Key Points
The effect of donor age on survival is negated by the effect of patient age.
Survival did not differ between sibling and offspring donor transplantation.
Abstract
We studied the association between non-HLA donor characteristics (age, sex, donor-recipient relationship, blood group [ABO] match, and cytomegalovirus [CMV] serostatus) and transplant outcomes after T-cell-replete HLA-haploidentical transplantation using posttransplantation cyclophosphamide (PT-Cy) in 928 adults with hematologic malignancy transplanted between 2008 and 2015. Siblings (n = 358) and offspring (n = 450) were the predominant donors, with only 120 patients having received grafts from parents. Although mortality risks were higher with donors aged 30 years or older (hazard ratio, 1.39; P < .0001), the introduction of patient age to the Cox regression model negated the effect of donor age. Two-year survival adjusted for CMV seropositivity, disease, and disease risk index was lower in patients aged 55 to 78 years after transplantation of grafts from donors younger than 30 years (53%) or aged at least 30 years (46%) compared with younger patients who received grafts from donors younger than 30 years (61%) and at least 30 years (60%; P < .0001). Similarly, 2-year survival in patients aged 55 to 78 years was lower after transplantation of grafts from siblings (45%) or offspring (48%) compared with patients aged 18 to 54 years after transplantation of grafts from siblings (62%), offspring (58%), and parents (61%; P < .0001). Graft failure was higher after transplantation of grafts from parents (14%) compared with siblings (6%) or offspring (7%; P = .02). Other non-HLA donor characteristics were not associated with survival or graft failure. The current analyses suggest patient and disease, rather than non-HLA donor characteristics, predominantly influence survival in adults.
Visual Abstract
Introduction
T-cell-replete HLA-haploidentical (haplo) hematopoietic cell transplantation with posttransplantation cyclophosphamide (PT-Cy) has become an increasingly used platform, given its easy application without the need for graft manipulation and the increasing number of studies showing its safety and efficacy.1-6 In the absence of definitive studies regarding the effects of donor characteristics on transplant outcomes for haplo T-cell-replete transplantation with PT-Cy, donor prioritization strategies are extrapolated from HLA-matched related and unrelated donor transplantation.7 The Baltimore group’s selection algorithm for haplo donors incorporates donor-specific antibodies, donor-recipient ABO match, donor age, donor sex, and cytomegalovirus (CMV) serostatus match. An earlier report from the Baltimore group concluded that greater HLA disparity between haplo donor-recipient pairs did not worsen event-free survival or graft-versus-host disease (GVHD).8
Another strategy for T-cell-replete haplo transplantation is transplantation of filgrastim-stimulated pooled bone marrow and peripheral blood from donors followed by intensified immune suppression including antithymocyte globulin.9 That report, based on 1210 donor-recipient pairs, recommended an offspring donor when available, based on improved survival with donors younger than 30 years. When an offspring is not available, their donor selection algorithm suggests, in descending order of priority, a male sibling mismatched at the noninherited maternal antigen, noninherited maternal antigen mismatched female sibling or father, a sibling mismatched at the noninherited paternal antigen, and mother.9 In contrast, recommendations for donor selection for T-cell-depleted haplo transplantation from the Perugia group conclude maternal donors are preferred.10 Despite these differences, in both reports,9,10 donor sex was not associated with survival and other transplant outcomes when maternal donors were excluded from the analysis.
With the increasing use of T-cell-replete haplo transplantation with PT-Cy for hematologic malignancy in adults worldwide, and given the absence of definitive studies on donor selection for this approach, we studied the effect of non-HLA donor characteristics on survival, graft failure, acute and chronic GVHD, nonrelapse mortality, and relapse in 928 donor-recipient pairs with hematologic malignancy. We hypothesized that survival is better with young donors who may be siblings or offspring.
Methods
Patients
The Center for International Blood and Marrow Transplant Research is a group of more than 400 transplant centers that contribute data prospectively on consecutive transplants. Patients are registered pretransplant with documentation of consent (obtained by transplant center) for participation in research studies. Patients are followed longitudinally until death or lost to follow-up. Sixty-five centers contributed patients, and transplants were performed between 2008 and 2015 in the United States. Eligible patients were aged 18 years and older with acute myeloid leukemia (AML), acute lymphoblastic leukemia, myelodysplastic syndrome, non-Hodgkin lymphoma, or Hodgkin lymphoma. Patients received bone marrow or peripheral blood from haplo first-degree relatives. Excluded were regimens that included in vivo T-cell depletion (n = 29). The Institutional Review Board of the National Marrow Donor Program approved this study.
Endpoints
The primary endpoint was overall survival. Death from any cause was considered an event. Primary and secondary graft failure were considered as a single outcome. Primary graft failure was defined as failure to achieve an absolute neutrophil count of at least 0.5 × 109/L for 3 consecutive days or less than 5% donor chimerism (peripheral blood CD3+ or bone marrow). Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent decline in the absolute neutrophil count (<0.5 × 109/L), loss of donor chimerism, or second transplant in patients with documented clinical remission.11 Grade II-IV acute GVHD and chronic GVHD were based on reports from each transplant center, using standard criteria.12,13 Relapse/progression was defined as disease recurrence (morphologic, cytogenetic, or molecular) or progression. Nonrelapse mortality was defined as death in remission. Surviving patients were censored at last follow-up.
Statistical methods
The cumulative incidences of graft failure at 1 year, acute GVHD at 6 months, and chronic GVHD at 2 years were calculated using the cumulative incidence estimator to accommodate competing risks.14 Multivariate models were built to examine the effect of donor characteristics on overall mortality, using Cox regression model, and on graft failure, nonrelapse mortality, relapse, or progression, and acute and chronic GVHD, using the Fine and Gray model.15,16
Exploratory analysis of the study population confirmed correlations between recipient age and donor-recipient relationship (correlation coefficient, 0.66; P < .0001) and donor age and donor-recipient relationship (correlation coefficient, −0.61; P < .0001), and none between recipient and donor age (correlation coefficient, 0.06; P = .06). We tested patient age and donor age in decades and separately for the primary outcome: survival. Subgroups that did not differ significantly were collapsed. Using this approach, we confirmed mortality risks were higher with donors aged 30 years or older (HR, 1.39; 95% CI, 1.12-1.73; P = .003) compared with younger than than 30 years; therefore, in subsequent analyses, donor age was dichotomized as younger than 30 vs at least 30 years. Similarly, exploratory analysis confirmed mortality risks were higher for patients aged 55 years and older (HR, 1.75; 95% CI, 1.45-2.11; P < .0001), and in subsequent analyses, patient age was dichotomized as 18 to 54 vs ≥55 years.
As donor age and donor-recipient relationship were strongly correlated, 2 separate multivariate models for outcomes of interest were built, with 1 model considering recipient age and donor-recipient relationship and the other considering recipient age and donor age. Other variables tested in multivariate models included donor sex and parity, donor-recipient blood group (ABO) match, donor and recipient CMV serostatus, hematopoietic comorbidity index, performance score, recipient race, disease, disease risk index, transplant conditioning regimen intensity, graft type, and transplant period. The probability of overall survival and incidences for nonrelapse mortality and relapse/progression were calculated from the final Cox model with adjustment for other factors that were associated with these outcomes. All variables tested met the assumptions for proportionality, and there were no first-order interactions between the variables for recipient age/donor-recipient relationship and recipient age/donor age and other variables held in the final multivariate model. Variables that attained P ≤ .05 were held in the final multivariate model. All P values are 2-sided, and analyses were performed using SAS version 9.4 (Cary, NC).
Results
Patient, disease, and transplant characteristics
The characteristics of the study population are shown in Table 1. The median age of donors was 38 years (range, 10-80 years), with only 7% of donors aged less than 21 years and 25% older than 50 years. Thirteen percent of donors were parents, 38% siblings, and 49% offspring. Fifty-eight percent of donors were male, and 49% were CMV seropositive. Donors were HLA mismatched with patients at 2 or more HLA loci. The median age of patients was 54 years (range, 18-78 years), and 62% were CMV seropositive. A third of patients had performance scores less than 90 and a hematopoietic comorbidity index score of at least 3.17 Most transplants were ABO matched (57%). AML was the predominant indication for transplantation. Disease risk index18 was intermediate risk for 62% and high/very high risk for 29% of patients. Bone marrow was the predominant graft, and there were no differences in use of bone marrow or peripheral blood by donor-recipient relationship (P = .78) or donor age (P = .64). The median total nucleated cell dose (3 × 108/kg; interquartile range, 2-4 × 108/kg) of bone marrow grafts and the median CD34 dose of peripheral blood (6 × 106/kg; interquartile range, 5-9 × 106/kg) did not differ by donor-recipient relationship (P = .16) or donor age (P = .81). The predominant transplant-conditioning regimen was total body irradiation 200 cGy with fludarabine and low-dose cyclophosphamide. All patients received a uniform GVHD prophylaxis with PT-Cy, tacrolimus or cyclosporine, and mycophenolate mofetil. As 70% of transplants occurred between 2012 and 2015, the median follow-up of the study population is 24 months (range, 3-97 months). Supplemental Table 1A shows patient, disease, and transplant characteristics by patient age group and donor-recipient relationship, and supplemental Table 1B by patient and donor age groups.
Table 1.
Donor, patient, disease and transplant characteristics
| Characteristics | Number (%) |
|---|---|
| Donor age, y | |
| 10-29 | 279 (30) |
| 30-49 | 420 (45) |
| 50-80 | 229 (25) |
| Donor-recipient relationship | |
| Parent | 120 (13) |
| Sibling | 358 (39) |
| Offspring | 450 (48) |
| Donor-recipient sex match | |
| Male donor/male recipient | 335 (36) |
| Male donor/female recipient | 208 (23) |
| Female donor/male recipient | 224 (24) |
| Female donor/female recipient | 161 (17) |
| Donor-recipient ABO match | |
| Matched | 530 (57) |
| Major mismatch | 147 (16) |
| Minor mismatch | 114 (12) |
| Not reported | 137 (15) |
| Donor-recipient cytomegalovirus serostatus match | |
| Donor negative/recipient negative | 232 (25) |
| Donor negative/recipient positive | 234 (25) |
| Donor positive/recipient negative | 119 (13) |
| Donor positive/recipient positive | 335 (36) |
| Not reported | 8 (1) |
| Donor sex and parity | |
| Male | 543 (59) |
| Female, nulliparous | 95 (10) |
| Female, parous | 223 (24) |
| Female, parity not reported | 57 (6) |
| Sex, not reported | 10 (< 1) |
| Patient age, y | |
| 18-54 | 480 (52) |
| 55-78 | 448 (48) |
| Recipient race | |
| White | 658 (71) |
| African American | 197 (21) |
| Other | 73 (8) |
| Performance score | |
| 90-100 | 572 (62) |
| <90 | 308 (33) |
| Not reported | 48 (5) |
| Hematopoietic cell transplant-comorbidity index | |
| 0-2 | 587 (63) |
| ≥3 | 341 (37) |
| Disease | |
| Acute myeloid leukemia | 415 (45) |
| Acute lymphoblastic leukemia | 136 (15) |
| Myelodysplastic syndrome | 117 (13) |
| Non-Hodgkin lymphoma | 199 (21) |
| Hodgkin lymphoma | 61 (6) |
| Disease risk index | |
| Low risk | 85 (9) |
| Intermediate risk | 573 (62) |
| High risk | 270 (29) |
| Graft type* | |
| Bone marrow | 632 (68) |
| Peripheral blood | 296 (32) |
| Conditioning regimen | |
| Myeloablative | |
| Total body irradiation + fludarabine | 80 (9) |
| Total body irradiation + other agents | 49 (5) |
| Busulfan + cyclophosphamide | 123 (13) |
| Busulfan + fludarabine | 28 (3) |
| Reduced intensity | |
| Total body irradiation + cyclophosphamide + fludarabine | 549 (59) |
| Total body irradiation + other agents | 29 (3) |
| Melphalan + fludarabine | 70 (8) |
| Transplant period | |
| 2008-2011 | 277 (30) |
| 2012-2015 | 651 (70) |
The median total nucleated cell dose for bone marrow grafts was 3 × 108/kg; interquartile range, 2 to 4 × 108/kg. The median CD34 dose of peripheral blood grafts was 6 × 106/kg; interquartile range, 5 to 9 × 106/kg.
Overall survival
Donor age was strongly correlated with patient age (P < .0001), as was donor age and donor-recipient relationship (P < .0001). Therefore, the effects of donor age and donor-recipient relationship were addressed separately in patients aged 18 to 54 years and 55 to 78 years. None of the donor characteristics studied was associated with survival after adjustment for patient age and other patient and disease characteristics (Table 2; Figure 1A-B). For patients aged 55 to 78 years, mortality risks did not differ by whether they received grafts from a sibling vs offspring (HR, 1.03; 95% CI, 0.78-1.36; P = .83) or by the age of the donor (HR, 1.16; 95% CI, 0.85-1.57; P = .35). Similarly, among patients aged 18 to 54 years, mortality risks did not differ by whether they received grafts from a sibling or offspring (HR, 1.05; 95% CI, 0.74-1.50; P = .78). Although 70% of donors were aged 30 years or older, we confirmed mortality risks did not differ between donors aged 30 to 49 and 50 to 80 years (HR, 0.80; 95% CI, 0.60-1.06; P = .12).
Table 2.
Effect of patient age, donor-recipient relationship, and donor age on overall mortality, nonrelapse mortality, and relapse
| Overall mortality, hazard ratio (95% CI)* | Nonrelapse mortality, hazard ratio (95% CI)† | Relapse, hazard ratio (95% CI)‡ | |
|---|---|---|---|
| Patient age, y/donor-recipient relationship | |||
| Age 18-54/parent donor | 1.00, P < .0001§ | 1.00, P = .003§ | 1.00, P = .18§ |
| Age 18-54/sibling donor | 0.87 (0.61-1.24), P = .44 | 0.96 (0.51-1.82), P = .90 | 0.75 (0.52-1.09), P = .14 |
| Age 18-54/offspring donor | 0.92 (0.61-1.38), P = .67 | 1.47 (0.75-2.88), P = .26 | 0.65 (0.41-1.03), P = .07 |
| Age 55-78/sibling donor | 1.53 (1.04-2.23), P = .030 | 2.36 (1.26-4.45), P = .007 | 0.84 (0.54-1.30), P = .43 |
| Age 55-78/offspring donor | 1.57 (1.13-2.20), P = .008 | 1.84 (1.04-3.25), P = .04 | 0.96 (0.66-1.39), P = .82 |
| Patient age/donor age, y | |||
| Age 18-54/donor age 10-29 | 1.00, P < .0001§ | 1.00, P = .001§ | 1.00, P = .28§ |
| Age 18-54/donor age 30-80 | 1.07 (0.79-1.44), P = .64 | 1.12 (0.67-1.86), P = .42 | 1.13 (0.82-1.57), P = .44 |
| Age 55-78/donor age 10-29 | 1.57 (1.09-2.26), P = .015 | 1.34 (0.76-2.56), P = .37 | 1.49 (0.99--2.24), P = .06 |
| Age 55-78/donor age 30-80 | 1.82 (1.38-2.39), P < .0001 | 2.09 (1.32-3.34), P = .002 | 1.24 (0.89-1.71), P = .19 |
Adjusted for recipient CMV seropositivity, disease risk index, and disease.
Adjusted for recipient CMV seropositivity and graft type.
‡Adjusted for disease risk index, disease and graft type.
This P value represents the level of significance for the overall Cox regression model. P values for paired comparisons within the model were considered significant only when the P value for the overall model was significant.
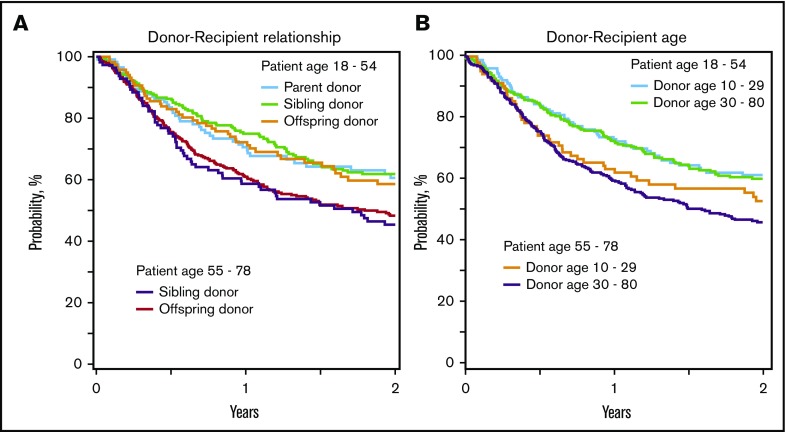
Figure 1.
Overall survival. (A) Overall survival by recipient age and donor-recipient relationship. The 2-year overall survival adjusted for recipient cytomegalovirus serostatus, disease, and DRI among recipients younger than 55 years was 61% (95% CI, 51%-69%) for parent donors, 62% (95% CI, 55%-68%) for sibling donors, and 59% (95% CI, 49%-67%) for offspring donors, and among recipients at least 55 years old, it was 45% (95% CI, 36%-54%) for sibling donors and 48% (95% CI, 43%-54%) for offspring donors. (B) Overall survival by recipient age and donor age. Two-year overall survival, adjusted for recipient cytomegalovirus serostatus, disease, and disease risk index among recipients younger than 55 years was 61% (95% CI, 53%-58%) with donors younger than 30 years and 60% (95% CI, 54%-66%) with donors at least 30 years old, and among recipients at least 55 years old, it was 53% (95% CI, 43%-62%) with donors younger than 30 years and 46% (95% CI, 41%-51%) with donors at least 30 years old.
Other factors associated with higher mortality included recipient CMV seropositivity (HR, 1.26; 95% CI, 1.04-1.53; P = .021) and intermediate disease risk index (HR, 1.65; 95% CI, 0.09-2.48; P = .017) and high/very high disease risk index (HR, 3.24; 95% CI, 2.09-5.01; P < .0001) compared with low disease risk index. Disease type also influenced mortality: Compared with AML, mortality was lower for acute lymphoblastic leukemia (HR, 0.68; 95% CI, 0.50-0.92; P = .017), myelodysplastic syndrome (HR, 0.71; 95% CI, 0.52-0.95; P = .022), and Hodgkin lymphoma (HR, 0.55; 95% CI, 0.35-0.87; P = .010), but not non-Hodgkin lymphoma (HR, 0.93, 95% CI, 0.73-1.19; P = .57). There were no differences in mortality risk by graft type (HR, 1.10; 95% CI 0.89-1.36; P = .37). The causes of death are shown in Table 3. Recurrent disease was the predominant cause of death.
Table 3.
Causes of death
| Recipient age 18-54 y | Recipient age 55-78 y | |||
|---|---|---|---|---|
| Donor age 10-29 y | Donor age 30-80 y | Donor age 10-29 y | Donor age 30-80 y | |
| Causes of death, n (%) | ||||
| Recurrent disease | 45 (67) | 84 (70) | 37 (70) | 119 (56) |
| Graft failure | 4 (6) | 1 (1) | __ | 2 (1) |
| GVHD | 2 (3) | 6 (5) | 1 (2) | 10 (5) |
| Infection | 5 (7) | 10 (8) | 4 (7) | 19 (9) |
| IPN/ARDS | 1 (2) | 4 (3) | 1 (2) | 4 (2) |
| Organ failure | 7 (10) | 11 (9) | 5 (9) | 25 (12) |
| Secondary malignancy | __ | 1 (1) | 1 (2) | 8 (4) |
| Hemorrhage | 1 (2) | __ | __ | 5 (2) |
| Other | __ | __ | 2 (4) | 4 (2) |
| Not reported | 2 (3) | 4 (3) | 2 (4) | 15 (7) |
ARDS, adult respiratory disease syndrome; IPN, interstitial pneumonitis.
Relapse and nonrelapse mortality
None of the donor characteristics studied except transplantation of peripheral blood was associated with relapse or nonrelapse mortality (Table 2). Relapse was lower (HR, 0.57; 95% CI, 0.44-0.74; P < .0001), and nonrelapse mortality was higher (HR, 1.45; 95% CI, 1.05-2.00; P = .024), after transplantation of peripheral blood, negating any benefits with regard to survival. In patients aged 55 to 78 years, nonrelapse mortality risks did not differ by whether they received grafts from a sibling vs offspring (HR, 0.78; 95% CI, 0.51-1.20; P = .25) or by donor age (HR, 1.56; 95% CI, 0.91-2.68; P = .11; Figure 2A-B). The trend was similar for relapse risks (HR, 1.14 [95% CI, 0.81-1.61; P = .45] and HR, 0.83 [95% CI, 0.59-1.18; P = .30], respectively. The 2-year incidence of relapse/progression in patients aged 18-54 years after transplantation of grafts from parents, siblings, or offspring and adjusted for disease, disease risk index, and graft type was 45% (95% CI, 35%-54%), 37% (95% CI, 30%-44%) and 30% (95% CI, 21%-38%), respectively. The corresponding incidences in patients aged 55 to 78 years after transplantation of grafts from siblings and offspring were 39% (95% CI, 30%-49%) and 43% (95% CI, 38%-49%), respectively. Disease and disease risk index were associated with relapse. Compared with AML, risks were lower for myelodysplastic syndrome (HR, 0.64; 95% CI, 0.45-0.91; P = .01) and Hodgkin lymphoma (HR, 0.49; 95% CI, 0.28-0.87; P = .015), but not non-Hodgkin lymphoma (HR, 0.83; 95% CI, 0.58-1.19; P = .30) or acute lymphoblastic leukemia (HR, 0.85; 95% CI, 0.63-1.14; P = .29). Compared with low disease risk index, risks were higher with intermediate (HR, 2.44; 95% CI, 1.34-4.45; P = .004) and high (HR, 4.33; 95% CI, 2.33-8.03; P < .0001) disease risk index.
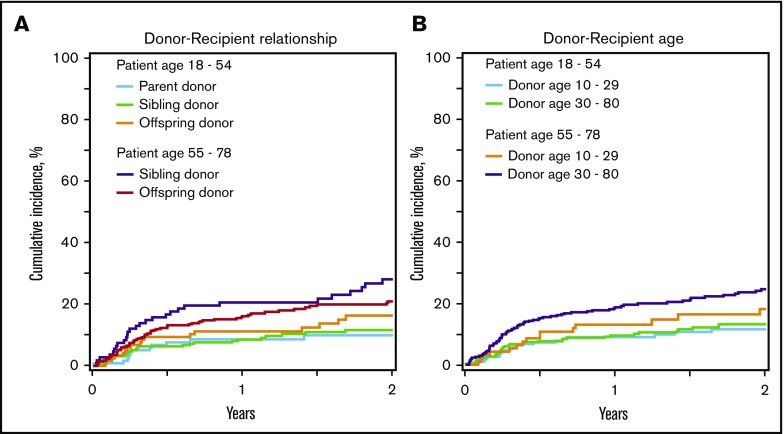
Figure 2.
Nonrelapse mortality. (A) Nonrelapse mortality by recipient age and donor-recipient relationship. The 2-year cumulative incidence of nonrelapse mortality adjusted for recipient cytomegalovirus serostatus and graft type among recipients younger than 55 years was 11% (95% CI, 6%-18%) for parent donors, 12% (95% CI, 8%-17%) for sibling donors, and 18% (95% CI, 11%-26%) for offspring donors, and among recipients at least 55 years old, it was 29% (95% CI, 20%-39%) for sibling donors and 21% (95% CI, 16%-26%) for offspring donors. (B) Nonrelapse mortality by recipient age and donor age. The 2-year cumulative incidence of nonrelapse mortality adjusted for recipient cytomegalovirus serostatus and graft type among recipients younger than 55 years was 13% (95% CI, 8%-19%) with donors younger than 30 years and 14% (95% CI, 10%-19%) with donors at least 30 years old, and among recipients at least 55 years old, it was 18% (95% CI, 11%-27%) with donors younger than 30 years and 25% (95% CI, 20%-30%) with donors at least 30 years old.
Graft failure
There were significant differences in graft failure risks by donor-patient relationship; graft failure was higher in patients who received grafts from their parents, but this was limited to patients aged 18 to 54 years, as donors in older patients were either siblings or offspring (Table 4; Figure 3A-B). Graft failure risks were not different between sibling and offspring donors (HR, 1.17; 95% CI, 0.49-2.79; P = .73) in patients aged 18 to 54 years and those aged 55 to 78 years (HR, 0.90; 95% CI, 0.48-1.69; P = .75). Use of bone marrow grafts did not differ by donor-recipient relationship, with bone marrow making up 69% of grafts from parents, 67% of grafts from siblings, and 69% of grafts from offspring (P = .78) donors. Graft failure did not differ by maternal vs paternal donors (14% [95% CI, 7%-23%] vs 15% [95% CI, 6%-27%]; P = .86).
Table 4.
Effect of patient age, donor-recipient relationship, and donor age on graft failure, acute and chronic GVHD
| Graft failure, hazard ratio (95% CI)* | Acute GVHD, hazard ratio (95% CI)† | Chronic GVHD, hazard ratio (95% CI)‡ | |
|---|---|---|---|
| Patient age, y/donor-recipient relationship | |||
| Age 18-54/parent donor | 1.00, P = .037§ | 1.00, P = .72§ | 1.00, P = .041§ |
| Age 18-54/sibling donor | 0.34 (0.16-0.70), P = .004 | 1.10 (0.77-1.59), P = .60 | 1.42 (0.93-2.17), P = .10 |
| Age 18-54/offspring donor | 0.39 (0.17-0.91), P = .029 | 0.84 (0.53-1.33), P = .46 | 1.18 (0.72-1.93), P = .52 |
| Age 55-78/sibling donor | 0.69 (0.33-1.46), P = .33 | 1.07 (0.67-1.69), P = .78 | 0.80 (0.46-1.39), P = .44 |
| Age 55-78/offspring donor | 0.62 (0.34-1.13), P = .12 | 0.98 (0.68-1.41), P = .93 | 0.70 (0.45-1.09), P = .12 |
| Patient age/donor age, y | |||
| Age 18-54/donor age 10-29 | 1.00, P = .10§ | 1.00, P = .24§ | 1.00, P = .001§ |
| Age 18-54/donor age 30-80 | 0.95 (0.40-2.34), P = .89 | 1.14 (0.83-1.57), P = .41 | 0.93 (0.67-1.29), P = .66 |
| Age 55-78/donor age 10-29 | 2.49 (1.11-5.56), P = .03 | 0.77 (0.48-1.24), P = .28 | 0.53 (0.31-0.92), P = .02 |
| Age 55-78/donor age 30-80 | 1.67 (0.86-3.26), P = .13 | 1.17 (0.85-1.60), P = .33 | 0.55 (0.38-0.80), P = .002 |
Adjusted for disease.
Adjusted for graft type.
‡Adjusted for graft type.
This P value represents the level of significance for the overall Fine and Gray model. P values for paired comparisons within the model were considered significant only when the P value for the overall model was significant.
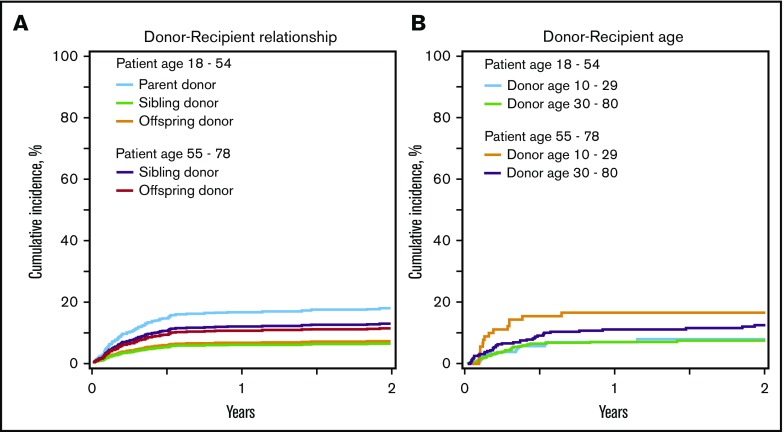
Figure 3.
Graft failure. (A) Graft failure by recipient age and donor-recipient relationship. Two-year cumulative incidence of graft failure adjusted for disease among recipients younger than 55 years was 17% (95% CI, 11%-26%) for parent donors, 6% (95% CI, 4%-10%) for sibling donors, and 7% (95% CI, 3%-13%) for offspring donors, and among recipients at least 55 years old, it was 13% (95% CI, 7%-20%) for sibling donors and 11% (95% CI, 8%-15%) for offspring donors. (B) Graft failure by recipient age and donor age. The 2-year cumulative incidence of graft failure adjusted for disease among recipients younger than 55 years was 7% (95% CI, 4%-12%) with donors younger than 30 years and 8% (95% CI, 5%-13%) with donors at least 30 years old, and among recipients at least 55 years old, it was 17% (95% CI, 9%-26%) with donors younger than 30 years and 13% (95% CI, 8%-18%) with donors at least 30 years old.
GVHD
None of the donor characteristics studied except graft type was associated with grade II-IV acute or chronic GVHD (Table 4). Risk for grade II-IV acute GVHD (HR, 1.57; 95% CI, 1.25-1.97; P = .0001) was higher with transplantation of peripheral blood. The 6-month incidence of grade II-IV acute GVHD after transplantation of peripheral blood and bone marrow were 41% (95% CI, 35%-47%) and 29% (95% CI, 26%-33%), respectively. Similarly, chronic GVHD risks were higher after transplantation of peripheral blood (HR, 2.46; 95% CI, 1.89-3.20; P < .0001). The 2-year incidences of chronic GVHD after transplantation of peripheral blood and bone marrow were 44% (95% CI, 38%-50%) and 19% (95% CI, 16%-22%), respectively. Among patients with chronic GVHD, 193 of 227 had severity grading reported: 65% were mild, 24% moderate, and 11% severe. GVHD severity did not differ by graft type (P = .83).
Discussion
The increasing application of T-cell-replete haplo transplantation with PT-Cy motivated us to study the effect of non-HLA donor characteristics on transplantation outcomes, adjusting for patient, disease, and transplantation characteristics. Our findings suggest that in adults, patient and disease characteristics are more important than either the age of the donor or donor-recipient relationship with regard to survival and GVHD. In young adults, transplantation of grafts from a parent was associated with higher graft failure rate. Although a thorough examination of immune mediated effects of shared maternal vs paternal antigens9,19 or priming/tolerizing immune effects of pregnancy in maternal donors20-23 is beyond the scope of our study, there were no differences in graft failure rates between maternal and paternal donors. Although siblings were older than offspring donors (44 vs 31 years; P < .0001), we did not observe an effect of donor age or donor-recipient relationship on graft failure. The higher risks for acute and chronic GVHD and the absence of a survival advantage with peripheral blood suggest that with the PT-Cy approach for haplo transplantation, peripheral blood should be reserved for patients at high risk for disease relapse.20 We observed lower chronic GVHD risks in patients aged 55 to 78 years, independent of donor age, and hypothesize that this is mitigated by higher early mortality in older patients. The rate of early mortality was higher in patients aged 55 to 78 years: it was 25% at 6 months and 40% at 1 year. The corresponding rates in younger patients were 15% and 26%.
All donor-recipient pairs in our study were mismatched at 2 or more HLA-loci; this, together with a modest sample size of 928 donor-recipient pairs, prevented us from exploring the effect of HLA disparity on transplant outcomes. To date, there are no reports that increasing HLA disparity is associated with transplant outcomes after T-cell-replete haplo transplantation with PT-Cy8 or antithymocyte globulin-based immunosuppression9, but these observations were also based on studies of modest numbers of donor-recipient pairs. There is broad agreement that presence of donor-specific antibodies in the recipient is associated with graft failure.21 The variability and thresholds that determine desensitization for patients with donor-specific antibodies vary between laboratories and centers and are best studied at individual centers to establish center-specific thresholds for desensitization.
Other donor characteristics such as sex, parity, CMV seropositivity, and blood group ABO match were not associated with transplant outcomes. Others have also shown the absence of an effect of either donor sex when maternal donors are excluded in the setting of haplo transplantation9,10 and ABO match in the setting of T-cell-replete haplo transplantation with PT-Cy2 on transplant outcomes. However, an EBMT report that included both T-cell-depleted and T-cell-replete haplo transplants demonstrated that acute GVHD risks were higher with bidirectional ABO mismatching only.22 In that report, other outcomes did not differ by ABO mismatching.
With regard to the effect of donor-recipient relationship and donor age, our observations differ from that reported by Wang and colleagues, who studied T-cell-replete haplo transplantation using intensive immune suppression.9 The observed differences may be attributed to the distinct strategies employed for haplo transplantation (intense immune suppression vs PT-Cy) and/or differences in the study population. Although both studies had a similar proportion of donors younger than 30 years compared with the Wang report, our population was older (median age, 54 vs 25 years) and had fewer transplants with parent donors (13% vs 59%). Consistent with the Wang report,9 we also observed higher mortality with donors aged at least 30 years (HR, 1.39; 95% CI, 1.12-1.73; P = .003), which also demonstrates that our study population was adequately powered to detect survival differences. However, when we considered patient and disease characteristics such as age, disease, and disease risk index, donor age was no longer significant. This implies that, in a predominantly older population, patient and disease characteristics are more important predictors for survival. Consistent with another report on patients older than 50 years who underwent haplo transplantation with PT-Cy, we also did not observe differences in survival between patients aged 60 to 69 years and those aged 70 to 78 years.23
Our relatively modest sample of 928 donor-recipient pairs prevented us from studying the effect of HLA disparity, including KIR ligand match status, as definitive studies on HLA matching for adult unrelated donor transplantation have required several thousands of donor-recipient pairs.24-26 Others have shown a noninherited maternal antigen effect with mismatched sibling donor transplantation,9,27 which we were unable to study, as parental donors only constituted 13% of donors, and detailed HLA typings of parents were not available. Similarly, others report higher graft failure when the recipient harbors donor-specific antibodies, which we were unable to study.21,28,29 Although transplantations occurred at 65 centers, we did not see differences in survival by transplant center (P = .98).30 Although our study is the largest of T-cell-replete haplo transplantation with PT-Cy in adults with hematologic malignancy, we only studied the effect of non-HLA donor characteristics on transplantation outcomes. In this context, survival is largely determined by patient and disease characteristics, rather than donor age or donor-recipient relationship. Higher graft failure with parental donor transplants merits further exploration.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Institutes of Health, National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; 5U10HL069294 from the National Institutes of Health, National Heart, Lung and Blood Institute and the National Cancer Institute; contract HHSH250201200016C with the Health Resources and Services Administration; and grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: S.R.M., M.-J.Z., A.S.M., E.J.F., and M.E. designed the study; A.S.M. and D.A.K. prepared the stud file for analyses; M.-J.Z. and A.S.M. analyzed the data; S.R.M., M.-J.Z., A.S.M., D.A.K., E.J.F., and M.E. summarized and interpreted the findings; S.R.M. drafted the manuscript; E.J.F. and M.E. critically reviewed the first draft of the manuscript; M.-J.Z., A.S.M., M.M.A.M., A.B., S.G., D.A.K., M.H., M.N., M.A.-P., R. Reshef, V.R., R. Romee, M.S., A.U.-I., and E.K.W. critically reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, the Center for International Blood and Marrow Transplant, Department of Medicine, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.
References
- 1.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunstein CG, Fuchs EJ, Carter SL, et al. ; Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31(10):1310-1316. [DOI] [PubMed] [Google Scholar]
- 5.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCurdy SR, Fuchs EJ. Selecting the best haploidentical donor. Semin Hematol. 2016;53(4):246-251. [DOI] [PubMed] [Google Scholar]
- 8.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Chang YJ, Xu LP, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014;124(6):843-850. [DOI] [PubMed] [Google Scholar]
- 10.Stern M, Ruggeri L, Mancusi A, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112(7):2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48(4):537-543. [DOI] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 13.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204-217. [DOI] [PubMed] [Google Scholar]
- 14.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. [DOI] [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187-220. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)–specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamaki S, Ichinohe T, Matsuo K, Hamajima N, Hirabayashi N, Dohy H; Japan Society of Hematopoietic Cell Transplantation. Superior survival of blood and marrow stem cell recipients given maternal grafts over recipients given paternal grafts. Bone Marrow Transplant. 2001;28(4):375-380. [DOI] [PubMed] [Google Scholar]
- 20.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35(26):3002-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canaani J, Savani BN, Labopin M, et al. Impact of ABO incompatibility on patients’ outcome after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia - a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017;102(6):1066-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104(7):1923-1930. [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 26.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rood JJ, Loberiza FR Jr, Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99(5):1572-1577. [DOI] [PubMed] [Google Scholar]
- 28.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839-2846. [DOI] [PubMed] [Google Scholar]
- 30.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489-1500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.