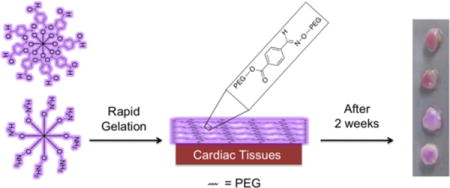
TOC Entry
Post-surgical cardiac adhesions increase surgery times as well as patient mortality and morbidity. We present a fast gelling oxime-crosslinked PEG hydrogel with tunable gelation time, degradation, and mechanical properties. This material is cytocompatible and prevents cellular adhesion. We demonstrate material retention on different cardiac tissues ex vivo over time and that functional group ratio alters material retention on different cardiac tissues.
Keywords: hydrogels, postsurgical adhesions, cardiac surgery
Materials that bind to tissues are widely used in the clinic for achieving homeostasis, sealing tissues, delivering exogenous substances locally, or preventing postsurgical adhesions.[1] Of these applications, there are a limited number of materials that have been used to prevent postsurgical adhesions in a clinical setting, the majority of which have been developed for abdominal and gynecological surgeries.[2] Postsurgical adhesions negatively impact organ function and removal increases reoperation times.[3, 4] This is especially true for cardiac surgeries where adhesions, or bands of scar tissue, can fuse the heart to neighboring tissues and organs. Postsurgical cardiac adhesions are a serious medical issue since thousands of cardiac surgeries are performed daily with reoperations constituting >20% of these surgeries.[5] Cardiac adhesions are a common problem in adults who experience multiple surgeries to repair or replace valves or to undergo coronary revascularization procedures,[6] as well as with pediatric patients who require repeated surgeries to repair congenital defects.[5, 7] The removal of adhesions poses a safety risk to patients since dissection increases reoperation times and can result in catastrophic hemorrhaging.[3, 4] Currently, only a handful of materials have been investigated to prevent postsurgical cardiac adhesions.[2, 3] Barrier membranes, such as REPEL-CV®, have been used in the clinic and while these can protect the heart upon resternotomy, they do not eliminate cardiac adhesions and have not been proven to reduce dissection times.[8] Retention of membranes on the tissue after placement can also be problematic due to postsurgical swelling and dynamic motion of the heart.[3, 9, 10]
A promising method to prevent postsurgical adhesions is to coat the tissue with a fast gelling polymer to prevent the susceptible tissue from adhering to other nearby tissue organs.[3, 9, 10] From a chemistry and material design perspective, adhesion prevention is challenging, especially in a cardiac surgery setting. The material must be easily applied, gel rapidly on the wet tissue surface, remain on the tissue for at least 2 weeks to overcome the initial inflammatory response post-surgery, and be biocompatible.[3, 11] This means that the pre-gel materials must be capable of reacting quickly and efficiently with themselves as well as with tissue, and the crosslinking functional groups must be biocompatible. Once gelled, the material must prevent cell adhesion and fibrin deposition since this leads to tissue adhesions.[3, 9] In addition, the material must exhibit minimal swelling since high swelling could result in cardiac tamponade (pressure on the heart). This has been an issue with materials such as CoSeal®,[12] which swells 300-400% after application.[10, 13]
We pursued application of oxime-crosslinked poly(ethylene glycol) (PEG) star polymers and assessed their potential for use in preventing cardiac adhesions. Oxime chemistry has been successfully used in a variety of in vitro and in vivo applications,[14] and PEG-coated surfaces have shown to minimize protein adsorption[15] and cellular adhesion.[16] We hypothesized an electron deficient aldehyde would be capable of reacting with amines on the tissue surface as well as with hydroxylamines to rapidly form a PEG-hydrogel on cardiac tissue (Figure 1a). Herein, we demonstrate that this material has the ability to have tunable gelation kinetics, inhibit cellular adhesion, have minimal swelling, and adhere to different cardiac tissues for over two weeks.
Figure 1.
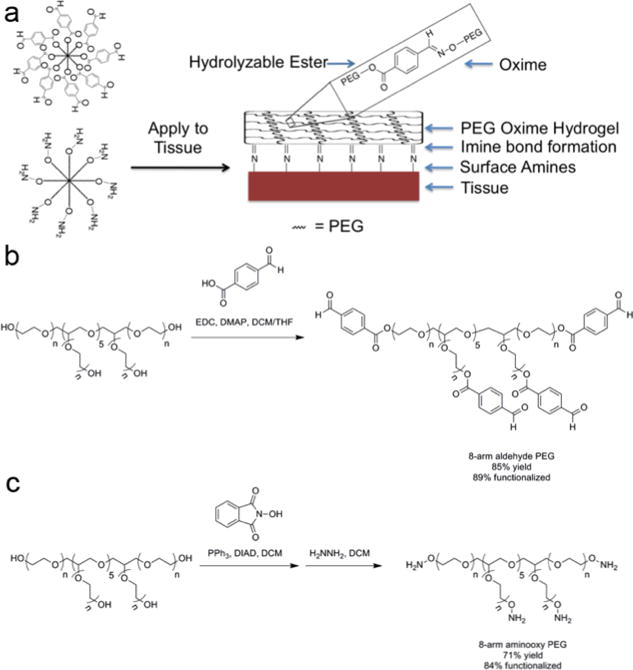
(a) Formation of oxime-crosslinked PEG-hydrogel onto tissue surface. (b) Synthesis of 8-arm aldehyde-PEG. (c) Synthesis of 8-arm aminooxy PEG. Abbreviations: N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC), 4-(dimethylamino)pyridine (DMAP), dichloromethane (DCM), tetrahydrofuran (THF), and diisopropyl azodicarboxylate (DIAD).
We chose to use materials crosslinked by oxime bonds because previous work has demonstrated it to be biocompatible, chemospecific, and bioorthogonal.[14] Since the equilibrium lies far toward the oxime product, these bonds exhibit excellent aqueous stability over imines and hydrazones. To achieve rapid gelation we used an 8-arm PEG containing an electron deficient aldehyde (ald-PEG, Figure 1b) and an 8-arm aminooxy-PEG (AO-PEG) (Figure 1c). The benzaldehyde group has been previously used to form Schiff base-crosslinked polymer networks.[17] Upon mixing the ald-PEG and AO-PEG in deionized water at 25 °C at equal concentrations, transparent hydrogels were rapidly formed (Figure 2a). The gelation rate was tunable based upon the weight percent of material from 400 seconds (25 mg/mL) to <2 seconds (100 mg/mL) (Figure 2b). For this system to be applicable in vivo, the hydrogel has to be hydrolytically stable for at least two weeks at physiological conditions. We characterized the percent mass loss over time for the 25, 50, and 100 mg/mL formulations at 37 °C (Figure 2c–e) that were incubated in 4× the gel volume of PBS, which was changed daily. For the 25 mg/mL gel, an increase in mass percent was observed at one week (likely due to increased swelling) followed by complete loss of the hydrogel after 18 days (Figure 2c). However, the 50 and 100 mg/mL gels only exhibited a mass loss of 26.5% and 19.7%, respectively, after 18 days (Figure 2d and e) indicating that at higher concentrations the oxime-crosslinked PEG-hydrogel system is hydrolytically stable at physiological conditions past two weeks.
Figure 2.
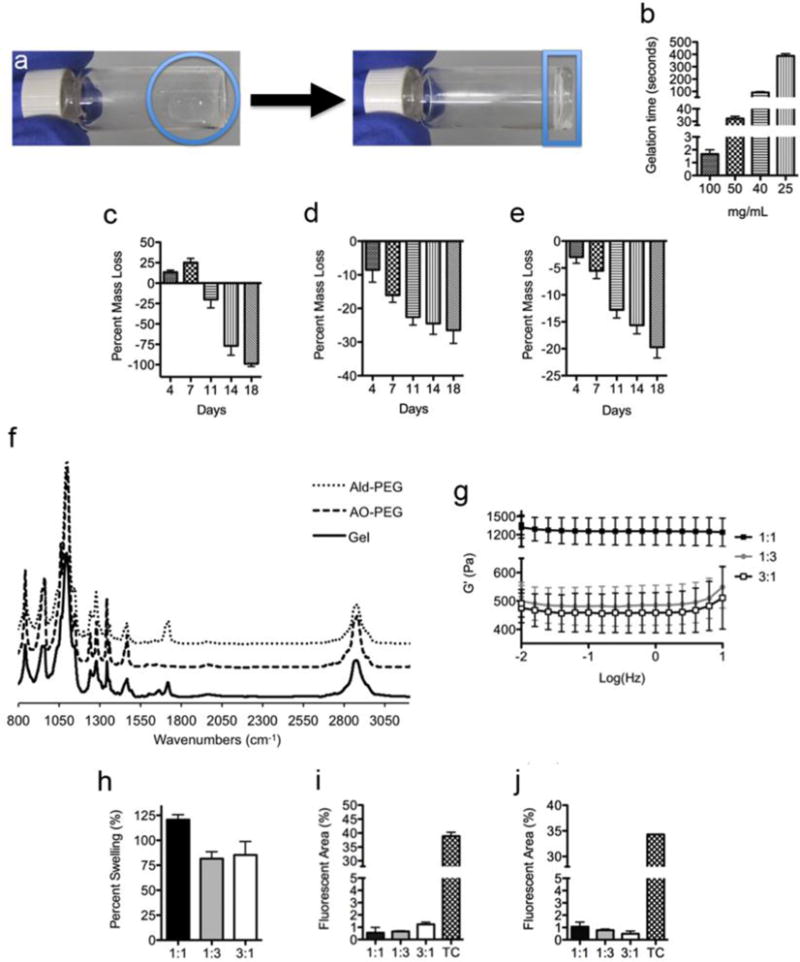
Formation of fast-gelling PEG hydrogels. (a) Upon mixing of ald-PEG and AO-PEG, transparent gels were formed in water at 25 °C. Left image: the two PEG components immediately after mixing is within the circle. Right image: the hydrogel that is formed from mixing the two PEG components together is within the rectangle. (b) Tunable gelation times based upon polymer concentration (mg/mL), demonstrating a decrease in gelation time with increasing concentration. Percent mass loss over time in PBS at 37 °C for (c) 25 mg/mL, (d) 50 mg/mL, and (e) 100 mg/mL gel formulations, demonstrating a decrease in degradation rate with increasing polymer concentration. (f) Infrared spectra of ald-PEG, AO-PEG, and oxime-crosslinked hydrogel, showing the formed oxime bond in the gel with the new peaks between 1600-1750 cm−1. (g) Storage moduli for 100 mg/mL gels. (h) Percent swelling for 100 mg/mL gels, demonstrating minimal swelling. Percent fluorescence area of membrane labeled (i) 3T3 fibroblasts and (j) RAW macrophages 24 h after seeding on 100 mg/mL gels, showing minimal cell adhesion to all PEG hydrogel formulations. Functional group ratio in g-j is aldehyde:aminooxy. TC = tissue culture plastic.
We pursued further characterization of the 100 mg/mL formulation given the rapid gelation time and slow degradation rate. Oxime bond formation was confirmed by infrared spectroscopy from the new peaks between 1600-1750 cm−1 for the gel versus the precursor polymers (Figure 2f). We next varied the functional groups ratios from 1:3 to 3:1 of ald-PEG:AO-PEG to investigate how excess functional groups affected gelation. These ratios were chosen since larger differences resulted in poor to incomplete gel formation. All three systems (1:1, 1:3, and 3:1 of ald-PEG:AO-PEG) gelled immediately upon mixing, which was confirmed by rheometry. The 100 mg/mL hydrogels had storage moduli (G’) ranging from ~450 to 1400 Pa (Figure 2g) and were highly hydrated with 94.7 ± 0.3, 96.5 ± 0.4, and 94.6 ± 0.5% water for 1:1, 1:3, and 3:1 hydrogels, respectively. All hydrogel formulations had minimal swelling (Figure 2h), with the 1:3 and 3:1 gels having a decrease in volume due to polymer loss during swelling, which was confirmed by mass measurements. Due to the rapid gel formation we expect these material properties to be maintained when these materials are applied in vivo.
Since functional group ratio affected gel stability we were interested to determine if an excess of either functional group would change the ability of cells to adhere to the material. Membrane labeled 3T3 fibroblasts or RAW macrophages were seeded on top of hydrogels or tissue culture plastic (TC) and the percent fluorescence area was quantified (Figure 2i and j). These cell types were chosen since they are involved in the inflammatory response. Both the fibroblasts (Figure 2i) and macrophages (Figure 2j) exhibited less than 2% fluorescent area for all three functional group ratios of the hydrogels, while TC plastic was between 30-40% fluorescent area for both cell types. This demonstrated that excessive amount of either functional group and the oxime bond did not alter the anti-cellular adhesive properties of PEG hydrogels.
We next examined if the elution products from the hydrogels exhibited any cytotoxicity by two different methods. First, we used an agar elution assay. A serum free agar gel was formed over a monolayer of cytosol labeled L929 fibroblasts and then the three different 100 mg/mL hydrogels (1:1, 1:3, and 3:1 of ald-PEG:AO-PEG), filter paper (negative control), or latex (positive cytotoxic control) were placed on top of the agar gel and cultured for an additional 24 h. We observed that there was a distinct difference in live cell number and morphology between the latex versus the filter paper and hydrogel groups (Figure 3b–d). The fibroblasts directly beneath the latex exhibited more rounded morphologies while cells beneath the hydrogels had similar cell morphology score and number of live cells as the nontoxic filter paper (Figure 3a). For the second cytocompatibilty assay the metabolic activity of monolayers of serum starved 3T3 fibroblasts using media doped (at 5, 25, or 50%) with the elution products from the hydrogels was quantified using Alamar Blue. At all concentrations, the 1:1 and 1:3 groups (aldehyde:aminooxy) were not significantly different than the group doped with an equivalent volume of PBS (Figure 3e–g). Both assays indicated that the 100 mg/mL PEG hydrogels were cytocompatible.
Figure 3.
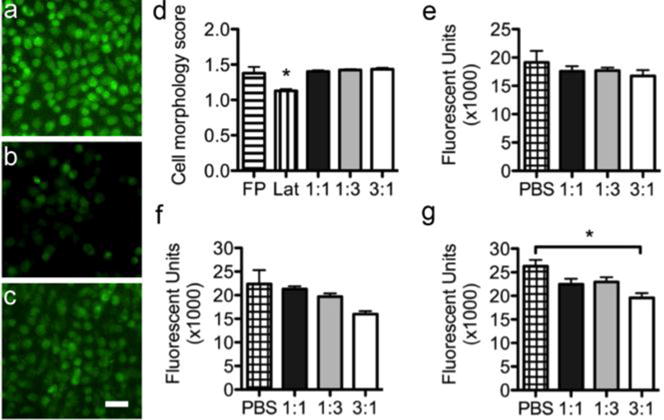
Fluorescent images of fibroblasts beneath (a) filter paper, (b) latex, and (c) 1:1 aldehyde:aminooxoy 100 mg/mL PEG hydrogel (scale bar is 50 μm). (d) Average cell morphology score of fibroblasts after 24 h beneath the materials (FP = filter paper, Lat = latex). Five pictures were taken per well (n=3) for a total of 15 pictures per group. Unlike the latex, which served as a positive cytotoxic control, the PEG hydrogels did not affect cell morphology. Metabolic activity of 3T3 fibroblasts after 24 h with elution product doped media at concentrations of (e) 5%, (f) 25%, and (g) 50% (n=3 per group); functional group ratio is aldehyde:aminooxy. At 5% and 25%, the elution products did not significantly affect metabolic activity. At 50%, the 3:1 group was significantly lower than the PBS control. Data are reported as mean ± SEM and analyzed with a one-way ANOVA with a Tukey’s post-hoc test (*p<0.05).
Finally, we examined the ability of these materials to adhere to cardiac tissue ex vivo. Previously, a sodium periodate oxidized dextran/PEG-amine system had been shown to exhibit different adhesion times to different tissues; however, this system was rapidly released from the tissue surface.[11] We tested the adhesion and retention time of oxime-crosslinked PEG gels on the various relevant tissue types. Fluorescently labeled ald-PEG and AO-PEG (see Supporting Information) solutions were loaded into pipetters and mixed directly on the surface of the biopsied tissue samples. Hydrogels were formed ex vivo on atrial, ventricular, cardiac adipose, and great vessel (aorta and superior vena cava) tissues (Figure 4a-d). For all tissues the 1:1 ald-PEG:AO-PEG gels were the slowest released from the tissue surface with 35%, 50%, 71%, 56%, and 65% material loss after two weeks for great vessels, atrium, adipose, ventricle, and PEG hydrogel alone, respectively (Figure 4e), and the gel remained visible on the tissue surfaces (Figure 4a-d). The 3:1 ald-PEG:AO-PEG gels had the medium amount of material loss over two weeks while the 1:3 ald-PEG:AO-PEG gels had the highest amount of material loss over two weeks (Figure 4e). The functional group ratio played a key role in overall gel stability, and ability to be retained on different tissue after two weeks. The excess of aminooxy groups (1:3 formulation) created the weakest gel and the least stable gel since 98% of the PEG was released into solution for the PEG on TC plastic (Figure 4e PEG groups). This lower stability was generally reflected in the release of PEG from the different cardiac tissues for the 1:3 group. We had hypothesized that the excess aldehyde groups would bind to amine residues present on the tissue surface and improve adhesion to tissue and therefore exhibit slower degradation from the tissue; however, the 1:1 formulation exhibited the slowest degradation over time once polymerized onto the different cardiac tissues. It is interesting to note that all the materials degraded slower on the vessels and fastest on the adipose tissue, while the myocardial tissues (atrial and ventricular) had similar degradation rates. This highlights the balance between functional group ratio, gel stability, and gel reactivity with tissue when designing materials to adhere to tissue.
Figure 4.
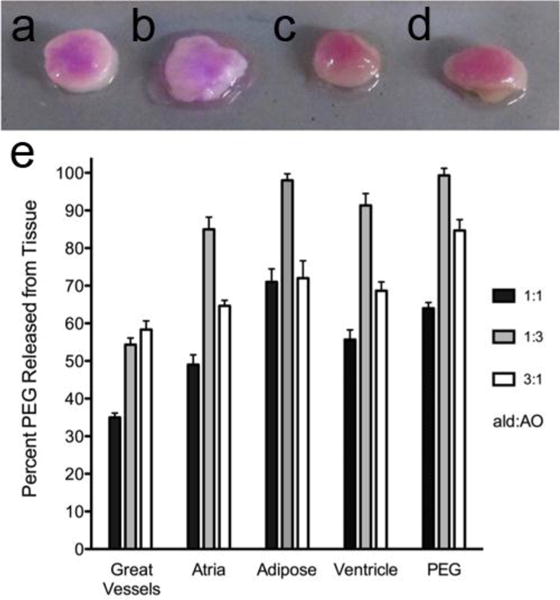
Oxime-crosslinked PEG adhesion to cardiac tissues. a) Great vessel, b) adipose, c) atrium, and d) cardiac adipose tissues coated with 1:1 aldehyde:aminooxy after 2 weeks. e) Percent PEG released from tissue surface after two weeks; functional group ratio is aldehyde:aminooxy (n=3 per group; Data is reported as mean ± SEM). Once adhered to the tissue, the PEG hydrogels degraded at different rates depending on the functional group ratio and the tissue, with the 1:1 gels having the slowest degradation as indicated by the lowest percent of PEG released from the tissue.
In conclusion, we have developed a fast-gelling oxime-crosslinked PEG-hydrogel, which is capable of rapidly adhering to various cardiac tissues. The gelation rate and degradation rate were tunable based upon the weight percent of the 8-arm aldehyde-PEG and aminooxy-PEG. Variation of the functional group ratio from 1:1, 1:3, and 3:1 ald-PEG:AO-PEG prevented adhesion of fibroblasts and macrophages, and the elution products were cytocompatible. We present that the cardiac tissue type and the functional group ratio of aldehyde:aminooxy directly impacted the ability of the material to adhere to the different cardiac tissue surfaces, and that the type of tissue and functional group ratio affected the rate of gel degradation. These results show that the types of tissue as well as the composition of the gels used to coat the tissue directly impact the retention of the material over time, which has important implications when designing materials for in vivo use. Given the minimal swelling, resistance to cell adhesion, and adherence to relevant cardiac tissues, oxime-crosslinked PEG hydrogels may have potential use in the prevention of postsurgical cardiac adhesions.
Supplementary Material
Acknowledgments
This work was funded in part by the UCSD CTRI (UL1 RR031980). GNG acknowledges an American Heart Association postdoctoral fellowship. JG acknowledges the UC LEADS program. MZ acknowledges the UCSD Department of Bioengineering REU summer research program.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.Spotnitz WD. Am Surgeon. 2012;78:1305. [PubMed] [Google Scholar]; Bouten PJM, Zonjee M, Bender J, Yauw STK, van Goor H, van Hest JCM, Hoogenboom R. Prog Polym Sci. 2014;39:1375. [Google Scholar]; Bre LP, Zheng Y, Pego AP, Wang WX. Biomater Sci-Uk. 2013;1:239. doi: 10.1039/c2bm00121g. [DOI] [PubMed] [Google Scholar]; Duarte AP, Coelho JF, Bordado JC, Cidade MT, Gil MH. Prog Polym Sci. 2012;37:1031. [Google Scholar]
- 2.Maciver AH, McCall M, Shapiro AMJ. Int J Surg. 2011;9:589. doi: 10.1016/j.ijsu.2011.08.008. [DOI] [PubMed] [Google Scholar]; Pados G, Venetis CA, Almaloglou K, Tarlatzis BC. Reprod Biomed Online. 2010;21:290. doi: 10.1016/j.rbmo.2010.04.021. [DOI] [PubMed] [Google Scholar]; Zawaneh PN, Putnam D. Tissue Eng Part B-Re. 2008;14:377. doi: 10.1089/ten.teb.2008.0226. [DOI] [PubMed] [Google Scholar]
- 3.Cannata A, Petrella D, Russo CF, Bruschi G, Fratto P, Gambacorta M, Martinelli L. Ann Thorac Surg. 2013;95:1818. doi: 10.1016/j.athoracsur.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Digest Surg. 2001;18:260. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]; Yeo Y, Kohane DS. Eur J Pharm Biopharm. 2008;68:57. doi: 10.1016/j.ejpb.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nkere UU. Asaio J. 2000;46:654. doi: 10.1097/00002480-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Morales D, Williams E, John R. Interact Cardiovasc Thorac Surg. 2010;11:277. doi: 10.1510/icvts.2009.232090. [DOI] [PubMed] [Google Scholar]
- 7.Salminen JT, Mattila IP, Puntila JT, Sairanen HI. Interact Cardiov Th. 2011;12:270. doi: 10.1510/icvts.2010.241448. [DOI] [PubMed] [Google Scholar]; Morales D, Williams E, John R. Interact Cardiov Th. 2010;11:277. doi: 10.1510/icvts.2009.232090. [DOI] [PubMed] [Google Scholar]; Seeger JM, Kaelin LD, Staples EM, Yaacobi Y, Bailey JC, Normann S, Burns JW, Goldberg EP. J Surg Res. 1997;68:63. doi: 10.1006/jsre.1996.4990. [DOI] [PubMed] [Google Scholar]
- 8.Lodge AJ, Wells WJ, Backer CL, O’Brien JE, Jr, Austin EH, Bacha EA, Yeh T, Jr, Decampli WM, Lavin PT, Weinstein S. Ann Thorac Surg. 2008;86:614. doi: 10.1016/j.athoracsur.2008.04.103. [DOI] [PubMed] [Google Scholar]
- 9.Duncan DA, Yaacobi Y, Goldberg EP, Mines M, O’Brien D, Congdon F, Carmichael MJ. J Surg Res. 1988;45:44. doi: 10.1016/0022-4804(88)90019-4. [DOI] [PubMed] [Google Scholar]
- 10.Marc Hendrikx M, Mees U, Hill AC, Egbert B, Coker GT, Estridge TD. Heart Surg Forum. 2001;4:204. [PubMed] [Google Scholar]
- 11.Artzi N, Shazly T, Baker AB, Bon A, Edelman ER. Adv Mater. 2009;21:3399. doi: 10.1002/adma.200900340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoleone CP, Valori A, Crupi G, Ocello S, Santoro F, Vouhe P, Weerasena N. G Gargiulo, in Interact Cardiov Th. 2009;9:978. doi: 10.1510/icvts.2009.212175. [DOI] [PubMed] [Google Scholar]
- 13.Alizzi AM, Summers P, Boon VH, Tantiongco JP, Thompson T, Leslie BJ, Williams D, Steele M, Bidstrup BP, Diqer AMA. Heart Lung Circ. 2012;21:22. doi: 10.1016/j.hlc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich S, Boturyn D, Marra A, Renaudet O, Dumy P. Chem-Eur J. 2014;2034 doi: 10.1002/chem.201302426. [DOI] [PubMed] [Google Scholar]; Grover GN, Lam J, Nguyen TH, Segura T, Maynard HD. Biomacromolecules. 2012;13 doi: 10.1021/bm301346e. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grover GN, Braden RL, Christman KL. Adv Mater. 2013;25:2937. doi: 10.1002/adma.201205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. Langmuir. 2001;17:5605. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 16.Zhang MQ, Desai T, Ferrari M. Biomaterials. 1998;19:953. doi: 10.1016/s0142-9612(98)00026-x. [DOI] [PubMed] [Google Scholar]
- 17.Cao LP, Cao B, Lu CJ, Wang GW, Yu L, Ding JD. J Mater Chem B. 2015;3:1268. doi: 10.1039/c4tb01705f. [DOI] [PubMed] [Google Scholar]; Ding C, Zhao L, Liu F, Cheng J, Gu J, Dan S, Liu C, Qu X, Yang Z. Biomacromolecules. 2010;11:1043. doi: 10.1021/bm1000179. [DOI] [PubMed] [Google Scholar]; Yang B, Zhang YL, Zhang XY, Tao L, Li SX, Wei Y. Polym Chem-Uk. 2012;3:3235. [Google Scholar]; Zhang Y, Tao L, Li S, Wei Y. Biomacromolecules. 2011;12:2894. doi: 10.1021/bm200423f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


