist AM1710. Furthermore, using intrathecal cannabinoid administration, this study identified a possible role for spinal CB2R in the antiallodynic actions of AM1710, as well as a reduction in proinflammatory cytokines, in paclitaxel-treated mice.47 Increased CB2R expression has also been reported in human peripheral nerves after injury, and CB2R agonist-mediated inhibition of capsaicin responses was observed in cultured human dorsal root ganglion sensory neurons.48 Our data demonstrating the antinociceptive and anti-inflammatory actions of CB2R activation in cornea, together with these studies, further support the utility of CB2R agonists for treating inflammatory pain.
Conclusion
Our study showed that topical application of the phytocannabinoids Δ8THC and CBD, and the cannabinoid derivative HU-308, reduced corneal hyperalgesia and neutrophil infiltration resulting from superficial chemical injury of corneal epithelium. The effects of these cannabinoids were mediated by distinct receptors, including CB1R and CB2R, as well as 5-HT1A receptors. This suggests that when used either as sole agents or in combination, these cannabinoids could be effective agents in the treatment of ocular pain and inflammation resulting from corneal surface injuries.
Abbreviations Used
- Δ8THC
Δ8-tetrahydrocannabinol
- Δ9THC
Δ9-tetrahydrocannabinol
- CBD
cannabidiol
- CB1R
cannabinoid 1 receptor
- CB2R
cannabinoid 2 receptor
- CNP
corneal neuropathic pain
- DMSO
dimethyl sulfoxide
- ECS
endocannabinoid system
- PBS
phosphate-buffered saline
- TRPV1
transient receptor potential cation channel subfamily V member 1
- WT
wild-type
Acknowledgments
This research was supported by the Canadian Institutes of Health Research (MOP-97768). D.T. was supported, in part, through the NSGS. The authors thank Janette Nason and Anjali Ghimire for their technical assistance.
Author Disclosure Statement
M.E.M.K. is the founder and director of Panag Pharma, Inc. Panag develops phytotherapeutics for local and regional treatment of pain and inflammation. D.T., E.A.C., J.T.T., A.-M.S., and M.D.C. have no existing competing financial interests.
References
- 1.Launay PS, Reboussin E, Liang H, et al. . Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol Dis. 2016;88:16–28 [DOI] [PubMed] [Google Scholar]
- 2.Belmonte C, Acosta MC, Merayo-Lloves J, et al. . What causes eye pain? Curr Ophthalmol Rep. 2015;3:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal S, Hamrah P. Understanding neuropathic corneal pain—gaps and current therapeutic approaches. Semin Ophthalmol. 2016;31:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Zhang W, Bi M, et al. . The molecular mechanisms of action of PPAR-gamma agonists in the treatment of corneal alkali burns (review). Int J Mol Med. 2016;38:1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voiculescu OB, Voinea LM, Alexandrescu C. Corneal neovascularization and biological therapy. J Med Life. 2015;8:444–448 [PMC free article] [PubMed] [Google Scholar]
- 6.Faktorovich EG, Melwani K. Efficacy and safety of pain relief medications after photorefractive keratectomy: review of prospective randomized trials. J Cataract Refract Surg. 2014;40:1716–1730 [DOI] [PubMed] [Google Scholar]
- 7.Maldonado R, Banos JE, Cabanero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157 Suppl 1:S23–S32 [DOI] [PubMed] [Google Scholar]
- 8.Huang WJ, Chen WW, Zhang X. Endocannabinoid system: role in depression, reward and pain control (review). Mol Med Rep. 2016;14:2899–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur R, Ambwani SR, Singh S. Endocannabinoid system: a multi-facet therapeutic target. Curr Clin Pharmacol. 2016;11:110–117 [DOI] [PubMed] [Google Scholar]
- 10.Pertwee RG, Howlett AC, Abood ME, et al. . International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;625:588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuda LA, Lolait SJ, Brownstein MJ, et al. . Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564 [DOI] [PubMed] [Google Scholar]
- 12.Wang ZY, McDowell T, Wang P, et al. . Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons. Neuroscience. 2014;277:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190 [DOI] [PubMed] [Google Scholar]
- 14.Onaivi ES, Ishiguro H, Gong JP, et al. . Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536 [DOI] [PubMed] [Google Scholar]
- 15.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65 [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Zhao R, Li J, et al. . Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. Eur J Pharmacol. 2016;786:128–136 [DOI] [PubMed] [Google Scholar]
- 17.Fine PG, Rosenfeld MJ. Cannabinoids for neuropathic pain. Curr Pain Headache Rep. 2014;18:45–1. [DOI] [PubMed] [Google Scholar]
- 18.Straiker AJ, Maguire G, Mackie K, et al. . Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol Vis Sci. 1999;40:2442–2448 [PubMed] [Google Scholar]
- 19.Toguri JT, Lehmann C, Laprairie RB, et al. . Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br J Pharmacol. 2014;171:1448–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toguri JT, Moxsom R, Szczesniak AM, et al. . Cannabinoid 2 receptor activation reduces leukocyte adhesion and improves capillary perfusion in the iridial microvasculature during systemic inflammation. Clin Hemorheol Microcirc. 2015;61:237–249 [DOI] [PubMed] [Google Scholar]
- 21.Toguri JT, Caldwell M, Kelly ME. Turning down the thermostat: modulating the endocannabinoid system in ocular inflammation and pain. Front Pharmacol. 2016;7:30–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin BR, Kallman MJ, Kaempf GF, et al. . Pharmacological potency of R- and S-3'-hydroxy-delta 9-tetrahydrocannabinol: additional structural requirement for cannabinoid activity. Pharmacol Biochem Behav. 1984;21:61–65 [DOI] [PubMed] [Google Scholar]
- 23.Formukong EA, Evans AT, Evans FJ. Analgesic and antiinflammatory activity of constituents of cannabis sativa L. Inflammation. 1988;12:361–371 [DOI] [PubMed] [Google Scholar]
- 24.Burstein SH, Hull K, Hunter SA, et al. . Cannabinoids and pain responses: a possible role for prostaglandins. FASEB J. 1988;2:3022–3026 [DOI] [PubMed] [Google Scholar]
- 25.Compton DR, Prescott WR, Jr, Martin BR, et al. . Synthesis and pharmacological evaluation of ether and related analogues of delta 8-, delta 9-, and delta 9,11-tetrahydrocannabinol. J Med Chem. 1991;34:3310–3316 [DOI] [PubMed] [Google Scholar]
- 26.Varvel SA, Bridgen DT, Tao Q, et al. . Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J Pharmacol Exp Ther. 2005;314:329–337 [DOI] [PubMed] [Google Scholar]
- 27.Linge R, Jimenez-Sanchez L, Campa L, et al. . Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26 [DOI] [PubMed] [Google Scholar]
- 28.Pertwee R. Pharmacological and therapeutic targets for Δ9tetrahydrocannabinol and cannabidiol. Euphytica. 2004;140:73–82 [Google Scholar]
- 29.Russo EB, Burnett A, Hall B, et al. . Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043 [DOI] [PubMed] [Google Scholar]
- 30.Hanus L, Breuer A, Tchilibon S, et al. . HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228–14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenk HN, Honda CN. Silver nitrate cauterization: characterization of a new model of corneal inflammation and hyperalgesia in rat. Pain. 2003;105:393–401 [DOI] [PubMed] [Google Scholar]
- 32.Wenk HN, Nannenga MN, Honda CN. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea. Pain. 2003;105:455–465 [DOI] [PubMed] [Google Scholar]
- 33.Marrazzo G, Bellner L, Halilovic A, et al. . The role of neutrophils in corneal wound healing in HO-2 null mice. PLoS One. 2011;6:e2118–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Yang H, Wang Z, et al. . Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury. Cell Signal. 2013;25:501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang F, Yang H, Wang Z, et al. . Transient receptor potential vanilloid 1 activation induces inflammatory cytokine release in corneal epithelium through MAPK signaling. J Cell Physiol. 2007;213:730–739 [DOI] [PubMed] [Google Scholar]
- 36.Mergler S, Valtink M, Coulson-Thomas VJ, et al. . TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp Eye Res. 2010;90:758–770 [DOI] [PubMed] [Google Scholar]
- 37.Murata Y, Masuko S. Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. Brain Res. 2006;1085:87–94 [DOI] [PubMed] [Google Scholar]
- 38.Belmonte C, Aracil A, Acosta MC, et al. . Nerves and sensations from the eye surface. Ocul Surf. 2004;2:248–253 [DOI] [PubMed] [Google Scholar]
- 39.McDowell TS, Wang ZY, Singh R, et al. . CB1 cannabinoid receptor agonist prevents NGF-induced sensitization of TRPV1 in sensory neurons. Neurosci Lett. 2013;551:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagüés A, Martín MI, Sánchez-Robles EM. Involvement of central and peripheral cannabinoid receptors on antinociceptive effect of tetrahydrocannabinol in muscle pain. Eur J Pharmacol. 2014;745:69–75 [DOI] [PubMed] [Google Scholar]
- 41.Agarwal N, Pacher P, Tegeder I, et al. . Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Resstel LB, Tavares RF, Lisboa SF, et al. . 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward SJ, McAllister SD, Kawamura R, et al. . Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol. 2014;171:636–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szczesniak A, Porter RF, Toguri JT, et al. . Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology. 2017;113, Part B:627–638 [DOI] [PubMed] [Google Scholar]
- 45.Kapellos TS, Recio C, Greaves DR, et al. . Cannabinoid receptor 2 modulates neutrophil recruitment in a murine model of endotoxemia. Mediators Inflamm. 2017;2017:431541–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinsey SG, Mahadevan A, Zhao B, et al. . The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng L, Guindon J, Cornett BL, et al. . Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anand U, Otto WR, Sanchez-Herrera D, et al. . Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680 [DOI] [PubMed] [Google Scholar]
References
Cite this article as: Thapa D, Cairns EA, Szczesniak A-M, Toguri JT, Caldwell MD, Kelly MEM (2018) The cannabinoids Δ8THC, CBD, and HU-308 act via distinct receptors to reduce corneal pain and inflammation, Cannabis and Cannabinoid Research 3:1, 11–20, DOI: 10.1089/can.2017.0041.