Abstract
The CXCR4 chemokine receptor plays a key regulatory role in many biological functions including embryonic development and controlling leukocyte functions during inflammation and immunity. CXCR4 has been also associated with multiple types of cancers where its overexpression/activation promotes metastasis, angiogenesis, and tumor growth and/or survival. Furthermore, CXCR4 is involved in HIV replication, as it is a co-receptor for viral entry into host cells. Altogether, these features make CXCR4 a very attractive target for the development of imaging and therapeutic agents. Here we report the in vivo evaluation of the MCoTI-based cyclotide, MCo-CVX-5c, for the development of imaging agents that target CXCR4. Cyclotide MCo-CVX-5c is a potent CXCR4 antagonist with a remarkable in vivo resistance to biological degradation in serum. A [64Cu]-DOTA-labeled version of this cyclotide demonstrated high and significant uptake in U87-stb-CXCR4 tumors compared to the control U87 tumors. Further, protracted imaging studies demonstrated radiotracer retention in the U87-stb-CXCR4 tumor at 24 h post injection. Uptake in U87-stb-CXCR4 tumors could be blocked by unlabeled MCo-CVX-5c, showing high in vivo specificity. Our results demonstrate the in vivo specificity and retention of a bioactive molecularly targeted cyclotide and highlight the potential of bioactive cyclotides for the development of new imaging agents that target CXCR4.
Keywords: CXCR4, cyclotide, GPCR, PET, molecular imaging
Graphical abstract
A 64Cu-labeled cyclotide with high specificity for receptor CXCR4 was used for in vivo PET-imaging of tumors with high CXCR4 expression level showing the potential of this microprotein molecular scaffold for in vivo bio-imaging applications.

INTRODUCTION
The G-protein coupled chemokine receptor 4 (CXCR4) has been shown to play an important role in several diseases including HIV, cancer and lupus.[1] CXCR4 and its ligand CXCL12, also known as stromal derived factor 1α (SDF1α), is important for the directional migration of cells during development and in normal physiology.[2] The interactions mediated by CXCL12 and it receptor CXCR4 are also important for homing of immune cells.[2b, 3] Activation of CXCR4 by CXCL12 promotes cell survival, proliferation and chemotaxis through ERK1/2, Akt effectors and mitogen-activated protein kinase (MAPK) pathways.[4] CXCR4 overexpression has been associated with multiple types of cancers where its activation promotes metastasis, angiogenesis, tumor growth and chemoresistance leading to poor prognosis.[5] Inhibition of CXCL12-mediated activation of CXCR4 has been shown to reduce metastasis and sensitize tumors for radiation, immuno and chemotherapy.[6] In addition, CXCR4 is involved in HIV replication, as it is a co-receptor for viral entry into host cells.[7] Altogether, these features have made CXCR4 a very attractive target for drug and imaging agent discovery.[8] Hence, several small molecules, small peptides/proteins and monoclonal antibodies have been developed to antagonize CXCR4 for anticancer and anti-HIV activity.[8c, 9]
CXCR4 overexpression has been also used as a biomarker in the field of diagnostic oncology.[10] Since CXCR4 is also expressed in several normal tissue, quantitative determination of its presence using non-invasive bioimaging techniques could provide benefit for therapeutic guidance.[10] Bioimaging of CXCR4 expression levels has been extensively studied with radiolabeled small molecules, peptides and antibodies.[10] In this work, we have explored the use of cylotides for CXCR4 bioimaging purposes. Cyclotides are globular microproteins (ranging from 28 to 37 amino acids) with a unique head-to-tail cyclized backbone, which is stabilized by three disulfide bonds forming a cystine-knot motif (Fig. 1).[11] The cyclic cystine-knot (CCK) molecular scaffold confers an extremely rigid molecular framework,[12] which provides cyclotides an extraordinary stability toward physical, chemical, and biological degradation.[11] Cyclotides can be chemically produced or encoded within standard cloning vectors, and expressed in bacteria or animal cells.[13] Cyclotides have up to 5 hypervariable loops that are amenable to substantial sequence variation,[14] and can be considered as a micro-antibody molecular framework to target intracellular and extracellular protein-protein interactions. Collectively, these features make the cyclotide scaffold an excellent molecular framework for the design of novel peptide-based therapeutics and potentially bioimaging tools.[15]
Figure 1.
Design and synthesis of MCo-CVX-based cyclotides to target the chemokine receptor CXCR4. A. Primary and tertiary structures of MCoTI-based peptides. Structure is based on a homology model using the solution structure of MCoTI-II as template (PDB: 1IB9).[26] The backbone cyclized peptide (connecting bond shown in green) is stabilized by the three-disulfide bonds (shown in red). Modified residues and or peptide segments are indicated by letters X and Z, respectively. Single letter codes B, J and p represent the amino acid, 2-naphthylalanine, citruline and D-proline, respectively. B. Analytical HPLC traces of and ES-MS characterization of purified cyclotides MCo-CVX-6 and MCo-CVX-6D. The expected average molecular weight is shown in parenthesis. Molecular graphics were built with Yasara (www.yasara.org).
Here, we report the synthesis, radiolabeling and biological activity of a 64Cu-radiolabeled cyclotide-based CXCR4 antagonist. We have demonstrated that, using positron emission tomography (PET), the bioactive cylotide specifically accumulates in subcutaneous glioblastoma tumors stably expressing CXCR4 (U87-stb-CXCR4) compared to control U87 tumors. We further supported those observations with ex vivo biodistribution studies. The higher target-to-non-target ratios and longer tumor retention times observed in the biodistribution studies support the potential of cyclotides as valuable bioimaging tools.
RESULTS AND DISCUSSION
Synthesis and characterization of [64Cu]MCo-CVX-6D cyclotide
We used the cyclotide MCo-CVX-5c previously designed in our laboratory to produce a radiolabeled cyclotide that can be detected by PET.[9a] This cyclotide is a potent CXCR4 antagonist that was designed using naturally occurring cyclotide MCoTI-I as molecular scaffold (Fig. 1A).[9a] To facilitate the chemoselective conjugation of the metal chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), the Lys residue in loop 6 was conservatively mutated to Arg to give cyclotide MCo-CVX-6 (Fig. 1A). The resulting cyclotide, MCo-CVX-6, was chemically synthesized using Fmoc-based solid-phase peptide synthesis on a sulfonamide resin as previously described.[9a] Activation of the sulfonamide linker with iodoacetonitrile, followed by cleavage with ethyl mercaptoacetate and acidolytic deprotection with trifluoroacetic acid (TFA), provided the fully deprotected linear peptide α-thioester. The corresponding peptide thioester precursors were efficiently cyclized and folded in a one-pot reaction using sodium phosphate buffer at pH 7.2 in the presence of 1 mM GSH. The cyclization/folding reactions were complete in 48 h (Fig. 1B and Fig. S1). The cyclization/folding yield for MCo-CVX-6 was estimated to be around 80% by HPLC (Fig. S1).
The ε-amine group of the Lys residue in loop 1 of MCo-CVX-6 was used to conjugate the chelator DOTA to provide MCo-CVX-6D, which was then utilized to prepare a non-radioactive Cu2+ analog ([Cu]MCo-CVX-6D) and a radiolabeled cyclotide with 64Cu2+ ([64Cu]MCo-CVX-6D). The resulting Cu-labeled and -unlabeled MCo-CVX-6D cyclotides were purified by HPLC, characterized by mass spectrometry (Fig. 1B), and subjected to in vitro biological evaluation.
In vitro binding and inhibition constants
IC50 and Ki values for the different cyclotides were measured relative to the binding of fluorescent CXCL12-Red to cells expressing the CXCR4 receptor as reported earlier.[16] Unlabeled MCo-CVX-cyclotides MCo-CVX-5c and MCo-CVX-6 showed IC50 values in the sub-nM range, similar to those previously reported (Fig. 2A).[9a] Cyclotide MCo-CVX-6 was about two times more potent than cyclotide MCo-CVX-5. Similarly, the conjugation of the DOTA chelator on the ε-amino group of the Lys residue provided a cyclotide, MCo-CVX-6D, with slightly better CXCR4 inhibitory activity, about 2 and 4 times more potent than MCo-CVX-6 and MCo-CVX-5c, respectively (Fig. 2A). These results demonstrate that replacement of the Lys at loop 6 by Arg and conjugation of DOTA to the side-chain of the Lys residue in loop 1 did not significantly affect the affinity of the resulting cyclotides towards the receptor CXCR4. In contrast, the control cyclotide MCoTI-I did not inhibit CXCL12 binding to CXCR4 (Fig. 2A). To test CXCR4 specificity in vitro, 1 μCi of the cyclotides [64Cu]MCo-CVX-6D or [64Cu]MCoTI-ID with high specific radioactivity (~0.256 mCi/μg) and radiochemical purity (>95%) (Fig. S2) were incubated with U87-stb-CXCR4 or U87 cell lines, with high and low CXCR4 expression, respectively. We have previously shown that nearly 95% of U87-stb-CXCR4 cells have surface CXCR4 expression while only ≈4% of U87 control cells show CXCR4 expression.[16] U87-stb-CXCR4 cells incubated with [64Cu]MCo-CVX-6D for 1 h showed > 40% uptake of the incubated dose and a 3-fold increase in bound radioactivity compared to the U87 control cells (Fig. 2B). In contrast, the MCoTI-I cyclotide conjugated with DOTA at the Lys residues in loop 1 and radiolabeled with 64Cu2+, [64Cu]MCoTI-ID, showed minimal uptake in both U87 cell lines (Fig. 2B). In vitro CXCR4 binding specificity of [64Cu]MCo-CVX-6D was also tested in the presence of 10 μM MCo-CVX-5c or 10 μM AMD3100 (Fig. 2B). We observed ≈30% reduction in bound [64Cu]MCo-CVX-6D in the presence of the excess non-radioactive inhibitor MCo-CVX-5c and AMD3100 (an FDA-approved CXCR4 inhibitor), further indicating that [64Cu]MCo-CVX-6D binding to CXCR4 is specific (Fig. 2B).
Figure 2.
In vitro biological activity of MCo-CVX-cyclotides. A. Representative curves of in vitro inhibition of CXCL12-Red binding to CXCR4 by MCo-CVX-5c, MCo-CVX-6 and MCo-CVX-6D demonstrating that substitution of Lys in MCo-CVX-5c and conjugation with DOTA to produce cyclotides MCo-CVX-6 and MCo-CVX-6D, respectively, do not affect their affinity towards CXCR4. B. In vitro binding of [64Cu]MCo-CVX-6D (CXCR4 targeting cyclotide) and [64Cu]MCoTI-ID (control cyclotide) to U87 and U87-stb-CXCR4 cells. In vitro binding of [64Cu]MCo-CVX-6D to U87-stb-CXCR4 cells is significantly reduced in the presence of CXCR4 inhibitors MCo-CVX-5c and AMD3100, both at 10 μM.
In vivo specificity and biodistribution of the CXCR4 targeting cyclotide
PET imaging
To gain insight into the in vivo specificity and biodistribution of [64Cu]MCo-CVX-6D, we performed PET-computed tomography (PET-CT) imaging studies in mice bearing U87 and U87-stb-CXCR4 glioblastoma xenografts on the left and right flanks, respectively. The PET imaging showed robust accumulation of [64Cu]MCo-CVX-6D in the U87-stb-CXCR4 tumor but not in the U87 tumor (Fig. 3). The increased uptake in the CXCR4-expressing tumor could be observed as early as 90 min and retained through 24 h post-injection (Fig. 3). In addition to the CXCR4-expressing U87-stb-CXCR4 tumor, high accumulation of radioactivity was also observed in liver, kidneys and spleen, and to less extension in the lungs. In contrast, the negative control cyclotide [64Cu]MCoTI-ID showed low uptake in both tumors (Fig. 3). [64Cu]MCoTI-ID showed accumulation only in the kidneys, which is consistent with renal clearance of this cyclotide (Fig. 3).
Figure 3.
Distribution of [64Cu]MCo-CVX-6D and [64Cu]MCoTI-ID in NOD/SCID mice bearing U87 and U87-stb-CXCR4 tumors with PET. Representative PET-CT volume rendered images of mice (n=3) injected with 250 μCi of [64Cu]MCo-CVX-6D or [64Cu]MCoTI-ID recorded 90 min and 24 h post tracers injection demonstrating high accumulation of [64Cu]MCo-CVX-6D in the U87-stb-CXCR4 tumor and [64Cu]MCoTI-ID showed mostly kidney uptake.
Biodistribution and blocking studies
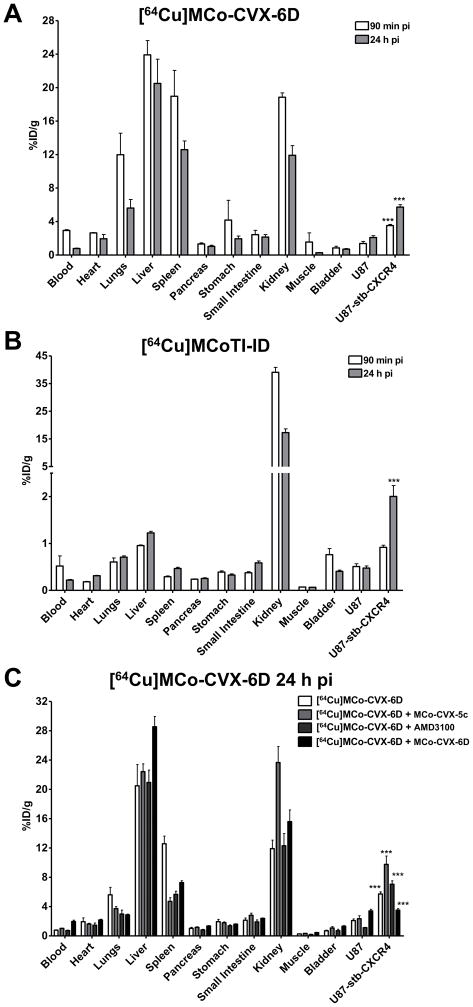
To confirm the observed results in PET images, biodistribution studies were conducted at 90 min and 24 h after the injection of [64Cu]MCo-CVX-6D or [64Cu]MCoTI-ID. In agreement with the imaging results, [64Cu]MCo-CVX-6D demonstrated uptake in CXCR4-expressing tumors in percentage of injected dose/g values of 5.73 ± 0.49% ID/g at 24 h. In contrast, the U87 control tumor uptake was 2.09 ± 0.38% ID/g (Fig. 4A). Accumulation of radioactivity in the liver, spleen and kidneys was also relatively high, with uptake values of 23.26 ± 2.1, 11.77 ± 1.59 and 11.91 ± 2.32% ID/g, respectively. Some accumulation was also observed in the lungs, although after 24 h was slightly smaller than the one observed in the CXCR4-expressing tumor (Fig. 4A).
Figure 4.
Ex vivo evaluation of [64Cu]MCo-CVX-6D and [64Cu]MCoTI-ID distribution and specificity in NOD/SCID mice bearing U87 and U87-stb-CXCR4 tumors. Ex vivo biodistribution analysis of [64Cu]MCo-CVX-6D (A) and [64Cu]MCoTI-ID (B) at 90 min and 24 h after injection. C. In vivo blocking experiments, in which mice were pre-injected with either 50 μg of MCo-CVX-5c or 250 μg AMD3100 1 h prior to or co-injected with 100 μg of cold MCo-CVX-6, with [64Cu]MCo-CVX-6D injection. These results validate CXCR4 mediated uptake of [64Cu]MCo-CVX-6D. *** denotes P < 0.001.
The tumor-to-muscle and tumor-to-blood ratios at 24 h for CXCR4-expressing tumors were 19.89 ± 4.68 and 7.51 ± 1.18, respectively, suggesting that cyclotide analogs are cleared relatively fast from the circulation. These results are in agreement with an estimated half-life of MCo-CVX-5c in mice of ≈ 36 min when dosed intra-venously (unpublished results), and they are also consistent with the ability of [64Cu]MCo-CVX-6D to provide CXCR4-specific images in live animal with high signal-to-noise ratios (Fig. 3). Co-injection of [64Cu]MCo-CVX-6D with non-radioactive MCo-CVX-6D (100 μg, 4 mg/kg) resulted in decreased radioactivity uptake in U87-stb-CXCR4 xenografts, validating CXCR4-mediated accumulation of [64Cu]MCo-CVX-6D in target tumors. We also observed reduced uptake of [64Cu]MCo-CVX-6D in spleen and lungs, and its enhanced accumulation liver and kidneys.
We additionally tested the specificity of [64Cu]MCo-CVX-6D in the presence of other CXCR4 inhibitors. This was accomplished by pre-injecting CXCR4 inhibitors AMD3100 (250 μg, 10 mg/kg) or cyclotide MCo-CVX-5c (50 μg, 2 mg/kg) before injection of radiolabeled [64Cu]MCo-CVX-6D (20 μCi). In all experiments biodistribution analysis was carried out at 24 h post radiotracer injection (Fig. 4C). In mice pre-injected with MCo-CVX-5c increased uptake of radioactivity in U87-stb-CXCR4 tumors was observed (10.75 ± 1.41% ID/g). No significant difference in [64Cu]MCo-CVX-6D uptake was observed in control U87 tumors. At the same time, significantly lower accumulation of [64Cu]MCo-CVX-6D in spleen (from 12.58 ± 2.07 to ≈5 % ID/g) and lungs (from 6.54 ± 1.07 to ≈3.7% ID/g) was detected. A 50% increase in [64Cu]MCo-CVX-6D uptake in kidneys was also observed indicating enhanced renal clearance. Analogs based on peptide CVX15 have been shown to bind mouse CXCR4[17] suggesting that at the pre-injection of low doses of cold analogs saturate the CXCR4 receptors thus allowing radioligand accumulation in tumors that have high CXCR4 expression. A similar approach is also utilized in radioimmunotherapy, where pre-treatment with non-radiolabeled antibody is used as a strategy to saturate the low density target expressing tissues prior to radiotherapeutic injection.[18] A significant decrease in spleen uptake of [64Cu]MCo-CVX-6D was observed in both pre-injection or co-injection of unlabeled cyclotide MCo-CVX-6D groups (Fig. 4C). Spleen is known to naturally express CXCR4, which could explain why pre-injection or co-injection of non-labeled CXCR4 competitors may reduce the uptake of the radiolabeled [64Cu]MCo-CVX-6D. Similar results to those found with cyclotide MCo-CVX-5c were also obtained when mice were pre-injected with AMD3100 (Fig. 4C). Altogether, these results demonstrate CXCR4 specific tumor targeting and pharmacokinetics of [64Cu]MCo-CVX-6D can be optimized by pre-injecting with CXCR4 inhibitors.
The liver also showed increased uptake that was most significant when [64Cu]MCo-CVX-6D was co-injected with the unlabeled cyclotide MCo-CVX-6 (kidney), or when [64Cu]MCo-CVX-6D was injected after a pre-injection of parent cyclotide MCo-CVX-5c (liver). Increased uptake in the liver, it is a trend often observed with 64Cu-based imaging agents,[19] which could be due to lipophilicity of the peptide or the dissociation of Cu2+ from the chelator and subsequent transchelation to plasma proteins such as albumin and ceruloplasmin[19] and may require further investigation.
The biodistribution of the control cyclotide [64Cu]MCoTI-ID showed major accumulation in the kidneys at 90 min and 24 h after injection, with values of 39.41 ± 4.34 and 18.52 ± 1.31 %ID/g, respectively (Fig. 4B). These values confirm the lack of non-specific binding of the control cyclotide MCoTI-I to animal tissue, and are consistent with renal clearance of the inactive cyclotide, which has been shown to be stable in serum.[9a] Similar results have been also recently reported for cyclotide MCoTI-II.[20]
Collectively, the PET-CT imaging and biodistribution studies demonstrate that [64Cu]MCo-CVX-6D binds rapidly and specifically to CXCR4-overexpression cells and can be utilized to image tumors with high CXCR4 expression.
CONCLUSIONS
We report for the first time the use of an engineered cyclotide for bioimaging purposes. To accomplish this, we used an engineered MCoTI-based cyclotide designed to bind and antagonize CXCL12-based activation of receptor CXCR4.[9a] This cyclotide was modified to facilitate the conjugation of a DOTA chelator to allow radiolabeling with PET-active 64Cu isotope. MCoTI-cyclotides share sequence and structural homology with linear cystine-knot squash trypsin inhibitors (also called knottins), which have been used as integrin bioimaging agents.[15a, 21] Other disulfide-rich microproteins derived from animal toxins, such as chlorotoxin (scorpion) and agatoxin (spider), which also contain Cys-knots, have been also used as bioimaging agents.[22] Although these microproteins also show high stability against proteases in general, due to their linear nature are susceptible to exoproteases. In contrast, cyclotides which are backbone cyclized in nature, show extreme resistance to biological degradation by both endo- and exoproteases. These features make cyclotides appear as highly promising alternative molecular frameworks for the design of peptide-based therapeutic and diagnostic tools.
In this work, we have shown that efficient detection of tumors containing CXCR4-expressing cells can be accomplished with the highly specific CXCR4-binding cyclotide [64Cu]MCo-CVX-6D in combination with PET-CT. The biodistribution of [64Cu]MCo-CVX-6D indicate that detection of tumors with high CXCR4 expression and demonstrate favorable kinetics by PET imaging. Rapid and non-invasive detection of CXCR4 overexpression in all malignant lesions in entirety provides unprecedented opportunities to stratify patients for CXCR4-based therapies as well as testing efficacy of the corresponding treatment.
MATERIALS AND METHODS
Analytical HPLC was performed on a HP1100 series instrument with 220 nm and 280 nm detection using a Vydac C18 column (5 mm, 4.6 × 150 mm) at a flow rate of 1 mL/min. All runs used linear gradients of 0.1% aqueous trifluoroacetic acid (TFA, solvent A) vs. 0.1% TFA, 90% acetonitrile in H2O (solvent B). Electrospray mass spectrometry (ES-MS) analysis was routinely applied to all cyclized peptides. ES-MS experiments were performed on an Applied Biosystems API 3000 triple quadrupole mass spectrometer.
Preparation of Fmoc-Tyr(tBu)-F
Fmoc-Tyr(tBu)-F was prepared using diethylaminosulfur trifluoride DAST as previously described[23] and quickly used afterwards. Briefly, DAST (160 μL, 1.2 mmol) was added drop wise at 25° C under nitrogen current to a stirred solution of Fmoc-Tyr(tBu)-OH (459.6 mg, 1 mmol) in 10 mL of dry dichloromethane (DCM), containing dry pyridine (81 μL, 1 mmol). After 20 minutes, the mixture was washed with ice-cold water (3 × 20 mL). The organic layer was separated and dried over anhydrous MgSO4. The solvent was removed under reduced pressure to give the corresponding Fmoc-amino acyl fluoride as white solid that was immediately used.
Loading of 4-sulfamylbutyryl AM resin with Fmoc-Tyr(tBu)-F
Loading of the first residue was accomplished using Fmoc-Tyr(tBu)-F as previously described.[23] Briefly, 4-sulfamylbutyryl AM resin (420 mg, 0.33 mmol) (Novabiochem) was swollen for 20 minutes with dry DCM and then drained. A solution of Fmoc-Tyr(tBu)-F (≈461 mg, 1 mmol) in dry DCM (2 mL) and N,N-diisopropylethylamine (DIEA) (180 μL, 1 mmol) was added to the drained resin and reacted at 25° C for 1 h. The resin was washed with dry DCM (5 × 5 mL), dried and kept at −20°C until use.
Chemical synthesis of MCo-CVX cyclotides
Solid-phase synthesis of all grafted peptides was carried out on an automatic peptide synthesizer ABI433A (Applied Biosystems) using the Fast-Fmoc chemistry with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU)/DIEA activation protocol at 0.1 mmole scale on a Fmoc-Tyr(tBu)-4-sulfamylbutyryl AM resin as previously described [9a, 24]. Side-chain protection compatible with Fmoc-chemistry was employed as previously described for the synthesis of peptide α-thioesters by the Fmoc-protocol,[25] except for the N-terminal Cys residue, which was introduced as Boc-Cys(Trt)-OH. Following chain assembly, the alkylation, thiolytic cleavage and side chain deprotection were performed as previously described.[23] Briefly, ≈100 mg of protected peptide resin were first alkylated two times with ICH2CN (174 μL, 2.4 mmol; previously filtered through basic alumina) and DIEA (82 μL, 0.46 mmol) in N-methylpyrrolidone (NMP) (2.2 mL) for 24 h. The resin was then washed with NMP (3 × 5 mL) and DCM (3 × 5 mL). The alkylated peptide resin was cleaved from the resin with HSCH2CO2Et (200 μL, 1.8 mmol) in the presence of a catalytic amount of sodium thiophenolate (NaSPh, 3 mg, 22 μmol) in N,N-dimethylformamide (DMF):DCM (1:2 v/v, 1.2 mL) for 24 h. The resin was then dried at reduced pressure. The side-chain protecting groups were removed by treating the dried resin with trifluoroacetic acid (TFA):H2O:tri-isopropylsilane (TIS) (95:3:2 v/v, 10 mL) for 3–4 h at room temperature. The resin was filtered and the linear peptide thioester was precipitated in cold Et2O. The crude material was dissolved in the minimal amount of H2O:MeCN (4:1) containing 0.1% TFA and characterized by HPLC and ES-MS as the desired grafted MCoTI-I linear precursor α-thioester (Fig. S1). Cyclization and folding was accomplished by flash dilution of the linear α-thioester TFA crude to a final concentration of ≈50 μM into freshly degassed 1 mM reduced glutathione (GSH), 0.1 M sodium phosphate buffer at pH 7.2 for 48 h (Fig. S1). Folded peptides were purified by semi-preparative HPLC using a linear gradient of 20–35% solvent B over 30 min. Purified peptides were characterized by HPLC and ES-MS confirming ≥95% purity (Fig. 1)
Conjugation of DOTA-NHS to MCo-cylotides
Around 700 μg of the corresponding MCo-cyclotide were dissolved in DMF (100 μL) and then a DOTA-NHS ester (Macrocyclics) (0.7 μmol, ≈ 5 equiv.) and DIEA (2% v/v) were added to start the acylation reaction of the ε-amino group of Lys residue in loop 1. The progress of reaction was monitored by HPLC and ES-MS. The reaction was complete in 18 h. The reaction crude was diluted in buffer A to less than 0.2% in DMF and desalted using a C18-solid-phase extraction cartridge, lyophilized and characterized by analytical HPLC and ES-MS (Fig. 1).
General procedure for radiolabeling of MCo-cyclotides with [64Cu]CuCl2
10 μg of DOTA-conjugated cyclotide (MCoTI-ID or MCo-CVX-6D) was dissolved in 100 μL of 0.1 M sodium acetate (pH 4.5) and 259 MBq (7 mCi) of 64Cu(OAc)2 was added to the cyclotide solutions. The resulting mixtures were heated at 65 °C for 0.5 h. Radio-HPLC purification was performed using a Phenomenex C-18 Luna 5 μm, 10 × 250 mm2 semi-preparative column using elution conditions described in the supplementary information. The UV absorbance was measured at 266 nm. The corresponding [64Cu]MCo-cyclotides were collected (Fig. S2), concentrated under vacuum and diluted in saline for in vitro and animal studies. [64Cu] MCo-CVX-6D and [64Cu] MCoTI-ID were obtained in ~45% yield with a specific activity of 9.55 MBq/μg (0.258 mCi/μg) and ~36% yield with a specific activity of 9.43 MBq/μg 0.255 mCi/μg, respectively with radiochemical purity higher than 95%.
Cell lines
All cell culture reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise specified. The human glioblastoma cell line U87 (CXCR4low) and CHO-K1 cell lines were purchased from American Type Culture Collection (ATCC) and cultured in our laboratory in MEM and F-12K medium respectively, both supplemented with 10% fetal bovine serum (FBS), 100 units/mL of penicillin, and 100 mg/mL of streptomycin. A U87 cell line stably transfected with human CD4 and CXCR4 (U87-stb-CXCR4, CXCR4high) was obtained from the NIH AIDS Research Reference Reagent Program and cultured in DMEM supplemented with 15% FBS, 1 μg/mL puromycin, 300 μg/mL G418, 100 units/mL of penicillin, and 100 mg/mL of streptomycin. Cell lines were maintained in a humidified incubator at 37 °C with 5% CO2. The CHO-K1 cell line stably expressing SNAP-CXCR4 was generated in our laboratory, and maintained in RPMI supplemented with 10% FBS, 1 μg/mL puromycin, 2 mg/mL G418, 100 units/mL of penicillin, and 100 mg/mL of streptomycin.
Competitive binding assays
Affinity of the cyclotides was determined by a FRET-based multi-concentration competitive binding assay using CHO-K1-SNAP-CXCR4 cells labeled with Lumi4-Tb against fluorescent CXCL12 as previously described.[16] Cylotide analog stocks were prepared in water and diluted with Tag-lite medium prior to their use in the assay. CHO-K1-SNAP-CXCR4 cells were prepared for in vitro assays according to manufacturer’s recommendation (Cisbio Bioassays). Briefly, subconfluent CHO-K1-SNAP-CXCR4 cells in T-175 cm2 flasks were rinsed with 10 mL of Tag-lite labeling medium and incubated with 100 nM SNAP-Lumi4-Tb in Tag-lite buffer for 1 h at 37 °C. 10,000 cells per well were used to carry out binding assays in 384-well plates. CHO-K1-SNAP-CXCR4-SNAP-Lumi4-Tb cells and SDF1α-Red (15 nM) were mixed with increasing concentrations of the corresponding cyclotide (1 × 10−4 M to 1 × 10−13 M) and incubated at room temperature for 2 h. Homogeneous time-resolved fluorescence (HTRF) with excitation at 340 nm and emissions at 620 and 665 nm (delay 50 μs, window time 400 μs, measurement time 1 s) were measured using a Perkin Elmer Victory3 1420 multi-label counter. Binding assays were done in triplicates and three independent experiments were performed. All calculations were done using GraphPad Prism 6 software (GraphPad Software, Inc., San Diego, CA). IC50 and Ki values were calculated by fitting the data to a sigmoidal dose response curve and the Cheng-Prusoff equation (with derived Kd = 19 for SDF1-α-Red used at the concentration of 15 nM), respectively.
Animal models
All experimental procedures using animals were conducted according to protocols approved by the Johns Hopkins Animal Care and Use Committee. Female NOD/SCID mice, six to eight weeks old, weighing between 25 and 30 g, were purchased from The Johns Hopkins Immune Compromised Animal Core. Mice were implanted subcutaneously (s.c.) with U87 and U87-stb-CXCR4 cells (4 × 106 cells/100 μL) in opposite flanks. Animals were used for biodistribution and PET/CT imaging experiments when the tumor size reached approximately 200–400 mm3.
PET-CT imaging and analysis
Whole-body PET and CT images were acquired on an eXplore VISTA small animal PET (GE) and an X-SPECT small SPECT/CT system (Gamma Medica Ideas, Northridge, CA), respectively. For imaging studies, mice were induced with 3% and maintained under 1.5% isoflurane (v/v). Whole body PET images (n = 3, 2 bed positions, 15-min emission/position) were acquired at 90 min after injection of ~9.25 MBq (250 μCi) of radiotracer, either [64Cu]MCo-CVX-6D or [64Cu]MCoTI-ID. After each PET scan, a CT scan was acquired in 512 projections for anatomic co-registration. PET data were corrected for decay and dead time, and reconstructed using the three-dimensional ordered subsets-expectation maximization algorithm (3D-OSEM). The percentage of injected dose per cc (%ID/cc) values was calculated based on a calibration factor using a known quantity of radioactivity. Volume-rendered images were generated using Amira 5.3.3 software (Visage Imaging Inc.).
Ex vivo biodistribution
NOD/SCID mice harboring U87 and U87-stb-CXCR4 xenografts were injected intravenously with 740 kBq (20 μCi) of [64Cu]MCo-CVX-6D in 200 μL of saline. Different groups of mice (n = 4 per group) were sacrificed 90 min and 24 h after injection of the tracer. Blood, tumors and selected tissues were harvested, weighed and the radioactivity in the tissues was measured in an automated gamma spectrometer. To demonstrate the in vivo specificity of [64Cu]MCo-CVX-6D, mice received blocking doses of AMD3100 (10 mg/kg) or cyclotide MCo-CVX-5c (2 mg/kg) subcutaneously 30 min before the injection of the radiotracer. In an independent experiment, mice (n = 3) were co-injected [64Cu]MCo-CVX-6D (740 kBq (20 μCi)) and MCo-CVX-6 (740 kBq (20 μCi)). Ex vivo biodistribution studies were performed 24 h after the radiotracer injection. Aliquots of the injected dose were counted as reference standards for the calculation of %ID/g values. All values were normalized and are reported as percent injected dose per gram of tissue (%ID/g).
Data analysis
Statistical analysis was performed using a Prism 6 Software (GraphPad Software, La Jolla, CA). P-values < 0.05 were considered to be significant using an unpaired two tailed t-test and the comparative reference was cell line or tumor with low CXCR4 expression.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Research Grants R01-GM113363 (J.A.C.) and R01CA166131(S.N), Department of Defense Congressionally Directed Medical Research Programs in Lung Cancer Grant LC150051 (J.A.C) and Breast Cancer BC121957. (S.N.), BROAD Medical Research Program-Crohn’s & Colitis Foundation of America Grant #483566 (J.A.C.), Lupus Research Institute (J.A.C) and Whittier Foundation (J.A.C), Alexander and Margaret Stewart Trust (S.N), and the resources provided by National Institutes of Health Research Grants P30-CA006973 and P50-CA103175.
References
- 1.Wong D, Korz W. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 2.a) Mackay CR. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]; b) Sallusto F, Baggiolini M. Nat Immunol. 2008;9:949–952. doi: 10.1038/ni.f.214. [DOI] [PubMed] [Google Scholar]
- 3.Moser B, Wolf M, Walz A, Loetscher P. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Teicher BA, Fricker SP. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 5.a) Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]; b) Balkwill F. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.a) Chatterjee S, Behnam Azad B, Nimmagadda S. Adv Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kang H, Watkins G, Douglas-Jones A, Mansel RE, Jiang WG. Breast. 2005;14:360–367. doi: 10.1016/j.breast.2004.12.007. [DOI] [PubMed] [Google Scholar]; c) Kato M, Kitayama J, Kazama S, Nagawa H. Breast Cancer Res. 2003;5:R144–150. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Feng Y, Broder CC, Kennedy PE, Berger EA. Science. 1996;272:872–877. [Google Scholar]; b) Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM, Clark-Lewis I, Legler DF, Loetscher M, Baggiolini M, Moser B. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 8.a) Mishan MA, Ahmadiankia N, Bahrami AR. Cell Biol Int. 2016;40:955–967. doi: 10.1002/cbin.10631. [DOI] [PubMed] [Google Scholar]; b) Xu C, Zhao H, Chen H, Yao Q. Drug Des Devel Ther. 2015;9:4953–4964. doi: 10.2147/DDDT.S84932. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Choi WT, Duggineni S, Xu Y, Huang Z, An J. J Med Chem. 2012;55:977–994. doi: 10.1021/jm200568c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Aboye TL, Ha H, Majumder S, Christ F, Debyser Z, Shekhtman A, Neamati N, Camarero JA. J Med Chem. 2012;55:10729–10734. doi: 10.1021/jm301468k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Burger JA, Peled A. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 10.a) Kuil J, Buckle T, van Leeuwen FW. Chem Soc Rev. 2012;41:5239–5261. doi: 10.1039/c2cs35085h. [DOI] [PubMed] [Google Scholar]; b) Woodard LE, Nimmagadda S. J Nucl Med. 2011;52:1665–1669. doi: 10.2967/jnumed.111.097733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Daly NL, Rosengren KJ, Craik DJ. Adv Drug Deliv Rev. 2009;61:918–930. doi: 10.1016/j.addr.2009.05.003. [DOI] [PubMed] [Google Scholar]; b) Gould A, Ji Y, Aboye TL, Camarero JA. Curr Pharm Des. 2011;17:4294–4307. doi: 10.2174/138161211798999438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Angew Chem Int Ed Engl. 2010;49:7030–7034. doi: 10.1002/anie.201002906. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Puttamadappa SS, Jagadish K, Shekhtman A, Camarero JA. Angew Chem Int Ed Engl. 2011;50:6948–6949. [Google Scholar]
- 13.a) Jagadish K, Camarero JA. Methods Mol Biol. 2017;1495:41–55. doi: 10.1007/978-1-4939-6451-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Li Y, Bi T, Camarero JA. Adv Bot Res. 2015;76:271–303. doi: 10.1016/bs.abr.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Jagadish K, Borra R, Lacey V, Majumder S, Shekhtman A, Wang L, Camarero JA. Angew Chem Int Ed Engl. 2013;52:3126–3131. doi: 10.1002/anie.201209219. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Austin J, Wang W, Puttamadappa S, Shekhtman A, Camarero JA. Chembiochem. 2009;10:2663–2670. doi: 10.1002/cbic.200900534. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Camarero JA, Kimura RH, Woo YH, Shekhtman A, Cantor J. Chembiochem. 2007;8:1363–1366. doi: 10.1002/cbic.200700183. [DOI] [PubMed] [Google Scholar]
- 15.a) Gould A, Camarero JA. Chembiochem. 2017 doi: 10.1002/cbic.201700153. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Craik DJ, Du J. Curr Opin Chem Biol. 2017;38:8–16. doi: 10.1016/j.cbpa.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Woodard LE, De Silva RA, Behnam Azad B, Lisok A, Pullambhatla M, GLW, Mease RC, Pomper MG, Nimmagadda S. Nucl Med Biol. 2014;41:552–561. doi: 10.1016/j.nucmedbio.2014.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesniak WG, Sikorska E, Shallal H, Behnam Azad B, Lisok A, Pullambhatla M, Pomper MG, Nimmagadda S. Mol Pharm. 2015;12:941–953. doi: 10.1021/mp500799q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson SM, Carrasquillo JA, Cheung NK, Press OW. Nat Rev Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson CJ, Ferdani R. Cancer Biother Radiopharm. 2009;24:379–393. doi: 10.1089/cbr.2009.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang CK, Stalmans S, De Spiegeleer B, Craik DJ. J Pept Sci. 2016;22:305–310. doi: 10.1002/psc.2862. [DOI] [PubMed] [Google Scholar]
- 21.Kintzing JR, Cochran JR. Curr Opin Chem Biol. 2016;34:143–150. doi: 10.1016/j.cbpa.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 22.a) Moore SJ, Leung CL, Norton HK, Cochran JR. PLoS One. 2013;8:e60498. doi: 10.1371/journal.pone.0060498. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Akcan M, Stroud MR, Hansen SJ, Clark RJ, Daly NL, Craik DJ, Olson JM. J Med Chem. 2011;54:782–787. doi: 10.1021/jm101018r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 23.Contreras J, Elnagar AY, Hamm-Alvarez SF, Camarero JA. J Control Release. 2011;155:134–143. doi: 10.1016/j.jconrel.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboye T, Kuang Y, Neamati N, Camarero JA. Chembiochem. 2015;16:827–833. doi: 10.1002/cbic.201402691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camarero JA, Mitchell AR. Protein Pept Lett. 2005;12:723–728. doi: 10.2174/0929866054864166. [DOI] [PubMed] [Google Scholar]
- 26.Felizmenio-Quimio ME, Daly NL, Craik DJ. J Biol Chem. 2001;276:22875–22882. doi: 10.1074/jbc.M101666200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.