Abstract
Purpose of review
To update advances in identifying factors affecting magnesium (Mg) status that assist in providing improved evidence-based clinical decision-making for assessing Mg status.
Recent findings
Findings from recent cohort studies, small randomized control trials, and multiple meta-analyses reinforce earlier work that serum Mg concentrations, urinary Mg excretion, and Mg dietary intakes are inversely associated with cardiovascular disease, chronic kidney disease, and diabetes. These studies indicate that the reference range for serum Mg needs updating, and that individuals with serum Mg in the range of 0.75–0.85 mmol/L and displaying changes in other factors associated with a low Mg status may be Mg deficient. Individuals with serum Mg concentrations below this range most likely are Mg deficient, and above this range, are most likely Mg sufficient.
Summary
The combined determination of serum Mg concentration, 24-hr urinary Mg excretion, and dietary Mg intake is currently the most practical method to obtain a sound assessment of Mg status. The strong correlations of Mg deficiency with increased risk of several chronic diseases, some of which exist as co-morbidities, indicate that Mg status should be ascertained in subjects presenting such pathology.
Keywords: Magnesium status, hypomagnesmia, chronic disease, risk factors
INTRODUCTION
Magnesium (Mg) is a cofactor for over 600 enzymatic reactions vital to metabolic pathways including DNA, RNA, protein and adenosine-5′-triphosphate synthesis; cellular energy production and storage; glycolysis; and cellular second messenger systems. Because it has so many critical functions, body Mg is well regulated through three main mechanisms: (i) absorption through the gastrointestinal tract, (ii) renal excretion after filtration-reabsorption, and (iii) exchange from the large pool in bone [1]. During low intakes of Mg, the percent absorbed from the diet is increased, the amount excreted in the urine is decreased, and bone reserves are used. When dietary intakes are adequate, the opposite occurs.
The strong response of the body to maintain Mg homeostasis has made it difficult to establish status indicators for Mg. In addition, mild and moderate Mg deficiencies (subclinical Mg deficiency) are mostly asymptomatic [2*]. As a result, subclinical Mg deficiency has not been routinely recognized in healthy population groups. Nonetheless, relatively low Mg intakes, serum Mg concentrations, and urinary Mg excretions have been associated with critical health issues, including cardiovascular disease [3, 4, 5*, 6], type 2 diabetes [7*, 8, 9], pre-diabetes [10*], and chronic kidney disease [11, 12]. These associations often did not correlate with a Mg deficiency as defined by the currently designated serum clinical reference range. The lack of correlation raises the question about the ability to assess Mg status to determine the presence of subclinical Mg deficiency. The lack justifies a review of current research to determine whether there is an improved method for evaluating Mg status, and a need for a revised clinical reference range for serum Mg
Measures to assess Mg status
A review of Mg assessment measures and their utility are described in Table 1 and those with clinical utility are the focus of this article.
Table 1.
Magnesium assessment measures and their utility1
| Biomarker | Type | Use | Utility | Factors to be considered | Assessment Methods |
|---|---|---|---|---|---|
| Dietary Mg | Exposure | Individual Population | Inadequate intake of dietary Mg contributes to etiology of Mg deficiency | Impacted by diets high in phytates, calcium, and phosphorus | Requires data on intake of phytates, calcium and possibly phosphorus as well as food preparation methods and extent of consumption of refined grains. Diet history and FFQ should be used to align with individuals; 24 hr recall and weighed or estimated diet records should be aligned with populations. |
| Serum / Plasma Mg Concentration | Exposure Status (deficiency or excess) | Individual Population | Responds to Mg restriction, wasting or supplementation. Generally not influenced by age or sex. | Feasible and inexpensive. Not reflective of total body stores. Impacted by time of day (circadian rhythm), inflammation, poor or compromised renal function, poorly controlled diabetes, drugs (diuretics, cancer therapies; PPIs). Hypoalbuminemia may lead to spuriously low magnesium values. | Assay Method of Choice: Atomic Absorption Spectrophotometry. Acceptable methods: Colorimetric is used by the majority of clinical laboratories; enzymatic also acceptable. NIST standard available Reference Range 0.75–0.95 mmol/L |
| 24 hr Urinary Mg Excretion | Exposure Status (deficiency or excess) | Individual Population | Responds to Mg restriction, wasting or supplementation. | Considerable subject burden. Susceptible to incomplete collection Impacted by time of day (circadian rhythm), inflammation, poor or compromised renal function, poorly controlled diabetes, drugs (diuretics) In adults, appears to be an effective biomarker. | Assay Method of Choice: Atomic Absorption Spectrophotometry. In presence of hypomagnesemia urinary Mg excretion <12 mg (0.5 mmol) is consistent with intact renal response and urinary Mg >24 mg (1 mmol) excretion indicates abnormal renal wasting. Reference Range: 3.0–5.0 mmol/24 hr |
| Fractional Excretion of Mg (FEMg) | Exposure Status for deficiency | Individual | Used to distinguish between GI and renal Mg losses | Impacted by time of day (circadian rhythm), inflammation, poor or compromised renal function, poorly controlled diabetes, drugs (diuretics, cancer therapies). | FEMg = [uMg × sCr)/sMg × uCr×0.7] × 100. U=urine, s=serum, Cr = creatinine. FEMg >2% indicates inappropriate renal wasting. FEMg <2% indicates appropriate renal handling. |
| Red Cell Mg Concentration | Exposure Status (deficiency or excess) | Individual | Responds to Mg restriction, wasting or supplementation. May be more accurate than serum measures. | Care must be taken not to hemolyze blood sample Unknown if reflective of body stores. | Assay Method of Choice: Atomic Absorption Spectrophotometry. Reference Range 1.65–2.65 mmol/L Test is not commercially available |
| Blood mononuclear cell Mg concentration | Exposure status for deficiency | Individual | Has been used in experimental human Mg deficiency and in patient populations. | May be more accurate than serum measures. | Test is not commercially available. |
| Serum Ionized Mg concentration | Exposure (deficiency or excess) | Individual | Responds to severe Mg restriction, wasting or supplementation. | Ion selective electrode probes susceptible to interference by calcium, lipophilic cations and thiocyanate in the blood. Unknown if reflective of body stores |
Ion selective electrodes can measure ionized Mg (70% of total Mg) in serum, plasma, and whole blood. Reference Range: 0.55–0.75 mmol/L. |
| Buccal cell Mg concentration | Exposure (deficiency or excess) | Individual | Relatively new. Few data from randomized trials | Unknown if reflective of body stores | Assay method yet to be validated. Commercial kits are available. |
| Mg Load Retention Test | Exposure (deficiency) | Individual | Based on 24-hr urine collection and ability to administer IV Mg Sulfate. | Invasive and cumbersome. Values correlate well with bone magnesium but have yet to be shown to correlate with other magnesium measures – such as serum or plasma blood levels. | Standard protocols exist. Based on amount of Mg retained post infusion of 15 mmol over 4 hour period. <20% retention – normal Mg status >20 and <50% - borderline deficient Mg status >50% - deficient Mg status |
Table compiled from Witkowski, Hubert, and Mazur, 2011 [13].
Serum Mg
A review of recent literature indicates that total serum Mg concentration is the predominant, but unreliable, clinical test used to assess Mg status. The reference interval used for total serum Mg concentration was determined by measuring Mg in 15,820 “healthy normal individuals” aged 18–74 years in the 1974 National Health and Nutrition Examination Survey 1 (NHANES 1). The central 95th percentile was established as the “normal” range for Mg, which resulted in a reference interval of 0.75–0.95 mmol/L [13]. This reference interval likely is not reflective of environmental conditions and eating habits of Americans today. For example, Mg intakes apparently have decreased because of such factors as lower amounts of Mg in processed foods and “fast foods” and a lower consumption of whole grains, and fruits and vegetables. In addition, over 99% of total body Mg (22 to 26 g for an adult) is extravascular; mostly in the bones (>50%), and <1% in the serum [1] [Figure 1]. Thus, serum Mg may not necessarily reflect true total body Mg content. This conclusion is supported by controlled metabolic unit studies with postmenopausal women that found serum Mg concentrations responded slowly (several weeks) with changes from deficiency to adequacy, or vice versa [14*]. Zhang and colleagues [15**] used a meta-analysis of 48 RCTs enrolling 2,131 subjects to quantify the overall responsiveness of serum Mg to oral Mg supplementation ranging from 197 to 994 mg/day for periods ranging from three weeks to five years (median 12 weeks). Significant dose and time responses of serum Mg concentration to oral Mg supplementation increased gradually until reaching a steady state at 300 mg/day after approximately 20 weeks (P-nonlinearity <0.001). High baseline serum Mg concentrations were associated with less or no changes in serum Mg. A recent extensive review found that serum Mg in relation with risk factors for chronic disease such as glucose intolerance, inflammation, and elevated blood pressure indicated that many individuals had what has been termed chronic latent Mg deficiency as defined by serum concentrations in the range of 0.75–0.85 mmol/L [2*]. These findings indicate that many physicians may assume that patients have “normal” Mg status when they may actually have chronic or subclinical deficiency.
Figure 1.
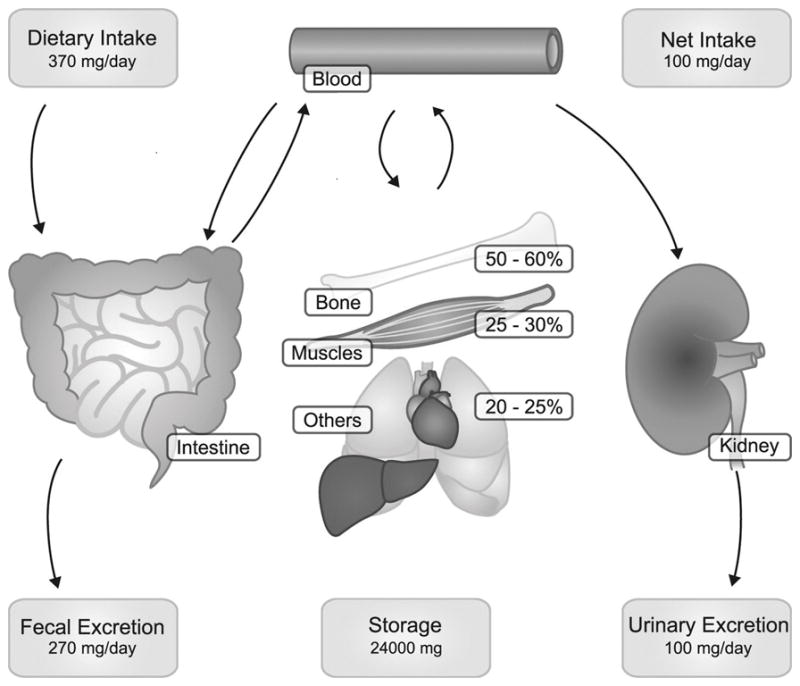
Magnesium homoeostasis. Panels represent the daily amount of Mg2+ intake and excretion. Daily the intestines absorb ≈120 mg and secrete 20 mg of Mg2+, resulting in a net absorption of 100 mg. In the kidney daily ≈2,400 mg Mg2+ is filtered by the glomerulus, of which 2,300 mg is reabsorbed along the kidney tubule. This results in a net excretion of 100 mg, which matches the intestinal absorption. Bone and muscle provide the most important Mg2+ stores. From de Baaji, Hoenderop, and Bindels, 2015 [1].
Dietary Mg
Dietary Mg also is used as a measure of Mg status. In the United States and Canada, Mg dietary reference intakes (DRIs) were set in 1997. For adults aged 19–30 years, and 31–70 years, respectively, recommended dietary allowances (RDAs) of 310 and 320 mg/d for women and 410 and 420 mg/d for men were set; estimated average requirements (EARs; average daily level of intake estimated to meet the requirements of 50% of healthy individuals) of 255 and 265 mg/d for women and 330 and 350 mg/d for men were set [16]. Controlled metabolic unit studies suggest that these DRIs need modification and that the DRIs vary with body weight [14*]. The studies indicated that the RDA and EAR may be 250 (or 3.57 mg/kg) and 175 mg/day (or 2.5 mg/kg), respectively, for a healthy 70-kg individual. In 2010, the average weights of American women and men were 166.2 and 195.5 (75.54 and 88.9 kg), respectively [17]. Basing the DRIs on body weight would result in an average EAR and RDA for women and men of 189 and 270 and 222 and 317 mg/day, respectively. Close to half of the US population has been shown to consume less than the daily requirement (1997 EAR) of Mg from foods [18*]. Table 2 provides a list of high Mg food sources.
TABLE 2.
Magnesium Content of Selected Foods1
| Food | Magnesium mg/serving |
|---|---|
| Almonds, dry roasted, 1 ounce | 80 (20% DV) |
| Spinach, boiled, ½ cup | 78 |
| Chocolate, dark, 70–85% cacao solids, 1 oz | 65 |
| Peanuts, oil roasted, ¼ cup | 63 |
| Oatmeal, regular and quick, cooked, 1 cup | 63 |
| Soymilk, plain or vanilla, 1 cup | 61 |
| Black beans, cooked, ½ cup | 60 |
| Peanut Butter, smooth, 2 TBSP | 54 |
| Edamame, shelled, cooked, ½ cup | 50 |
| Bread, whole wheat, 2 slices | 48 |
| Potato, baked with skin, 1 medium, 173 g | 48 |
| Avocado, cubed, 1 cup | 44 |
| Rice, brown, cooked, ½ cup | 42 |
| Soymilk, original and vanilla with added calcium, vitamins A&D, 1 cup | 36 |
| Milk, reduced fat, 2% milk fat, 1 cup | 34 |
| Cereal, cheerios, 1 cup | 32 |
| Banana, 1 medium, 118g | 32 |
| Yogurt, plain, low fat, 8 oz’ | 27 |
| Kale, cooked, boiled, 1 cup | 23 (6% DV) |
Source: USDA, National Nutrient Database for Standard Reference, Release 28 [19].
DV=Daily Value
The efficiency of a Mg uptake after ingestion is affected by its chemical form, when given as a supplement and by dietary factors that can enhance (lactose, fructose, and glucose) or inhibit absorption (fiber, free fatty acids, oxalate, phytate, and high levels of zinc). Also, net Mg absorption increases with increasing Mg intake; however fractional Mg absorption falls. Humans studies using diets ranging from low to high have found absorption to range from 35% to 70%. However, with normal intakes, 30% to 40% of dietary Mg is absorbed [16].
Urinary Mg
Although no standard has been set for urinary Mg excretion indicating a deficiency, controlled metabolic experiments indicate 40 to 80 mg (1.65 to 3.29 mmol) is the range of daily Mg excretion when Mg intakes are <250 mg/day, and 80 to 160 mg is the daily range when intakes are >250 mg/day independent of gender [14*]. However, it was found that urinary Mg excretion changed rapidly between these two ranges (within a few days) upon changing from below to equal or above the current RDA, or vice versa. This rapid change indicates that a single determination of 24-hour urinary Mg would be best used to support other Mg status indicators, or in the evaluation of Mg status of population groups. Analysis of datasets from subsets the Nurses’ Health Study I and II and the Health Professionals Follow-up Study found that for the population 24-hour urinary excretion of Mg, among other urinary biomarkers measured, was stable upon repeated measurements within one year. The investigators calculated intraclass correlation coefficients (ICC) of urinary magnesium in multiple 24-hour urine samples to evaluate reproducibility. They further calculated a reliability index (RI; the mean of a very large number of repeat measurements) to further evaluate reproducibility. For magnesium excretion the ICC was found to be ≥ 0.70 with an RI of ≥ 0.80. Thus these investigators suggest that biomarkers such as urinary magnesium measured on several timepoints are potentially useful for the assessment of various nutritional and environmental exposures in epidemiologic studies [19**].
Mg status indicators and their association with cardiovascular diseases
In the vast majority of prospective and cross-sectional studies, serum, urinary, and dietary Mg have been inversely associated with hypertension and blood pressure. This is exemplified by a recent systematic review and meta-analysis of prospective cohort studies that found a dose-response between dietary Mg and hypertension with intakes below 250 mg/day by visual inspection of the displayed figure [20]. A longitudinal analysis for of heart failure diagnosis in the Jackson Heart Study cohort involving 4,916 African Americans free of heart failure but with other co-morbidities found the median gap from the baseline visit to initiation of heart failure was 1,837 days with 270 incident cases being reported. Participants in the first quartile of Mg intake (0.52 – 2.30 mg/day/kg body wt approx.181 mg/day) had an increased risk of heart failure hospitalization compared to those in the remaining quartiles (>2.30 mg/day/kg body wt) [HR of 0.66 and a 95% CI of 0.47 to 0.94]. In the Atherosclerosis Risk in Communities Study, a low serum Mg (<70 mmol/L) was associated with a 70% higher risk of incident heart failure [21]. An extensive review of cohort studies of cardiovascular disease outcomes found that the risk of mortality and morbidity of several cardiovascular diseases decreased as Mg status markers improved (dietary intake >250 mg/day; serum concentration >0.75 mmol/L; urinary excretion >100 mg/day) [2*].
Over 30 randomized controlled trials using a range of formulations and doses of oral Mg in normotensives and hypertensives provides a rich source of data on serum and urinary Mg with changes in blood pressure. As noted for the cohort studies, these studies similarly show increases in serum or urinary Mg with oral Mg supplementation along with variable results on blood pressure. In the meta-analysis by Zhang and colleagues [3], after a median dose of 368 mg/day for a median duration of 3 months in 2028 subjects, SBP was reduced by 2.0 mmHg (95% CI 0.43 to 3.58) and DBP by 1.78 mmHg (95% CI 0.73 to 2.82) with a noted rise of 0.05 mmol/L in serum Mg. A more targeted meta-analysis that included 11 studies (543 subjects) enrolling individuals with insulin resistance, prediabetes, or noncommunicable chronic disease found that Mg supplementation (365 – 450 mg/day) for a median of 3.6 months resulted in a mean reduction in SBP of 4.18 mmHg (standardized mean difference, −0.20; 95% CI −0.37 to −0.03) and 2.27 mmHg in DBP (−0.29; 95% CI −0.46 to −0.12) [22]. The authors of this meta-analysis concluded that the magnitude of blood pressure reduction is of great clinical significance when applied at the public health level.
Mg status indicators and their association with the metabolic syndrome and diabetes
Magnesium deficiency is not only a feature of diabetes but also frequently observed in individuals with prediabetes and/or the metabolic syndrome. Most recent support of this relationship is provided by the Canadian Health Measures Survey cycle 3 conducted between 2012 and 2013 that determined serum Mg concentrations in subjects aged 3–79 years. Type I or type 2 diabetes was associated with 0.04 to 0.07 mmol/L lower serum Mg compared to not having diabetes. Serum Mg concentration was negatively associated with diabetes, BMI, serum glucose, serum insulin, HbA1c, and HOMA-IR [23**]. This study also found substantial proportions of the adult sex-age groups (9.5%–16.6%) and adolescents 12–19 years (15.8–21.8%) had serum Mg concentrations <0.75 mmol/L, currently accepted as an indication of Mg deficiency.
Another recent systematic review and meta-analysis of observational studies found a less robust association between serum Mg and the metabolic syndrome (mean difference: −0.19; 95% CI −0.36 to −0.03; P=0.023) [24*]. However, increased Mg intake was highly associated with a lower risk of the metabolic syndrome (OR: 0.73, 95% CI 0.62 to 0.86; P<0.001).
An analysis of data from 8,555 participants enrolled in the population-based Rotterdam Study found, in the fully adjusted statistical model, that participants with hypomagnesaemia (not defined) had an increased risk of developing diabetes over a median follow-up of 5.7 years (HR of 1.79 and a 95% CI of 1.16 to 2.77). Hypomagnesemic subjects were at risk for developing prediabetes over a median follow-up of 5.7 years, which was not statistically significant in fully adjusted models. A 0.1 mmol/L decrease in serum Mg concentrations was associated with an increased risk of both prediabetes and diabetes. This analysis also found that common genetic variants in magnesium-regulating genes influenced diabetes risk and that the risk was mediated through serum Mg [10*] with the authors suggesting a potential causal role of Mg in the development of diabetes.
Analysis of dietary Mg intake data from 25 prospective cohort studies involving 637,922 subjects with 26,828 incident cases of type 2 diabetes found, after adjusting for age and body mass index, the risk of developing diabetes was reduced by 8–13% per 100 mg/day increment in dietary Mg intake [7*].
Mg status indicators and their association with bone health
Mg totals about 1.0% and is in constant equilibrium with serum Mg and plays an important role in bone and mineral homeostasis. Deficient Mg can directly affect bone by altering the structure of apatite crystals. Mg deficiency is also associated with reductions of PTH and 1,25(OH2) D levels and inflammation, all of which can lead to bone loss.
Both experimental and epidemiological studies suggest that both low and high serum Mg concentrations are associated with changes in bone health [25]. Postmenopausal women with osteoporosis are noted to have consistently lower serum Mg concentrations across studies than their healthy controls. Men, but not women, enrolled in the European Prospective Investigation into Cancer and Nutrition-Norfolk cohort showed significant inverse trends in fracture risk across serum Mg concentration groups for spine fractures (P=0.02) and total hip, spine, and wrist fractures (P=0.02). The mean serum Mg for men was 0.81 ± 0.12 mmol/L [26]. Similarly, for 2245 men aged 42–61 participating in the Kuopio Ischemic Heart Disease prospective cohort study found that low serum Mg levels with comparable dietary intakes were strongly and independently associated (HR 2.10, 0.74 mmol/L bottom quartile of serum Mg to top quartile, 0.89 mmol/L; 95% CI 1.30 to 3.41) with increased risk of total and femoral fractures [27].
The effect of Mg intake on bone indices such as bone mineral density (BMD) is unclear and the data are mixed. Magnesium intake was evaluated with respect to BMD and fractures, measured at multiple sites, in a systematic review of 24 studies that included both males and females (118,664 subjects 0.8 mos. to 67.7 yrs) and in an accompanying meta-analysis that included 12 studies. These authors found that high intakes of Mg (not defined) were not significantly associated with an increased risk of hip (summary effect size 1.92; 95% CI 0.81 to 4.55) and total fractures (1.01; 0.94 to 1.07). However, a high degree of heterogeneity was present in this small subgroup dataset. Additionally, they noted a positive and marginally significant correlation between Mg intake and femoral neck and total hip BMD based on 9 studies.
Mg status indicators and their association with chronic kidney disease
Hypomagnesemia has been shown to be an emerging risk factor for incident chronic kidney disease [11, 12]. Dietary intake of Mg was associated with a rapid decline in kidney function independent of multiple kidney disease risk factors as documented in The Healthy Aging in Neighborhoods of Diversity across the Life Span Study enrolling 3,720 healthy adults (estimated GFR >60 ml/min/1.73m2). Magnesium intakes ranged from 46.9 mg/1000 kcal in tertile 1 to a high of 468.2 mg/1,000 kcal in tertile 3. After a median of 5 years of follow-up, participants in the lowest tertile [OR for tertile 1 vs 3: 2.02, 95% CI 1.05 to 3.86, p=0.02 in fully adjusted model) of dietary Mg intake had a 2-fold greater odds of rapid decline in kidney function [28**].
The finding that Mg affects kidney function decline suggests that a kidney function measurement may be useful in assessing Mg status. For example, urinary fibroblast growth factor-23 (FGF-23) might be such an indicator. FGF-23 is a potent regulator of vitamin D and phosphate metabolism. In a cross-sectional study which pooled three populations of young adults (n=1411) with normal kidney function and of African ancestry, but from diverse geographical regions, a linear regression analysis showed that after adjusting for covariates and calorie-adjusted dietary factors, a significant trend of increased serum FGF-23 levels occurred with decreasing quartiles of Mg intake (P<0.001) [29**].
Low serum Mg concentrations with FGF-23 dysregulation also have been deemed as a risk factor for cardiovascular mortality in diabetic patients with mild-to-moderate chronic kidney disease because these variables were associated with increased mitral valve calcification and intima media thickness [30*]. Additionally, decreased serum Mg and FGF-23 concentrations were significantly and independently associated with higher pulse pressure in pre-dialysis diabetic patients with chronic kidney disease [31].
Summary
Magnesium has been characterized as a shortfall nutrient of public health concern in America [18*]. Data from prospective cohort studies and small focused randomized controlled trials show strong correlation of Mg deficiency with increased risk of several chronic diseases, some of which exist as co-morbidities. The data are convincing that there may be a relationship, but we do not know the type of relationship. The papers linking low Mg to disease in this review are all associative data (except for blood pressure). What is needed to advance the field are Mg supplementation studies to test whether the association is causal. However, a simple, rapid, and reliable single measurement to determine the presence of Mg deficiency is lacking. Each of the three most commonly used indicators of magnesium status has shortcomings. The serum reference range is questionable in individuals with Mg concentrations between 0.75 and 0.85 mmol/L as possibly deficient. Dietary Reference Intakes apparently need revision and likely change with body weight. Urinary Mg responds too quickly to changes in dietary Mg intakes. However, because each status indicator can be strengthened by the addition of one or both of the other indicators, a combination of all three indicators would be the most practical method to assess Mg status.
Conclusion
At present, a combined determination of serum Mg concentration and urinary Mg excretion along with a dietary Mg history seems to be the propitious choice to get a valid assessment of magnesium status. Magnesium deficiency should be considered as contributing to some chronic diseases and associated morbidities if subjects exhibit a combined serum Mg <0.85 mmol/L; urinary Mg excretion <80.0 mg/day; and/ Mg intakes <250 mg/day.
Key Points.
A simple, rapid, and accurate single measurement to assess total body Mg status is lacking.
A combination of serum, urinary, and dietary Mg may the most practical method to assess Mg status at present.
Improved, evidence based Mg DRIs and serum reference range are needed.
Magnesium is a shortfall nutrient of public health concern.
Assessment of Mg status is important because deficiency has an impact on multiple chronic diseases, morbidity and mortality
Acknowledgments
None
Financial support and sponsorship: RBC (scientific consultant). This work was supported by the Office of Dietary Supplements of the National Institutes of Health, Department of Health and Human Services. FHN - None
Abbreviations
- Mg
magnesium
- RDA
recommended dietary allowance
- DRI
dietary reference intake
- EAR
estimated average requirement
Footnotes
Conflicts of Interest: RBC–Ad Hoc Consultant, RMJHoldings Biotech firm; FHN-None
All authors read and approved the final manuscript.
References
- 1.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 2*.Costello RB, Elin RJ, Rosanoff A, Wallace TC, Guerrero-Romero F, Hruby A, et al. Perspective: the case for an evidence-based reference interval for serum magnesium: the time has come. Adv Nutr. 2016;7(6):977–93. doi: 10.3945/an.116.012765. This article provides an extensive review of serum Mg values across an array of population groups and its association with chronic disease risk factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, et al. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68(2):324–33. doi: 10.1161/HYPERTENSIONAHA.116.07664. [DOI] [PubMed] [Google Scholar]
- 4.Posadas-Sanchez R, Posadas-Romero C, Cardoso-Saldana G, Vargas-Alarcon G, Villarreal-Molina MT, Perez-Hernandez N, et al. Serum magnesium is inversely associated with coronary artery calcification in the Genetics of Atherosclerotic Disease (GEA) study. Nutr J. 2016;15:22. doi: 10.1186/s12937-016-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Taveira TH, Ouellette D, Gulum A, Choudhary G, Eaton CB, Liu S, et al. Relations of magnesium intake with cardiac function and heart failure hospitalizations in African American adults: the Jackson Heart Study. Circ Heart Fail. 2016;9(4):e002698. doi: 10.1161/CIRCHEARTFAILURE.115.002698. This analysis of longitudinal study data found an increased risk of heart failure admissions for those with intakes at or less than 181 mg/d compared to those with intakes greater than 181 mg/d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Wang K, Han D, He X, Wei J, Zhao L, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210. doi: 10.1186/s12916-016-0742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Fang X, Han H, Li M, Liang C, Fan Z, Aaseth J, et al. Dose-response relationship between dietary magnesium intake and risk of type 2 diabetes mellitus: a systematic review and meta-regression analysis of prospective cohort studies. Nutrients. 2016;8(11):E739. doi: 10.3390/nu8110739. This paper provides a comprehensive review and impact of dietary Mg and incidence of type 2 diabetes in a range of prospective cohort studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65(1):3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 9.Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2017;176(1):11–9. doi: 10.1530/EJE-16-0517. [DOI] [PubMed] [Google Scholar]
- 10*.Kieboom BC, Ligthart S, Dehghan A, Kurstjens S, de Baaij JH, Franco OH, et al. Serum magnesium and the risk of prediabetes: a population-based cohort study. Diabetologia. 2017;60(5):843–53. doi: 10.1007/s00125-017-4224-4. This cohort study demonstrated an increased risk of both prediabetes and diabetes with a lower serum Mg concentration coupled with a corresponding common genetic variant in Mg-regulating genes that influence diabetes risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joosten MM, Gansevoort RT, Bakker SJ. Low plasma magnesium and risk of developing chronic kidney disease: results from the PREVEND Study. Kidney Int. 2015;87(6):1262–3. doi: 10.1038/ki.2015.33. [DOI] [PubMed] [Google Scholar]
- 12.Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, et al. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015;87(4):820–7. doi: 10.1038/ki.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenstein FW, Stanton MF. Serum magnesium levels in the United States, 1971–1974. J Am Coll Nutr. 1986;5(4):399–414. doi: 10.1080/07315724.1986.10720143. [DOI] [PubMed] [Google Scholar]
- 14*.Nielsen FH, Johnson LA. Data from controlled metabolic ward studies provide guidance for the determination of status indicators and dietary requirements for magnesium. Biol Trace Elem Res. 2017;177(1):43–52. doi: 10.1007/s12011-016-0873-2. Data from classic metabolic balance studies highlight the need for assessing multiple parameters of Mg status. [DOI] [PubMed] [Google Scholar]
- 15**.Zhang X, Del Gobbo LC, Hruby A, Rosanoff A, He K, Dai Q, et al. The circulating concentration and 24-h urine excretion of magnesium dose-and time-dependently respond to oral magnesium supplementation in a meta-analysis of randomized controlled trials. J Nutr. 2016 doi: 10.3945/jn.115.223453. This paper presents the most comprehensive review of the effect of Mg intake on parameters of serum and urinary Mg over a wide range of intake levels and duration of intake further demonstrating that those with low serum Mg levels had a more robust response to supplemental Mg. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press (US); 1997. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention National Center for Health Statistics. Obesity and Overweight. 2016 [Available from: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm.
- 18*.US Department of Health and Human Services and US Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. (8) 2015 [Available from: http://health.gov/dietaryguidelines/2015/guidelines/This compendium on the eating habits of Americans notes that Mg, among other nutrients, is identified as a “nutrient of concern” as many groups are undercoming this nutrient.
- 19**.Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105(1):159–68. doi: 10.3945/ajcn.116.139758. This analysis of cohort datasets found that 24-hr urinary Mg excretion to be a suitable and stable biomarker for magnesium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han H, Fang X, Wei X, Liu Y, Jin Z, Chen Q, et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr J. 2017;16(1):26. doi: 10.1186/s12937-017-0247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutsey PL, Alonso A, Michos ED, Loehr LR, Astor BC, Coresh J, et al. Serum magnesium, phosphorus, and calcium are associated with risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2014;100(3):756–64. doi: 10.3945/ajcn.114.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dibaba DT, Xun P, Song Y, Rosanoff A, Shechter M, He K. The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017 Jul 19; doi: 10.3945/ajcn.117.155291. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Bertinato J, Wang KC, Hayward S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients. (9) 2017;(3) doi: 10.3390/nu9030296. This national survey provides the most recent assessment of serum Mg concentrations and its relationship to several chronic diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016;32(4):409–17. doi: 10.1016/j.nut.2015.09.014. This review paper found a strong association of lower risk of metabolic syndrome with increased Mg intakes but not with serum Mg concentrations. [DOI] [PubMed] [Google Scholar]
- 25.Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos Int. 2016;27(4):1389–99. doi: 10.1007/s00198-015-3400-y. [DOI] [PubMed] [Google Scholar]
- 26.Hayhoe RP, Lentjes MA, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015;102(2):376–84. doi: 10.3945/ajcn.114.102723. [DOI] [PubMed] [Google Scholar]
- 27.Kunutsor SK, Whitehouse MR, Blom AW, Laukkanen JA. Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol. 2017 doi: 10.1007/s10654-017-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Rebholz CM, Tin A, Liu Y, Kuczmarski MF, Evans MK, Zonderman AB, et al. Dietary magnesium and kidney function decline: The Healthy Aging Neighborhoods of Diversity across the Life Span Study. Am J Nephrol. 2016;44(5):381–7. doi: 10.1159/000450861. In this prospective cohort study, a significant relationship between low dietary intakes of Mg and the risk of rapid decline in kidney function was demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Kosk D, Kramer H, Luke A, Camacho P, Bovet P, Rhule JP, et al. Dietary factors and fibroblast growth factor-23 levels in young adults with African ancestry. J Bone Miner Metab. 2016 doi: 10.1007/s00774-016-0804-5. This cross-sectional study links urinary fibroblast growth factor-23, declining kidney function, and low Mg intake suggesting a key role for Mg in the kidney function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Silva AP, Gundlach K, Buchel J, Jeronimo T, Fragoso A, Silva C, et al. Low magnesium levels and FGF-23 dysregulation predict mitral valve calcification as well as intima media thickness in predialysis diabetic patients. Int J Endocrinol. 2015;2015:308190. doi: 10.1155/2015/308190. This paper demonstrated the contribution of low serum Mg levels coupled with FGF-23 and the presence of vascular risk factors in diabetic patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragoso A, Silva AP, Gundlach K, Buchel J, Neves PL. Magnesium and FGF-23 are independent predictors of pulse pressure in pre-dialysis diabetic chronic kidney disease patients. Clin Kidney J. 2014;7(2):161–6. doi: 10.1093/ckj/sfu003. [DOI] [PMC free article] [PubMed] [Google Scholar]


