Abstract
Scrub typhus is an emerging, insect-transmitted disease caused by Orientia tsutsugamushi, a Gram- and LPS-negative bacterium that replicates freely within professional phagocytes and endothelial cells (EC). Scrub typhus is prevalent with high mortality rates, but information regarding its molecular pathogenesis, microbial virulence determinants, and key immune responses is limited. Improved animal models have recently developed that respectively resemble the pathological features of self-limiting or severe scrub typhus in humans. Strong activation of Th1 and CD8, but not Th2 and Treg, immune responses, accompanied by altered angiopoietin/Tie2-related regulation, are hallmarks of lethal infection in murine models. This review, based primarily on recent advances from clinical and experimental studies, highlights tissue- and EC-specific biomarkers that are indicative of immune dysregulation. The potential roles of neutrophils and damage-associated molecular pattern (DAMP) molecules at late stages of disease are discussed in the context of vascular leakage, pulmonary and renal injury, and scrub typhus pathogenesis.
Keywords: Orientia, Vascular responses, Scrub typhus, Pathogenesis
Scrub typhus is a zoonotic and life-threatening disease. More than one million new cases are diagnosed every year, mostly in a broad geographic region or “tsutsugamushi triangle” in Southeast Asia, with one-third of the world’s population at risk of infection [reviewed in (1, 2)]. Under-reporting and misdiagnosis in endemic regions and potential emerging of this infection in Africa and South America are major concerns for this neglected tropical disease (1–3). The disease is initiated by the bite of a larval Leptotrombidium mite or chigger, which transmits Orientia tsutsugamushi (formerly named Rickettsia tsutsugamushi), an obligate intracellular bacterium.
Orientia comprises numerous strains of differing virulence, and approximately 50% and 25% of human infections are associated with infection with Karp- and Gilliam-related strains, respectively (4). After an incubation period of 7–14 days, some patients develop eschar, a unique and localized pathological skin lesion (5), followed by fever, rash, myalgia, headache, and non-specific flu-like symptoms. Pathological characteristics of fatal scrub typhus include diffuse interstitial pneumonia, hepatic lesions, meningoencephalitis, and coagulation disorders (6, 7). In some endemic areas, scrub typhus is a leading cause of non-malaria febrile illness (8, 9), but diagnosis of this infection is relatively difficult, due to initial non-specific clinical presentation and other challenges (10). The endothelial tropism of Orientia can lead to vasculitis that affects all organs, especially in severe cases (11–13). Patients can have fast and unpredictable progression and multi-organ failure, with up to 70% mortality, depending on bacterial strains involved and receipt of an accurate diagnosis (8, 14). Scrub typhus is treatable, as antibiotics like azithromycin and doxycycline are effective, if given at the onset of disease (6). The bacteriostatic nature of antibiotics in use, delayed diagnosis, persistent infection, and the lack of efficient vaccines are major issues (2, 15, 16). Adaptive immunity or cross-species protection in humans is short-lived and bacterial strain-related (17, 18), but the mechanism of waning immunity is unclear, which increases the challenge for developing effective vaccines for the control of scrub typhus.
This review focuses primarily on advances from recent in vitro studies in Orientia-infected target cells, clinical findings, and animal models of scrub typhus, highlighting tissue- and cell-specific biomarkers that are indicative of immune dysregulation. The potential mechanisms underlying alterations in immune responses and the possible roles of neutrophils and DAMPs during infection in murine models are discussed in the context of vascular leakage, pulmonary and renal injury, and severe scrub pathogenesis. Key players in scrub typhus pathogenesis and potential therapeutics are also discussed.
Bacterial Replication and Cellular Activation
Orientia has a small genome of ~1,500 genes with no pathogenicity islands or plasmids, but its biology remains poorly understood [reviewed in Refs.(19, 20)], because the organisms are osmotically sensitive and hard to propagate in cell cultures (21). For this genetically un-tractable bacterium, electron microscopic analyses or immunostaining have been the most important approaches, although labeling of bacteria with fluorescent probes has some success (22). Orientia entry into non-phagocytic cells can be divided into the adhesion and invasion stages, which involve bacterial 56-kDa type-specific antigens and surface cell antigens, as well as host fibronectin- and clathrin-dependent endocytic pathways (23–25). The bacterium can activate, but quickly escape from, cellular autophagy (26) and replicate freely but slowly in the cytosol (9 h for the dividing time). Bacteria can form perinuclear micro-colonies or exit from host cells via a budding-like process (20). The cytopathic effects of Orientia in human endothelial cells (EC) are milder than other endothelial-target pathogens such as spotted fever and typhus group of Rickettsiae (27) and hantaviruses [reviewed in (28)]. Orientia can destabilize the Golgi and ER and alter host secretory and apoptosis pathways via ankyrin repeat-containing protein-mediated mechanisms and other undefined processes (29–31).
Innate recognition of Orientia remains unclear (19, 32, 33). In contrast to other Gram-negative bacteria or Rickettsia spp. (LPS positive), Orientia lacks the classical lipopolysaccharide and peptidoglycan in its cell wall, although a peptidoglycan-like structure has been identified recently (34). TLR2 and nucleotide-binding oligomerization domain-containing protein 1 (NOD1)/IL-32-related pathway partially mediate Orientia recognition and inflammasome activation (32). In mouse macrophages, live Orientia (but not heat- or UV-inactivated bacteria) can trigger ASC inflammasome activation leading to IL-1β production, which is a critical innate immune response for effective host defense (35). Live Orientia are also highly competent in stimulating a M1-polarized phenotype in human monocytes/macrophages (36, 37). High levels of cytokines (TNF-α, IL-1β, IL-6, IL-12p40, IL-23p19), CXCR3-related chemokines (especially CXCL11), apoptosis-related genes, as well as type I IFN and its related genes, resemble host responses commonly elicited against viruses (36, 37). Unexpectedly, Orientia can propagate well in LPS-activated macrophages via NO-mediated pathways (38); however, the implication of these in vitro findings needs to be carefully evaluated in the context of in disease progress versus control (39).
The eschar biopsies have revealed that Orientia are mainly associated with dendritic cells (DCs, positive for CD1a/DCSIGN/S100/FXIIa and CD163), monocytes (positive for CD14/LSP-1/CD68), and vascular endothelium; these cells may contribute to local immunity and bacterial dissemination (12, 31). In cultured DCs or neutrophils, Orientia can rapidly escape from autophagy and replicate in DC cytosol (40), but neutrophils are not effective host cells (41). Infection stimulates DC activation, with increased expression of MHC II and costimulatory molecules (CD80, CD83, CD86, CD40), CCR7 (the receptor for CCL19 and CCL21), and inflammatory cytokines (IL-6, IL-12, TNF-α), as well as the potential of priming of IFN-γ-producing Th1 cells in vitro (40, 42). Compared with LPS-stimulated DCs, Orientia-infected DCs have much weaker levels of maturation, migration, and T cell-priming potential (40). But, Orientia is more potent to activate DCs than bacteria that reside in vacuolar compartments, including Coxiella burnetii (the agent of Q fever) and Brucella abortus (the agent of brucellosis) (43). Overall, Orientia infection stimulates sub-optimally activated, DC1-like phenotypes that preferentially induce Th1 cell activation in vitro (42).
Given the above findings, it may not be surprising that scrub typhus patients develop strong inflammatory immune responses, with unremarkable activation of Th2 cytokines (IL-4 and IL-13) (44) and peripheral Treg cells (45). IFN-γ-mediated deprivation of tryptophan partially contributes to Orientia growth restriction (46). However, massive T cell apoptosis, CD4+ T lymphopenia, and neutrophilia in the acute phase of infection, followed by preferential increase of NK and CD8+ T cells, may contribute to severe outcomes, as well as impaired immunological memory (45, 47). Disease severity is positively linked to the presence of eschar, the duration of skin rash, and the levels of serum cytokines (TNF-α, IL-8, IL-10) and circulating IFN-γ-producing NK cells (44, 48, 49). Disease severity is also positively linked to endothelial-related markers, including soluble L-selectin (50) and sCD163, VCAM-1 (51), and von Willebrand factor(52). Patients with low IL-10 and miR-155 levels are more vulnerable to a cytokine storm and severe pathology (53). Thrombocytopenia observed in severe scrub typhus patients (52, 54–56) and the failure of platelet count normalization, even after a general improvement of other markers of multi-organ dysfunction, imply for immune-mediated thrombocytopenia mechanism (57). These clinical studies collectively indicate immune system dysregulation in scrub typhus patients, but the nature and the mechanisms of immune dysfunction have not been investigated. Since the lung is the major organ for Orientia infection in humans and in animal infection models (see below), as well as the site of hematopoietic progenitors and platelet formation (58), a better understanding of tissue- and cell-specific alterations is crucially important for scrub typhus biology and vaccine development.
Animal Models of Self-Limited Scrub Typhus
Host-Orientia interactions in animal models are understudied and require biosafety level 3 facilities. Non-human primates, especially Macaca fascicularis (cynomolgus macaques) and Presbytis cristatus (silvered leaf monkeys), have been used to study pathological changes and immunological responses to Orientia infections (5, 59). The Rhesus macaques intradermally-inoculated with O. tsutsugamushi Karp strain are excellent models for detailed analyses of initial target cells in the eschars, local immune responses and adaptive immunity, as well as for vaccine-based studies (60). The development of an Orientia-specific ELISpot assay for measuring IFN-γ-mediated cellular immunity is of relevance to monitor the control of scrub typhus in Rhesus macaques and humans (61) and in vaccine-based studies for protective immunity (60, 62). While non-human primates closely mimic human scrub typhus, they are not widely used in laboratories due to the high expense and other logistical issues.
Since 2012, several groups have established improved murine models for scrub typhus, which better mimic infection and pathology than the previously used, intraperitoneal-inoculation models that have several inherent drawbacks [reviewed in (28)]. The use of outbred mice fed with different mite lines or inoculated intradermally with different Orientia strains has the advantage of modeling natural infection, bacterial dissemination, and clinical features (63, 64); however, these models are technically challenging with a high possibility of variations in infection outcomes. The use of BALB/c and C57BL/6 inbred mice inoculated subcutaneously or intradermally with Orientia Karp strain can model self-limited scrub typhus in humans (39, 65). However, these skin-inoculated outbred or inbred mice fail to form eschar lesions. Nevertheless, inbred mouse models permit detailed immunological analyses, because of their defined kinetics of bacterial dissemination from injection sites to visceral organs, as well as the highest bacterial loads that are detected in the lungs, as in the case of infection in humans and non-human primates.
Following intradermal inoculation, C57BL/6 mice develop fever, marked hypothermia, moderate weight loss, and then gradually recover in three weeks. However, infectious bacteria can be detected in the kidneys and other organs for months, even though mice can maintain high titers of serum Ag-specific antibodies (>1:65,000) at 12 weeks post-infection (65). Analyses of serum and lung samples have revealed a Th1/Th2-mixed response profile, with marked elevations of inflammatory markers (IL-6, IL-12, IFN-γ, G-CSF, CCL5, CCL11, IL-1α/β, IL-2, TNF-α, GM-CSF), as well as modulatory cytokines (IL-9 and IL-13) (65). IL-9 is a pleiotropic cytokine mostly produced by a special subset of CD4+ T (Th9) cells following TGF-β and IL-4 stimulation, and can modulate host immune responses and promote the resolution of inflammation (66, 67). Although IL-9 is involved in Th2-type inflammatory responses (68, 69), its role in bacterial infections remains unclear. Regardless of the source or the role of IL-9, it seems that skin-inoculated mice can mount Th1/Th2-balanced immune responses at acute stages of infection (65). Since these infected mice also have transient thrombocytopenia, with signs of platelet alterations and anemia, this model can be used to examine subclinical and persistent infections that are often observed in humans (16).
Functional CD8+ T cells are required for the restriction of Orientia growth at the acute and chronic stages of infection, as MHC I−/− and CD8−/− mice are highly susceptible to ordinarily sublethal doses of Karp stain and have lethal outcomes, regardless of the inoculation routes (70, 71). Depletion of CD8+ T cells via neutralizing antibodies at the chronic stages can reactivate bacterial growth (70); however, antigen-primed CD8+ cells can also mediate acute tissue injury (70, 71), implying a complex role of CD8+ T cells at different stages of disease. Also, the role of NO-mediated responses in Orientia infection may be more complex than we previously appreciated, because 1) MHC I−/− and CD8−/− mice have significantly higher IFN-γ and iNOS levels, as well as bacterial loads, than their wild-type controls (70, 71); 2) NO-inducing conditions can enhance Orientia replication in macrophages in a bacterium strain-dependent manner (38, 72); and 3) high levels of iNOS in the lungs have been observed in autopsies of severely infected cases (73). At present, information on the roles of NK, NKT, and CD4+ T cell subsets during infection in naïve or antigen-immunized mice is very limited (70, 74). There are no reports for the kinetics of expansion or retraction of Orientia-specific CD8+ and CD4+ T cell subsets in any murine models of scrub typhus. The use of cell-specific knockout and trackable transgenic mice will help define immune mechanisms for infection control.
Th1-Skewed, but Th2-suppressed, Responses during Severe Scrub Typhus
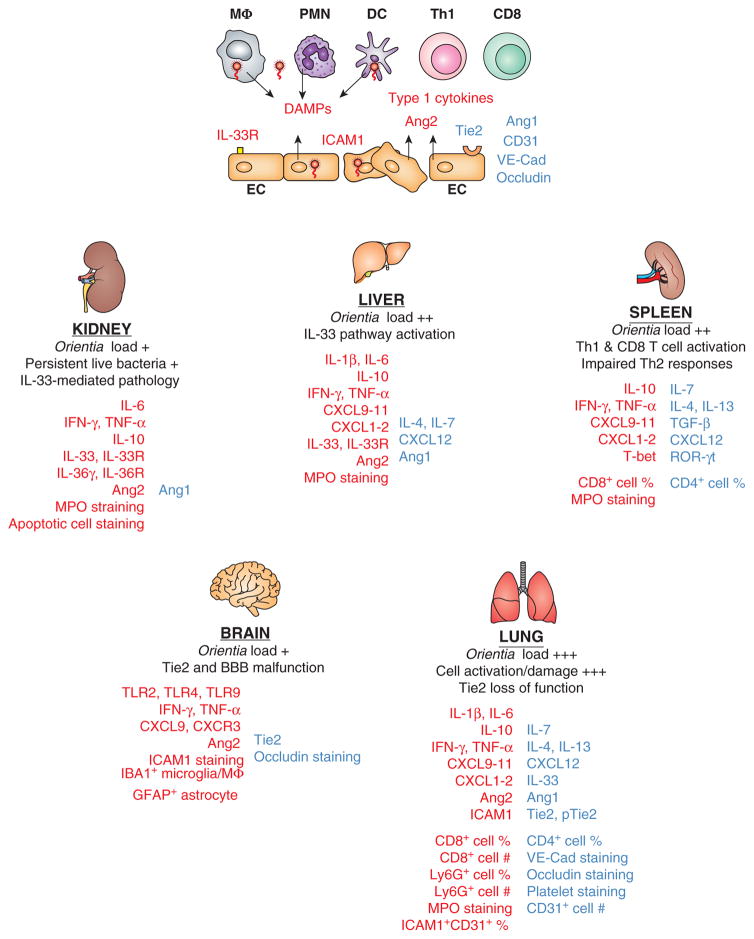
Following intravenous inoculation of O. tsutsugamushi Karp strain, C57BL/6 mice can have lethal or sublethal outcomes, depending on infection doses and host immune status (71, 75, 76). These mice develop disseminated endothelial infection with a spectrum of vasculitis, hemorrhage, interstitial pneumonia, or meningioencephalitis (75, 77), which resemble severe scrub typhus in humans. For lethal infection, mouse tissue bacteria reach peaks within a week and then come under control; however, body weight loss progresses, with 100% mortality in two weeks. PCR- and protein-based studies have revealed tissue-specific immunological features and argue for immunopathogenesis, rather than bacterial over-growth, in late-stage pathology and mortality (57). Firstly, Th1-skewed, but Th2-suppressed, immune profiles are consistently detected in the lung, liver, spleen, kidney, heart, and brain tissues during the course of infection (65, 78). As illustrated in Figure 1, the expression levels of inflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ, CXCL9-11, etc.) and IL-10 are significantly elevated, but there are no signs for activation of, or even reduced baseline levels for, Th2 markers (IL-4, IL-13, and CXCL12) or regulatory molecules (IL-7, GATA3, and ROR-γt). At this stage, it remains unclear as to whether this polarized gene expression profile is the result of selective cellular recruitment or just an up-regulation of specific mediators by resident cells. Nevertheless, these trends are consistent with in vitro findings of M1- and DC1-type immune phenotypes (36, 37, 40, 42), implying a strong skew to Th1 immune responses during the course of disease progression (65, 76, 78).
Figure 1. Dysregulated Th1 immune responses, accompanied by impaired Th2 responses, contribute to Orientia tsutsugamushi-induced acute tissue injury and vascular dysfunction.
Biomarkers or cell subsets that are up-regulated (in red) or down-regulated (in blue) during in vitro infection of human cell cultures and lethal or sublethal infection in C57BL/6 mice are illustrated. At the cellular level (top panel of the figure), Orientia coccobacilli within target cells such as monocytes/macrophages (MΦ), dendritic cells (DC), neutrophils (PMN), and endothelial cells (EC) preferentially stimulate the activation of Th1 and CD8+ T cells, but not Th2 cells, with high levels of type 1/inflammatory cytokines. Infected target cells can also release or activate damage-associated molecular pattern (DAMP) molecules such as IL-33 and other host factors. ECs can contribute to immune activation via surface expression of IL-33 receptors and ICAM1, or the release of vascular destabilizing factors such as angiopoietin protein-2 (Ang2). Sustained infection can trigger EC apoptosis and down-regulate the expression of Ang1, CD31 (also known as platelet endothelial cell adhesion molecule-1), occludin (a tight junction-associated protein), and VE-cadherin (endothelial adhesion junctions) via yet-undefined mechanisms. At the tissue level (lower panels of the figure), bacterial loads at the acute or chronic stages of disease are illustrated comparatively, together with hallmarks of pathological changes in the examined organs. The common immunological changes revealed from recent publications (65, 71, 75–78) include the sustained production of Th1 cytokines, selective activation of CD8+ T cells and Ly6G/MPO-positive PMNs, as well as organ-specific differences in the IL-33-like DAMPs. Vascular dysfunction is presented as reduced expression of Ang1, Tie2 (a tyrosine-protein kinase receptor for Ang1 and Ang2), CD31, occludin, and VE-cadherin at the severe stages of infection in mice. Data for lung flow cytometry and vascular staining, as well as kidney IL-36γ/IL-36R analysis, are unpublished. Collectively, these cell- and tissue-specific alterations contribute to the loss of vascular integrity, excessive tissue damage, and host mortality.
Secondly, there are no overt signs of neutrophil recruitment/activation at early hours or days of infection. However, myeloperoxidase (MPO)-positive and CD63+Ly6G+ neutrophils become steadily evident around day 6 and are most extensive around day 10 in the lungs, liver, spleen, and heart (77, 78), which is consistent with the low recovery of CD31+ and VE-cadherin+ endothelial cells (EC) from infected lungs (manuscript in preparation). The loss of positive staining for EC-specific markers, accompanied with increased accumulation and activation of neutrophils, is one of the most important features for this lethal infection mode (Figure 1), and is highly relevant for the understanding of the pathogenesis of vasculitis in severe infections in humans (11–13).
These immunological changes are positively correlated with cellular apoptosis, vascular leakage, and multi-organ failure, as mice receiving lethal or sublethal doses succumb to infection in 2–3 weeks (77, 78). Although the intravenous inoculation models have intrinsic shortcomings as they bypass skin- and draining lymph node-mediated immune regulation, they are valuable tools for examining severe scrub typhus. Neutrophil-based studies are important for understanding Orientia biology (79), as it is still debatable as to whether neutrophils are target cells for Orientia infection in vivo. Neutrophil-based studies are clinically relevant, as neutrophilia and thrombocytopenia are positively associated with severe scrub typhus in humans (52, 54–56) and in experimental mice (65, 75). Also, neutrophil gelatinase-associated lipocalin can contribute to kidney injury in scrub typhus patients (80). Although neutrophils are short-lived, they can regulate EC and platelet functions and local immune responses via their released cytotoxic effector molecules, cytokines, and endogenous DAMP molecules.
DAMP Molecules in Scrub Typhus Pathogenesis
DAMP molecules can initiate and perpetuate an immune response under both noninfectious and infectious inflammatory conditions. Many molecules in the IL-1 super family, including IL-1α, IL-1β, IL-33, IL-36, and high mobility group box 1 (HMGB1), are considered as alarmins [as reviewed by (81, 82)]. IL-1 family alarmins share structural similarities and have dual functionality, depending on their secretion and activation process and intracellular location. For example, pro-IL-33 can be released from stressed and necrotic cells and processed by neutrophil elastase and cathepsin G. IL-33 binding to its IL-33R/ST2L receptor can regulate innate immunity and CD8+ T-cell responses in a tissue-specific manner (83–85), or play deleterious roles in infectious diseases and models (86).
In Orientia-infected mice, IL-33 contributes to scrub typhus pathogenesis via exacerbating infection-triggered vascular activation and tissue damage (76). Organ-specific differences in IL-33 and IL-33R/ST2L expression profiles (Figure 1) suggest selective regulation of the IL-33/ST2L axis. Indeed, IL-33−/− mice show attenuated renal pathology after lethal infection, while rIL-33 treatment augments renal injury following a sublethal infection, via increasing CXCL1 and CXCL2, but altering anti-apoptotic gene BCL2 in the kidneys (76). At present, the tissue/cellular sources of IL-33 and the maturation process of released IL-33 are unclear. Given that infectious Orientia can persist in humans and mice for months or years (16, 65, 71), and that serum IL-33/ST2 levels serve as a surrogate of endothelial dysfunction in human diseases (87), monitoring IL-33/ST2 levels during acute and persistent infection is desirable.
HMGB1 is another alarmin of potential interest, as serum HMGB1 levels are positively linked to the severity of human scrub typhus (52, 88). HMGB1 release in infection, inflammation, and cell death is well documented; its mature proteins can stimulate TLR expression and bind to the TLR4/RAGE receptor on the surface of activated EC and other cells [see reviews in (82, 89)]. In dengue virus infection, viral proteins translocated in the nucleus can trigger monocytes to release HMGB1; the binding of HMGB1 to TLR4/RAGE on the surface of EC contributes to the loss of vascular integrity and increased inflammatory responses (90). The role of HMGB1 in scrub typhus remains unknown (52, 88); however, Orientia infection selectively activates TLR2/4/9 expression in mouse brains during lethal and sublethal infection, especially at late stages of disease, which correlates with neuroinflammation and vascular stress (77). The breakdown of the blood-brain barrier (BBB) is featured by a marked loss of occludin+ tight junction staining, as well as an increased trafficking of T cells, in the cortex and cerebellum of infected mice (77). Since Orientia are sparse in the brains, it is speculated that inflammatory cytokines like IFN-γ and TNF-α can synergize with DAMP molecules to promote BBB opening (77). It will be important to examine the involvement of IL-1 family DAMP molecules, their receptors, and TLR2/4/9 at acute and convalescence stages via population-based genetic studies of scrub typhus patients (91) or pathway-focused studies in mouse models (33).
Vascular Responses and Dysfunction during severe Orientia Infection
The endothelium provides a crucial interface between tissues and circulating inflammatory cells. Sepsis and severe infections caused by viruses (dengue, influenza, West Nile viruses) and parasites (Toxoplasma gondii, Plasmodium falciparum) can lead to vascular dysfunction (92, 93). Orientia, like Rickettsia, is an endothelium-targeting pathogen, readily detectable within lectin+ ECs in the mouse brains (77) and other organs (75, 77). While molecular details of Rickettsia-EC interaction are becoming clear [see reviews in (94, 95)], there is a paucity of studies for Orientia-triggered vascular dysfunction. Recent studies of Orientia infection in primary HUVEC cultures and in C57BL/6 mice have revealed marked alterations in the levels of angiopoietins (the vascular growth factors important for embryonic and postnatal angiogenesis) and Tie2 (an endothelial tyrosine kinase receptor) (76, 77). Angiopoietin-1 (Ang1) is constitutively produced by vascular support cells, specialized pericytes in the kidney, and hepatic stellate cells in the liver; Ang1/Tie2 binding and Tie2 phosphorylation (pTie2) are critical for vessel maturation, adhesion, migration, and survival. Ang2, on the other hand, promotes cell death and disrupts vascularization, via competing for Tie2; increased Ang2 production from activated/damaged EC also promotes inflammation (93, 96). Orientia infection suppresses Ang1 expression, but greatly increases Ang2 expression and Ang1/Ang2 ratios, in HUVEC cultures and in mouse tissues (76–78). Impaired levels of Tie2 mRNA, as well as total Tie2 and pTie2 proteins, are found in Orientia-infected mouse brain and lungs at late stages of lethal infection (77). Dysregulated Ang/Tie2 axis is positively correlated with reduced tight junctions (occludin) and adherence junctions (VE-cadherin, Figure 1), contributing to scrub typhus pathogenesis.
Orientia-infected endothelium also contributes to immune regulation via other mechanisms, including cytokine production (IL-1β, IL-6, IL-8), leukocyte recruitment (ICAM-1, MCP-1/CCL2), and immune regulation (cellular apoptosis, receptor expression) (32, 97, 98). Infection dose/time-dependent expression of IL-33, soluble ST2, and membrane-bound ST2L in primary HUVEC cultures supports findings from Orientia-infected mice (76). Given the secretion of IL-33 by damaged and living cells and its important role in endothelial activation (99, 100), one can speculate that Orientia-primed endothelium can be a source of DAMPs, and the latter can further amplify vascular damage and inflammation (76).
Conclusions and Future Studies
Our understanding of the pathophysiology of scrub typhus is still limited, and there are sizable knowledge gaps as to how the host immune system responds to O. tsutsugamushi at the cellular or molecular level. It has become clear that strong Th1- and CD8-mediated immune responses are essential for restricting intracellular replication of bacteria. Relatively high levels of inflammatory cytokines and IFN-γ-inducible chemokines are common immunological features observed in Orientia-infected humans and in animal models. However, dysregulated immune responses, especially sustained activation of CD8+ T cells and neutrophils after the control of bacterial growth, may contribute to acute tissue damage and host mortality. New evidence has emerged from clinical and experiential studies that suggests pathogenic roles of host cytokines (TNF-α, IL-8) and endogenous DAMPs (HMGB1, IL-33), neutrophil/platelet alterations, and their associations with endothelial dysregulation in severe scrub typhus. The identification of tissue/cell-specific gene regulation profiles and pathogenic biomarkers (Figure 1) will expedite mechanistic and translational research in the future.
Recent progress also raises several fundamental questions. What are the molecular mechanisms underlying Th1/CD8-skewed, but Th2/Treg-suppressed, immune responses during severe infection? What are the roles of neutrophils and platelets during Orientia infection? What is the critical window of time to restore immune balance? What is the therapeutic option to minimize vascular damage? Since waning adaptive immunity has been noticed in scrub typhus patients, a better understanding of effect and memory CD4+ versus CD8+ T cells is highly relevant for vaccine development. The establishment of biomarkers will help examine how a given DAMP and/or cytokine promotes bacterial clearance versus cellular injury. Monitoring serum levels of and genetic variations in pathogenic biomarkers will help assess disease progression and risk factors, as reported for other diseases (101–103). Such knowledge is essential for the development of effective vaccines or therapeutics for controlling scrub typhus.
Acknowledgments
This work was supported partly by NIH/NIAID grants (AI117368 and AI126343), as well as a Data Generation Award from the UTMB Institute of Human Infections and Immunity.
The author thanks Dr. Wei-Mei Ching (at the Naval Medical Research Center, Maryland), Dr. David Walker (at the University of Texas Medical Branch), and Dr. Linsey Yeager (at the University of Texas Medical Branch) for critical reading and scientific editing of the manuscript, as well as key members in the laboratory (Dr. Thomas Shelite, Brandon Trent) and other members in the Immunology Joint Group (Drs. Yingzi Cong, Janice Endsley, Haitao Hu, Ricardo Rajsbaum, Robin Stephens, and Jiaren Sun) for helpful discussion.
References
- 1.Kelly DJ, Fuerst PA, Ching WM, Richards AL. Scrub typhus: the geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;48(Suppl 3):S203–230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- 2.Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. 2013;89:301–307. doi: 10.4269/ajtmh.13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weitzel T, Dittrich S, Lopez J, Phuklia W, Martinez-Valdebenito C, Velasquez K, Blacksell SD, Paris DH, Abarca K. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954–961. doi: 10.1056/NEJMoa1603657. [DOI] [PubMed] [Google Scholar]
- 4.Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med. 2012;5:261–264. doi: 10.1016/S1995-7645(12)60036-4. [DOI] [PubMed] [Google Scholar]
- 5.Walsh DS, Delacruz EC, Abalos RM, Tan EV, Jiang J, Richards AL, Eamsila C, Rodkvantook W, Myint KS. Clinical and histological features of inoculation site skin lesions in cynomolgus monkeys experimentally infected with Orientia tsutsugamushi. Vector Borne Zoonotic Dis. 2007;7:547–554. doi: 10.1089/vbz.2006.0642. [DOI] [PubMed] [Google Scholar]
- 6.Jeong YJ, Kim S, Wook YD, Lee JW, Kim KI, Lee SH. Scrub typhus: clinical, pathologic, and imaging findings. Radiographics. 2007;27:161–172. doi: 10.1148/rg.271065074. [DOI] [PubMed] [Google Scholar]
- 7.Paris DH, Chansamouth V, Nawtaisong P, Lowenberg EC, Phetsouvanh R, Blacksell SD, Lee SJ, Dondorp AM, van der Poll T, Newton PN, Levi M, Day NP. Coagulation and inflammation in scrub typhus and murine typhus--a prospective comparative study from Laos. Clin Microbiol Infect. 2012;18:1221–1228. doi: 10.1111/j.1469-0691.2011.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi) PLoS Negl Trop Dis. 2015;9:e0003971. doi: 10.1371/journal.pntd.0003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis. 2017;11:e0005838. doi: 10.1371/journal.pntd.0005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–370. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moron CG, V, Popov L, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–759. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 12.Kim DM, Lim SC, Won KJ, Choi YJ, Park KH, Jang WJ. Severe scrub typhus confirmed early via immunohistochemical staining. Am J Trop Med Hyg. 2007;77:719–722. [PubMed] [Google Scholar]
- 13.Tseng BY, Yang HH, Liou JH, Chen LK, Hsu YH. Immunohistochemical study of scrub typhus: a report of two cases. Kaohsiung J Med Sci. 2008;24:92–98. doi: 10.1016/S1607-551X(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 14.Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8:379–383. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Panpanich R, Garner P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2002:CD002150. doi: 10.1002/14651858.CD002150. [DOI] [PubMed] [Google Scholar]
- 16.Chung MH, Lee JS, Baek JH, Kim M, Kang JS. Persistence of Orientia tsutsugamushi in humans. J Korean Med Sci. 2012;27:231–235. doi: 10.3346/jkms.2012.27.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay S, Richards AL. Scrub typhus vaccines: past history and recent developments. Hum Vaccin. 2007;3:73–80. doi: 10.4161/hv.3.3.4009. [DOI] [PubMed] [Google Scholar]
- 18.Mansueto P, Vitale G, Di Lorenzo G, Arcoleo F, Mansueto S, Cillari E. Immunology of human rickettsial diseases. J Biol Regul Homeost Agents. 2008;22:131–139. [PubMed] [Google Scholar]
- 19.Ge Y, Rikihisa Y. Subversion of host cell signaling by Orientia tsutsugamushi. Microbes Infect. 2011;13:638–648. doi: 10.1016/j.micinf.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Kim MK, Kang JS. Involvement of lipid rafts in the budding-like exit of Orientia tsutsugamushi. Microb Pathog. 2013;63:37–43. doi: 10.1016/j.micpath.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Giengkam S, Blakes A, Utsahajit P, Chaemchuen S, Atwal S, Blacksell SD, Paris DH, Day NP, Salje J. Improved quantification, propagation, purification and storage of the obligate intracellular human pathogen Orientia tsutsugamushi. PLoS Negl Trop Dis. 2015;9:e0004009. doi: 10.1371/journal.pntd.0004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atwal S, Giengkam S, VanNieuwenhze M, Salje J. Live imaging of the genetically intractable obligate intracellular bacteria Orientia tsutsugamushi using a panel of fluorescent dyes. J Microbiol Methods. 2016;130:169–176. doi: 10.1016/j.mimet.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho BA, Cho NH, Seong SY, Choi MS, Kim IS. Intracellular invasion by Orientia tsutsugamushi is mediated by integrin signaling and actin cytoskeleton rearrangements. Infect Immun. 2010;78:1915–1923. doi: 10.1128/IAI.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha NY, Cho NH, Kim YS, Choi MS, Kim IS. An autotransporter protein from Orientia tsutsugamushi mediates adherence to nonphagocytic host cells. Infect Immun. 2011;79:1718–1727. doi: 10.1128/IAI.01239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu H, Lee JH, Han SH, Kim SY, Cho NH, Kim IS, Choi MS. Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes. Infect Immun. 2006;74:4246–4253. doi: 10.1128/IAI.01620-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko Y, Choi JH, Ha NY, Kim IS, Cho NH, Choi MS. Active escape of Orientia tsutsugamushi from cellular autophagy. Infect Immun. 2013;81:552–559. doi: 10.1128/IAI.00861-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura A. Invasion and intracellular growth of Rickettsia tsutsugamushi. Microbiol Sci. 1988;5:228–232. [PubMed] [Google Scholar]
- 28.Valbuena G, Walker DH. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol. 2013;2:170. doi: 10.3389/fcimb.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tay ST, Rohani MY, Ho TM, Devi S. In vitro demonstration of the hemolytic, cytotoxic activities and induction of apoptosis in Orientia tsutsugamushi infected L929 mouse fibroblast cells. Southeast Asian J Trop Med Public Health. 2003;34:352–356. [PubMed] [Google Scholar]
- 30.Beyer AR, Rodino KG, VieBrock L, Green RS, Tegels BK, Oliver LD, Jr, Marconi RT, Carlyon JA. Orientia tsutsugamushi Ank9 is a multifunctional effector that utilizes a novel GRIP-like Golgi localization domain for Golgi-to-endoplasmic reticulum trafficking and interacts with host COPB2. Cell Microbiol. 2017:19. doi: 10.1111/cmi.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M, Ferguson DP, Blacksell SD, Newton PN, Day NP, Turner GD. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:e1466. doi: 10.1371/journal.pntd.0001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho KA, Jun YH, Suh JW, Kang JS, Choi HJ, Woo SY. Orientia tsutsugamushi induced endothelial cell activation via the NOD1-IL-32 pathway. Microb Pathog. 2010;49:95–104. doi: 10.1016/j.micpath.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Gharaibeh M, Hagedorn M, Lilla S, Hauptmann M, Heine H, Fleischer B, Keller C. Toll-like receptor 2 recognizes Orientia tsutsugamushi and increases susceptibility to murine experimental scrub typhus. Infect Immun. 2016;84:3379–3387. doi: 10.1128/IAI.00185-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atwal S, Giengkam S, Chaemchuen S, Dorling J, Kosaisawe N, VanNieuwenhze M, Sampattavanich S, Schumann P, Salje J. Evidence for a peptidoglycan-like structure in Orientia tsutsugamushi. Mol Microbiol. 2017;105:440–452. doi: 10.1111/mmi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS One. 2012;7:e39042. doi: 10.1371/journal.pone.0039042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tantibhedhyangkul W, Prachason T, Waywa D, El Filali A, Ghigo E, Thongnoppakhun W, Raoult D, Suputtamongkol Y, Capo C, Limwongse C, Mege JL. Orientia tsutsugamushi stimulates an original gene expression program in monocytes: relationship with gene expression in patients with scrub typhus. PLoS Negl Trop Dis. 2011;5:e1028. doi: 10.1371/journal.pntd.0001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tantibhedhyangkul W, Ben Amara A, Textoris J, Gorvel L, Ghigo E, Capo C, Mege JL. Orientia tsutsugamushi, the causative agent of scrub typhus, induces an inflammatory program in human macrophages. Microb Pathog. 2012;55:55–63. doi: 10.1016/j.micpath.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa M, Satoh M, Kataoka M, Ando S, Saijo M. Nitric oxide enhanced the growth of an obligated intracellular bacterium Orientia tsutsugamushi in murine macrophages. Microb Pathog. 2017;107:335–340. doi: 10.1016/j.micpath.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Keller CA, Hauptmann M, Kolbaum J, Gharaibeh M, Neumann M, Glatzel M, Fleischer B. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl Trop Dis. 2014;8:e3064. doi: 10.1371/journal.pntd.0003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JH, Cheong TC, Ha NY, Ko Y, Cho CH, Jeon JH, So I, Kim IK, Choi MS, Kim IS, Cho NH. Orientia tsutsugamushi subverts dendritic cell functions by escaping from autophagy and impairing their migration. PLoS Negl Trop Dis. 2013;7:e1981. doi: 10.1371/journal.pntd.0001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rikihisa Y, Ito S. Entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect Immun. 1982;38:343–350. doi: 10.1128/iai.38.1.343-350.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu H, Park SM, Cheon IS, Park MY, Shim BS, Gil BC, Jeung WH, Hwang KJ, Song KD, Hong KJ, Song M, Jeong HJ, Han SH, Yun CH. Orientia tsutsugamushi infection induces CD4+ T cell activation via human dendritic cell activity. J Microbiol Biotechnol. 2013 doi: 10.4014/jmb.1303.03019. [DOI] [PubMed] [Google Scholar]
- 43.Gorvel L, Textoris J, Banchereau R, Ben Amara A, Tantibhedhyangkul W, von Bargen K, Ka MB, Capo C, Ghigo E, Gorvel JP, Mege JL. Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PLoS One. 2014;9:e99420. doi: 10.1371/journal.pone.0099420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astrup E, Janardhanan J, Otterdal K, Ueland T, Prakash JA, Lekva T, Strand OA, Abraham OC, Thomas K, Damas JK, Mathews P, Mathai D, Aukrust P, Varghese GM. Cytokine network in scrub typhus: high levels of interleukin-8 are associated with disease severity and mortality. PLoS Negl Trop Dis. 2014;8:e2648. doi: 10.1371/journal.pntd.0002648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho BA, Ko Y, Kim YS, Kim S, Choi MS, Kim IS, Kim HR, Cho NH. Phenotypic characterization of peripheral T cells and their dynamics in scrub typhus patients. PLoS Negl Trop Dis. 2012;6:e1789. doi: 10.1371/journal.pntd.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prachason T, Konhan K, Pongnarin P, Chatsiricharoenkul S, Suputtamongkol Y, Limwongse C. Activation of indoleamine 2,3-dioxygenase in patients with scrub typhus and its role in growth restriction of Orientia tsutsugamushi. PLoS Negl Trop Dis. 2012;6:e1731. doi: 10.1371/journal.pntd.0001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang SJ, Jin HM, Cho YN, Kim SE, Kim UJ, Park KH, Jang HC, Jung SI, Kee SJ, Park YW. Increased level and interferon-gamma production of circulating natural killer cells in patients with scrub typhus. PLoS Negl Trop Dis. 2017;11:e0005815. doi: 10.1371/journal.pntd.0005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramme S, An le V, Khoa ND, Trin le V, Tannich E, Rybniker J, Fleischer B, Drosten C, Panning M. Orientia tsutsugamushi bacteremia and cytokine levels in Vietnamese scrub typhus patients. J Clin Microbiol. 2009;47:586–589. doi: 10.1128/JCM.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwasaki H, Mizoguchi J, Takada N, Tai K, Ikegaya S, Ueda T. Correlation between the concentrations of tumor necrosis factor-alpha and the severity of disease in patients infected with Orientia tsutsugamushi. Int J Infect Dis. 2010;14:e328–333. doi: 10.1016/j.ijid.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Paris DH, Jenjaroen K, Blacksell SD, Phetsouvanh R, Wuthiekanun V, Newton PN, Day NP, Turner GD. Differential patterns of endothelial and leucocyte activation in ‘typhus-like’ illnesses in Laos and Thailand. Clin Exp Immunol. 2008;153:63–67. doi: 10.1111/j.1365-2249.2008.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otterdal K, Janardhanan J, Astrup E, Ueland T, Prakash JA, Lekva T, Abraham OC, Thomas K, Damas JK, Mathews P, Mathai D, Aukrust P, Varghese GM. Increased endothelial and macrophage markers are associated with disease severity and mortality in scrub typhus. J Infect. 2014 doi: 10.1016/j.jinf.2014.06.018. pii: S0163-4453(14)00196-0. doi:10.1016. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Ning Z, Qiu Y, Liao Y, Chang H, Ai Y, Wei Y, Deng Y, Shen Y. Elevated levels of vWF and HMGB1 are associated with disease severity and clinical outcome of scrub typhus. Int J Infect Dis. 2017;61:114–120. doi: 10.1016/j.ijid.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 53.Tsai MH, Chang CH, Tsai RK, Hong YR, Chuang TH, Fan KT, Peng CW, Wu CY, Hsu WL, Wang LS, Chen LK, Yu HS. Cross-regulation of proinflammatory cytokines by interleukin-10 and miR-155 in Orientia tsutsugamushi-infected human macrophages prevents cytokine storm. J Invest Dermatol. 2016;136:1398–1407. doi: 10.1016/j.jid.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 54.Abhilash K, Mannam PR, Rajendran K, John RA, Ramasami P. Chest radiographic manifestations of scrub typhus. J Postgrad Med. 2016;62:235–238. doi: 10.4103/0022-3859.184662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Zhao Z, Bi Z, Kou Z, Zhang M, Yang L, Zheng L. Risk factors associated with severe scrub typhus in Shandong, northern China. Int J Infect Dis. 2014;29:203–207. doi: 10.1016/j.ijid.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Zhao D, Zhang Y, Yin Z, Zhao J, Yang D, Zhou Q. Clinical predictors of multiple organ dysfunction syndromes in pediatric patients with scrub typhus. J Trop Pediatr. 2016;63:167–173. doi: 10.1093/tropej/fmw066. [DOI] [PubMed] [Google Scholar]
- 57.Ittyachen AM, Abraham SP, Krishnamoorthy S, Vijayan A, Kokkat J. Immune thrombocytopenia with multi-organ dysfunction syndrome as a rare presentation of scrub typhus: a case report. BMC Res Notes. 2017;10:496. doi: 10.1186/s13104-017-2826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegue E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacMillan JG, Rice RM, Jerrells TR. Development of antigen-specific cell-mediated immune responses after infection of cynomolgus monkeys (Macaca fascicularis) with Rickettsia tsutsugamushi. J Infect Dis. 1985;152:739–749. doi: 10.1093/infdis/152.4.739. [DOI] [PubMed] [Google Scholar]
- 60.Paris DH, Chattopadhyay S, Jiang J, Nawtaisong P, Lee JS, Tan E, Dela Cruz E, Burgos J, Abalos R, Blacksell SD, Lombardini E, Turner GD, Day NP, Richards AL. A nonhuman primate scrub typhus model: protective immune responses induced by pKarp47 DNA vaccination in cynomolgus macaques. J Immunol. 2015;194:1702–1716. doi: 10.4049/jimmunol.1402244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sumonwiriya M, Paris DH, Sunyakumthorn P, Anantatat T, Jenjaroen K, Chumseng S, Im-Erbsin R, Tanganuchitcharnchai A, Jintaworn S, Blacksell SD, Chowdhury FR, Kronsteiner B, Teparrukkul P, Burke RL, Lombardini ED, Richards AL, Mason CJ, Jones JW, Day NPJ, Dunachie SJ. Strong interferon-gamma mediated cellular immunity to scrub typhus demonstrated using a novel whole cell antigen ELISpot assay in rhesus macaques and humans. PLoS Negl Trop Dis. 2017;11:e0005846. doi: 10.1371/journal.pntd.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi S, Jeong HJ, Hwang KJ, Gill B, Ju YR, Lee YS, Lee J. A Recombinant 47-kDa Outer Membrane Protein Induces an Immune Response against Orientia tsutsugamushi Strain Boryong. Am J Trop Med Hyg. 2017;97:30–37. doi: 10.4269/ajtmh.15-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lurchachaiwong W, Chan TC, Richards AL, McCardle W, Schuster AL. Establishment of Orientia tsutsugamushi Lc-1 (Rickettsiales: Rickettsiaceae) infection in ICR outbred mice (Rodentia: Muridae) by needle challenge. J Med Entomol. 2014;51:658–660. doi: 10.1603/me13025. [DOI] [PubMed] [Google Scholar]
- 64.Sunyakumthorn P, Paris DH, Chan TC, Jones M, Luce-Fedrow A, Chattopadhyay S, Jiang J, Anantatat T, Turner GD, Day NP, Richards AL. An intradermal inoculation model of scrub typhus in Swiss CD-1 mice demonstrates more rapid dissemination of virulent strains of Orientia tsutsugamushi. PLoS One. 2013;8:e54570. doi: 10.1371/journal.pone.0054570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soong L, Mendell NL, Olano JP, Rockx-Brouwer D, Xu G, Goez-Rivillas Y, Drom C, Shelite TR, Valbuena G, Walker DH, Bouyer DH. An intradermal inoculation mouse model for immunological investigations of acute scrub typhus and persistent infection. PLoS Negl Trop Dis. 2016;10:e0004884. doi: 10.1371/journal.pntd.0004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, Kaplan MH, Weigmann B, Zaiss MM, Fearon U, Veale DJ, Canete JD, Distler O, Rivellese F, Pitzalis C, Neurath MF, McKenzie ANJ, Wirtz S, Schett G, Distler JHW, Ramming A. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med. 2017;23:938–944. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, Kaplan MH. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–440 e431. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, Kaplan MH, Zhou B. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38:360–372. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauptmann M, Kolbaum J, Lilla S, Wozniak D, Gharaibeh M, Fleischer B, Keller CA. Protective and pathogenic roles of CD8+ T lymphocytes in murine Orientia tsutsugamushi infection. PLoS Negl Trop Dis. 2016;10:e0004991. doi: 10.1371/journal.pntd.0004991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu G, Mendell NL, Liang Y, Shelite TR, Goez-Rivillas Y, Soong L, Bouyer DH, Walker DH. CD8+ T cells provide immune protection against murine disseminated endotheliotropic Orientia tsutsugamushi infection. PLoS Negl Trop Dis. 2017;11:e0005763. doi: 10.1371/journal.pntd.0005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukuhara M, Fukazawa M, Tamura A, Nakamura T, Urakami H. Survival of two Orientia tsutsugamushi bacterial strains that infect mouse macrophages with varying degrees of virulence. Microb Pathog. 2005;39:177–187. doi: 10.1016/j.micpath.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Hsu YH, Chen HI. Pulmonary pathology in patients associated with scrub typhus. Pathology. 2008;40:268–271. doi: 10.1080/00313020801911488. [DOI] [PubMed] [Google Scholar]
- 74.Ha NY, Shin HM, Sharma P, Cho HA, Min CK, Kim HI, Yen NT, Kang JS, Kim IS, Choi MS, Kim YK, Cho NH. Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J Nanobiotechnology. 2016;14:76. doi: 10.1186/s12951-016-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shelite TR, Saito TB, Mendell NL, Gong B, Xu G, Soong L, Valbuena G, Bouyer DH, Walker DH. A hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus. PLoS Negl Trop Dis. 2014;8:e2966. doi: 10.1371/journal.pntd.0002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shelite TR, Liang Y, Wang H, Mendell NL, Trent BJ, Sun J, Gong B, Xu G, Hu H, Bouyer DH, Soong L. IL-33-dependent endothelial activation contributes to apoptosis and renal injury in Orientia tsutsugamushi-infected mice. PLoS Negl Trop Dis. 2016;10:e0004467. doi: 10.1371/journal.pntd.0004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soong L, Shelite TR, Xing Y, Kodakandla H, Liang Y, Trent BJ, Horton P, Smith KC, Zhao Z, Sun J, Bouyer DH, Cai J. Type 1-skewed neuroinflammation and vascular damage associated with Orientia tsutsugamushi infection in mice. PLoS Negl Trop Dis. 2017;11:e0005765. doi: 10.1371/journal.pntd.0005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soong L, Wang H, Shelite TR, Liang Y, Mendell NL, Sun J, Gong B, Valbuena GA, Bouyer DH, Walker DH. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PLoS Negl Trop Dis. 2014;8:e3191. doi: 10.1371/journal.pntd.0003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rikihisa Y, Ito S. Effect of antibody on entry of Rickettsia tsutsugamushi into polymorphonuclear leukocyte cytoplasm. Infect Immun. 1983;39:928–938. doi: 10.1128/iai.39.2.928-938.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun IO, Shin SH, Cho AY, Yoon HJ, Chang MY, Lee KY. Clinical significance of NGAL and KIM-1 for acute kidney injury in patients with scrub typhus. PLoS One. 2017;12:e0175890. doi: 10.1371/journal.pone.0175890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. 2017;198:1395–1402. doi: 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- 82.Bertheloot D, Latz E. HMGB1, IL-1alpha, IL-33 and S100 proteins: dual-function alarmins. Cell Mol Immunol. 2017;14:43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, Sun J. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol. 2013;190:5666–5675. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garth JM, Reeder KM, Godwin MS, Mackel JJ, Dunaway CW, Blackburn JP, Steele C. IL-33 signaling regulates innate IL-17A and IL-22 production via suppression of prostaglandin E2 during lung fungal infection. J Immunol. 2017;199:2140–2148. doi: 10.4049/jimmunol.1602186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rostan O, Arshad MI, Piquet-Pellorce C, Robert-Gangneux F, Gangneux JP, Samson M. Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases. Infect Immun. 2015;83:1738–1748. doi: 10.1128/IAI.02908-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gungor O, Unal HU, Guclu A, Gezer M, Eyileten T, Guzel FB, Altunoren O, Erken E, Oguz Y, Kocyigit I, Yilmaz MI. IL-33 and ST2 levels in chronic kidney disease: Associations with inflammation, vascular abnormalities, cardiovascular events, and survival. PLoS One. 2017;12:e0178939. doi: 10.1371/journal.pone.0178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pilzweger C, Holdenrieder S. Circulating HMGB1 and RAGE as clinical biomarkers in malignant and autoimmune diseases. Diagnostics (Basel) 2015;5:219–253. doi: 10.3390/diagnostics5020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. doi: 10.1111/imr.12601. [DOI] [PubMed] [Google Scholar]
- 90.Ong SP, Lee LM, Leong YF, Ng ML, Chu JJ. Dengue virus infection mediates HMGB1 release from monocytes involving PCAF acetylase complex and induces vascular leakage in endothelial cells. PLoS One. 2012;7:e41932. doi: 10.1371/journal.pone.0041932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janardhanan J, Joseph Martin S, Astrup E, Veeramanikandan R, Aukrust P, Abraham OC, Varghese GM. Single-nucleotide polymorphisms in Toll-like receptor (TLR)-2, TLR4 and heat shock protein 70 genes and susceptibility to scrub typhus. J Hum Genet. 2013;58:707–710. doi: 10.1038/jhg.2013.89. [DOI] [PubMed] [Google Scholar]
- 92.Aslan A, van Meurs M, Moser J, Popa ER, Jongman RM, Zwiers PJ, Molema G, Zijlstra JG. Organ-specific differences in endothelial permeability-regulating molecular responses in mouse and human sepsis. Shock. 2017;48:69–77. doi: 10.1097/SHK.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh CC, David S, Zhang R, Berghelli A, Milam K, Higgins SJ, Hunter J, Mukherjee A, Wei Y, Tran M, Suber F, Kobzik L, Kain KC, Lu S, Santel A, Yano K, Guha P, Dumont DJ, Christiani DC, Parikh SM. Gene control of tyrosine kinase TIE2 and vascular manifestations of infections. Proc Natl Acad Sci U S A. 2016;113:2472–2477. doi: 10.1073/pnas.1519467113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valbuena G, Walker DH. Infection of the endothelium by members of the order Rickettsiales. Thromb Haemost. 2009;102:1071–1079. doi: 10.1160/TH09-03-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahni SK, Narra HP, Sahni A, Walker DH. Recent molecular insights into rickettsial pathogenesis and immunity. Future Microbiol. 2013;8:1265–1288. doi: 10.2217/fmb.13.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frye M, Dierkes M, Kuppers V, Vockel M, Tomm J, Zeuschner D, Rossaint J, Zarbock A, Koh GY, Peters K, Nottebaum AF, Vestweber D. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med. 2015;212:2267–2287. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kee SH, Cho KA, Kim MK, Lim BU, Chang WH, Kang JS. Disassembly of focal adhesions during apoptosis of endothelial cell line ECV304 infected with Orientia tsutsugamushi. Microb Pathog. 1999;27:265–271. doi: 10.1006/mpat.1999.0304. [DOI] [PubMed] [Google Scholar]
- 98.Cho NH, Seong SY, Huh MS, Kim NH, Choi MS, Kim IS. Induction of the gene encoding macrophage chemoattractant protein 1 by Orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infect Immun. 2002;70:4841–4850. doi: 10.1128/IAI.70.9.4841-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–6948. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pollheimer J, Bodin J, Sundnes O, Edelmann RJ, Skanland SS, Sponheim J, Brox MJ, Sundlisaeter E, Loos T, Vatn M, Kasprzycka M, Wang J, Kuchler AM, Tasken K, Haraldsen G, Hol J. Interleukin-33 drives a proinflammatory endothelial activation that selectively targets nonquiescent cells. Arterioscler Thromb Vasc Biol. 2013;33:e47–55. doi: 10.1161/ATVBAHA.112.253427. [DOI] [PubMed] [Google Scholar]
- 101.Su L, Zhai R, Sheu CC, Gallagher DC, Gong MN, Tejera P, Thompson BT, Christiani DC. Genetic variants in the angiopoietin-2 gene are associated with increased risk of ARDS. Intensive Care Med. 2009;35:1024–1030. doi: 10.1007/s00134-009-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo L, Zhou X, Guo X, Zhang X, Sun Y. Association of interleukin-33 gene single nucleotide polymorphisms with ischemic stroke in north Chinese population. BMC Med Genet. 2013;14:109. doi: 10.1186/1471-2350-14-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stremitzer S, Zhang W, Yang D, Ning Y, Stintzing S, Sebio A, Sunakawa Y, Yamauchi S, Matsusaka S, El-Khoueiry R, Stift J, Wrba F, Gruenberger T, Lenz HJ. Genetic variations in angiopoietin and pericyte pathways and clinical outcome in patients with resected colorectal liver metastases. Cancer. 2015;121:1898–1905. doi: 10.1002/cncr.29259. [DOI] [PMC free article] [PubMed] [Google Scholar]