Abstract
Abstract Prostate cancer is the second leading cause of cancer deaths in the USA. The challenge in managing castration-resistant prostate cancer (CRPC) stems not from the lack of therapeutic options but from the limited duration of clinical and survival benefit offered by treatments in this setting due to primary and acquired resistance. The remarkable molecular heterogeneity and tumor adaptability in advanced prostate cancer necessitate optimization of such treatment strategies. While the future of CRPC management will involve newer targeted therapies in deliberately biomarker-selected patients, interventions using current approaches may exhibit improved clinical benefit if employed in the context of optimal sequencing and combinations. This review outlines our current understanding of mechanisms of therapeutic resistance in progression to and after the development of castration resistance, highlighting targetable and reversible mechanisms of resistance.
Keywords: Therapeutic resistance, Prostate cancer, Castration-resistant prostate cancer
Introduction
In 2016, there will be over 180,000 new cases of prostate cancer in the USA alone, accounting for over one in five new cancer diagnoses [1]. While it is also a leading cause of cancer deaths, prostate cancer patients display a wide spectrum of clinical trajectories and outcomes, where some achieve remission yet others rapidly develop lethal disease. Our understanding of the use of systemic therapies to impair prostate tumor growth stems from Huggins’ seminal discovery in 1941 on the therapeutic response of prostate cancer to castration-induced androgen deprivation [2], groundbreaking work which led to his receipt of the Nobel Prize in 1966. Since then, while we have amassed more knowledge on the intricacies of androgen signaling axis and the androgen receptor (AR), the treatment paradigm for recurrent and advanced prostate cancer, as well as an adjuvant to localized prostate cancer, remains dependent on androgen deprivation in the form of surgical or medical castration, the latter via an approach known as androgen deprivation therapy (ADT) [3]. Today, ADT is deployed for men with both metastatic and high-risk localized diseases and is frequently utilized alongside other modalities.
First-line ADT, most frequently administered in the form of luteinizing hormone-releasing hormone (LHRH) agonists/antagonists with or without antiandrogens, suppresses prostate-specific antigen (PSA) levels in up to 90% of patients whose disease has spread beyond the prostate [4]. While dramatic initial responses to ADT are not uncommon, these responses are seldom sustained in the long term, with the median duration of response of up to 18months [5]. Virtually, all patients eventually progress to castration resistance, during which biochemical and radiographic progression is observed despite castrate levels of serum testosterone [6]. This key characteristic of continued tumor progression despite seemingly “starved” androgen conditions led to the initial impression that castration-resistant prostate cancer (CRPC) was a hormone-independent entity. The realization that CRPC remains fueled by androgen signaling, albeit with an increased utility of nontraditional pathways and alterations involving both the androgenic ligand (dihydrotestosterone (DHT)) and the AR [7], led to newer AR-directed agents abiraterone and enzalutamide deployed in metastatic CRPC (mCRPC) that are direct efficacious strategies targeting the continued dependence of prostate cancer on androgens [8, 9]. These therapeutic manipulations, however, are also met with resistance which remains an issue in the second-, third-, and fourth-line therapies—or until the patient finally succumbs to his illness. Today, the median overall survival for patients upon developing CRPC is in the range of 14–26 months [10].
Considering the augmentation of FDA-approved options for men with CRPC from only docetaxel in 2004 to six [8, 9, 11–14] different therapies in 2016, there is great need to understand the selective pressures that drive resistance to identify actionable targets that prolong the duration of benefit offered by these treatments. In addition, questions surrounding optimal treatment sequencing and combinations remain [15, 16] and are currently being examined by both prospective and retrospective studies. Indeed, the issue of resistance extends beyond patients developing refractory disease to continued therapy with one agent to other agents in the form of cross-resistance occurring not only in the context of drugs targeting the same pathway but also between drug classes [17–19]. With newer treatments in the developmental pipeline, we are faced with a rapidly evolving clinical landscape but a lagging understanding of how each treatment complements another and their sequencing optimization platforms to maximize clinical efficacy. This review will examine the known pathways of therapeutic resistance to systemic therapies, focusing on potentially targetable resistance reversal strategies in advanced lethal CRPC.
Resistance to Androgen Ablation in Hormone-Sensitive Disease
Resistance to ADT is virtually inevitable but occurs after a unique timeline of therapy for each patient after biochemical recurrence or diagnosis of advanced disease [20]. The clinical heterogeneity in progression to metastatic CRPC reflects the diversity of molecular adaptations present in this setting. The deprivation of serum testosterone initially leads to reduced levels of DHT, the primary mediator of AR signaling in prostate tissue. Upon withdrawal of these ligands, the androgen-dependent tumor cells undergo dramatic apoptosis, while a subset may maintain a dormant state [21]. Cancer stem cells have also been appreciated in this setting in promoting progression to CRPC [22]. Eventually, a population of such arrested cells is able to adapt to these states of low androgen, resuming proliferation with progression manifesting in the form of rising PSA that may or may not be coupled with osseous, soft tissue, or visceral metastases [23]. Progression to CRPC thus represents a cumulation of resistance strategies deployed by cells against castration. These critical adaptations involving the AR, ligand, as well as signaling regulators of the AR mechanistic pathway are described in the following sections (and illustrated in Fig. 1).
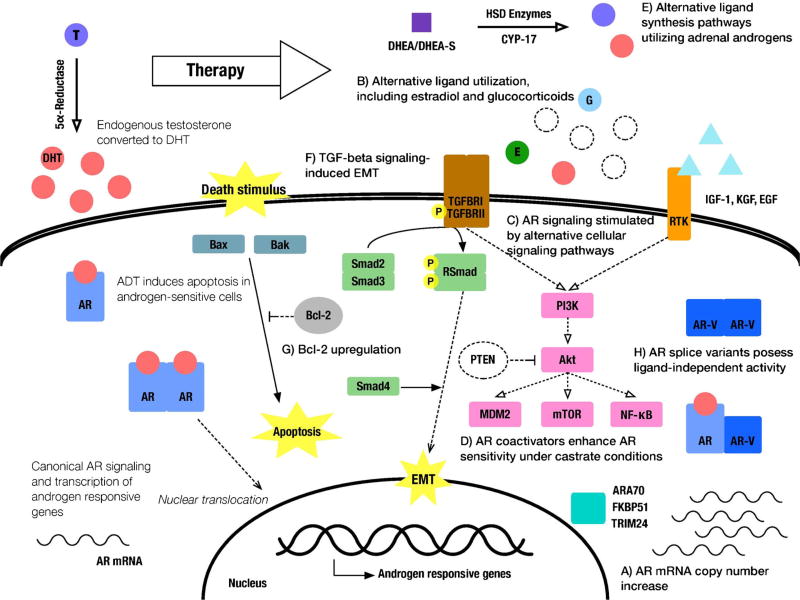
Fig. 1.
Major mechanisms of therapeutic resistance to androgen axis-targeting therapies in prostate cancer. A AR gene and mRNA amplifications to increase ligand sensitivity. B Utilization of nonandrogen ligands such as estradiol and glucocorticoids. C AR activation via alternative survival signaling pathways including PI3K/AKT, NF-κB, and RTKs. D AR coactivators can enhance AR sensitivity to various alternative ligands. E Utilization of alternative ligand synthesis pathways for conversion of adrenal androgen precursors. F Activation of TGF-β signaling pathway in EMT-to-MET interconversions. G Overexpression of prosurvival molecules such as Bcl-2 contributing to evasion of apoptosis activation. H AR splice variants with ligand-independent activation promote AR transcriptional programs in the absence of ligand
AR Adaptations Towards Alternative Activation Mechanisms
Among the strategies deployed by tumor cells to evade apoptosis induction by ADT, several mechanisms fall under functional adaptations of AR actions. One of the earlier breakthroughs in understanding the development of hormone-refractory disease came with the observation of AR gene amplification [24, 25], detected in almost a quarter of CRPC tissue specimens but virtually nonexistent in hormone-sensitive tissue [26]. In addition, studies have shown that resistance to antiandrogens is consistently linked to upregulation of AR expression [27], reflecting adaptations to increase sensitivity to low androgen (ligand) levels in sustaining AR programs (Fig. 1(A)). Antiandrogens used in combined androgen blockade in hormone-sensitive disease traditionally utilize bicalutamide, and less commonly flutamide and nilutamide. An important observation in this setting was that 15–30% [28] of tumors, upon becoming resistant to androgen blockade, would exhibit regressions after discontinuation of therapy, a phenomenon clinically defined as antiandrogen withdrawal syndrome (AWS) [29]. Now, we appreciate that certain AR mutations are well known to reactivate AR signaling; the T877A mutation, for example, confers resistance to hydroxyflutamide, the active form of flutamide [30]. W741C/L, another mutation of the AR ligand binding domain (LBD), confers resistance to bicalutamide [31], both of which illustrate the molecular basis of AWS. Additionally, the previously mentioned T877A mutation coupled with another AR mutation L701H functionally confers a promiscuous activation of the AR by glucocorticoids [32] (Fig. 1(B)). Comparisons between AR-dependent target genes in androgen-independent and androgen-dependent cells reveal that the AR-regulated transcriptional program is notably altered in castration-resistant disease, especially in the context of cell cycle genes, some of which result in the inactivation of cell cycle checkpoints [33].
AR activities can be stimulated by alternative signaling pathways, many of which play significant roles in the development of other human malignancies [34]. The NF-κB transcription factor signaling pathway has an established role in the progression to CRPC by maintaining AR activity [35] and sustaining AR messenger RNA (mRNA) and protein expression levels [36]. Further, its gene signature is sufficient in predicting prostate cancer-specific survival in clinical samples [37]. Other prominent survival signaling pathways (bypassing AR-driven mechanisms) such as the PI3K/AKT pathway have also been examined in the context of progression to metastatic CRPC and established as contributors to advanced metastatic disease [38]. The loss of phosphatase and tensin homolog deleted on chromosome ten (PTEN), tumor suppressor, and negative regulator of this pathway is one of the most frequent of molecular alterations in human prostate cancer. Indeed, PTEN loss allows the promotion of growth independent of AR signaling, and given PTEN loss, the development of castration resistance is intrinsic and not contingent on sustained AR activity [39, 40]. Further, PTEN status at diagnosis is predictive of not only time to CRPC, metastasis, and prostate cancer-specific survival but also response to ADT [41]. Finally, growth factors including insulin-like growth factor 1 (IGF-1), keratinocyte growth factor (KGF), and epidermal growth factor (EGF) have been shown to directly activate AR independent of androgen [42]. A schematic representation of these pathways is depicted in Fig. 1(C).
The AR is known to interact with a plethora of coactivators and corepressors (over 150) [43], many of which play a role in the transition to castration-resistant disease. Co-activators enhancing AR activity may functionally contribute to the AR’s heightened sensitivity to alternative ligands in the absence of endogenous androgen [44]. For example, the coactivator ARA70 can increase AR responsiveness to estradiol in prostate cancer cell lines [45] (Fig. 1(B)). FKBP51, another coactivator, stabilizes the HSP90-AR complex, enhancing the ability of AR molecules to bind to androgen [46]. Finally, TRIM24 is a transcriptional activator that has been shown to contribute to AR signaling under castrate androgen levels in SPOP mutants and in CRPC [47] (Fig. 1(D)).
Ligand Synthesis Adaptations
Castration-induced androgen withdrawal is known to deplete levels of circulating testosterone by 90–95% and achieve castrate levels of serum testosterone (less than 20 ng/dL) in most patients [48]; however, there is evidence to suggest that intratumoral levels of DHT in prostate tissue remain around 25% and could be as high as 40% of baseline prior to therapy [49]. These levels of intratumoral androgen are sufficient to sustain androgen signaling [50, 51] and can be accounted for in part by the contribution of androgen precursors from the adrenal gland, namely dehydroepiandrosterone (DHEA) and DHEA-S, its sulfated form. DHEA can be converted to DHT within the tumor via enzymes 3β-hydroxysteroid dehydrogenase (3βHSD), steroid 5α-reductase (SRD5A), and 17β-hydroxysteroid dehydrogenase (17βHSD) [52]. Emergence of prostate cancer cells to a castration-resistant state involves the upregulation of various steroidogenic enzymes, including HSD3B1, HSD3B2, HSD17B3, SRD5A1, and CYP17A1 for higher production of intratumoral androgen [53, 54]. A gain of function mutation has been identified in 3βHSD, conferring resistance to ubiquitination and degradation [55]. From a clinical standpoint, the efficacy of cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17), inhibiting agents abiraterone and now the less commonly used ketoconazole, is reflective of the dependence of CRPC on androgen metabolism [56]. Moreover, other nontraditional pathways for DHT synthesis that can bypass biochemical synthesis of testosterone utilize precursors such as acetic acid and progesterone [57]. A schematic representation of these pathways is shown in Fig. 1(E).
Impact of the Prostate Tumor Microenvironment: Can Epithelial-Mesenchymal Transition Circumvent Apoptosis?
While there have been significant molecular advances in genomic classification of human cancer during disease progression to metastasis, our understanding of the biology of individual tumors as an independent phenotypic entity is still profoundly limited; tumor behavior and biological properties depend largely on cancer epithelial cells and the adjacent stromal cells that comprise the tumor microenvironment [58]. It is in the very context of shaping the tumor microenvironment landscape that epithelial-mesenchymal transition (EMT) [59], a critical phenotypic process that confers invasive and migratory capacity to localized primary tumor cells [60, 61] towards metastasis, has been implicated as a contributing force (under androgenic control) to prostate cancer development and progression. While androgens can induce EMT in prostate epithelial cells, low AR levels, conditions reflective of ADT, seem crucial in sensitizing prostate cancer cells to androgen-mediated EMT [62]. EMT induces tumor stem cell-like properties [63], contributing to therapeutic resistance as well as evasion of apoptosis and immune surveillance [64]. While EMT involves a variety of interactive signaling pathways, transforming growth factor β (TGF-β) is recognized as the master orchestrator of this process, with hallmark consequences of loss of the cell adhesion molecule E-cadherin [65] (Fig. 1(F)). Rapidly growing evidence implicates EMT in human prostate cancer progression, with a significant association with poorer outcomes and aggressive disease [66, 67]. Since ADT has been shown to promote EMT, it may play an unorthodox role in fueling progression to lethal prostate cancer. ADT can significantly alter the dynamics of the prostate tumor microenvironment, affecting stromal, endothelial, and immune cells [68].
Prostate cancer has a lower proliferative capacity compared to other solid tumors, and this hallmark feature of growth kinetics renders apoptosis induction as a critical therapeutic avenue for targeted therapies directed at the apoptotic signaling mechanisms responsible for apoptosis evasion [69]. In the simplest terms, tumor progression results from an imbalance in cell proliferation and death. While resisting cell death is a classic hallmark of neoplasms [70], few compounds specifically targeting the apoptotic pathway have progressed in the developmental pipeline to phase III clinical studies in prostate cancer [71]. AR as a critical transcription factor in the regulation of prostate cell growth acts as a repressor for pro-apoptotic genes; initiation of ADT triggers apoptotic cascades in hormone-sensitive prostate cancer cells and prostatic glandular epithelium [72]. In the subset of cells capable of evading apoptosis, the cellular machinery to activate the cascade is retained but severely altered. In the development of CRPC, molecular changes within prostate cancer cells may prevent the transcription of genes involved in the programmed cell death in castrate conditions [73]. Further, overexpression of Bcl-2, a protagonist antiapoptotic molecule belonging to the Bcl-2 family of proand antiapoptotic signaling effectors [74] (Fig. 1(G)), is associated with prostate cancer progression and confers resistance to ADT [75]. Bcl-2 has long attracted interest as a viable target in various tumors, most notably in the form of antisense oligonucleotides and small molecule inhibitors [76].
Anoikis describes a related modality of cell death, whereby cells undergo apoptosis upon detachment from the extracellular matrix (ECM), a process that must be evaded for tumor cells to spread [77]. This is an essential tactic deployed by tumor cells as both a means of metastatic spread and therapeutic resistance. Tumor cells undergoing EMT are able to circumvent anoikis through cellular reprogramming; pro-EMT molecules including but not limited to transcriptional repressors SNAIL and SLUG, as well as cell adhesion molecules including E-cadherin, have been known to confer resistance to anoikis [78]. Furthermore, prostate cancer cells are able to alter their integrin expression profiles which leads to an anoikis-resistant phenotype. Integrin αvβ3 in prostate as well as other cancers confers a migratory phenotype and is overexpressed in the androgen-independent human prostate cancer cells (PC3) [79]. Regarded as a characteristic consequence and a necessary component to EMT, the processes of anoikis and EMT jointly contribute to the overall properties of chemoresistance, immune evasion, and metastasis.
Therapeutic Resistance in CRPC
The clinical landscape of CRPC has evolved rapidly, particularly in the past half decade. Patients today have FDA-approved options ranging from next-generation AR signaling inhibitors abiraterone and enzalutamide, taxanes docetaxel and cabazitaxel, bone targeting radiopharmaceutical radium-223, and dendritic cell vaccine sipuleucel-T, with additional promising therapeutics in the developmental pipeline. Despite the explosion of options, resistance to therapies in CRPC remains a critical area of unmet clinical need as evidenced by the modest survival benefits offered by each of these treatments. On top of the unknowns regarding optimal drug sequencing, the exact placement of drugs whose mechanisms of action do not involve the AR or AR signaling axis, namely radium-223 and sipuleucel-T, remains undetermined.
The mechanisms contributing to the emergence of CRPC likely continue in the castrate state as patients progress through additional lines of therapy. Instead of revisiting each of these mechanisms, this section will highlight findings on resistance to therapies administered for CRPC. Representative mechanisms are summarized in Table 1. In recent years, AR splice variants (AR-V) have been uncovered as a novel mechanism via which AR signaling is dynamically sustained in advanced lethal tumors. The AR-Vs are truncated forms of the AR lacking the LBD, some of which are constitutively active and allow for the ligand-independent activation of AR target genes [92, 93]. The AR-V7, a clinically relevant variant due to its ligand-independent activity, along with abundance, heightened expression in CRPC tissues, and its detectable protein product has recently attracted interest as a potential therapy-selecting biomarker [94–96] (Fig. 1(H)). Preclinical [97, 98] as well as clinical evidence is suggestive of its role in resistance to novel AR-directed therapies abiraterone and enzalutamide [84••] but not to taxane chemotherapies [83••]. The differential response of AR-V7-positive patients to these two classes of therapy supports its use as a predictive biomarker, yet important details on the precise role of AR-Vs still need to be elucidated.
Table 1.
Drug-specific mechanisms of resistance in CRPC therapy. Notably, non-drug-specific mechanisms driving the development of hormone refractory disease continue to play a role in disease progression in CRPC
| Drug-specific mechanism(s) of resistance | |
|---|---|
| Enzalutamide | |
| Abiraterone | |
| Docetaxel | |
| Cabazitaxel | |
| Sipuleucel-T |
|
| Radium-223 |
|
| Apalutamide |
Even after the emergence of CRPC, the tumor microenvironment remains a remarkable contributor to tumor cell behavior at local and distal sites of invasion and metastasis. With a higher likelihood of tumor dissemination in the CRPC state, each compartment within which the tumor resides is a nurturing microenvironment—prostate, lymph node, bone, and so on. Notably, since approximately 90% [99] of patients with advanced disease develop metastases to the bone, the leading cause of disease morbidity [100], there is great momentum in characterizing the bone microenvironment dynamics and the targeting of bone metastases in patients and in preclinical models towards overcoming therapeutic resistance. While not strictly a resistance mechanism in and of itself, tumor establishment in the bone niche is a known detriment to survival and quality of life [101, 102]. Interactions between tumor cells and resident cells of the bone microenvironment contribute to the development of bone metastases; tumor cells compete with hematopoietic stem cells (HSCs) for the occupation of the osteoblastic niche. Particularly, metastasis to the bone was promoted if the niche was altered in a way to eliminate HSCs from the bone microenvironment [103]. Clinically, denosumab, a human monoclonal antibody against receptor activator of NF-κB (RANK), ligand has been shown to delay the risk of skeletal complications in those with bone metastasis [104] as well as the development of bone metastatic disease in nonmetastatic CRPC [105]. RANK ligand, an active player in the bone microenvironment involved in normal bone turnover, has been shown to activate various transcription factors regulating EMT, stem cell properties, neuroendocrine differentiation, and osteomimicry, as well as promote bystander cell involvement in bone metastasis formation [106]. The radiopharmaceutical radium-223 offers improvement in overall survival, further lending support to the idea that the bone is a viable and important therapeutic target [107].
Resistance to Androgen Axis Inhibition/AR-Directed Therapies
Enzalutamide is a second-generation antiandrogen initially approved in 2012 for patients with mCRPC in the post-docetaxel setting, but its indication was later expanded in 2014 to include chemotherapy-naïve men with mCRPC [108]. Enzalutamide binds the LBD of the AR with high affinity, reducing AR nuclear translocation efficiency and disrupting binding to androgen response elements (AREs) on DNA, and impairs the recruitment of AR coactivators [109]. While the deployment of enzalutamide in clinical practice has significantly altered the treatment paradigm of CRPC, resistance is inevitable as evidenced by the modest improvement in overall survival. In addition, 25% of patients display primary resistance to the drug [110]. The missense mutation F876L in the LBD is known to confer resistance to enzalutamide by bestowing agonist properties [80••, 111], a common issue encountered with first-generation antiandrogens. Further, apalutamide (previously known as ARN-509), a newer second-generation antiandrogen, is also known to behave as an AR agonist in the presence of this mutation. Interestingly, there is evidence to suggest that mutants harboring F876L may respond to first-generation antiandrogens bicalutamide and flutamide [81]. Such findings may warrant clinical investigation of rechallenging certain patients with a previously failed agent. It also appears that GR induction, whose role is appreciated in the progression to CRPC, is a bypass mechanism that leads to transcription of AR-targeted genes and resistance to enzalutamide [112]. In the context of alternative AR activation, there is evidence that the aforementioned NF-κB pathway, particularly the upregulation of NF-κB2/p52, reactivates AR signaling through both full-length AR (AR-FL) and AR-V [82].
Abiraterone acetate is a selective potent inhibitor of CYP17 [113] approved in 2011 for mCRPC in the post-docetaxel setting, but with expanded indication in 2012 to include chemotherapy-naïve men with CRPC [114]. The efficacy of abiraterone reflects the contribution of intratumoral and adrenal androgens in sustaining prostate cancer growth in CRPC. As with enzalutamide, resistance to abiraterone is inevitable and up to a third of patients display primary resistance [110]. Studies have revealed that several genes in androgen biosynthesis pathways, including CYP17A1, the primary target of abiraterone, are upregulated following treatment with abiraterone, representing an adaptation of tumors to evade the effects of inhibition [98]. Further, CYP17 inhibition results in the accumulation of upstream substrates, which may be utilized in alternative steroid biosynthesis pathways that ultimately lead to DHT [115]. Given the relatively recent approvals of both enzalutamide and abiraterone, much of their resistance mechanisms remain under exploration.
Resistance to Taxane Chemotherapies
Recent advances in genomic cloning and phosphoproteomic profiling of advanced prostate tumors have fueled great excitement in prostate cancer therapeutics towards the development and implementation of personalized targeted therapies [85, 116••, 117]. Nevertheless, the therapeutic impact of taxane-based first- and second-line chemotherapies (docetaxel and cabazitaxel, respectively) on patient survival is still essential in the management of advanced prostate cancer, both in hormonesensitive disease and CRPC [118••, 119••]. Docetaxel, first approved for CRPC in 2004, was the only chemotherapeutic drug to improve overall survival in patients with CRPC for the greater half of the following decade [120]. It is a microtubule-targeting (stabilizing) agent that binds to β-tubulin subunits, resulting in apoptosis and G2/M cell cycle arrest [121, 122]. Microtubules are essential structures for facilitating AR trafficking to the nucleus [86, 123], and the activity of docetaxel significantly depletes nuclear AR [87]. In the CRPC setting, taxanes are an important therapeutic option for those refractory to AR-targeting agents, but resistance develops after a median PSA response duration of 7–8 months [14]. One well-documented mechanism of docetaxel resistance involves the overexpression of ATP-binding cassette (ABC) transporter molecules such as P-glycoprotein contributing to increased drug efflux from the tumor cells [124]. Additionally, structural changes in β-tubulin, such as the class III β-tubulin isoform, can affect docetaxel binding and activity, diminishing docetaxel efficacy [88]. Notably, class III β-tubulin expression is heightened as a result of androgen ablation [125] and is predictive of response to docetaxel [126]. The former observation highlights the tumor’s ability to adapt to therapeutic interventions. Furthermore, docetaxel resistance is also associated with EMT induction, as evidenced by decreased E-cadherin expression and increased mesenchymal markers [90], reaffirming the significance of tumor microenvironment phenotypic landscape in therapeutic resistance.
Given the relatively shorter period since its approval, resistance mechanisms to cabazitaxel, a second-generation microtubule-stabilizing agent, are less understood. Mechanistically, it was recently demonstrated that while cabazitaxel does not result in the depletion of nuclear AR (as docetaxel), it reduces AR expression with the overall outcome of apoptosis [127]. Significantly enough, despite their similar targeting action against microtubules, the mechanisms of docetaxel and cabazitaxel resistance (dissected so far) are not entirely overlapping, implicating differential mechanisms of resistance. Thus, cabazitaxel has weaker affinity for P-glycoprotein, and it is less susceptible to resistance via drug efflux compared to docetaxel [91]. However, much like docetaxel, elevated class III β-tubulin and altered EMT markers do confer resistance to cabazitaxel treatment in advanced tumors [89]. In the context of cross-resistance with antiandrogens, the therapeutic landscape becomes more intriguing as the action of cabazitaxel as a second-line chemotherapy is unhindered in enzalutamide-resistant prostate cancer, whereas docetaxel shows blunted efficacy [17]. Given that docetaxel is the standard first-line chemotherapy drug for CRPC and cabazitaxel’s performance in this setting is unknown, questions regarding sequencing remain, especially when coupled with implementation of AR-directed agents.
Considering the potential impact of transient interconversions of EMT to its reverse process mesenchymal-epithelial transition (MET) in the sequencing of taxane chemotherapy with antiandrogens to impair lethal prostate cancer, recent efforts focus on profiling the EMT phenotypic landscape towards building personalized signatures to predict therapeutic response/resistance in patients with advanced disease. As discussed above, microtubule-targeting taxane chemotherapy offers a critical therapeutic avenue for those patients who have failed AR-targeting strategies. The detection of AR-V7 in clinical specimens has not been shown to predict resistance to taxanes [83••]; there is evidence that taxane chemotherapy may even contribute to the reversion of theAR-V7phenotype [128]. Further, recent work from our group provided exciting new evidence that in a preclinical model of prostate tumor progression, cabazitaxel treatment induces prostate tumor cell redifferentiation by reversing EMT to MET into the epithelial phenotype among therapy-resistant prostate cancer cells [129]. Moreover, synergism of microtubule-targeting chemotherapy, docetaxel, with the novel AR N-terminal domain (NTD) inhibitor EPI-001/002, can navigate serial cycling of EMT to the reverse process of MET, supporting the possibility of reprogramming the EMT profile in advanced disease, even in those tumors harboring the AR variants [130]. Despite its temporal nature, the plasticity of EMT emerges as an attractive target for resensitizing prostate tumors resistant to AR-directed therapies and/or first- or second-line microtubule-targeting chemotherapies.
The Future of Clinical Management of Advanced CRPC
Apparent from both the non-therapy-specific and therapy-specific mechanisms of resistance in CRPC, tumor cells display remarkable adaptability. The clinical heterogeneity in disease course is a reflection of the marked molecular heterogeneity observed in prostate cancer, demanding a personalized approach to treatment [131]. Newer techniques of noninvasive liquid biopsies including circulating tumor cells (CTCs) and circulating tumor DNA (ctDNA) provide opportunities to examine tumor adaptations in real time and has profound implications for precision medicine [132]. Significantly enough, liquid biopsies allow prostate cancer to be followed temporally to identify resistant clones and driver mutations that may account for therapeutic failure [116••]. As we become better acquainted with the underlying molecular mechanisms that drive resistance and associated biomarkers, serial biopsies are likely to provide additional insights into clinical decision-making.
Evidence from a series of combination approaches assessing the clinical benefit, including abiraterone and enzalutamide, NCT01949337; Rad-223 and abiraterone, NCT02043678; enzalutamide and docetaxel, NCT01565928, among many others, provides promise as to the therapeutic sequencing of existing combination strategies. Particularly, with the heightened interest surrounding immunotherapies, combination strategies involving sipuleucel-T are being considered as well for additive clinical benefits. For example, the potential approach of combining sipuleucel-T and abiraterone, drugs with nonoverlapping toxicities, may overcome the initial lag in objective clinical benefit often seen in sipuleucel-T monotherapy [133]. In metastatic androgen-sensitive disease, the combination of docetaxel and ADT has provided a significant survival advantage over ADT alone and is considered the standard of care today [118••]. Considering that certain therapies together may have synergistic effects preclinically [130], the shift towards strategic combination therapies for CRPC in the future is certainly possible as well [134]. Preclinical and early clinical studies reveal promising leads in novel therapeutic targets. A recent phase II study involving the PARP inhibitor olaparib benefitted men with advanced CRPC harboring DNA repair mutations [135], which occur at a frequency of 11.8% in patients with mCRPC [136]. These findings point at not only a potential biomarker for treatment selection but also a therapeutic target in those who have failed multiple lines of therapy. In the context of the tumor microenvironment, the process of EMT is a potential target, given the relevance of EMT-to-MET cycling. TGF-β inhibition necessitates a cautious undertaking due to its ubiquity and complex intersecting signaling pathways but stands to open an alternative avenue to overcoming therapeutic resistance to currently employed antiandrogen and taxane-based strategies [137], potentially in the appropriately designed sequencing approach. Galunisertib (LY2157299), a small molecule inhibitor targeting TGF-β receptor I, biologically impacting the EMT outcomes in prostatic tumors is currently being investigated alongside enzalutamide (NCT02452008) in a phase II study as well in preclinical models of advanced tumor progression to metastasis.
Conclusions
The seemingly simple reliance of prostate cells on androgens belies the complexities that arise in advanced prostate cancer, all of which directly reflect the intricacies of the mechanisms of resistance responsible for therapy failure in patients. Critically, further tumor adaptations arise in the CRPC state driven by the specific selective pressures of next-generation AR-directed therapies and cytotoxic chemotherapies, many of which were unknown until recently. Promisingly, however, resistance to these interventions may be reversible by employing strategies such as EMT-to-MET cycling, yet our understanding of how to optimize such synergism between preexisting and newer therapeutics is still limited. Clinical management of advanced CRPC is challenging due to the diversity of resistant clones, especially in those refractory to multiple lines of therapies. The future of prostate cancer management in these patients will likely involve not only serial liquid biopsies to assess disease state and probe for additional actionable targets but also strategic combination strategies that induce reversal of the resistant phenotype. Ongoing studies on not only newer therapeutic targets but also novel combination strategies will be crucial in controlling the dynamic process of tumor clone evolution and prolonging survival in lethal prostate cancer.
Acknowledgments
We acknowledge the support of this work through funding from the James F. Hardymon Endowment in Urologic Research at the University of Kentucky (NK), NIH grant K23CA197526 (CP); the National Center for Advancing Translational Sciences, UL1TR000117 (MN); and the Dean of the College of Medicine at the University of Kentucky.
Abbreviations
- ADT
Androgen deprivation therapy
- CRPC
Castration-resistant prostate cancer
- EMT
Epithelial-mesenchymal transition
- MET
Mesenchymal-epithelial transition
- ECM
Extracellular matrix
- TGF-β
Transforming growth factor β
- LBD
Ligand binding domain
- AR
Androgen Receptor
- PTEN
Phosphatase and tensin homolog deleted on chromosome ten
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest Mary Nakazawa, Channing Paller, and Natasha Kyprianou declare that they have no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–23. [Google Scholar]
- 3.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 4.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 6.Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011;29:S1–8. doi: 10.1016/j.urolonc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–9. [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 10.Halabi S, Lin C-Y, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–7. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 14.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 15.Valenca LB, Sweeney CJ, Pomerantz MM. Sequencing current therapies in the treatment of metastatic prostate cancer. Cancer Treat Rev. 41:332–40. doi: 10.1016/j.ctrv.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Sartor O, Gillessen S. Treatment sequencing in metastatic castrate-resistant prostate cancer. Asian Journal of Andrology. 2014;16:426–31. doi: 10.4103/1008-682X.126378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Soest RJ, de Morree ES, Kweldam CF, de Ridder CM, Wiemer EA, Mathijssen RH, et al. Targeting the androgen receptor confers in vivo cross-resistance between enzalutamide and docetaxel, but not cabazitaxel, in castration-resistant prostate cancer. Eur Urol. 2015;67:981–5. doi: 10.1016/j.eururo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 18.Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–9. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Dhawan MS, Healy P, George DJ, Harrison MR, Oldan J, et al. Exploring the clinical benefit of docetaxel or enzalutamide after disease progression during abiraterone acetate and prednisone treatment in men with metastatic castration-resistant prostate cancer. Clinical Genitourinary Cancer. 2015;13:392–9. doi: 10.1016/j.clgc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clinical Advances in Hematology & Oncology: H&O. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 21.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, et al. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–76. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 22.Yun EJ, Zhou J, Lin CJ, Hernandez E, Fazli L, Gleave M, et al. Targeting cancer stem cells in castration-resistant prostate cancer. Clinical Cancer Research. 2016;22:670–9. doi: 10.1158/1078-0432.CCR-15-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 24.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–6. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 25.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. [PubMed] [Google Scholar]
- 26.Bubendorf L, Kononen J, Koivisto P, Schraml P, Moch H, Gasser TC, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–6. [PubMed] [Google Scholar]
- 27.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Vida A, Bianchini D, Van Hemelrijck M, Hughes S, Malik Z, Powles T, et al. Is there an antiandrogen withdrawal syndrome with enzalutamide? BJU Int. 2015;115:373–80. doi: 10.1111/bju.12826. [DOI] [PubMed] [Google Scholar]
- 29.Hara T, Miyazaki J, Araki H, Yamaoka M, Kanzaki N, Kusaka M, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–53. [PubMed] [Google Scholar]
- 30.Bohl CE, Miller DD, Chen J, Bell CE, Dalton JT. Structural basis for accommodation of nonsteroidal ligands in the androgen receptor. J Biol Chem. 2005;280:37747–54. doi: 10.1074/jbc.M507464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci U S A. 2005;102:6201–6. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate-resistance and novel therapeutic approaches. Oncogene. 2013;32:5501–11. doi: 10.1038/onc.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin RJ, Lho Y, Connelly L, Wang Y, Yu X, Saint Jean L, et al. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68:6762–9. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang L, Altuwaijri S, Deng F, Chen L, Lal P, Bhanot UK, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175:489–99. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin R, Yi Y, Yull FE, Blackwell TS, Clark PE, Koyama T, et al. NF-kappaB gene signature predicts prostate cancer progression. Cancer Res. 2014;74:2763–72. doi: 10.1158/0008-5472.CAN-13-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulholland David J, Tran Linh M, Li Y, Cai H, Morim A, Wang S, et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19:792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian Journal of Andrology. 2014;16:378–86. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mithal P, Allott E, Gerber L, Reid J, Welbourn W, Tikishvili E, et al. PTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancer. Int J Urol. 2014;21:1209–14. doi: 10.1111/iju.12571. [DOI] [PubMed] [Google Scholar]
- 42.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 43.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 44.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223–38. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: a new pathway for sex hormones in prostate. Proc Natl Acad Sci U S A. 1998;95:5527–32. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ni L, Yang CS, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–53. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groner Anna C, Cato L, de Tribolet-Hardy J, Bernasocchi T, Janouskova H, Melchers D, et al. TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell. 29:846–58. doi: 10.1016/j.ccell.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzman DL, Antonarakis ES. Does degree of androgen suppression matter in hormone-sensitive. Journal of Clinical Oncology. 2015;33:1098–100. doi: 10.1200/JCO.2014.60.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. American Association for Cancer Research. 2004;10:7121–6. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 50.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clinical Cancer Research. 2004;10:440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 51.Titus MA, SchellM J, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clinical Cancer Research. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 52.Chang K, Ercole CE, Sharifi N. Androgen metabolism in prostate cancer: from molecular mechanisms to clinical consequences. Br J Cancer. 2014;111:1249–54. doi: 10.1038/bjc.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 55.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu H, Garcia JA. Targeting the adrenal gland in castration-resistant prostate cancer: a case for orteronel, a selective CYP-17 17,20-lyase inhibitor. Curr Oncol Rep. 2013;15:105–12. doi: 10.1007/s11912-013-0300-1. [DOI] [PubMed] [Google Scholar]
- 57.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 58.Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res. 2012;4:183–93. doi: 10.2147/CMAR.S32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Wang B-E, Leong KG, Yue P, Li L, Jhunjhunwala S, et al. Androgen deprivation causes epithelial–mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 60.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 61.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 62.Zhu M-L, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–77. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cellular and Molecular Life Sciences: CMLS. 2010;67:2605–18. doi: 10.1007/s00018-010-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48:37–43. doi: 10.1016/j.bone.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 65.Nakazawa M, Kyprianou N. Epithelial-mesenchymal-transition regulators in prostate cancer: androgens and beyond. J Steroid Biochem Mol Biol. 2016;166:84–90. doi: 10.1016/j.jsbmb.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Jaggi M, Nazemi T, Abrahams NA, Baker JJ, Galich A, Smith LM, et al. N-cadherin switching occurs in high Gleason grade prostate cancer. Prostate. 2006;66:193–9. doi: 10.1002/pros.20334. [DOI] [PubMed] [Google Scholar]
- 67.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–11. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 68.Nouri M, Ratther E, Stylianou N, Nelson CC, Hollier BG, Williams ED. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention. Frontiers in Oncology. 2014;4 doi: 10.3389/fonc.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kyprianou N. Molecular exploitation of apoptosis pathways in prostate cancer. World Scientific; 2012. [Google Scholar]
- 70.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Zielinski RR, Eigl BJ, Chi KN. Targeting the apoptosis pathway in prostate cancer. Cancer Journal. 2013;19:79–89. doi: 10.1097/PPO.0b013e3182801cf7. [DOI] [PubMed] [Google Scholar]
- 72.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28:251–65. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 73.Isaacs JT. Apoptosis: translating theory to therapy for prostate cancer. J Natl Cancer Inst. 2000;92:1367–9. doi: 10.1093/jnci/92.17.1367. [DOI] [PubMed] [Google Scholar]
- 74.McKenzie S, Kyprianou N. Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance. J Cell Biochem. 2006;97:18–32. doi: 10.1002/jcb.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kajiwara T, Takeuchi T, Ueki T, Moriyama N, Ueki K, Kakizoe T, et al. Effect of Bcl-2 overexpression in human prostate cancer cells in vitro and in vivo. Int J Urol. 1999;6:520–5. doi: 10.1046/j.1442-2042.1999.00102.x. [DOI] [PubMed] [Google Scholar]
- 76.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clinical Cancer Research. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;65:11230–5. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 79.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–64. [PubMed] [Google Scholar]
- 80••.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3:1030–43. doi: 10.1158/2159-8290.CD-13-0142. The first evidence to suggest that the AR mutation F876L confers agonist properties to enzalutamide. While several activating mutations have been identified in the context of first-generation antiandrogens, this is the first mutation to be identified in a second-generation antiandrogen. [DOI] [PubMed] [Google Scholar]
- 81.Lallous N, Volik SV, Awrey S, Leblanc E, Tse R, Murillo J, et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 2016;17 doi: 10.1186/s13059-015-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nadiminty N, Tummala R, Liu C, Yang J, Lou W, Evans CP, et al. NF-kappaB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12:1629–37. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83••.Antonarakis ES, Lu C, Luber B, et al. ANdrogen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncology. 2015;1:582–91. doi: 10.1001/jamaoncol.2015.1341. This follow-up study showed that the presence of AR-V7 does not predict a response to taxane chemotherapies, challenging the predictive value of AR-V7 as a treatment selection biomarker for therapeutic resistance to non-antiandrogen treatments in CRPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84••.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38. doi: 10.1056/NEJMoa1315815. Clinical evidence defining the association of AR splice variant-7 (AR-V7) with therapeutic resistance to abiraterone and enzalutamide in men with CRPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–25. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–5. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu Q, Luduena RF. Removal of beta III isotype enhances taxol induced microtubule assembly. Cell Struct Funct. 1993;18:173–82. doi: 10.1247/csf.18.173. [DOI] [PubMed] [Google Scholar]
- 89.Duran GE, Wang YC, Francisco EB, Rose JC, Martinez FJ, Coller J, et al. Mechanisms of resistance to cabazitaxel. Mol Cancer Ther. 2015;14:193–201. doi: 10.1158/1535-7163.MCT-14-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, et al. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol. 2012;181:2188–201. doi: 10.1016/j.ajpath.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 91.Paller CJ, Antonarakis ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Design, Development and Therapy. 2011;5:117–24. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakazawa M, Antonarakis ES, Luo J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Hormones & Cancer. 2014;5:265–73. doi: 10.1007/s12672-014-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocrine-Related Cancer. 2011;18:R183–96. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is upregulated during prostate cancer progression and promotes androgen-depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71:1656–67. doi: 10.1002/pros.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–9. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clinical Cancer Research. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–94. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 100.Logothetis CJ, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5:21–8. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 101.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–7. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 102.Halabi S, Vogelzang NJ, Kornblith AB, Ou SS, Kantoff PW, Dawson NA, et al. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. Journal of Clinical Oncology. 2008;26:2544–9. doi: 10.1200/JCO.2007.15.0367. [DOI] [PubMed] [Google Scholar]
- 103.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor microenvironment complexity: emerging roles in cancer therapy. Cancer Res. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith MR, Coleman RE, Klotz L, Pittman K, Milecki P, Ng S, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26:368–74. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chu GCY, Zhau HE, Wang R, Rogatko A, Feng X, Zayzafoon M, et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocrine-related cancer. 2014;21:311–26. doi: 10.1530/ERC-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El-Amm J, Aragon-Ching JB. Targeting bone metastases in metastatic castration-resistant prostate cancer. Clinical Medicine Insights Oncology. 2016;10:11–9. doi: 10.4137/CMO.Ss30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ning Y-M, Brave M, Maher VE, Zhang L, Tang S, Sridhara R, et al. U.S. Food and Drug Administration approval summary: enzalutamide for the treatment of patients with chemotherapy-naïve metastatic castration-resistant prostate cancer. Oncologist. 2015;20:960–6. doi: 10.1634/theoncologist.2015-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buttigliero C, Tucci M, Bertaglia V, Vignani F, Bironzo P, Di Maio M, et al. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev. 2015;41:884–92. doi: 10.1016/j.ctrv.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 111.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, et al. Aclinically relevant androgen receptormutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–9. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 112.Arora Vivek K, Schenkein E, Murali R, Subudhi Sumit K, Wongvipat J, Balbas Minna D, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. Journal of Clinical Oncology. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 114.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–16. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 116••.Carreira S, Romanel A, Goodall J, Grist E, Ferraldeschi R, Miranda S, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med. 2014;6:254ra125. doi: 10.1126/scitranslmed.3009448. A critical study revealing the complexity of therapeutically resistant clones in CRPC associated with regional and temporal heterogeneity, which can be monitored by sequential plasma and tumor biopsies to target lethal disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Drake JM, Paull EO, Graham NA, Lee JK, Smith BA, Titz B, et al. Phosphoproteome integration reveals patient-specific networks in prostate cancer. Cell. 166:1041–54. doi: 10.1016/j.cell.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118••.Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46. doi: 10.1056/NEJMoa1503747. Shows a significant overall survival benefit in the addition of docetaxel to first-line ADT in metastatic hormone-sensitive prostate cancer, supporting the importance of targeting androgen sensitive and not CRPC by taxane chemotherapy to overcome cross-resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119••.James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77. doi: 10.1016/S0140-6736(15)01037-5. Shows a significant overall survival benefit in the addition of docetaxel to first-line ADT in metastatic hormone-sensitive prostate cancer, supporting the importance of targeting androgen sensitive and not CRPC by taxane chemotherapy to overcome cross-resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petrylak DP, Tangen CM, Hussain MHA, Lara PNJ, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 121.Pienta KJ. Seminars in Oncology. Elsevier; 2001. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer; pp. 3–7. 2001. [DOI] [PubMed] [Google Scholar]
- 122.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 123.Darshan MS, Loftus MS, Thadani-Mulero M, Levy BP, Escuin D, Zhou XK, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O’Neill AJ, Prencipe M, Dowling C, Fan Y, Mulrane L, Gallagher WM, et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer. 2011;10:1–13. doi: 10.1186/1476-4598-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Terry S, Ploussard G, Allory Y, Nicolaiew N, Boissiere-Michot F, Maille P, et al. Increased expression of class III beta-tubulin in castration-resistant human prostate cancer. Br J Cancer. 2009;101:951–6. doi: 10.1038/sj.bjc.6605245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ploussard G, Terry S, Maillé P, Allory Y, Sirab N, Kheuang L, et al. Class III β-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70:9253–64. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin SK, Kyprianou N. Chapter three—Exploitation of the androgen receptor to overcome taxane resistance in advanced prostate ancer. In: Paul BF, Kenneth DT, editors. Advances in cancer research. Academic; 2015. pp. 123–58. [DOI] [PubMed] [Google Scholar]
- 128.Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, Eisenberger MA, et al. Serial blood-based analysis of ARV7 in men with advanced prostate cancer. Annals of Oncology. 2015;26:1859–65. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin SK, Pu H, Penticuff JC, Cao Z, Horbinski C, Kyprianou N. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin SK, Banuelos CA, Sadar MD, Kyprianou N. N-terminal targeting of androgen receptor variant enhances response of castration resistant prostate cancer to taxane chemotherapy. Mol Oncol. 2015;9:628–39. doi: 10.1016/j.molonc.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wyatt AW, Mo F, Wang Y, Collins CC. The diverse heterogeneity of molecular alterations in prostate cancer identified through next-generation sequencing. Asian Journal of Andrology. 2013;15:301–8. doi: 10.1038/aja.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discovery. 2016;6:479–91. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 133.Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, McCoy C, et al. A randomized phase II trial of sipuleucel-T with concurrent versus sequential abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer. American Association for Cancer Research. 2015;21:3862–9. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 134.Paller CJ, Bradbury PA, Ivy SP, Seymour L, LoRusso PM, Baker L, et al. Design of phase I combination trials: recommendations of the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee. Clinical Cancer Research. 2014;20:4210–7. doi: 10.1158/1078-0432.CCR-14-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. doi: 10.1056/NEJMoa1603144. 0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao Z, Kyprianou N. Mechanisms navigating the TGF-β pathway in prostate cancer. Asian Journal of Urology. 2015;2:11–8. doi: 10.1016/j.ajur.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]