Abstract
Krabbe disease (KD; also called globoid cell leukodystrophy) is a genetic disorder involving demyelination of the central (CNS) and peripheral (PNS) nervous systems. The disease may be subdivided into three types; an infantile form which is most common and severe, a juvenile form, and a rare adult form. KD is an autosomal recessive disorder caused by a deficiency of galactocerebrosidase (GALC) activity in lysosomes leading to accumulation of galactoceramide and neurotoxic galactosylsphingosine (psychosine) in macrophages (globoid cells) as well as neural cells especially in oligodendrocytes and Schwann cells. This ultimately results in damage to myelin in both CNS and PNS with associated morbidity and mortality. Accumulation of psychosine, a lysolipid with detergent-like properties, over a threshold level could trigger membrane destabilization leading to cell lysis. Moreover, sub-threshold concentrations of psychosine trigger cell signaling pathways that induce oxidative stress, mitochondrial dysfunction, apoptosis, inflammation, endothelial/vascular dysfunctions, and neuronal and axonal damage. Since the proposed “psychosine hypothesis” considerable efforts have been made in search for effective therapy for lowering psychosine load using pharmacological, gene and stem cell approaches to attenuate psychosine-induced neurotoxicity. In this review, we focus on the recent advances and prospective research on understanding of disease mechanisms and therapeutic approaches for KD.
Keywords: globoid cell leukodystrophy, Krabbe disease, myelin, therapy
1. Etiology of Krabbe disease
Krabbe disease (KD; OMIM #245200) is a fatal autosomal recessive lysosomal storage disorder (LSD) caused by genetic deficiency or abnormalities of galactocerebrosidase (GALC; EC 3.2.1.46) and thus accumulation of its substrates galactosylceramide (GalCer) and galactosylsphingosine (psychosine/PSY) in macrophages and neural tissue leading to progressive loss of myelin (Kolodny 1996; Miyatake and Suzuki 1972; Wenger et al. 2001b). The disease was described first by the Danish neurologist Knud H. Krabbe in 1916 (Krabbe 1916) and PSY-mediated disease mechanisms were established first by Miyatake and Suzuki in 1972 as “the psychosine hypothesis” (Miyatake and Suzuki 1972). KD is also known as globoid leukodystrophy (GLD) because of its characteristic pathological features of large multinucleated macrophages (globoid cells) in brain white matter and demyelination and gliosis (leukodystrophy) (Collier and Greenfield 1924; Wenger et al. 2001b). The disease most often affects infants with onset before age of 6 months (more than 85–90% of cases) with irritability, fever, limb-stiffness, seizures, feeding difficulty, slowing of mental and motor development, and growth retardation. Approximately 10 to 15% of patients have a later onset; these include late infantile (6 months to 3 years), adolescence (3 to 8 years) or adulthood which is clinically more heterogeneous and less severe (Wenger et al. 2000). Adults may present with mild symptoms, or even be asymptomatic despite the same enzyme abnormalities (Kolodny et al. 1991; Lyon et al. 1991; Turazzini et al. 1997).
2. Genetic causes of Krabbe disease
In 1970 deficiency of GALC enzyme activity in lysosomes in KD was first reported by Malone (Malone 1970) and further confirmed by Suzuki and Suzuki (Suzuki and Suzuki 1970). GALC was mapped to chromosome 14 by Zlotogora et al. (Zlotogora et al. 1990) and its cDNA was cloned by Chen et al. and Sakai et al (Chen and Wenger 1993; Sakai et al. 1994). The human GALC gene (OMIM #606890) encodes a precursor form of GALC (80kDa), a single chain peptide of 669 amino acid residues (Sakai et al. 1994). The precursor form of GALC is processed to the mature form by cleavage to 50 kDa N-terminal fragment and 30 kDa C-terminal fragment in lysosomes (Nagano et al. 1998). So far, more than 130 mutations have been identified in the Human Gene Mutation Database (HGMD), 147 in Online Molecular and Metabolic Bases of Inherited Diseases (OMMBID), and 149 in Leiden Open Variation Database (LOVD). Based on HGMD, at least 128 have been reported to cause KD by deletions, frameshifts, and missense mutations in GALC gene (De Gasperi et al. 1996; Fiumara et al. 2011; Fu et al. 1999; Furuya et al. 1997; Lissens et al. 2007; Rafi et al. 1996; Tappino et al. 2010; Wenger et al. 1997; Xu et al. 2006). The genetic features of these mutations have been reviewed previously (Graziano and Cardile 2015; Wenger et al. 2000). Among the mutations, a 30 kb deletion that encodes nonfunctional protein is the most common (35–50%) (Rafi et al. 1995; Wenger et al. 1997). In addition, the majority of other GALC mutations associated with KD are missense mutations that cause catalytic inactivation, protein truncation, misfolding, mistargeting, and degradation (Lee et al. 2010).
3. Metabolism of psychosine and galactocerebrosides
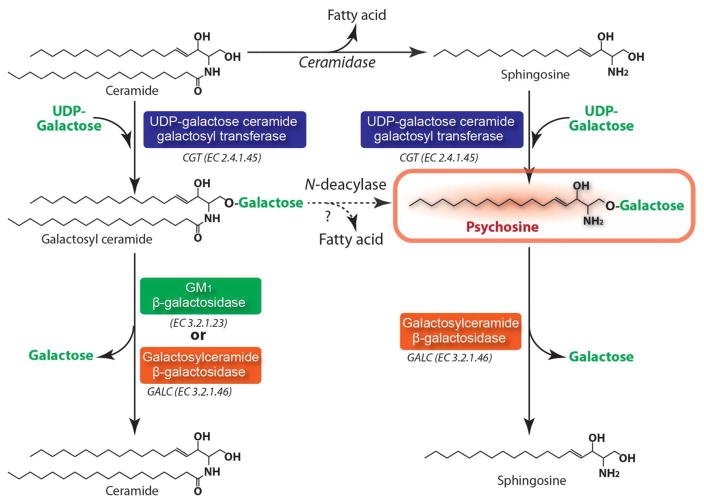
PSY, a cytotoxic lysolipid, is a dead-end metabolic byproduct in the metabolism of sphingolipids under GALC deficient conditions. PSY is generated by galactosylation of sphingosine by UDP-galactose ceramide galactosyl transferase (CGT E.C. 2.4.1.45) (Cleland and Kennedy 1960) (Fig. 1). Alternatively, studies have also reported that PSY may also be produced by deacylation of galactosylceramide (GalCer) by N-deacylase (Kanazawa et al. 2000; Svennerholm et al. 1980) (Fig. 1). Under normal conditions, the cellular and tissue levels of PSY are extremely low. However, under the GALC deficient conditions, PSY accumulates to high levels in tissues, especially in the brain (up to ~50% of total brain cerebrosides) (Suzuki 1998; Suzuki 2003). Similar to psychosine, GALC is synthesized by galactosylation of ceramide by CGT. Unlike most other lysosomal diseases, the primary substrate of GALC (GalCer) is not found at such high levels in KD tissue due to its alternative hydrolysis by GM1 ganglioside β-galactosidase (β-Gal; EC 3.2.1.23) (Knaap and Valk 2005) (Fig. 1).
Figure 1. Anabolic and catabolic pathways of psychosine and galactosylceramide.
Galctosylceramide is one of major glycosphingolipids of myelin. It is synthesized by galactosylation of ceramide by the action of UDP-galactose ceramide galactosyl transferase (CGT). Galactosylceramide is degraded to ceramide by degalactosylation by both GM1 β-galactosidase and galactosylceramide β-galactosidase (GALC). Psychosine is synthesized by galactosylation of sphingosine which is generated from deacylation of ceramide by ceramidase. Alternatively, psychosine may also be synthesized from galctosylceramide by the action of N-deacylase. Psychosine is a neurotoxic lysolipid and present at extremely low levels in the cells and tissues under normal conditions. However, under the GALC deficient conditions, PSY accumulates to high levels in tissues, especially in the brain. Unlike most other lysosomal diseases, the primary substrate of GALC (galctosylceramide) is not found at such high levels in Krabbe disease tissues due to its alternative hydrolysis by GM1 ganglioside β-galactosidase.
4. Pathological role of psychosine in oligodendrocytes
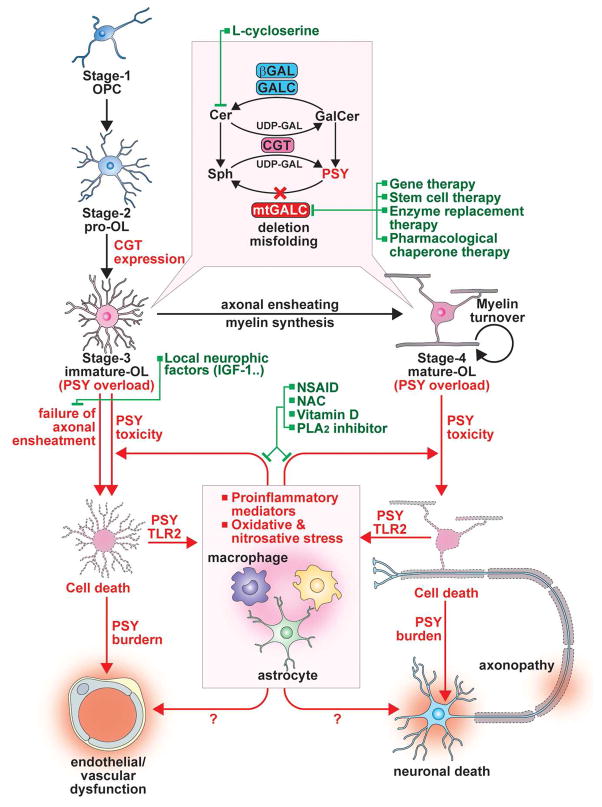
CGT is highly expressed in oligodendrocytes (OLs) and Schwann cells but to a lower degree in neurons and astrocytes (Castelvetri et al. 2011; Pernber et al. 2002; Schaeren-Wiemers et al. 1995). Accordingly, CGT expressing cells are highly vulnerable to the PSY-induced neurotoxicity under GALC deficient conditions (Ida et al. 1990; Nagara et al. 1986; Suzuki 1998). Under normal conditions, CGT in OLs and Schwann cells participates in synthesis of myelin lipids (e.g. GalCer and sulfatide) (Dupree et al. 1999; Marcus et al. 2002; Marcus et al. 2006) and thus plays a critical role in not only myelin formation but also functional and structural maintenance of myelin and other cellular membranes (Schaeren-Wiemers et al. 1995; Schulte and Stoffel 1993; Stahl et al. 1994). In support, cgt knockout mice showed functional abnormality in myelin (Coetzee et al. 1996). OLs express CGT and thus GalCer (O1 antigen (Bansal et al. 1989)) is synthesized at late processes of OL progenitor cell (OPC) differentiation into OLs (Gard and Pfeiffer 1989; Suzuki and Suzuki 1970). OL differentiation is divided into four distinct stages [stage 1, OL progenitors (OPCs); stage 2, pro-OLs; stage 3, immature OLs; stage 4, mature OLs] according to the cell proliferation capacity, mobility, temporal expression of cell markers, and morphology (Fig. 2) (Gard and Pfeiffer 1990). Fig. 2 shows that CGT and GalCer (O1) are expressed in immature OLs (stage 3) where the cells no longer divide and show maturation of their arborization but do not form myelin yet (Berg and Schachner 1982; Gard and Pfeiffer 1990), suggesting that PSY accumulation under the GALC deficient conditions is initiated at stage 3 in immature OLs. In support, in vitro cell culture studies reported that GALC deficient OPCs did not have any obvious cytotoxicity under normal conditions (Won et al. 2013) while they showed increased susceptibility to exogenous PSY induced cytotoxicity (Graziano et al. 2016). Accordingly, following initiation of OPC differentiation, in the absence of exogenous PSY, the cells experienced increased cytotoxicity in parallel with increased expression of CGT (Won et al. 2013). Although the OL differentiation stage specific for PSY cytotoxicity under GALC deficient conditions should be further elucidated in vivo, these studies suggest a possible role of CGT expression and synthesis of GalCer in degeneration of differentiating OLs under GALC deficient conditions (Won et al. 2013).
Figure 2. Mechanisms underlying demyelination and dysmyelination and mechanism-based therapeutic targets for Krabbe disease.
PSY is a byproduct of metabolism of galactosylceramide (GalCer), one of major glycosphingolipids of myelin. Therefore, expression of GalCer synthesizing enzyme UDP-galactose ceramide galactosyl transferase (CGT) leading to production of GalCer is tightly linked to PSY synthesis and thus PSY-induced OL toxicity under GALC deficient conditions. In OL progenitor cells (OPCs) the expression level of CGT is low but its expression is upregulated during the differentiation into immature and mature OLs. Therefore, psychosine may accumulate in OLs during the late stages of differentiation and resulting in failure of myelin formation. In addition, myelin turnover process may result in further accumulation of PSY in survived mature OLs and thus ultimately lead to loss of OLs and demyelination. Based on the critical role of PSY accumulation in the disease processes, numerous therapeutic approaches have been proposed and tested to reduce the PSY load. These includes pharmacological inhibition of PSY synthesis (L-cycloserine), enzyme replacement therapy, gene and stem cell therapies. Recent studies also described the idea of stabilizing the misfolded GALC enzyme in KD by pharmacological chaperone and tested in cell culture studies. The loss of OLs may induce neuroinflammation via activation of innate immune responses mediated by TLR2 and at the same time, the induced neuroinflammation may be amplified by PSY released from degenerated OLs. In addition to brain resident cells, such as astrocytes and microglia, the infiltrating peripheral immune cells, such as macrophages and lymphocytes, are also involved in PSY-induced mechanisms of neuroinflammatory, oxidative, and nitrosative stress pathway. In addition, these secondary disease processes may involve other disease processes, such as endothelial vascular complications and neuronal/axonal loss and thus neuro-muscular dysfunction. Therefore, drugs targeting to decrease the PSY load and neuroinflammation (e.g. NSAID, sPLA2 inhibitors) and antioxidants (NAC and vitamin D) could be potential alternative therapy or combination therapy with other approaches, such as enzyme replacement therapy, gene therapy, and stem cell therapy.
Initially, KD was reported as a demyelinating disease in which progressive accumulation of PSY in OLs and myelin causes loss of OLs and myelin (Suzuki 2003; Wenger et al. 2001a). However, presence of severe hypomyelination with lack of large diameter axons in the PNS of Twi-5J mice bearing missense mutation of galc (E130K) (Potter et al. 2013) and incomplete myelin pattern and associated decrease in conduction velocities of PNS nerves in primate model of KD (Weimer et al. 2005) suggest that KD may also involve dysmyelination, formation of defective myelin or failure of myelin development. Although the underlying mechanisms for dysmyelination under GALC deficient conditions are not known at present, PSY accumulation during the OL differentiation and its inhibition of final maturation and survival of differentiating OLs were described as factors contributing to myelin loss (Won et al. 2013) (Fig. 2). OPCs and immature OLs are greatly overproduced during normal development. Among them, only immature OLs that succeed in ensheathing axons survive (~50%), whereas those that fail degenerate (Fig. 2) (Barres et al. 1992; Trapp et al. 1997). Since immature OLs seem to accumulate PSY under GALC deficient conditions, the spontaneous degeneration of immature OLs which fail ensheathment of axons could cause a greater PSY burden on neighboring cells (e.g. myelinating OLs, astrocytes, microglia, and neurons) and thus accelerate disease pathology.
At the molecular level, degeneration of immature OLs during normal differentiation is induced as a result of failed or limited binding of OLs to axonal neuregulin and laminin which are known to promote their survival and maturation via activation of membrane bound EGF receptor (ErbB) and integrin α6β1 [see review (Simons and Trajkovic 2006)]. In addition, locally limited amounts of neurotrophic factors, such as insulin-like growth factor-1 (IGF-1), neurotrophin-3 (NT-3), and ciliary neurotrophic factor (CNTF), produced by neurons and astrocytes are also known to play pivotal role(s) in these processes (Simons and Trajkovic 2006). Proinflammatory cytokines and chemokines produced by inflammatory cells (astrocytes, microglia, and macrophages) are also known to influence OL differentiation and myelin formation [see review (Schmitz and Chew 2008)] and thus PSY associated inflammatory responses in KD may also influence OL differentiation and myelin formation. Direct impact of PSY load on neuronal survival and axonal functions and structural integrities is also described recently (Cantuti-Castelvetri et al. 2015; Cantuti-Castelvetri et al. 2012; Cantuti Castelvetri et al. 2013; Castelvetri et al. 2011), however, the role of PSY-induced neuronal and axonal defects in OL differentiation and myelin formation has not been understood yet. Moreover, the participation of other brain resident cells (e.g. astrocytes and microglia) and infiltrating immune cells (e.g. macrophages and lymphocytes), via expression of neurotrophic and proinflammatory factors, in regulation of OL differentiation and myelin formation needs further investigation.
5. Implication of active myelination processes in the CNS pathology of KD
In humans, most dramatic changes in myelination occur between mid-gestation and the end of the second postnatal year even though in some areas of the CNS (e.g. cortical fibers of the cerebral cortex), the myelination continues 30~40 years after birth (Sampaio and Truwit 2001). Although spatiotemporal pattern of myelin abnormalities of KD patients during gestation and infancy is not fully defined, magnetic resonance (MR) imaging studies have shown that spinal cord involvement precedes intracranial abnormalities in infantile KD (Given et al. 2001; Vasconcellos and Smith 1998). Spinal cord is one of the first CNS areas acquiring myelin (20 weeks of gestation) and its myelination is not completed until the second year postnatally (Dambska and Wisniewski 1999; Tanaka et al. 1995). This suggests that KD pathology may involve actively myelinating areas. Accordingly, MR signal abnormalities were also reported in other active myelinating areas in early-onset KD (< 2 years) and late-onset KD (>2 years). These include corticospinal projection fibers (pyramidal tract), posterior corpus callosum, and parieto-occipital white matter (Loes et al. 1999). Pyramidal tract myelination begins at 25 weeks of gestation (Wozniak and O’Rahilly 1982) and it continues till 2~3 years of age (Kinney et al. 1988; Yakovlev and Lecours 1967). Myelination of corpus callosum posterior begins 3~8 months after birth (Barkovich et al. 1988; Kinney et al. 1988; Yakovlev and Lecours 1967) and continues through adolescence and adulthood (Bartzokis et al. 2001; Courchesne et al. 2000). Myelination of parieto-occipital white matter begins 4–6 months after birth (Kinney et al. 1988; Yakovlev and Lecours 1967) and achieves 50% level by 11–14 months of age (Deoni et al. 2011; Dietrich et al. 1988). These studies indicate that brain areas of pyramidal tract, posterior corpus callosum, and parieto-occipital white matter are under active myelination during the course of early onset (< 2 years) and late onset (> 2 years) KD, thus supporting involvement of active myelinating CNS areas in KD pathology. Accordingly, the abnormalities of cerebellar white matter were observed only in early-onset but not in late-onset KD (Loes et al. 1999). Myelination of cerebellar white matter begins during gestation (Kinney et al. 1988) and reaches adult levels at 3~18 months of age (Barkovich et al. 1988). Therefore, myelination of cerebellar white matter, for the most part, reached adult levels before the onset of late-onset KD pathology (> 2 years). Although the mechanisms underlying these findings are not known at present, it will be of interest to study whether the CNS areas that have already reached adult levels of myelination before onset on KD are less vulnerable to late onset KD pathology. Since MR studies did not fully reflect degrees of myelination/demyelination to local levels of PSY in different CNS regions of KD patients, detailed studies of regional PSY accumulation to degree of myelination/demyelination may provide insights into mechanism(s) of regional specific KD pathologies.
6. Mechanisms underlying psychosine toxicity in the CNS and PNS
Based on the its detergent-like properties, PSY induced changes in membrane architecture were initially believed to be the cause of OL death and loss of myelin in KD pathology (Suzuki 1998). Recent studies indeed described that PSY induces membrane disruption in a liposome-based in vitro study and altered structure and function of lipid-rafts critical for many membrane processes (Hawkins-Salsbury et al. 2013; White et al. 2011). However, studies demonstrating the apoptotic loss of OLs in postmortem brain tissue of KD patients and an in vitro studies supporting exogenous PSY-induced apoptosis of C6 rat glioma cells suggest a role for PSY induced proapoptotic cell signaling mechanisms (Jatana et al. 2002). Though the mechanisms underlying PSY-induced apoptosis are not fully understood at present, a number of studies from various laboratories indicate the participation of multiple cell signaling pathways in PSY-induced toxicities as summarized in table 1.
TABLE 1.
Mechanisms underlying psychosine toxicity.
| Pathways Involved | Selected Citations |
|---|---|
| Membrane stability and functions | |
| Membrane disruption and altered lipid-raft function | White et al., 2009; White et al., 2011; Hawkins-Salsbury et al., 2013 |
| Stress and Proapoptotic Pathways | |
| Inhibition of cell survival signaling (e.g. Akt and Erk): | Hamanoue et al. 2004; Zaka et al., 2005; Won et al. 2013; |
| Activation of proapoptotic pathways (e.g. JNK, p53, caspases 3/9, and cytochrome C release) | Haq et al. 2003; Giri et al., 2006; Ribbens et al., 2014; Smith et al., 2011 |
| Transcription factors (up-and down-regulation of AP-1 and NFκB) | Haq et al. 2003 |
| Immune and Inflammatory Responses | |
| Cellular involvement in the CNS | |
| Astrocytes | Kobayashi et al. 1986; Jesionek-Kupnicka et al. 1997; Mohri et al. 2006; Ransohoff and Brown 2012; Hawkins-Salsbury et al. 2012; Snook et al. 2014 |
| Microglia | Ohno et al. 1993; LeVine and Brown 1997; Bashir and Haq 2011; Ransohoff and Brown 2012; Ijichi et al. 2013; Claycomb et al. 2014; Snook et al. 2014 |
| Peripheral immune cells (e.g. macrophages and lymphocytes) | Kobayashi et al. 1986; Wu et al. 2000; Ohno et al. 1993; Kondo et al. 2011 |
| Pro-or anti-Inflammatory mediators | |
| Proinflammatory cytokines (e.g. IL-1β, IL-6, and TNF-α) | LeVine and Brown 1997; Pedchenko and LeVine 1999; Giri et al. 2002 |
| Chemokines (e.g. MCP-1, MIP-1α, and MIP-1β) | Wu et al. 2000; Wu et al. 2001 |
| Anti-inflammatory cytokine (e.g. IL-10 and LIF) | Wu et al. 2000; Potter et al. 2013 |
| MMP-3 expression and altered ECM composition | Ijichi et al. 2013; Claycomb et al. 2014 |
| Related transcriptional regulation | |
| AP-1, C/EBP, and Oct-1 (no alteration in NFκB) | Giri et al. 2002; Haq et al., 2003 |
| Innate immune responses | |
| OL death induced TLR2 activation of microglia and macrophage. | Snook et al. 2014 |
| Systemic immune/inflammatory responses | |
| Functional impairment of hematopoietic stem/progenitor cells | Visigalli et al. 2010 |
| Thymic atrophy by autonomic denervation | Galbiati et al. 2007 |
| Increased apoptosis of peripheral blood lymphocytes and proinflammatory responses of peripheral blood mononuclear cells. | Formichi et al. 2007 |
| Oxidative and Nitrosative Stress | |
| Increased oxidative stress | Haq et al. 2003; Khan et al. 2005; Giri et al. 2008; Hawkins-Salsbury et al. 2012; Voccoli et al. 2014 |
| Decreased levels of reduced glutathione | Khan et al. 2005; Hawkins-Salsbury et al. 2012 |
| Increased iNOS expression | Giri et al. 2002; Borda et al. 2008; Ripoll et al. 2011; Bashir and Haq 2011; Hawkins-Salsbury et al. 2012 |
| Efficacy of antioxidant treatment (N-acetylcysteine) | Haq et al. 2003; Khan et al. 2005; Giri et al. 2008; Hawkins-Salsbury et al. 2012 |
| Cellular Metabolic Processes | |
| Mitochondrial dysfunctions and decreased ATP levels | Haq et al. 2003; Khan et al. 2005; Formichi et al., 2007; Voccoli et al. 2014 |
| Peroxysomal dysfunction | Khan et al. 2005; Haq et al. 2006 |
| Abnormal lipid metabolism | |
| Upregulated biosynthesis of cholesterol and free fatty acids | Giri et al. 2008 |
| sPLA2 activation and increases in AA and LPC | Giri et al. 2006; Giri et al. 2008 |
| AMPK inactivation | Giri et al. 2008 |
| Neuro-and axono-pathies and Neuromuscular Dysfunctions | |
| Axonal defects and neuronal death | Castelvetri et al. 2011 |
| Decrease peripheral nerve conduction and skeletal muscles atrophy | Dehkharghani et al. 1981; Marjanovic et al. 1996; Dolcetta et al. 2005 |
| Neuromuscular junctional abnormalities by dysregulations of caspases and Akt | Cantuti-Castelvetri et al. 2015 |
| α-synuclein inclusion in neurons | Smith et al. 2014 |
| Neurofilament abnormality by deregulation of PP1 and PP2A and dysregulation of axonal transport by GSK3β activation | Cantuti-Castelvetri et al. 2012; Cantuti Castelvetri et al. 2013 |
| Endothelial and Vascular Dysfunction | |
| Abnormalities in brain microvasculature | |
| Enlargement of perivascular space, dialed lumens, and altered endothelium and smooth muscle cell morphologies. | Belleri et al. 2013; Giacomini et al. 2015; Kondo and Suzuki 1993 |
| Increased brain vascular permeability, vessel fragmentation. | |
| Decreased microvascular density and total vessel lengths. | |
| Underlying mechanism | |
| Decreased endothelial proliferation and migration and thus decreased neovascularization | Belleri et al. 2013 |
| Actin cytoskeleton disruption and decreased tight junction protein | Belleri et al. 2013 |
i) Psychosine induced stress and proapoptotic signaling pathways
Studies using differentiating human OL cells (MO3.13) describe that exogenous PSY induces oxidative stress and their apoptosis via reducing mitochondrial action potentials, releasing mitochondrial cytochrome C, and activating caspase 3 and 9 (Haq et al. 2003). PSY was also reported to activate JNK (Haq et al. 2003), a stress-activated protein kinase which plays a pivotal role in apoptotic cell death in OLs (Jurewicz et al. 2006), and induces activities and expression of cell death signaling, such as caspase-3, PTEN, and Bad, while inhibiting cell survival signaling, such as PI3K, Akt, and Erk activities (Arai and Lo 2010; Graziano et al. 2016; Haq et al. 2003; Won et al. 2013). In addition, the observed p53 immunoreactivity in postmortem brain tissue of KD patients (Jatana et al. 2002), p53 mediated apoptotic death of PSY-treated cultured OLs (Pannuzzo et al. 2010), and PSY-induced decreased activity of NF-κB (which prevents apoptotic death of OLs) (Hamanoue et al. 2004) indicate that multiple signaling pathways participate in PSY induced OL apoptosis. Exogenous PSY was internalized quickly (minutes) in in-vitro OL culture (personal communication) but the mechanism for its internalization is not known at present. T cell death-associated gene 8 (TDAG8) was reported as a receptor for PSY (Im et al. 2001), but subsequent studies revealed that TDAG8 is not involved in PSY-induced OL death (Giri et al. 2006).
ii) Role of psychosine in immune and inflammatory responses
Immune and inflammatory responses mediated by brain resident microglia and astrocytes as well as infiltrating peripheral immune cells have been implicated in the pathogenesis of various CNS disorders (Ransohoff and Brown 2012). Implication of brain resident immune cells in KD pathology was first recognized by the observation of globoid cell, a multinucleated activated microglia or macrophage, in the CNS of KD patients and in brains of twitcher mice (twi), an authentic animal model of KD which lacks galc gene. Although the precise mechanism for formation of globoid cells in response to inflammatory reactions is not understood at present, role of astroglial MMP-3 expression (Ijichi et al. 2013) and altered extracellular matrix composition were reported as possible mechanisms (Claycomb et al. 2014). In the CNS of KD/twi, activation of astrocytes, as characterized by increased proliferation and enhanced expression of glial fibrillary acidic protein (GFAP), is prominent in demyelinating areas of white matter (Jesionek-Kupnicka et al. 1997; Kobayashi et al. 1986). Interestingly, the activation of astrocytes coincided chronologically and topographically with infiltration of macrophages (Kobayashi et al. 1986). Studies with twi mice reported increased expression of chemoattractant protein (e.g. MCP-1) in the brain and increased infiltration of hematogenous lineage cells into the CNS (Wu et al. 2000), thus suggesting interactions between CNS and PNS immune systems in KD/twi pathophysiology. GALC deficiency in twi mice was also reported to induce functional impairment of hematopoietic stem/progenitor cells (Visigalli et al. 2010) and autonomic denervation of lymphoid organs and thus irreversible thymic atrophy (Galbiati et al. 2007) and altered immune-competency. PSY is present in nanomolar concentrations in blood and thymus (Chuang et al. 2013; Galbiati et al. 2007; Zhu et al. 2012) and reported to directly affect survival and inflammatory responses of peripheral blood lymphocytes and peripheral blood mononuclear cells (Formichi et al. 2007). These studies indicate that GALC deficiency systemically affects immune/inflammatory responses.
In the brains of KD and twi mice, the activation of astrocytes and microglia and infiltration of vascular immune cells in demyelinating white matter areas are associated with expression of cytokines (e.g. TNF-α, IL-1β, and IL-6), chemokines (e.g. MCP-1, MIP-1α, and MIP-1β), and iNOS (Borda et al. 2008; Giri et al. 2002; Hawkins-Salsbury et al. 2012; LeVine and Brown 1997; Pedchenko and LeVine 1999; Ripoll et al. 2011; Wu et al. 2001). Therefore, induction of neuroinflammation is one of key events in PSY-mediated OL toxicity in twi mice and KD pathology. Proinflammatory cytokines, such as TNFα and IL-1β, and iNOS-induced oxidative/nitrosative stress was implicated in OL death under demyelinating disease conditions (Buntinx et al. 2004; Smith et al. 1999; Takahashi et al. 2003). However, studies also reported that mice cross-bred from twi and macrophage-deficient osteopetrotic mutant showed a more severe clinical phenotype as compared to the twi mice and thus suggest that macrophages in KD/twi may also play a protective role (Kondo et al. 2011).
Mechanisms underlying the PSY-induced neuroinflammation in the CNS are not fully understood at present. Previous studies with cultured astrocytes reported that PSY itself is unable to induce expression of proinflammatory genes (e.g. IL-1β, IL-6, and TNF-α, and iNOS ) due to its inability to activate NF-κB (Giri et al. 2002), a transcription factor critical for proinflammatory gene expression (Lawrence 2009). Rather, PSY was able to enhance proinflammatory gene expression induced by proinflammatory stimulators, such as lipopolysaccharide or IFNγ and IL-1β by further activation of transcription factors, such as AP-1, C/EBP, and Oct-1 (Giri et al. 2002), thereby suggesting the role of PSY in upregulation of pre-existing inflammation (Fig. 2). It is of interest to note that twi mice exhibited increased expression of TLR2, a member of toll-like-receptor involved in innate immune system, in microglia/macrophages and its activation by PYS-induced OL death resulted in astrocyte activation and up-regulation of cytokine/chemokine gene expressions (Snook et al. 2014). These observations suggest that PSY is directly/indirectly involved in induction and amplification of neuroinflammation in twi mice. However, recent study with another KD mouse model (twi-5J) did not support the relationship of degree of PSY load to inflammatory disease (Potter et al. 2013; Tappino et al. 2010). Twi-5J mice showed higher PSY levels in the CNS than PNS, but hypomyelination and axonal loss were more severe in the PNS (Potter et al. 2013). Accordingly, PNS expressed higher levels of proinflammatory cytokines (e.g. IL-1β) but lower levels of anti-inflammatory and myelin protective cytokine (e.g. LIF) (Marriott et al. 2008; Potter et al. 2013). Although these studies support the critical role of neuroinflammation in OL/Schwann cell survival and myelin stability under KD conditions, the mechanisms underlying differential PSY accumulation and thus regulation of CNS vs. PNS inflammation and demyelination in twi vs. twi-5J mice are not understood at present.
iii) Involvement of secretory phospholipase A2 (sPLA2) in PSY-induced cytotoxicity
PLA2 (EC 3.1.1.4) is a family of enzymes catalyzing the hydrolysis of glycerophospholipids at sn-2 to generate free fatty acids and lyso-phospholipids. Three main categories or types of sPLA2 are; secretory (sPLA2), cytosolic (cPLA2), and calcium-independent PLA2 (iPLA2). Previous studies with brains of KD patients and twi mice and in vitro studies of OLs treated with PSY reported significant increase in levels of PLA2 metabolites, such as arachidonic acid (AA) and lysophosphatidylcholine (LPC) (Giri et al. 2006). Additional cell culture studies described that PSY induced AA and LPC release from glycerophospho lipids in OLs by activation of sPLA2 (Giri et al. 2006). sPLA2 activation under pathological conditions is known to cause excessive production of free fatty acids (e.g. AA) and their metabolites (e.g. prostaglandins, leukotriens, and epoxides) leading to inflammatory reaction, mitochondrial depolarization, oxidative stress, and subsequent cell/tissue injuries (Adibhatla et al. 2003; Boyanovsky and Webb 2009; Buckland et al. 1998; Okuda et al. 1994; Titsworth et al. 2008; Toborek et al. 1999). In addition, concomitant increase in lyso-phospholipids (e.g. LPC) may exert deleterious effects, such as OL apoptosis and myelin breakdown (Blakemore et al. 2002; Shields et al. 1999; Wallace et al. 2003). The observed low levels of reduced glutathione (GSH) in brains of twi mice and OLs treated with PSY, and attenuation of PSY-induced cell death by treatment with pharmacological sPLA2 inhibitor or antioxidant N-acetylcysteine (NAC) (Giri et al. 2008; Giri et al. 2006; Haq et al. 2003; Hawkins-Salsbury et al. 2012; Khan et al. 2005) indicate a pathological role of sPLA2 pathway in PSY-induced oxidative stress and cytotoxicity to OLs.
iv) Psychosine-induced mitochondrial and peroxisomal defects
Mitochondria are the cellular site for energy production as well as site for a number of essential metabolic reactions. Studies have shown that PSY accumulation induces mitochondrial dysfunction, such as loss of action potential, generation of reactive oxygen species (ROS), and release of cytochrome c (Giri et al. 2006; Haq et al. 2003; Voccoli et al. 2014), and thus depletion of ATP as well as GSH (Khan et al. 2005). Although the mechanism underlying PSY-induced mitochondrial dysfunction is not understood, pathognomonic increase in mitochondrial calcium has been recently suggested to be one of mechanisms for PSY-induced mitochondrial dysfunction (Voccoli et al. 2014). In addition, the abnormal increase in sPLA2 activity induced by PSY could also participate in mitochondrial damage (Haq et al. 2003) since LPC, a product of sPLA2, was reported to inhibit mitochondrial enzyme activities (Kalous et al. 1992) and disruption of mitochondrial integrity and thus function (Hollie et al. 2014).
Peroxisomes, another subcellular organelle, discovered in 1950s are now recognized to perform critical cellular functions, such as lipid metabolism as specific fatty acid specific β-oxidation system (Wanders and Waterham 2006), detoxification of hydrogen peroxide by catalase (Schrader and Fahimi 2006), biosynthesis of polyunsaturated fatty acids (PUFAs) and plasmalogens for OLs differentiation and myelin synthesis (Contreras and Rapoport 2002; Hajra and Das 1996; Sprecher 2000). The role of peroxisomes in the formation and maintenance of myelin is underscored by the observed demyelination in peroxisomal disorders of single enzyme deficiency of X-linked adrenoleukodystrophy and deficiency of peroxisomal biogenesis in Zellweger syndrome (Adamo et al. 1986; Houdou et al. 1993; Lazo et al. 1991). The deleterious role of PSY in peroxisomal functions was first reported by Khan et al (Khan et al. 2005) describing PSY-mediated inhibition of peroxisomal β-oxidation, reduced acyl-CoA oxidase expression, and decreased plasmalogen synthesis. Cellular homeostasis of peroxisomes is maintained via a “growth and division” process where nuclear transcription factor peroxisome proliferator-activated receptors (PPARs) play a critical role in peroxisomal biosynthesis (Kersten et al. 2000). PPAR belongs to the nuclear receptor super family, and is composed of three subtypes; PPARα, PPARβ, and PPARγ. PSY was reported to downregulate PPARα activity in OLs via activating sPLA2 pathway (Haq et al. 2006), and thus biogenesis and proliferation of peroxisomes. These observations indicate that excessive PSY accumulation in KD inhibits not only mitochondrial functions but also peroxisomal functions and thus causes disruption of both metabolic and energy homeostasis.
v) PSY-induced AMPK dysregulation and defects in metabolic and energy homeostasis
AMP-activated protein kinase (AMPK) is an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism. As named, AMPK is activated when cells are under conditions of energy deprivation (high AMP and low ATP) (Carling 2005; Hardie 2004). Upon activation (its phosphorylation), it phosphorylates and inactivates a number of anabolic enzymes to save ATP (e.g. acetyl-CoA carboxylase and HMG-CoA reductase) and activates catabolic processes (e.g. glucose uptake and fatty acid oxidation) to produce ATP (Carling 2005; Hardie 2004). Therefore, PSY induced mitochondrial dysfunction causes ATP depletion (Haq et al. 2003; Khan et al. 2005; Voccoli et al. 2014). Nevertheless, AMPK activity in OLs was down-regulated under KD conditions and biosynthesis of lipids (e.g. cholesterol and free fatty acids), which consumes the cellular ATP, was up-regulated (Giri et al. 2008). These observations suggest that AMPK inactivation by PSY under KD conditions may produce severe metabolic imbalance that accelerates ATP depletion in OLs. Pharmacological activation of AMPK, by 5-aminoimidazole-4-carboxamide-1- β-4-ribofuranoside (AICAR) an analogue of AMP, attenuated the PSY-induced down-regulation of AMPK and restored the altered biosynthesis of lipids (Giri et al. 2008). However, AICAR was not able to rescue mitochondrial dysfunction and ROS generation induced by PSY (Giri et al. 2008), suggesting that PSY-induced AMPK down-regulation and mitochondrial dysfunction are irreversible and/or caused by different cell signaling pathways.
vi) Neuro- and axono-pathy and neuromuscular dysfunctions
The atrophy of skeletal muscles in patients with KD is one of the major debilitating manifestations (Dehkharghani et al. 1981; Marjanovic et al. 1996) and demyelination of PNS has been regarded as one of mechanisms in muscle dysfunction (Duchen et al. 1980; Kondo et al. 1988; Powell et al. 1983; Tanaka et al. 1988). However, a growing body of evidence suggests that neuro-muscular pathology in KD may involve PSY-mediated direct abnormality and degeneration of neurons, axons, and neuromuscular junctions (Cantuti-Castelvetri et al. 2015; Castelvetri et al. 2011; Dolcetta et al. 2005; Smith et al. 2014). PSY was reported to induce neuronal inclusions of α-synuclein (Smith et al. 2014), neurofilament abnormalities by deregulation of PP1 and PP2A phosphatases (Cantuti-Castelvetri et al. 2012), and dysregulation of axonal transport induced by activation of glycogen synthase kinase 3β (GSK3β) (Cantuti Castelvetri et al. 2013) and thus axonal defects and neuronal death (Castelvetri et al. 2011). In addition, PSY is also known to decrease peripheral nerve conduction (Dolcetta et al. 2005) and increase neuromuscular junctional abnormalities by activation of caspase and repression of Akt activity (Cantuti-Castelvetri et al. 2015). Another study reported autonomic denervation of lymphoid organs leading to immune atrophy in twi mice (Galbiati et al. 2007). These studies summarize the role of PSY-mediated toxicity in neuro-/axono-pathy and neuromuscular and immune dysfunctions.
vii) Brain microvascular dysfunction
Alterations of brain micro-vasculatures and disruption of blood-brain barrier (BBB) are critical features of many neurological disorders (Belleri and Presta 2016; Greenberg and Jin 2005; Rosenberg 2012; Segura et al. 2009). The abnormalities in brain microvasculature in KD were initially reported by Kondo and Suzuki (Kondo and Suzuki 1993) and later by other research groups (Belleri and Presta 2016; Belleri et al. 2013; Giacomini et al. 2015). Key features of the microvasculature abnormalities in the brains of KD patients or twi mice include enlargement of perivascular space, increased brain vascular permeability, macrophage infiltration, decreased microvessel density, reduced vessel length, increased vessel fragmentation, dialed lumens with altered endothelium morphology, and discontinuous endothelial wrapping by smooth muscle cells (Belleri et al. 2013; Giacomini et al. 2015; Kondo and Suzuki 1993). Mechanisms underlying microvasculature abnormalities in KD/twi brains are largely illusive but recent studies by Belleri et al. (Belleri et al. 2013) suggested the involvements of PSY-induced reductions in endothelial proliferation and migration and decreased response to proangiogenic factors (e.g. VEGF and FGF2), and thus impeded neovascularization. In addition, PSY-induced disruption of actin cytoskeleton and decreased expression of tight junction protein (e.g. ZO-1) (Belleri et al. 2013) may also contribute to the alterations in microvascular ultrastructure and its permeability. Overall, these studies suggest that functional and structural deficits in brain microvasculatures are a novel pathogenic mechanism in KD/twi and thus a potential therapeutic target.
7. Therapeutic approaches for Krabbe disease
Since Miyatake and Suzuki proposed “psychosine hypothesis” in 1972 (Miyatake and Suzuki 1972), numerous therapies have been attempt in human and animal models to lower the PSY burden in the CNS and PNS and thus to reduce the PSY-induced neural toxicity. These include pharmacological, gene, stem cell, enzyme replacement, pharmacological chaperon, and anti-inflammatory and anti-oxidant therapies.
i) Pharmacological inhibitor of sphingolipid synthesis
Progressive accumulation of neurotoxic PSY is a key hallmark of KD. Therefore, intensive efforts, by numerous investigators, focused on development of therapeutic approaches for reducing the synthesis of PSY using galactoshingolipid synthesis inhibitors. At present there is no known specific inhibitor for CGT. Rather, pharmacological inhibitor of sphingolipid synthesis (L-cycloserine) was tested in twi mice for its therapeutic potential for KD. L-cycloserine is an irreversible inhibitor of 3-ketodihydrosphingosine synthetase which is the first enzyme of the sphingolipid pathway. Treatment of twi mice with L-cycloserine was reported to reduce psychosine burden in neural tissue and disease pathology along with some increase in life-span but the tested mice eventually died with KD pathologies (Biswas and LeVine 2002; LeVine et al. 2000). In spite of the observed efficacy, clinical use of L-cycloserine for KD remains a concern because of potential adverse effect of inhibition of sphingolipid biosynthesis. Sphingolipid synthesis is required for normal brain development (Hirabayashi and Furuya 2008) and, therefore, L-cycloserine-mediated global inhibition of sphingolipid synthesis could potentially disturb normal brain development.
ii) Enzyme replacement therapy (ERT)
The theoretical basis of ERT for KD is that lysosomal enzymes including GALC secreted from enzyme-producing cells are taken up by surrounding enzyme-deficient cells, a phenomenon known as cross-correction (Sands and Davidson 2006). As compared to other therapies (e.g. gene and stem cell therapies), ERT is readily applicable in the general clinical setting, and suitable not only for stand-alone therapy but also in combination with other therapeutic approaches. ERT has been successful with lysosomal disorders (LSDs) of Gaucher disease and Fabry disease (Baldo 2015; Germain 2002; Schiffmann et al. 1997). The molecular basis of ERT for LSDs is that the secreted lysosomal hydrolases tagged with unique mannose-6-phosphate (M6P) group are taken up by cells with specific M6P receptors (Coutinho et al. 2012). Human GALC protein possesses 4 potential N-glycosylation sites (Deane et al. 2011) which play a role in M6P receptor mediated targeting of GALC to lysosomes (Kornfeld 1986). In cell culture studies, GALC deficient OLs were able to uptake exogenous GALC enzyme (Kondo et al. 2005; Luddi et al. 2001). In addition, systemic GALC treatments of twi mice as well as human mutant GALC knock-in mice were reported to reduce the CNS psychosine accumulation (Lee et al. 2005; Matthes et al. 2015). However, current ERT therapy based on recombinant GALC ERT was not successful in stopping the death of animals from KD pathologies (Lee et al. 2005; Matthes et al. 2015). The reasons for the limited efficacy of recombinant GALC ERT are reported to be its inability to cross the BBB and low cross-correction efficiency of recombinant wild type GALC (Kim 2014; Meng et al. 2005; Wenger et al. 2000). Recently, studies described increased GALC cross-activity and BBB permeability by using a fusion protein [GALC with HIV-1 transactivator of transcription (Tat) protein transduction domain (PTD)] (Meng et al. 2013; Zhang et al. 2008). Tat-PTD is a short peptide known to efficientlty cross plasma membranes (Choi et al. 2006; Derossi et al. 1996; Derossi et al. 1998; Derossi et al. 1994; Fu et al. 2006; Kim et al. 2005; McConnell et al. 2007; Meng et al. 2013; Wu et al. 2006; Zhang et al. 2008). GALC fusion with Tat-PTD increased its cellular uptake in cultured skin fibroblasts from KD patients as compared to wild type GALC lacking Tat domain (Meng et al. 2013; Zhang et al. 2008). This increased cellular uptake correlated with increased lysosomal accumulation of Tat-GALC fusion protein in lysosomes as well as in other subcellular compartments (Meng et al. 2013; Zhang et al. 2008). Since GALC has optimal enzyme activity at lysosomal low pH (Hill et al. 2013), additional or alternative approaches for specific targeting or enrichment of GALC to lysosomes is required for successful ERT in KD.
iii) Gene therapy
Gene therapy for sustained delivery of functional GALC gene was tested using viral delivery systems (e.g. adenovirus, lentivirus, and adeno-associated virus) in animal models of KD. Transfer of GALC gene using viral vectors significantly inhibited CNS accumulation of psychosine and prolonged the life span of tested twi mice, but again all tested animals eventually died (Galbiati et al. 2009; Rafi et al. 2012; Rafi et al. 2015a; Shen et al. 2001). For instance, administration of twi mice with GALC expressing adeno-associated-virus vector of serotype rh10 (AAVrh10), which cross the blood–brain barrier (BBB) (Zhang et al. 2011), resulted in increased GALC activities in brain as well as in other organs and significantly improved the weight gain, lifespan, fertility, neuropathology, and myelination (Rafi et al. 2012; Rafi et al. 2015a). However, the mice eventually suffered hind leg weakness and paralysis (Rafi et al. 2012; Rafi et al. 2015a). The gene therapy using current viral vectors is highly efficient and can transduce a wide variety of cell types and tissues. However, its requirement for high dosage(s) of viral vectors for global replacement of GALC gene in human brain is a critical limitation of the approache.
iv) Stem cell therapy
Stem cell therapy, which can provide healthy cells with intact GALC activity to allow remyelination, has been suggested as a promising alternative approach for treatment of KD. Transplantation of hematopoietic stem cells (HSCs) and umbilical cord blood-derived stem cells (UCBSCs) from compatible healthy donors attenuated the disease course but the patients eventually developed deterioration of motor and language skills (Escolar et al. 2005; Krivit et al. 1998; Pastores 2009). Several factors may be contributing for this inefficacy of stem cell transplantation, including broad distribution of cells in the body, and relatively large size of the targeted human brain where stem cells may access and correct only limited area(s) of brain. In addition, low efficiency of stem cell differentiation into mature OLs is another drawback of stem cell therapy. To overcome these limitations, embryonic stem cell-derived OLs were transplanted into twi mice (Kuai et al. 2015). However, there was no significant improvement in brain GALC activity as well as disease pathology of twi mice possibly due to poor cell survival and limited migration ability of transplanted OLs (Kuai et al. 2015). Immune rejection of the transplanted cells is potentially another critical drawback of HSC and UCBSC transplantation. Genetically engineered stem cells (patient derived HSCs) with lentiviral vectors expressing functional GALC gene (Biffi et al. 2013) or multipotent progenitor cells or induced pluripotent stem cells (iPSCs) (Giri and Bader 2015) are interesting. These approaches allow to produce large quantities of genetically engineered stem cells which are compatible with the patient immune system. However, relatively longer preparation time for sufficient numbers of genetically engineered stem cells and related technical difficulties allow only experienced centers with needed technical facilities to perform these types of studies (Herberts et al. 2011). However, a study has described that forced GALC expression to higher levels is also toxic to hematopoietic stem and progenitor cells (HSPC) (Gentner et al. 2010). To avoid HSPC cytotoxicity induced by GALC overexpression, GALC gene in lentiviral vector was incorporated with specific target sequence for microRNAs (e.g. miR-126 and miR-130a) which are specifically expressed in HSPCs to inhibit GALC gene over-expression (Gentner et al. 2010; Ungari et al. 2015). Transplantation of these genetically modified HSPCs increased the life span of twi mice and ameliorated phenotype as compared to untreated siblings but again the mice eventually developed the disease (Gentner et al. 2010; Ungari et al. 2015). So far, no successful stem cell approach has extended the life span of twi mice similar to that of wild type controls. Recent studies described that combination of stem cell transplantation and gene therapy greatly extended the lifespan of twi mice with normal behavior and improved CNS and PNS findings (Rafi et al. 2015b; Ricca et al. 2015). Therefore, combination of different strategies for restoring GALC activity would be ideal approach for efficient therapy for KD.
v) Pharmacological chaperone therapy
Nearly 70% of disease-associated missense mutations are predicted to cause instability and misfolding of GALC (Deane et al. 2011; Wenger et al. 1997) and some of these mutations observed in late-onset KD still retain partial GALC activity (Conzelmann and Sandhoff 1983). Recently, pharmacological chaperone therapy (PCT) has emerged as an alternative strategy for treating LSDs caused by partially defective enzyme activities (see review (Parenti et al. 2015)). Pharmacological chaperone is a small molecule that specifically binds to the catalytic site of the misfolded enzyme and thus facilitates its proper folding, maturation, and transport to final cellular destination (Parenti et al. 2015). The first such study of PCT on KD was reported by Lee et al. where treatment of cells expressing human mutant GALC (D528N) with α-lobeline, a novel PCT compound for GALC, increased the cellular GALC enzyme activity (Lee et al. 2010). However, this compound was ineffective on other GALC mutations (e.g. I234T and L629R). Later, N-octyl-4-epi-β-valienamine (NOEV), a synthetic β-galactose-type unsaturated carbasugar amine, was tested and reported to stabilize enzyme activity of mutant GALC (e.g. G270D and G352R) in skin fibroblasts (Hossain et al. 2015). However, such efficacy was not observed in skin fibroblasts bearing different GALC mutations from other patients (Hossain et al. 2015). Recently, a study discussed that azasugar derivatives (iso-galactofagomine and aza-galacto-fagomine) specifically bind at the GALC active site and stabilize GALC (Hill et al. 2015) but their efficacies on different types of GALC mutants are not validated at present. The PCT is an innovative approach for KD especially for late-onset forms bearing misfolding mutations but with major drawbacks. In most cases, pharmacological chaperones are reversible competitive inhibitors of target enzymes and bind to the active site of target proteins, the net increase in enzyme activity is variable with different enzyme variants bearing different mutations (Parenti et al. 2015).
vi) Anti-inflammatory and anti-oxidant therapies
Anti-inflammatory therapy
In brains of KD patients and twi mice, inflammatory activities are commonly associated with PSY-induced demyelination/dysmyelination processes (Adachi and Chiba 2008; Formichi et al. 2007; Giri et al. 2002; Giri et al. 2006; Haq et al. 2003; Kagitani-Shimono et al. 2005; Kappos et al. 2010; LeVine and Brown 1997; Luzi et al. 2009; O’Sullivan and Dev 2015; Pasqui et al. 2007). The inflammation in the white matter seems to be the secondary process to PSY-induced OL death since proinflammatory cytokines and associated disease mechanisms are potentially harmful to myelin (Peterson and Fujinami 2007). Therefore, targeting the mechanism responsible for expression of proinflammatory mediators is regarded as one of the strategies to delay or suppress the disease process. Treatment of twi mice with ibudilast (MN-166), an anti-inflammatory phospho-diesterase inhibitor (Fujimoto et al. 1999; Suzumura et al. 1999; Taguchi et al. 1999), reduced microglial activation, apoptotic loss of OLs, and myelin loss but failed to extend the life span (Kagitani-Shimono et al. 2005). Another study evaluated efficacies of nonsteroidal anti-inflammatory drugs (NSAIDs), such as minocycline, indomethacin, and ibuprofen, on mice expressing mutant GALC (H168C) and reported that these NSAIDs extended life span of the tested mice to some degree but the tested mice died with the disease (Luzi et al. 2009; Luzi et al. 2001).
Anti-oxidant therapy
CNS disease of twi mice is associated with severe PSY-induced oxidative stress and mitochondrial dysfunction (Giri et al. 2008; Haq et al. 2003; Hawkins-Salsbury et al. 2012; Khan et al. 2005). OLs are more sensitive to oxidative stress than other brain cells, seemingly due to a low capacity of antioxidant defenses (Smith et al. 1999). Therefore, normalization of the redox potential of OLs is important in attenuation of OL loss in the CNS of KD patients and twi mice. A study using diet-based therapy showed that combination of galactose free soy isoflavones and antioxidant mixture (Lglutathione, Coenzyme Q10, and xanthophylls) significantly attenuated disease progression in twi mice (Pannuzzo et al. 2010). The cell culture study also showed that each antioxidant component and their combination inhibited PSY-induced OL death (Pannuzzo et al. 2010). N-acetyl cysteine (NAC) is a thiol-based anti-oxidant and it functions either directly as a reactive oxygen species (ROS) scavenger or indirectly as a glutathione precursor (Atkuri et al. 2007). In cell culture studies, NAC treatment protected against PSY-induced OL death (Giri et al. 2008; Haq et al. 2003; Khan et al. 2005). In twi mice, NAC treatment was effective in decreasing oxidative stress and improving immunohistochemical markers of disease (Hawkins-Salsbury et al. 2012), but without any clinical improvements (Hawkins-Salsbury et al. 2012). The brains of KD patients as well as twi mice showed impaired vitamin D homeostasis and thus vitamin D deficiency (Paintlia et al. 2015). Vitamin D, a member of fat-soluble secosteroids responsible for intestinal absorption of calcium and phosphate and a potent membrane antioxidant (Wiseman 1993), was also tested in twi mouse model. The supplementation of twi pups with vitamin D3 (cholecalciferol, a precursor of vitamin D) via breast milk from the nursing mother before weaning and its continuous supplementation with chow after weaning significantly delayed the neurological disabilities with modest increase in lifespan of twi mice (Paintlia et al. 2015). Although the present anti-oxidant therapy as a stand-alone therapy had limited efficacy in KD pathology, combining the anti-oxidant/anti-inflammatory therapy with therapy targeting PSY accumulation is a potentially attractive approach for control of KD disease processes.
8. Summary and conclusion
KD, a fatal neurological disorder, is caused by genetic inheritance of mutant GALC causing progressive accumulation of PSY and demyelination/neurodegeneration. PSY is a neurotoxic metabolic byproduct of galactosphingolipids which are the major lipids of OLs and myelin, hence, loss of OLs and myelin are the primary underlying mechanisms in KD. Since Twi mice express significant levels of myelin (Paintlia et al. 2015), this suggests that not all OLs are seemingly affected by GALC deficiency and PSY toxicity and that some of the OLs undergo complete differentiation into myelin producing mature-OLs. The differentiation stage specific expression of CGT and GalCer in OLs and their death under GALC deficient conditions and the observed MR abnormalities in active myelinating area of KD brains suggests that KD pathology may involve both dysmyelinating and demyelinating mechanisms and that timely targeting of these pathologies in different brain area is critical for effective therapeutic approach for KD. There is also significant evidence that KD involves multiple secondary disease processes, such as immune, inflammatory, vascular, and neuronal, and axonal pathologies. Therefore, combination of approaches to decrease PSY load with antioxidant/anti-inflammatory therapies may potentially be effective against spatiotemporal disease processes of KD.
Significance Statement.
Inherited deficiency of lysosomal enzyme activity of galactocerebrosidase (GALC) and subsequent pathognomonic accumulation of psychosine and psychosine-induced loss of oligodendrocytes and myelin are the “hallmark” of Krabbe disease (KD) which mostly affects infants. Over the years, considerable efforts have been made in search of effective therapy using pharmacological, recombinant enzyme, gene and stem cell approaches to reduce endogenous psychosine load and to attenuate psychosine-induced neurotoxicity. However, KD continues to remain without a cure. The aim of this review is to provide an up-to-date discussion of the disease mechanisms and evaluation of prospective therapeutic approaches for KD.
Acknowledgments
The authors declare no conflict of interests regarding the publication of this paper. This work was supported by NIH research grant NS064195.
References
- Adachi K, Chiba K. FTY720 story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspectives in medicinal chemistry. 2008;1:11–23. [PMC free article] [PubMed] [Google Scholar]
- Adamo AM, Aloise PA, Pasquini JM. A possible relationship between concentration of microperoxisomes and myelination. Int J Dev Neurosci. 1986;4(6):513–517. doi: 10.1016/0736-5748(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, Dempsey RJ. Phospholipase A2, hydroxyl radicals, and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal. 2003;5(5):647–654. doi: 10.1089/152308603770310329. [DOI] [PubMed] [Google Scholar]
- Arai K, Lo EH. Astrocytes protect oligodendrocyte precursor cells via MEK/ERK and PI3K/Akt signaling. Journal of neuroscience research. 2010;88(4):758–763. doi: 10.1002/jnr.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Current opinion in pharmacology. 2007;7(4):355–359. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA. Enzymes approved for human therapy: indications, mechanisms and adverse effects. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2015;29(1):31–55. doi: 10.1007/s40259-015-0116-7. [DOI] [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. Journal of neuroscience research. 1989;24(4):548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1 Pt 1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Belleri M, Presta M. Endothelial cell dysfunction in globoid cell leukodystrophy. Journal of neuroscience research. 2016 doi: 10.1002/jnr.23744. [DOI] [PubMed] [Google Scholar]
- Belleri M, Ronca R, Coltrini D, Nico B, Ribatti D, Poliani PL, Giacomini A, Alessi P, Marchesini S, Santos MB, Bongarzone ER, Presta M. Inhibition of angiogenesis by beta-galactosylceramidase deficiency in globoid cell leukodystrophy. Brain. 2013;136(Pt 9):2859–2875. doi: 10.1093/brain/awt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G, Schachner M. Immunoelectron microscopic characterization of galactocerebroside and nervous system antigen-1 (NS-1) positive oligodendrocytes in culture. Neurosci Lett. 1982;28(1):75–80. doi: 10.1016/0304-3940(82)90211-7. [DOI] [PubMed] [Google Scholar]
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, Baldoli C, Martino S, Calabria A, Canale S, Benedicenti F, Vallanti G, Biasco L, Leo S, Kabbara N, Zanetti G, Rizzo WB, Mehta NA, Cicalese MP, Casiraghi M, Boelens JJ, Del Carro U, Dow DJ, Schmidt M, Assanelli A, Neduva V, Di Serio C, Stupka E, Gardner J, von Kalle C, Bordignon C, Ciceri F, Rovelli A, Roncarolo MG, Aiuti A, Sessa M, Naldini L. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- Biswas S, LeVine SM. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatric research. 2002;51(1):40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Chari DM, Gilson JM, Crang AJ. Modelling large areas of demyelination in the rat reveals the potential and possible limitations of transplanted glial cells for remyelination in the CNS. Glia. 2002;38(2):155–168. doi: 10.1002/glia.10067. [DOI] [PubMed] [Google Scholar]
- Borda JT, Alvarez X, Mohan M, Ratterree MS, Phillippi-Falkenstein K, Lackner AA, Bunnell BA. Clinical and immunopathologic alterations in rhesus macaques affected with globoid cell leukodystrophy. Am J Pathol. 2008;172(1):98–111. doi: 10.2353/ajpath.2008.070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanovsky BB, Webb NR. Biology of secretory phospholipase A2. Cardiovasc Drugs Ther. 2009;23(1):61–72. doi: 10.1007/s10557-008-6134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Kinkaid AR, Wilton DC. Cardiolipin hydrolysis by human phospholipases A2. The multiple enzymatic activities of human cytosolic phospholipase A2. Biochim Biophys Acta. 1998;1390(1):65–72. doi: 10.1016/s0005-2760(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. Journal of neuroscience research. 2004;76(6):834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Maravilla E, Marshall M, Tamayo T, D’Auria L, Monge J, Jeffries J, Sural-Fehr T, Lopez-Rosas A, Li G, Garcia K, van Breemen R, Vite C, Garcia J, Bongarzone ER. Mechanism of neuromuscular dysfunction in Krabbe disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(4):1606–1616. doi: 10.1523/JNEUROSCI.2431-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Zhu H, Givogri MI, Chidavaenzi RL, Lopez-Rosas A, Bongarzone ER. Psychosine induces the dephosphorylation of neurofilaments by deregulation of PP1 and PP2A phosphatases. Neurobiology of disease. 2012;46(2):325–335. doi: 10.1016/j.nbd.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti Castelvetri L, Givogri MI, Hebert A, Smith B, Song Y, Kaminska A, Lopez-Rosas A, Morfini G, Pigino G, Sands M, Brady ST, Bongarzone ER. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(24):10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D. AMP-activated protein kinase: balancing the scales. Biochimie. 2005;87(1):87–91. doi: 10.1016/j.biochi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Castelvetri LC, Givogri MI, Zhu H, Smith B, Lopez-Rosas A, Qiu X, van Breemen R, Bongarzone ER. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta neuropathologica. 2011;122(1):35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Wenger DA. Galactocerebrosidase from human urine: purification and partial characterization. Biochimica et biophysica acta. 1993;1170(1):53–61. doi: 10.1016/0005-2760(93)90175-9. [DOI] [PubMed] [Google Scholar]
- Choi HS, Lee SH, Kim SY, An JJ, Hwang SI, Kim DW, Yoo KY, Won MH, Kang TC, Kwon HJ, Kang JH, Cho SW, Kwon OS, Choi JH, Park J, Eum WS, Choi SY. Transduced Tat-alpha-synuclein protects against oxidative stress in vitro and in vivo. Journal of biochemistry and molecular biology. 2006;39(3):253–262. doi: 10.5483/bmbrep.2006.39.3.253. [DOI] [PubMed] [Google Scholar]
- Chuang WL, Pacheco J, Zhang XK, Martin MM, Biski CK, Keutzer JM, Wenger DA, Caggana M, Orsini JJ., Jr Determination of psychosine concentration in dried blood spots from newborns that were identified via newborn screening to be at risk for Krabbe disease. Clinica chimica acta; international journal of clinical chemistry. 2013;419:73–76. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Claycomb KI, Winokur PN, Johnson KM, Nicaise AM, Giampetruzzi AW, Sacino AV, Snyder EY, Barbarese E, Bongarzone ER, Crocker SJ. Aberrant production of tenascin-C in globoid cell leukodystrophy alters psychosine-induced microglial functions. Journal of neuropathology and experimental neurology. 2014;73(10):964–974. doi: 10.1097/NEN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland WW, Kennedy EP. The enzymatic synthesis of psychosine. J Biol Chem. 1960;235:45–51. [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86(2):209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Collier J, Greenfield JG. The encephalitis periaxialis of Schilder: A clinical and pathological study, with an account of two cases, one of which was diagnosed during life. Brain. 1924;47:489–519. [Google Scholar]
- Contreras MA, Rapoport SI. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr Opin Lipidol. 2002;13(3):267–272. doi: 10.1097/00041433-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Conzelmann E, Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Developmental neuroscience. 1983;6(1):58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Coutinho MF, Prata MJ, Alves S. Mannose-6-phosphate pathway: a review on its role in lysosomal function and dysfunction. Molecular genetics and metabolism. 2012;105(4):542–550. doi: 10.1016/j.ymgme.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Dambska M, Wisniewski KE. Normal and Pathologic Development of the Human Brain and Spinal Cord. London, England: John Libbey & Company Ltd; 1999. [Google Scholar]
- De Gasperi R, Gama Sosa MA, Sartorato EL, Battistini S, MacFarlane H, Gusella JF, Krivit W, Kolodny EH. Molecular heterogeneity of late-onset forms of globoid-cell leukodystrophy. American journal of human genetics. 1996;59(6):1233–1242. [PMC free article] [PubMed] [Google Scholar]
- Deane JE, Graham SC, Kim NN, Stein PE, McNair R, Cachon-Gonzalez MB, Cox TM, Read RJ. Insights into Krabbe disease from structures of galactocerebrosidase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15169–15173. doi: 10.1073/pnas.1105639108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkharghani F, Sarnat HB, Brewster MA, Roth SI. Congenital muscle fiber-type disproportion in Krabbe’s leukodystrophy. Archives of neurology. 1981;38(9):585–587. doi: 10.1001/archneur.1981.00510090079010. [DOI] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. The Journal of biological chemistry. 1996;271(30):18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends in cell biology. 1998;8(2):84–87. [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. The Journal of biological chemistry. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- Dietrich RB, Bradley WG, Zaragoza EJt, Otto RJ, Taira RK, Wilson GH, Kangarloo H. MR evaluation of early myelination patterns in normal and developmentally delayed infants. AJR American journal of roentgenology. 1988;150(4):889–896. doi: 10.2214/ajr.150.4.889. [DOI] [PubMed] [Google Scholar]
- Dolcetta D, Amadio S, Guerrini U, Givogri MI, Perani L, Galbiati F, Sironi L, Del Carro U, Roncarolo MG, Bongarzone E. Myelin deterioration in Twitcher mice: motor evoked potentials and magnetic resonance imaging as in vivo monitoring tools. Journal of neuroscience research. 2005;81(4):597–604. doi: 10.1002/jnr.20574. [DOI] [PubMed] [Google Scholar]
- Duchen LW, Eicher EM, Jacobs JM, Scaravilli F, Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103(3):695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. The Journal of cell biology. 1999;147(6):1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. The New England journal of medicine. 2005;352(20):2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- Fiumara A, Barone R, Arena A, Filocamo M, Lissens W, Pavone L, Sorge G. Krabbe leukodystrophy in a selected population with high rate of late onset forms: longer survival linked to c.121G>A (p.Gly41Ser) mutation. Clinical genetics. 2011;80(5):452–458. doi: 10.1111/j.1399-0004.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- Formichi P, Radi E, Battisti C, Pasqui A, Pompella G, Lazzerini PE, Laghi-Pasini F, Leonini A, Di Stefano A, Federico A. Psychosine-induced apoptosis and cytokine activation in immune peripheral cells of Krabbe patients. Journal of cellular physiology. 2007;212(3):737–743. doi: 10.1002/jcp.21070. [DOI] [PubMed] [Google Scholar]
- Fu AL, Wu SP, Dong ZH, Sun MJ. A novel therapeutic approach to depression via supplement with tyrosine hydroxylase. Biochemical and biophysical research communications. 2006;351(1):140–145. doi: 10.1016/j.bbrc.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Fu L, Inui K, Nishigaki T, Tatsumi N, Tsukamoto H, Kokubu C, Muramatsu T, Okada S. Molecular heterogeneity of Krabbe disease. Journal of inherited metabolic disease. 1999;22(2):155–162. doi: 10.1023/a:1005449919660. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Sakoda S, Fujimura H, Yanagihara T. Ibudilast, a phosphodiesterase inhibitor, ameliorates experimental autoimmune encephalomyelitis in Dark August rats. Journal of neuroimmunology. 1999;95(1–2):35–42. doi: 10.1016/s0165-5728(98)00251-3. [DOI] [PubMed] [Google Scholar]
- Furuya H, Kukita Y, Nagano S, Sakai Y, Yamashita Y, Fukuyama H, Inatomi Y, Saito Y, Koike R, Tsuji S, Fukumaki Y, Hayashi K, Kobayashi T. Adult onset globoid cell leukodystrophy (Krabbe disease): analysis of galactosylceramidase cDNA from four Japanese patients. Human genetics. 1997;100(3–4):450–456. doi: 10.1007/s004390050532. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Basso V, Cantuti L, Givogri MI, Lopez-Rosas A, Perez N, Vasu C, Cao H, van Breemen R, Mondino A, Bongarzone ER. Autonomic denervation of lymphoid organs leads to epigenetic immune atrophy in a mouse model of Krabbe disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(50):13730–13738. doi: 10.1523/JNEUROSCI.3379-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Givogri MI, Cantuti L, Rosas AL, Cao H, van Breemen R, Bongarzone ER. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. Journal of neuroscience research. 2009;87(8):1748–1759. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE. Two proliferative stages of the oligodendrocyte lineage (A2B5+O4− and O4+GalC−) under different mitogenic control. Neuron. 1990;5(5):615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- Gentner B, Visigalli I, Hiramatsu H, Lechman E, Ungari S, Giustacchini A, Schira G, Amendola M, Quattrini A, Martino S, Orlacchio A, Dick JE, Biffi A, Naldini L. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Science translational medicine. 2010;2(58):58ra84. doi: 10.1126/scitranslmed.3001522. [DOI] [PubMed] [Google Scholar]
- Germain DP. Fabry disease: recent advances in enzyme replacement therapy. Expert opinion on investigational drugs. 2002;11(10):1467–1476. doi: 10.1517/13543784.11.10.1467. [DOI] [PubMed] [Google Scholar]
- Giacomini A, Ackermann M, Belleri M, Coltrini D, Nico B, Ribatti D, Konerding MA, Presta M, Righi M. Brain angioarchitecture and intussusceptive microvascular growth in a murine model of Krabbe disease. Angiogenesis. 2015;18(4):499–510. doi: 10.1007/s10456-015-9481-6. [DOI] [PubMed] [Google Scholar]
- Giri S, Bader A. A low-cost, high-quality new drug discovery process using patient-derived induced pluripotent stem cells. Drug discovery today. 2015;20(1):37–49. doi: 10.1016/j.drudis.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK. Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16(7):661–672. doi: 10.1096/fj.01-0798com. [DOI] [PubMed] [Google Scholar]
- Giri S, Khan M, Nath N, Singh I, Singh AK. The role of AMPK in psychosine mediated effects on oligodendrocytes and astrocytes: implication for Krabbe disease. Journal of neurochemistry. 2008;105(5):1820–1833. doi: 10.1111/j.1471-4159.2008.05279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S, Khan M, Rattan R, Singh I, Singh AK. Krabbe disease: psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. Journal of lipid research. 2006;47(7):1478–1492. doi: 10.1194/jlr.M600084-JLR200. [DOI] [PubMed] [Google Scholar]
- Given CA, 2nd, Santos CC, Durden DD. Intracranial and spinal MR imaging findings associated with Krabbe’s disease: case report. AJNR American journal of neuroradiology. 2001;22(9):1782–1785. [PMC free article] [PubMed] [Google Scholar]
- Graziano AC, Cardile V. History, genetic, and recent advances on Krabbe disease. Gene. 2015;555(1):2–13. doi: 10.1016/j.gene.2014.09.046. [DOI] [PubMed] [Google Scholar]
- Graziano AC, Parenti R, Avola R, Cardile V. Krabbe disease: involvement of connexin43 in the apoptotic effects of sphingolipid psychosine on mouse oligodendrocyte precursors. Apoptosis. 2016;21(1):25–35. doi: 10.1007/s10495-015-1183-4. [DOI] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438(7070):954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- Hajra AK, Das AK. Lipid biosynthesis in peroxisomes. Ann N Y Acad Sci. 1996;804:129–141. doi: 10.1111/j.1749-6632.1996.tb18613.x. [DOI] [PubMed] [Google Scholar]
- Hamanoue M, Yoshioka A, Ohashi T, Eto Y, Takamatsu K. NF-kappaB prevents TNF-alpha-induced apoptosis in an oligodendrocyte cell line. Neurochemical research. 2004;29(8):1571–1576. doi: 10.1023/b:nere.0000029571.39497.56. [DOI] [PubMed] [Google Scholar]
- Haq E, Contreras MA, Giri S, Singh I, Singh AK. Dysfunction of peroxisomes in twitcher mice brain: a possible mechanism of psychosine-induced disease. Biochemical and biophysical research communications. 2006;343(1):229–238. doi: 10.1016/j.bbrc.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Haq E, Giri S, Singh I, Singh AK. Molecular mechanism of psychosine-induced cell death in human oligodendrocyte cell line. Journal of neurochemistry. 2003;86(6):1428–1440. doi: 10.1046/j.1471-4159.2003.01941.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. Journal of cell science. 2004;117(Pt 23):5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Parameswar AR, Jiang X, Schlesinger PH, Bongarzone E, Ory DS, Demchenko AV, Sands MS. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. Journal of lipid research. 2013;54(12):3303–3311. doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins-Salsbury JA, Qin EY, Reddy AS, Vogler CA, Sands MS. Oxidative stress as a therapeutic target in globoid cell leukodystrophy. Experimental neurology. 2012;237(2):444–452. doi: 10.1016/j.expneurol.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. Journal of translational medicine. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CH, Graham SC, Read RJ, Deane JE. Structural snapshots illustrate the catalytic cycle of beta-galactocerebrosidase, the defective enzyme in Krabbe disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20479–20484. doi: 10.1073/pnas.1311990110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CH, Viuff AH, Spratley SJ, Salamone S, Christensen SH, Read RJ, Moriarty NW, Jensen HH, Deane JE. Azasugar inhibitors as pharmacological chaperones for Krabbe disease. Chemical science. 2015;6(5):3075–3086. doi: 10.1039/c5sc00754b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Furuya S. Roles of l-serine and sphingolipid synthesis in brain development and neuronal survival. Progress in lipid research. 2008;47(3):188–203. doi: 10.1016/j.plipres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Hollie NI, Cash JG, Matlib MA, Wortman M, Basford JE, Abplanalp W, Hui DY. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochimica et biophysica acta. 2014;1841(6):888–895. doi: 10.1016/j.bbalip.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Higaki K, Saito S, Ohno K, Sakuraba H, Nanba E, Suzuki Y, Ozono K, Sakai N. Chaperone therapy for Krabbe disease: potential for late-onset GALC mutations. Journal of human genetics. 2015;60(9):539–545. doi: 10.1038/jhg.2015.61. [DOI] [PubMed] [Google Scholar]
- Houdou S, Takashima S, Suzuki Y. Immunohistochemical expression of peroxisomal enzymes in developing human brain. Mol Chem Neuropathol. 1993;19(3):235–248. doi: 10.1007/BF03160002. [DOI] [PubMed] [Google Scholar]
- Ida H, Eto Y, Maekawa K. Biochemical pathogenesis of demyelination in globoid cell leukodystrophy (Krabbe’s disease): the effects of psychosine upon oligodendroglial cell culture. Acta Paediatr Jpn. 1990;32(1):20–26. doi: 10.1111/j.1442-200x.1990.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Ijichi K, Brown GD, Moore CS, Lee JP, Winokur PN, Pagarigan R, Snyder EY, Bongarzone ER, Crocker SJ. MMP-3 mediates psychosine-induced globoid cell formation: implications for leukodystrophy pathology. Glia. 2013;61(5):765–777. doi: 10.1002/glia.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Heise CE, Nguyen T, O’Dowd BF, Lynch KR. Identification of a molecular target of psychosine and its role in globoid cell formation. The Journal of cell biology. 2001;153(2):429–434. doi: 10.1083/jcb.153.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatana M, Giri S, Singh AK. Apoptotic positive cells in Krabbe brain and induction of apoptosis in rat C6 glial cells by psychosine. Neurosci Lett. 2002;330(2):183–187. doi: 10.1016/s0304-3940(02)00655-9. [DOI] [PubMed] [Google Scholar]
- Jesionek-Kupnicka D, Majchrowska A, Krawczyk J, Wendorff J, Barcikowska M, Lukaszek S, Liberski PP. Krabbe disease: an ultrastructural study of globoid cells and reactive astrocytes at the brain and optic nerves. Folia Neuropathol. 1997;35(3):155–162. [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Andrzejak S, Selmaj K. TRAIL-induced death of human adult oligodendrocytes is mediated by JNK pathway. Glia. 2006;53(2):158–166. doi: 10.1002/glia.20249. [DOI] [PubMed] [Google Scholar]
- Kagitani-Shimono K, Mohri I, Fujitani Y, Suzuki K, Ozono K, Urade Y, Taniike M. Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model. Journal of neuroinflammation. 2005;2(1):10. doi: 10.1186/1742-2094-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalous M, Rauchova H, Drahota Z. The effect of lysophosphatidylcholine on the activity of various mitochondrial enzymes. Biochimica et biophysica acta. 1992;1098(2):167–171. doi: 10.1016/s0005-2728(05)80332-9. [DOI] [PubMed] [Google Scholar]
- Kanazawa T, Nakamura S, Momoi M, Yamaji T, Takematsu H, Yano H, Sabe H, Yamamoto A, Kawasaki T, Kozutsumi Y. Inhibition of cytokinesis by a lipid metabolite, psychosine. The Journal of cell biology. 2000;149(4):943–950. doi: 10.1083/jcb.149.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P, Group FS. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. The New England journal of medicine. 2010;362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Khan M, Haq E, Giri S, Singh I, Singh AK. Peroxisomal participation in psychosine-mediated toxicity: implications for Krabbe’s disease. Journal of neuroscience research. 2005;80(6):845–854. doi: 10.1002/jnr.20529. [DOI] [PubMed] [Google Scholar]
- Kim DW, Eum WS, Jang SH, Kim SY, Choi HS, Choi SH, An JJ, Lee SH, Lee KS, Han K, Kang TC, Won MH, Kang JH, Kwon OS, Cho SW, Kim TY, Park J, Choi SY. Transduced Tat-SOD fusion protein protects against ischemic brain injury. Molecules and cells. 2005;19(1):88–96. [PubMed] [Google Scholar]
- Kim SU. Lysosomal storage diseases: Stem cell-based cell- and gene-therapy. Cell transplantation. 2014 doi: 10.3727/096368914X681946. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Knaap MSvd, Valk J. Magnetic Resonance of Myelination and Myelin Disorders. New York: Springer; 2005. [Google Scholar]
- Kobayashi S, Chiu FC, Katayama M, Sacchi RS, Suzuki K, Suzuki K. Expression of glial fibrillary acidic protein in the CNS and PNS of murine globoid cell leukodystrophy, the twitcher. Am J Pathol. 1986;125(2):227–243. [PMC free article] [PubMed] [Google Scholar]
- Kolodny EH. Globoid leukodystrophy. In: Vinken PJ, GWB, editors. Handbook of Clinical Neurology: Neurodystrophies and Neurolipidoses. Amsterdam: Elsevier; 1996. pp. 187–210. [Google Scholar]
- Kolodny EH, Raghavan S, Krivit W. Late-onset Krabbe disease (globoid cell leukodystrophy): clinical and biochemical features of 15 cases. Dev Neurosci. 1991;13(4–5):232–239. doi: 10.1159/000112166. [DOI] [PubMed] [Google Scholar]
- Kondo A, Hoogerbrugge PM, Suzuki K, Poorthuis BJ, Van Bekkum DW, Suzuki K. Pathology of the peripheral nerve in the twitcher mouse following bone marrow transplantation. Brain research. 1988;460(1):178–183. doi: 10.1016/0006-8993(88)91220-6. [DOI] [PubMed] [Google Scholar]
- Kondo A, Suzuki K. The blood brain barrier in human leukodystrophies and allied diseases. Ultrastructural and morphometric studies on the capillaries in brain biopsies. Clin Neuropathol. 1993;12(3):169–174. [PubMed] [Google Scholar]
- Kondo Y, Adams JM, Vanier MT, Duncan ID. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(10):3610–3624. doi: 10.1523/JNEUROSCI.6344-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Wenger DA, Gallo V, Duncan ID. Galactocerebrosidase-deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18670–18675. doi: 10.1073/pnas.0506473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. The Journal of clinical investigation. 1986;77(1):1–6. doi: 10.1172/JCI112262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe KA. New familial, infantile form of diffuse brain-sclerosis. Brain. 1916;39:74–114. doi: 10.1093/brain/awt232. [DOI] [PubMed] [Google Scholar]
- Krivit W, Shapiro EG, Peters C, Wagner JE, Cornu G, Kurtzberg J, Wenger DA, Kolodny EH, Vanier MT, Loes DJ, Dusenbery K, Lockman LA. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. The New England journal of medicine. 1998;338(16):1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- Kuai XL, Ni RZ, Zhou GX, Mao ZB, Zhang JF, Yi N, Liu ZX, Shao N, Ni WK, Wang ZW. Transplantation of mouse embryonic stem cell-derived oligodendrocytes in the murine model of globoid cell leukodystrophy. Stem cell research & therapy. 2015;6:30. doi: 10.1186/s13287-015-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 2009;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo O, Singh AK, Singh I. Postnatal development and isolation of peroxisomes from brain. J Neurochem. 1991;56(4):1343–1353. doi: 10.1111/j.1471-4159.1991.tb11431.x. [DOI] [PubMed] [Google Scholar]
- Lee WC, Courtenay A, Troendle FJ, Stallings-Mann ML, Dickey CA, DeLucia MW, Dickson DW, Eckman CB. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(11):1549–1551. doi: 10.1096/fj.05-3826fje. [DOI] [PubMed] [Google Scholar]
- Lee WC, Kang D, Causevic E, Herdt AR, Eckman EA, Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(16):5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine SM, Brown DC. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. Journal of neuroimmunology. 1997;73(1–2):47–56. doi: 10.1016/s0165-5728(96)00166-x. [DOI] [PubMed] [Google Scholar]
- LeVine SM, Pedchenko TV, Bronshteyn IG, Pinson DM. L-cycloserine slows the clinical and pathological course in mice with globoid cell leukodystrophy (twitcher mice) Journal of neuroscience research. 2000;60(2):231–236. doi: 10.1002/(SICI)1097-4547(20000415)60:2<231::AID-JNR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Lissens W, Arena A, Seneca S, Rafi M, Sorge G, Liebaers I, Wenger D, Fiumara A. A single mutation in the GALC gene is responsible for the majority of late onset Krabbe disease patients in the Catania (Sicily, Italy) region. Human mutation. 2007;28(7):742. doi: 10.1002/humu.9500. [DOI] [PubMed] [Google Scholar]
- Loes DJ, Peters C, Krivit W. Globoid cell leukodystrophy: distinguishing early-onset from late-onset disease using a brain MR imaging scoring method. AJNR Am J Neuroradiol. 1999;20(2):316–323. [PMC free article] [PubMed] [Google Scholar]
- Luddi A, Volterrani M, Strazza M, Smorlesi A, Rafi MA, Datto J, Wenger DA, Costantino-Ceccarini E. Retrovirus-mediated gene transfer and galactocerebrosidase uptake into twitcher glial cells results in appropriate localization and phenotype correction. Neurobiology of disease. 2001;8(4):600–610. doi: 10.1006/nbdi.2001.0407. [DOI] [PubMed] [Google Scholar]
- Luzi P, Abraham RM, Rafi MA, Curtis M, Hooper DC, Wenger DA. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain research. 2009;1300:146–158. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi P, Rafi MA, Zaka M, Curtis M, Vanier MT, Wenger DA. Generation of a mouse with low galactocerebrosidase activity by gene targeting: a new model of globoid cell leukodystrophy (Krabbe disease) Molecular genetics and metabolism. 2001;73(3):211–223. doi: 10.1006/mgme.2001.3194. [DOI] [PubMed] [Google Scholar]
- Lyon G, Hagberg B, Evrard P, Allaire C, Pavone L, Vanier M. Symptomatology of late onset Krabbe’s leukodystrophy: the European experience. Dev Neurosci. 1991;13(4–5):240–244. doi: 10.1159/000112167. [DOI] [PubMed] [Google Scholar]
- Malone MJ. Deficiency in degradative enzyme system in globoid leukodystrophy. Trans Am Soc Neurochem. 1970;1:56. [Google Scholar]
- Marcus J, Dupree JL, Popko B. Myelin-associated glycoprotein and myelin galactolipids stabilize developing axo-glial interactions. The Journal of cell biology. 2002;156(3):567–577. doi: 10.1083/jcb.200111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53(4):372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Marjanovic B, Cvetkovic D, Dozic S, Todorovic S, Djuric M. Association of Krabbe leukodystrophy and congenital fiber type disproportion. Pediatric neurology. 1996;15(1):79–82. doi: 10.1016/0887-8994(96)00092-6. [DOI] [PubMed] [Google Scholar]
- Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, Wu Q, Kolbe S, Gordon IR, Wang H, Egan G, Murray S, Butzkueven H, Kilpatrick TJ. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;56(6):686–698. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- Matthes F, Andersson C, Stein A, Eistrup C, Fogh J, Gieselmann V, Wenger DA, Matzner U. Enzyme replacement therapy of a novel humanized mouse model of globoid cell leukodystrophy. Experimental neurology. 2015;271:36–45. doi: 10.1016/j.expneurol.2015.04.020. [DOI] [PubMed] [Google Scholar]
- McConnell KW, Muenzer JT, Chang KC, Davis CG, McDunn JE, Coopersmith CM, Hilliard CA, Hotchkiss RS, Grigsby PW, Hunt CR. Anti-apoptotic peptides protect against radiation-induced cell death. Biochemical and biophysical research communications. 2007;355(2):501–507. doi: 10.1016/j.bbrc.2007.01.180. [DOI] [PubMed] [Google Scholar]
- Meng XL, Eto Y, Schiffmann R, Shen JS. HIV Tat Domain Improves Cross-correction of Human Galactocerebrosidase in a Gene- and Flanking Sequence-dependent Manner. Molecular therapy Nucleic acids. 2013;2:e130. doi: 10.1038/mtna.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XL, Shen JS, Watabe K, Ohashi T, Eto Y. GALC transduction leads to morphological improvement of the twitcher oligodendrocytes in vivo. Molecular genetics and metabolism. 2005;84(4):332–343. doi: 10.1016/j.ymgme.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochemical and biophysical research communications. 1972;48(3):539–543. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- Nagano S, Yamada T, Shinnoh N, Furuya H, Taniwaki T, Kira J. Expression and processing of recombinant human galactosylceramidase. Clinica chimica acta; international journal of clinical chemistry. 1998;276(1):53–61. doi: 10.1016/s0009-8981(98)00095-3. [DOI] [PubMed] [Google Scholar]
- Nagara H, Ogawa H, Sato Y, Kobayashi T, Suzuki K. The twitcher mouse: degeneration of oligodendrocytes in vitro. Brain Res. 1986;391(1):79–84. doi: 10.1016/0165-3806(86)90009-x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan C, Dev KK. Galactosylsphingosine (psychosine)-induced demyelination is attenuated by sphingosine 1-phosphate signalling. Journal of cell science. 2015;128(21):3878–3887. doi: 10.1242/jcs.169342. [DOI] [PubMed] [Google Scholar]
- Okuda S, Saito H, Katsuki H. Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience. 1994;63(3):691–699. doi: 10.1016/0306-4522(94)90515-0. [DOI] [PubMed] [Google Scholar]
- Paintlia MK, Singh I, Singh AK. Effect of vitamin D3 intake on the onset of disease in a murine model of human Krabbe disease. Journal of neuroscience research. 2015;93(1):28–42. doi: 10.1002/jnr.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannuzzo G, Cardile V, Costantino-Ceccarini E, Alvares E, Mazzone D, Perciavalle V. A galactose-free diet enriched in soy isoflavones and antioxidants results in delayed onset of symptoms of Krabbe disease in twitcher mice. Molecular genetics and metabolism. 2010;100(3):234–240. doi: 10.1016/j.ymgme.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Parenti G, Andria G, Valenzano KJ. Pharmacological Chaperone Therapy: Preclinical Development, Clinical Translation, and Prospects for the Treatment of Lysosomal Storage Disorders. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(7):1138–1148. doi: 10.1038/mt.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqui AL, Di Renzo M, Auteri A, Federico G, Puccetti L. Increased TNF-alpha production by peripheral blood mononuclear cells in patients with Krabbe’s disease: effect of psychosine. European journal of clinical investigation. 2007;37(9):742–745. doi: 10.1111/j.1365-2362.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Pastores GM. Krabbe disease: an overview. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S75–81. doi: 10.5414/cpp47075. [DOI] [PubMed] [Google Scholar]
- Pedchenko TV, LeVine SM. IL-6 deficiency causes enhanced pathology in Twitcher (globoid cell leukodystrophy) mice. Experimental neurology. 1999;158(2):459–468. doi: 10.1006/exnr.1999.7125. [DOI] [PubMed] [Google Scholar]
- Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P. Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. Journal of neuroscience research. 2002;69(1):86–93. doi: 10.1002/jnr.10264. [DOI] [PubMed] [Google Scholar]
- Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple sclerosis. Journal of neuroimmunology. 2007;184(1–2):37–44. doi: 10.1016/j.jneuroim.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Santos M, Davisson MT, Rowitch DH, Marks DL, Bongarzone ER, Petryniak MA. Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease. Human molecular genetics. 2013;22(17):3397–3414. doi: 10.1093/hmg/ddt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HC, Knobler RL, Myers RR. Peripheral neuropathy in the Twitcher mutant. A new experimental model of endoneurial edema. Laboratory investigation; a journal of technical methods and pathology. 1983;49(1):19–25. [PubMed] [Google Scholar]
- Rafi MA, Luzi P, Chen YQ, Wenger DA. A large deletion together with a point mutation in the GALC gene is a common mutant allele in patients with infantile Krabbe disease. Human molecular genetics. 1995;4(8):1285–1289. doi: 10.1093/hmg/4.8.1285. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Luzi P, Zlotogora J, Wenger DA. Two different mutations are responsible for Krabbe disease in the Druze and Moslem Arab populations in Israel. Human genetics. 1996;97(3):304–308. doi: 10.1007/BF02185759. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Curtis MT, Wenger DA. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20(11):2031–2042. doi: 10.1038/mt.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Luddi A, Curtis MT, Wenger DA. Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Molecular genetics and metabolism. 2015a;114(3):459–466. doi: 10.1016/j.ymgme.2014.12.300. [DOI] [PubMed] [Google Scholar]
- Rafi MA, Rao HZ, Luzi P, Wenger DA. Long-term Improvements in Lifespan and Pathology in CNS and PNS After BMT Plus One Intravenous Injection of AAVrh10-GALC in Twitcher Mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2015b;23(11):1681–1690. doi: 10.1038/mt.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. The Journal of clinical investigation. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca A, Rufo N, Ungari S, Morena F, Martino S, Kulik W, Alberizzi V, Bolino A, Bianchi F, Del Carro U, Biffi A, Gritti A. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Human molecular genetics. 2015;24(12):3372–3389. doi: 10.1093/hmg/ddv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll CB, Flaat M, Klopf-Eiermann J, Fisher-Perkins JM, Trygg CB, Scruggs BA, McCants ML, Leonard HP, Lin AF, Zhang S, Eagle ME, Alvarez X, Li YT, Li SC, Gimble JM, Bunnell BA. Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe’s disease. Stem Cells. 2011;29(1):67–77. doi: 10.1002/stem.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(7):1139–1151. doi: 10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Inui K, Fujii N, Fukushima H, Nishimoto J, Yanagihara I, Isegawa Y, Iwamatsu A, Okada S. Krabbe disease: isolation and characterization of a full-length cDNA for human galactocerebrosidase. Biochemical and biophysical research communications. 1994;198(2):485–491. doi: 10.1006/bbrc.1994.1071. [DOI] [PubMed] [Google Scholar]
- Sampaio RC, Truwit CL. In: Myelination in the developing human brain. Nelson CA, Luciana M, Collins ML, editors. Cambridge: MIT Press; 2001. [Google Scholar]
- Sands MS, Davidson BL. Gene therapy for lysosomal storage diseases. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;13(5):839–849. doi: 10.1016/j.ymthe.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, van der Bijl P, Schwab ME. The UDP-galactose:ceramide galactosyltransferase: expression pattern in oligodendrocytes and Schwann cells during myelination and substrate preference for hydroxyceramide. Journal of neurochemistry. 1995;65(5):2267–2278. doi: 10.1046/j.1471-4159.1995.65052267.x. [DOI] [PubMed] [Google Scholar]
- Schiffmann R, Heyes MP, Aerts JM, Dambrosia JM, Patterson MC, DeGraba T, Parker CC, Zirzow GC, Oliver K, Tedeschi G, Brady RO, Barton NW. Prospective study of neurological responses to treatment with macrophage-targeted glucocerebrosidase in patients with type 3 Gaucher’s disease. Annals of neurology. 1997;42(4):613–621. doi: 10.1002/ana.410420412. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Chew LJ. Cytokines and myelination in the central nervous system. Scientific World Journal. 2008;8:1119–1147. doi: 10.1100/tsw.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta. 2006;1763(12):1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Schulte S, Stoffel W. Ceramide UDP galactosyltransferase from myelinating rat brain: purification, cloning, and expression. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):10265–10269. doi: 10.1073/pnas.90.21.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I, De Smet F, Hohensinner PJ, Ruiz de Almodovar C, Carmeliet P. The neurovascular link in health and disease: an update. Trends Mol Med. 2009;15(10):439–451. doi: 10.1016/j.molmed.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Shen JS, Watabe K, Ohashi T, Eto Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene therapy. 2001;8(14):1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- Shields SA, Gilson JM, Blakemore WF, Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28(1):77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Simons M, Trajkovic K. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. Journal of cell science. 2006;119(Pt 21):4381–4389. doi: 10.1242/jcs.03242. [DOI] [PubMed] [Google Scholar]
- Smith BR, Santos MB, Marshall MS, Cantuti-Castelvetri L, Lopez-Rosas A, Li G, van Breemen R, Claycomb KI, Gallea JI, Celej MS, Crocker SJ, Givogri MI, Bongarzone ER. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. The Journal of pathology. 2014;232(5):509–521. doi: 10.1002/path.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain pathology. 1999;9(1):69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG, Peterson KE, Lackner AA, Bunnell BA. Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am J Pathol. 2014;184(2):382–396. doi: 10.1016/j.ajpath.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000;1486(2–3):219–231. doi: 10.1016/s1388-1981(00)00077-9. [DOI] [PubMed] [Google Scholar]
- Stahl N, Jurevics H, Morell P, Suzuki K, Popko B. Isolation, characterization, and expression of cDNA clones that encode rat UDP-galactose: ceramide galactosyltransferase. Journal of neuroscience research. 1994;38(2):234–242. doi: 10.1002/jnr.490380214. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Twenty five years of the “psychosine hypothesis”: a personal perspective of its history and present status. Neurochem Res. 1998;23(3):251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Globoid cell leukodystrophy (Krabbe’s disease): update. Journal of child neurology. 2003;18(9):595–603. doi: 10.1177/08830738030180090201. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe’s disease): deficiency of galactocerebroside beta-galactosidase. Proceedings of the National Academy of Sciences of the United States of America. 1970;66(2):302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain research. 1999;837(1–2):203–212. doi: 10.1016/s0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21(1):53–64. [PubMed] [Google Scholar]
- Taguchi I, Oka K, Kitamura K, Sugiura M, Oku A, Matsumoto M. Protection by a cyclic AMP-specific phosphodiesterase inhibitor, rolipram, and dibutyryl cyclic AMP against Propionibacterium acnes and lipopolysaccharide-induced mouse hepatitis. Inflammation research : official journal of the European Histamine Research Society [et al] 1999;48(7):380–385. doi: 10.1007/s000110050475. [DOI] [PubMed] [Google Scholar]
- Takahashi JL, Giuliani F, Power C, Imai Y, Yong VW. Interleukin-1beta promotes oligodendrocyte death through glutamate excitotoxicity. Annals of neurology. 2003;53(5):588–595. doi: 10.1002/ana.10519. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nagara H, Kobayashi T, Goto I. The twitcher mouse: accumulation of galactosylsphingosine and pathology of the sciatic nerve. Brain research. 1988;454(1–2):340–346. doi: 10.1016/0006-8993(88)90835-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Mito T, Takashima S. Progress of myelination in the human fetal spinal nerve roots, spinal cord and brainstem with myelin basic protein immunohistochemistry. Early human development. 1995;41(1):49–59. doi: 10.1016/0378-3782(94)01608-r. [DOI] [PubMed] [Google Scholar]
- Tappino B, Biancheri R, Mort M, Regis S, Corsolini F, Rossi A, Stroppiano M, Lualdi S, Fiumara A, Bembi B, Di Rocco M, Cooper DN, Filocamo M. Identification and characterization of 15 novel GALC gene mutations causing Krabbe disease. Human mutation. 2010;31(12):E1894–1914. doi: 10.1002/humu.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titsworth WL, Liu NK, Xu XM. Role of secretory phospholipase a(2) in CNS inflammation: implications in traumatic spinal cord injury. CNS Neurol Disord Drug Targets. 2008;7(3):254–269. doi: 10.2174/187152708784936671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999;73(2):684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137(2):459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turazzini M, Beltramello A, Bassi R, Del Colle R, Silvestri M. Adult onset Krabbe’s leukodystrophy: a report of 2 cases. Acta Neurol Scand. 1997;96(6):413–415. doi: 10.1111/j.1600-0404.1997.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Ungari S, Montepeloso A, Morena F, Cocchiarella F, Recchia A, Martino S, Gentner B, Naldini L, Biffi A. Design of a regulated lentiviral vector for hematopoietic stem cell gene therapy of globoid cell leukodystrophy. Molecular therapy Methods & clinical development. 2015;2:15038. doi: 10.1038/mtm.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcellos E, Smith M. MRI nerve root enhancement in Krabbe disease. Pediatric neurology. 1998;19(2):151–152. doi: 10.1016/s0887-8994(98)00033-2. [DOI] [PubMed] [Google Scholar]
- Visigalli I, Ungari S, Martino S, Park H, Cesani M, Gentner B, Sergi Sergi L, Orlacchio A, Naldini L, Biffi A. The galactocerebrosidase enzyme contributes to the maintenance of a functional hematopoietic stem cell niche. Blood. 2010;116(11):1857–1866. doi: 10.1182/blood-2009-12-256461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voccoli V, Tonazzini I, Signore G, Caleo M, Cecchini M. Role of extracellular calcium and mitochondrial oxygen species in psychosine-induced oligodendrocyte cell death. Cell death & disease. 2014;5:e1529. doi: 10.1038/cddis.2014.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23(8):3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders RJ, Waterham HR. Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem. 2006;75:295–332. doi: 10.1146/annurev.biochem.74.082803.133329. [DOI] [PubMed] [Google Scholar]
- Weimer MB, Gutierrez A, Baskin GB, Borda JT, Veazey RS, Myers L, Phillippi-Falkenstein KM, Bunnell BA, Ratterree MS, England JD. Serial electrophysiologic studies in rhesus monkeys with Krabbe disease. Muscle Nerve. 2005;32(2):185–190. doi: 10.1002/mus.20350. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Human mutation. 1997;10(4):268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Rafi MA, Luzi P, Datto J, Costantino-Ceccarini E. Krabbe disease: genetic aspects and progress toward therapy. Molecular genetics and metabolism. 2000;70(1):1–9. doi: 10.1006/mgme.2000.2990. [DOI] [PubMed] [Google Scholar]
- Wenger DA, Suzuki K, Suzuki Y, Suzuki K. In: Chapter 147: Galactosylceramide Lipidosis: Globoid Cell Leukodystrophy (Krabbe disease) Scriver CR, Beauder AL, Sly WS, Valle D, editors. New York: McGraw-Hill; 2001a. [Google Scholar]
- Wenger DA, Suzuki K, Suzuki Y, Suzuki K. Galactosylceramide Lipidosis: Globoid cell Leukodystrophy (Krabbe Disease) In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001b. pp. 3669–3694. [Google Scholar]
- White AB, Galbiati F, Givogri MI, Lopez Rosas A, Qiu X, van Breemen R, Bongarzone ER. Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease. Journal of neuroscience research. 2011;89(3):352–364. doi: 10.1002/jnr.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS letters. 1993;326(1–3):285–288. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- Won JS, Kim J, Paintlia MK, Singh I, Singh AK. Role of endogenous psychosine accumulation in oligodendrocyte differentiation and survival: implication for Krabbe disease. Brain research. 2013;1508:44–52. doi: 10.1016/j.brainres.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak W, O’Rahilly R. An electron microscopic study of myelination of pyramidal fibres at the level of the pyramidal decussation in the human fetus. J Hirnforsch. 1982;23(3):331–342. [PubMed] [Google Scholar]
- Wu SP, Fu AL, Wang YX, Yu LP, Jia PY, Li Q, Jin GZ, Sun MJ. A novel therapeutic approach to 6-OHDA-induced Parkinson’s disease in rats via supplementation of PTD-conjugated tyrosine hydroxylase. Biochemical and biophysical research communications. 2006;346(1):1–6. doi: 10.1016/j.bbrc.2006.03.247. [DOI] [PubMed] [Google Scholar]
- Wu YP, Matsuda J, Kubota A, Suzuki K, Suzuki K. Infiltration of hematogenous lineage cells into the demyelinating central nervous system of twitcher mice. Journal of neuropathology and experimental neurology. 2000;59(7):628–639. doi: 10.1093/jnen/59.7.628. [DOI] [PubMed] [Google Scholar]
- Wu YP, McMahon EJ, Matsuda J, Suzuki K, Matsushima GK, Suzuki K. Expression of immune-related molecules is downregulated in twitcher mice following bone marrow transplantation. Journal of neuropathology and experimental neurology. 2001;60(11):1062–1074. doi: 10.1093/jnen/60.11.1062. [DOI] [PubMed] [Google Scholar]
- Xu C, Sakai N, Taniike M, Inui K, Ozono K. Six novel mutations detected in the GALC gene in 17 Japanese patients with Krabbe disease, and new genotype-phenotype correlation. Journal of human genetics. 2006;51(6):548–554. doi: 10.1007/s10038-006-0396-3. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. In: The myelogenetic cycles of regional maturation of the brain. Minkowski A, editor. Oxford: Blackwell; 1967. [Google Scholar]
- Zhang H, Yang B, Mu X, Ahmed SS, Su Q, He R, Wang H, Mueller C, Sena-Esteves M, Brown R, Xu Z, Gao G. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19(8):1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Dinh A, Cronin J, Li SC, Reiser J. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. Journal of neurochemistry. 2008;104(4):1055–1064. doi: 10.1111/j.1471-4159.2007.05030.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Lopez-Rosas A, Qiu X, Van Breemen RB, Bongarzone ER. Detection of the neurotoxin psychosine in samples of peripheral blood: application in diagnostics and follow-up of Krabbe disease. Archives of pathology & laboratory medicine. 2012;136(7):709–710. doi: 10.5858/arpa.2011-0667-LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogora J, Chakraborty S, Knowlton RG, Wenger DA. Krabbe disease locus mapped to chromosome 14 by genetic linkage. American journal of human genetics. 1990;47(1):37–44. [PMC free article] [PubMed] [Google Scholar]