SUMMARY
Mast cells are hematopoietic progenitor-derived, granule-containing immune cells that are widely distributed in tissues that interact with the external environment, such as the skin and mucosal tissues. It is well-known that mast cells are significantly involved in IgE-mediated allergic reactions, but because of their location, it has also been long hypothesized that mast cells can act as sentinel cells that sense pathogens and initiate protective immune responses. By using mast cell or mast cell protease deficient murine models, recent studies by our groups and others indicate that mast cells have pleiotropic regulatory roles in immunological responses against pathogens. In this review, we discuss studies that demonstrate that mast cells can either promote host resistance to infections caused by bacteria and fungi or contribute to dysregulated immune responses that can increase host morbidity and mortality. Overall, these studies indicate that mast cells can influence innate immune responses against bacterial and fungal infections via multiple mechanisms. Importantly, the contribution of mast cells to infection outcomes depends in part on the infection model, including the genetic approach used to assess the influence of mast cells on host immunity, hence highlighting the complexity of mast cell biology in the context of innate immune responses.
Keywords: mast cells, infection, fungi, bacteria, innate immunity
INTRODUCTION
Mast cells are particularly abundant at host-environment interfaces, such as the skin and intestinal mucosa. Because of their location, it has been hypothesized that mast cells can act as sentinel cells that sense pathogen attacks and initiate a protective immune response. Initial studies with the use of c-kit mutant mice confirmed this hypothesis and helped provide further insight into how mast cells contribute to the host’s response against parasites, bacteria, fungus and viruses.
The evolution of our understanding of the role of mast cells in innate immunity is perhaps best described by what we learned from mast cells in parasitic infections. The earliest studies determined that mast cell numbers increase during certain parasitic infections and degranulate when exposed to parasite-derived antigens.(1, 2) Since then, many groups have used c-Kit mutant KitW/W-v and/or KitWsh/Wsh mice as indicative of how mast cell deficiency, amongst other abnormalities in these mice, may affect host immunity against primary infections with various parasites, including Nippostrongylus brasiliensis,(3, 4) Strongyloides ratti,(5) Strongyloides venezuelensis,(6–8) Trichinella spiralis,(9, 10) and Trichinella muris.(11, 12) Most of these studies showed that such c-kit mutant mast cell-deficient mice have a delay in intestinal worm clearance during a primary infection. However, to what extent the delays in parasite clearance detected in these c-kit mast cell-deficient mice reflected their lack of mucosal mast cells vs. one or more of their other phenotypic abnormalities (including their intestinal cells of Cajal deficiency, which results in abnormal gut motility)(13) was not determined by these studies. This is because mast cell-dependency in these observations cannot not be confirmed by systemic adoptive transfer of mast cells(14–17) due to the inability to engraft intestinal mucosal mast cells in c-kit mutant mice. This issue was recently addressed with the generation of c-Kit independent mast cell-deficient mice. The strategy for the generation of c-Kit independent mast cell-specific conditional mice was recently reviewed by Galli SJ et al.(18) Mukai K et al. used two types of mast cell-deficient mice that have normal c-kit levels ("Hello Kitty" and MasTRECK mice) to confirm that mast cells play an important role in S. venezuelensis egg clearance in primary infections.(19) The use of c-Kit-independent mice also assisted in settling conflicting results for the role of mast cells in leishmaniasis. In fact, experiments with c-Kit mutant mice led to conclusions ranging from no contribution(20) to pro-pathogenic(21) to protective(22) roles of mast cells in leishmaniasis. Paul et al. used Cpa-Cre mice to provide evidence that the involvement of mast cells in the clinical development of cutaneous leishmaniasis is unlikely.(23)
Similar discrepancies were observed when c-Kit mutant mast cell-deficient mice were used to investigate the contribution of mast cells to infection immunity against bacteria and fungi. While some of these discrepancies can be attributed to abnormalities associated with the c-Kit mutation and/or mouse background, it became clear to us and other researchers in the field that the protective role of mast cells in bacteria and fungal immunity is not as clear cut as originally thought. The relative high abundance of data generated by us and others in the field of bacterial and fungal infections allows us to challenge ourselves to draw some conclusions on the mechanisms by which mast cells influence host immunity and the environmental factors that may impact these interactions. These topics are the focus of this review. We also speculate on potential new lines of research based on our own pressing questions that we and others expect to address in the near future.
MAST CELLS AND BACTERIAL INFECTIONS
Since the publication of two landmark studies published back-to-back in 1996 showing that mast cells were crucial for protection against enterobacteria infection in the cecal ligation and puncture (CLP) model of sepsis(24) and against i.p.-injected Klebsiella pneumoniae and Escherichia coli,(25) several additional studies have shown that mast cells protect against infections caused by a variety of bacterial pathogens.(26–32) Much of this work provided a better understanding of how mast cells detect and respond to bacteria products.
Mechanisms of mast cell activation during bacterial infections
How do mast cells recognize bacteria and/or bacteria products to undergo activation? Mast cells express a variety of pattern recognition receptors, including Toll-like receptors (TLRs) that allow mast cells to respond to TLR ligands by secreting cytokines, chemokines, and lipid mediators.(33) Moreover, it was shown that TLR4 expression on mast cells is required for mast cell protection during CLP.(34) Mast cell activation can also be modulated by factors that are bacteria unrelated. For example, the protective effects mediated by mast cells in CLP can be enhanced by growth factors, such as stem cell factor (SCF).(35)
Despite the involvement of TLRs in mast cell activation, early in vitro studies led to the consensus that mast cells do not degranulate in response to TLR ligands. These studies contradicted the fact that the release of mast cell pre-formed mediators, such as histamine and proteases, was detected during CLP(36–39) and that peritoneal mast cells show morphological evidence of degranulation after LPS i.p. administration.(39) One plausible explanation for this phenomenon is that mast cells release pre-formed mediators in response to endogenous peptides that are generated during CLP or after LPS administration, such as complement components, endothelin-1, and neurotensin.(37, 40, 41)
It is important to note that conventional mast cell degranulation may not be a prerequisite for pre-formed mast cell mediators to exert a protective effect during bacterial infections. For example, we recently demonstrated that mast cell protease (MCPT)4, the functional mouse homologue of chymase,(42) protects against systemic infection caused by a strain of Group B Streptococcus that does not induce beta hexosaminidase release.
Mast cell-mediated bactericidal and protective pro-inflammatory effects during bacterial infections
There is some evidence that mast cells can exert a direct killing effect against bacteria. It has been shown that intracellular IL-15 expression in mast cells can transcriptionally limit their MCPT2 levels, resulting in decreased mast cell-associated chymotrypsin-like activity in vitro, decreased mast cell antibacterial properties, and reduced survival of mice subjected to CLP.(43) Moreover, it has been shown that mast cells can produce antimicrobial peptides (AMPs), such as cathelicidins, that have direct bactericidal activity against Group A Streptococcus skin infection.(44, 45)
Despite this evidence, the ability for mast cells to induce the recruitment of inflammatory cells to the focus of infection has been proposed as the main mechanism by which mast cells exert their protective effects against bacteria. Moreover, for some pathogens, it has been possible to identify the mast cell mediators involved in inflammatory cell recruitment. For example, it was demonstrated that MCPT6(46) and IL-6(47) are protective against Klebsiella pneumoniae, and that mast cell-derived tumor necrosis factor (TNF) can amplify the inflammatory response against uropathogenic E. coli.(48)
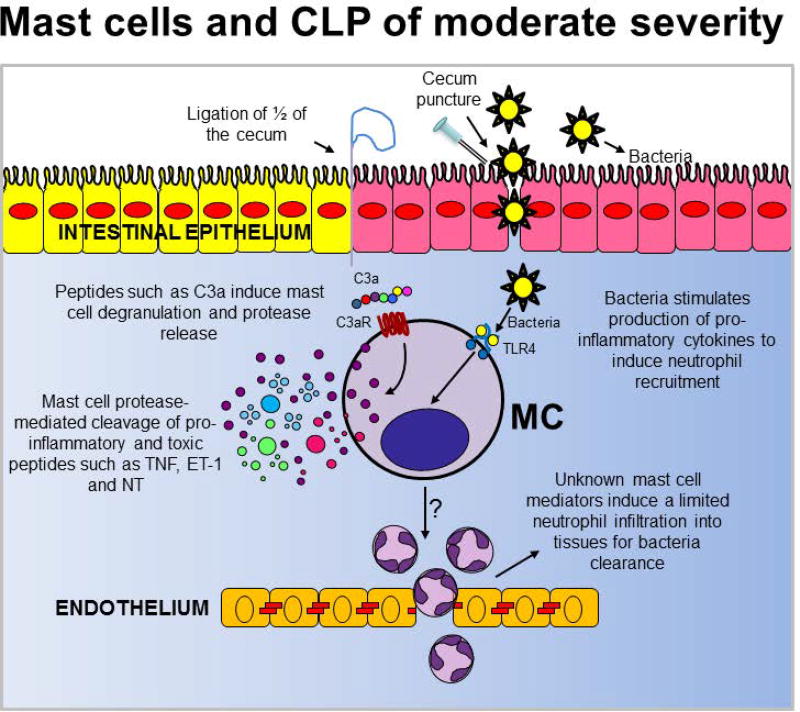
CLP of moderate severity is one of the most studied models in which the contribution of mast cells to innate immunity has been investigated. CLP is a model of traumatic or iatrogenic intestinal perforation that results in a polymicrobial infection of the peritoneum. The CLP model involves ligation of the cecum immediately below the ileocecal valve (to produce a distal ischemia) followed by needle puncture.(49)
Studies conducted with c-Kit mutant mice subjected to CLP that causes a bacterial infection that results in a relatively low mortality rate in normal mice, indicate that mast cells contribute to host defense by promoting inflammation and/or the ability for myeloid cells to clear bacteria.(25, 40, 50) (Fig. 1). Despite these studies, it is still unclear how mast cells exert their pro-inflammatory effects in the CLP model.
Figure 1.
The contribution of mast cells in the moderately severe cecal ligation and puncture (CLP) model (which results in less than 50% mortality rates) in wild type mice.
TNF, Tumor necrosis factor; C3a, complement component 3a; C3R, C3a receptor; TLR4, toll-like receptor 4; ET-1, endothelin-1; NT, neurotensin
Several groups have proposed but not demonstrated the potential role of mast cell-derived TNF in moderately severe CLP. This is because the results obtained with TNF-deficient mice(35) or TNF neutralizing antibody treated mice(51) have clearly demonstrated that TNF can have protective functions during some bacterial infections, and that such TNF-dependent effects may include the enhancement of neutrophil recruitment and/or function as well as promote bacterial clearance. However, by using knock-in mice in which only mast cells do not produce TNF, we clearly demonstrated that mast cells are not the main cell source of TNF required to trigger inflammation in response to infection induced by moderately severe CLP.(50)
IL-6 also has been proposed as a mast cell-derived cytokine that can contribute to a positive outcome after CLP. In contrast to TNF, mast cells have been shown to contribute to increased IL-6 levels at the infection site (peritoneum) at very early stages following CLP. More importantly, it has been shown that mast cell-derived IL-6 significantly contributes to mouse survival after CLP. Although it has been shown that the pro-inflammatory properties of IL-6 can enhance bacteria clearance during infection,(52, 53) mast cell-derived IL-6 seems not to protect mice from CLP by this mechanism.
It is not surprising that it has been so difficult to determine which mast cell mediators contribute to protection in CLP by enhancing the inflammatory response. CLP is a polymicrobial model of infection of high complexity in which multiple mediators may exert overlapping roles. Therefore, the effects of the deletion of a specific mediator in mast cells may be compensated by a different mediator with an overlapping function. Moreover, the effects of a deletion in mast cell mediators may be masked by c-Kit mutation-associated abnormalities, such as alterations in neutrophil numbers. Regarding the later, the use of new c-Kit independent mast cell deficient mice that lack these abnormalities has already shown promise for a better understanding of how mast cells may contribute to inflammation during a bacterial infection. For example, by inducing ablation of connective tissue mast cells in Mcpt5-Cre; iDTR+ mice after diphtheria toxin A injection, it was shown that mast cells and CXCL1/2 contribute to neutrophil recruitment into the peritoneal cavity after LPS-induced endotoxemia.(39) It is unknown whether mast cell-derived CXCL1/2 plays a beneficial role in CLP, but these studies are underway.
Protective effects of mast cell-restricted proteases
The defining morphological feature of mast cells is their electron-dense secretory granules, which contain large amounts of pre-formed mediators, such as biogenic amines, proteoglycans, and cytokines.(54) These granules also contain several mast cell-specific proteases, most notably, chymase, tryptase, and mast cell carboxypeptidase A3 (CPA3), whose release is induced by either IgE-dependent mast cell activation(54) or IgE-independent mechanisms.(41, 55)
The overall substrate specificities of the mast cell proteases have been conserved for over 150–200 million years of mammalian evolution.(56, 57) This suggests the presence of a strong selective pressure for maintaining mast cell protease specificity and an important role for mast cell proteases in innate immunity. Early studies on the contribution of mast cell proteases to innate immunity were focused on how these mast cell mediators can modulate the host immune response to infection as an important regulatory mechanism to prevent sepsis development. Specifically, bacterial infections can trigger a host immune response that includes the production of endogenous mediators that can induce many of the physiological symptoms observed during sepsis, such as hypotension. Using pharmacological and genetic approaches, it has been shown that mast cell proteases can contribute to “detoxification” of these endogenous peptides via proteolytic degradation and inactivation. For example, CPA3 and mast cell-associated neurolysin (a non-specific mast cell protease) promote homeostasis through the down-regulation of endothelin (ET)-1 and neurotensin levels, respectively (Fig. 1).(37, 41)
Early studies on the role of mast cell proteases in parasitic infections provided strong evidence that mast cell proteases can have an impact on host-pathogen interactions and hence infection outcomes. In these seminal studies, Knight et al. showed that expulsion of T. spiralis was significantly delayed in mice lacking MCPT1, which suggests an important contribution of intestinal mucosal mast cells and MCPT1 in the clearance of this infection.(58) Later on, it was found that MCPT1-mediated degradation of the tight junction protein, occludin, is a mechanism by which mast cells increase intestinal permeability and hence contribute to expel the parasite.(59) Despite these reports, there are a relatively small number of studies addressing similar mechanisms during bacterial infections. One of the main reasons for the scarcity of this type of study is that mice with deficiencies in mast cell restricted proteases were generated only recently.(18) This is an essential tool to identify the potential substrates for mast cell proteases during a bacterial infection. Two recent studies highlight the use of these mice to investigate the role of mast cell proteases in protection against bacterial infections by disrupting host-bacteria interactions. These studies were performed by using Mcpt4−/− mice, which do not exhibit any marked defect in the expression of proteases with trypsin-like or CPA activity, e.g. MCPT6 or CPA3, respectively.(42) In the first study, Choi HW et al. investigated how mast cells contribute to protection against urinary tract infections (UTIs) caused by uropathogenic E. coli.(60) Uropathogenic E. coli gain access to the bladder and rapidly invade superficial bladder epithelial cells to avoid being flushed out when urine is voided. When epithelial cells become overburdened with pathogenic bacteria, they initiate self-destruction processes resulting in reduced microbial burden. Choi HW et al. provided evidence that mast cells recruited to the infection site via IL-1β mediate the infected bladder epithelial cell exfoliation. Cell death and exfoliation is caused by uptake of granule-associated MCPT4 by the infected cells, which triggers the disruption of lysosomal vesicles and hence lytic cell death.
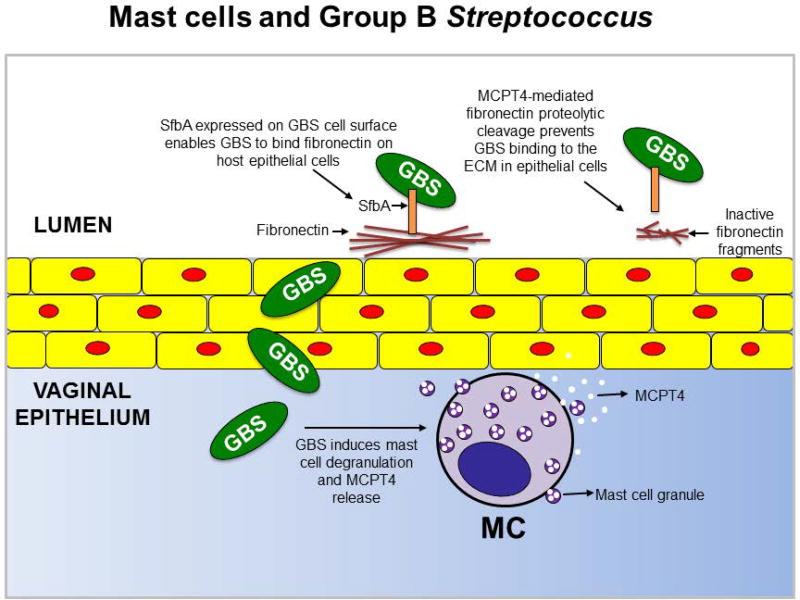
The second study was performed by our own group. By using mice with c-Kit independent mast cell deficiency, we showed that mast cells are required for an effective immune response during systemic GBS infections.(61) GBS are Gram-positive bacteria that frequently colonize the lower genital tract of healthy women and can cause severe infections during pregnancy, leading to preterm birth, stillbirth, or early-onset newborn infections. In a recent study, we demonstrated that MCPT4 decreases the severity of systemic GBS infection and the preterm birth rates. We found that this can be attributed in part to the ability of MCPT4 to downregulate GBS-extracellular matrix (ECM) interactions via proteolytic degradation of fibronectin into inactive fragments (Fig. 2). (62)
Figure 2.
Proposed mechanism for the protective effect of MCPT4 against Group B Streptococcus (GBS) dissemination and preterm birth.
MCPT4, Mast cell protease 4; MC, mast cells; SfbA, streptococcal fibronectin binding protein; ECM, extracellular matrix
Mast cells can be detrimental to the outcomes of certain bacterial infections
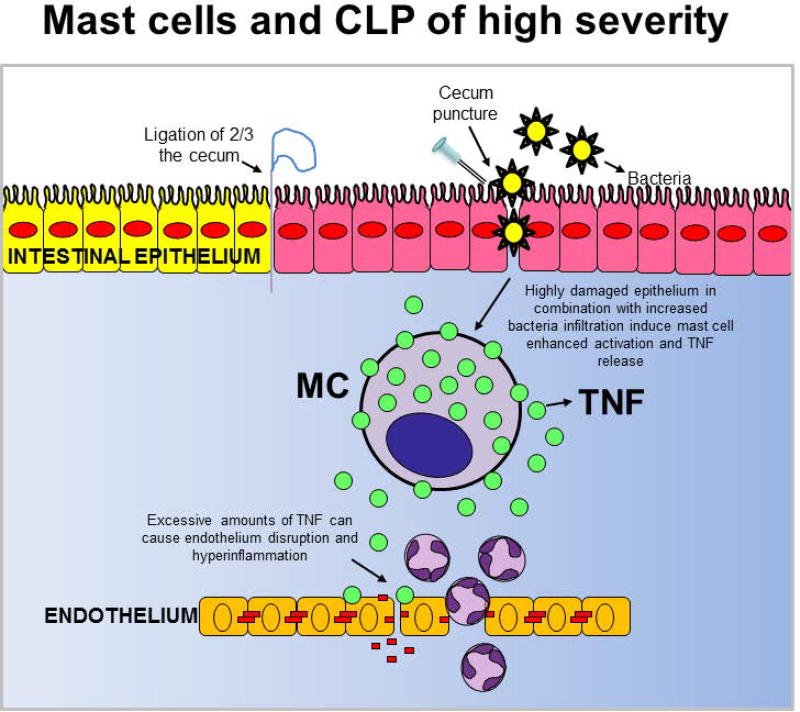
Studies with a CLP model of high severity, which induces more than 50% mortality in wild type mice after surgery, were the first to show that mast cells can be detrimental to the outcomes of a severe bacterial infection.(50) The high severity CLP model is widely recognized among sepsis researchers as the “gold standard for polymicrobial sepsis” (63–65) because it closely mimics the cytokine-storm mediated response to severe infection and the multiple organ dysfunction syndrome (MODS) associated with severe sepsis.(66)
In the high severity CLP model, hyper-inflammation is a main indicator of a dysregulated host response to infection that can cause severe sepsis and shock irrespective of the host’s bacterial clearance efficiency. By using knock-in mice, we found that mast cell-derived TNF is one of the main drivers of hyper-inflammation and death following severe CLP. (50) We confirmed our findings with a mast cell-specific TNF deficient mouse that we generated by crossing a mouse in which Cre recombinase is expressed under the control of the CPA3 promoter (Cpa3-Cre mouse) (67) with a Tnf “floxed” mouse (Fig. 3).(68) Additional supportive evidence came from the MCPT4-deficient mice, which are impaired in their ability to down-regulate TNF levels via proteolytic degradation. Hence, these mice exhibit the phenotype of a mouse subjected to severe CLP, with increased inflammation and mortality, when they are subjected to moderate CLP.(38)
Figure 3.
The contribution of mast cells in the high severity cecal ligation and puncture (CLP) of model (which induces more than 50% mortality rates) in wild type mice after surgery.
TNF, Tumor necrosis factor
A detrimental role for mast cells during high severity CLP (< 50% of control mice survived at 4 d after surgery) was also observed with a c-Kit independent knock-in mouse model called the red mast cell and basophil (RMB) mouse. In these mice, the 3′-UTR of the Ms4a2 gene encoding the FcεRI β chain includes a cassette composed of an internal ribosomal entry site, a sequence coding for the bright red td-Tomato (tdT) fluorescent protein, a 2A cleavage sequence, and the human diphtheria toxin receptor (hDTR). By deleting mast cells after diphteria toxin A injection, Dahdah A et al. showed that mast cells and mast cell-derived IL-4 aggravate sepsis by impairing the ability for macrophages to clear bacteria. It is unclear as to why mast cells seem to play a suppressive instead of a pro-inflammatory role in the CLP model used by Dahdah A et al. However, we should point out that it is well-known that subtle differences in the way CLP is induced can have a great impact on the outcome of the model.(49) For example, we usually observe twenty times more neutrophils in the peritoneal cavity of wild type mice subjected to severe CLP than what was reported for wild type mice in Dahdah et al’s study. According to this observation, we think that it would be informative to perform CLPs of different severities in the RMB mouse to evaluate whether the contribution of mast cells to infection outcomes varies with the CLP model severity to the same extent as it was observed in other mast cell deficient mouse strains.
High severity CLP is not the only model in which mast cells were shown to play a detrimental role. Mast cells and mast cell-derived TNF can enhance bacterial growth and hasten death after intraperitoneal inoculation of Salmonella typhimurium.(50) Furthermore, Chan et al. demonstrated that mast cell-derived IL-10 contributed importantly to the suppression of E. coli-specific antibody production during experimental UTI in mice, which accounted for, at least in part, E. coli persistence in the bladder.(69)
MAST CELLS AND FUNGAL INFECTIONS
Protective and non-protective roles of mast cells against fungi
Fungi are associated with a wide spectrum of diseases in humans and animals, ranging from acute self-limiting manifestations in immunocompetent individuals to allergy and severe life-threatening infections in immunocompromised patients.(70) Considering the harmful effects of mast cells in connection with allergic reactions, mast cell degranulation by the cell wall polysaccharides of Candida albicans, a commensal fungus of the skin and mucosal surfaces,(71) was meant to contribute to sensitization against food antigens by affecting the mucosal barrier in mice.(72) Controversy still exists as to whether gastrointestinal colonization by C. albicans contributes to atopic dermatitis aggravation. By promoting food allergy, Candida colonization may likely contribute to a pathogenic response in atopic dermatitis.
Similarly, mast cell activation by Aspergillus lectins is known to activate mast cell degranulation(73) and contribute to the allergic response in vivo.(74) Mature A. fumigatus hyphae but not conidia induced mast cell degranulation in the absence of IgE. Interestingly, hyphae of less pathogenic Aspergillus species, such as flavus, niger, and nidulans induced much less mast cell degranulation.(75) However, this simplistic view is currently being extensively modified, and it is becoming more and more clear that mast cells have a complex array of functions in response to fungi. In addition to being detrimental, mast cells also carry out a number of beneficial functions, most notably in connection with innate antifungal resistance and promotion of immune tolerance.
Studies have suggested that mast cells participate in a number of ways to the Candida/host interaction at mucosal surfaces.(76, 77) Murine mast cells phagocytose C. albicans, produce nitric oxide by mechanisms involving TLR2 and Dectin-1(78), and kill the fungus through secreted granular components.(79) Moreover, as protease-activated receptors are involved in the inflammatory response to fungi,(80) the participation of mast cells may also likely occur through their proteases. In vitro, human MCs mount a specific temporal pattern of responses towards C. albicans that includes an initial phase characterized by the secretion of granular proteins, neutrophil recruitment, and reduced fungal viability followed by a late stage, which includes the release of mediators with known anti-inflammatory activity, such as IL-1ra.(76) Thus, for their strategical location at vascularized mucosal surfaces combined with a unique versatility,(81) mast cells are well positioned to respond to fungi and/or fungal allergens.
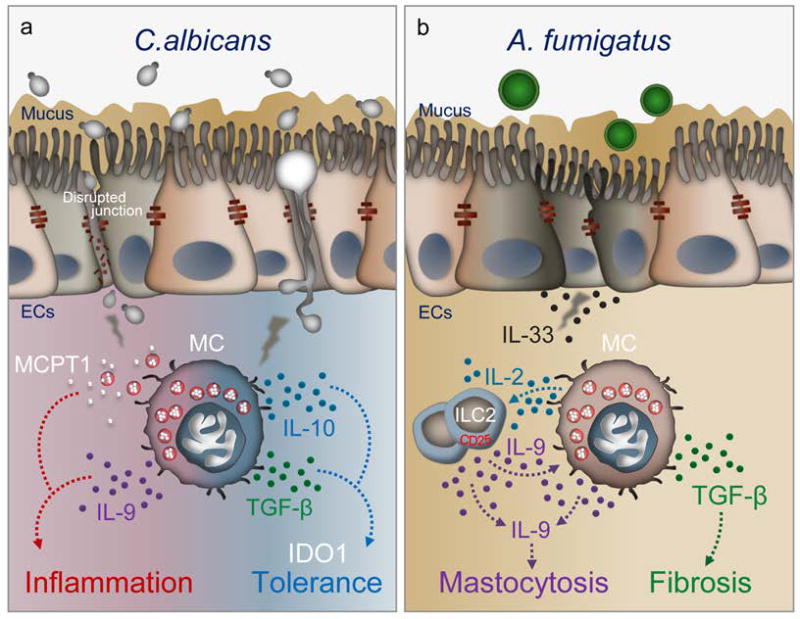
We have recently found that mast cells are key players of Candida commensalism and pathogenicity at mucosal surfaces (Renga et al., manuscript under revision) (Fig. 4A). Despite being implicated in gut immunopathology(82) and sensitization to food antigens,(72) C. albicans colonization protects against local(83, 84) and distant(85) immune pathologies in mice. Mast cells appear to decode the dual pathogenic vs. protective roles of the fungus by integrating multiple signals and mechanisms in the gastrointestinal tract. Both mucosal and stromal mast cells(86, 87) were expanded in the stomach of C. albicans-infected mice and they mediated different infection outcomes. The different mast cell types, despite being able to phagocytose unopsonized yeasts, exhibited different candidacidal activity. As suggested,(79) mucosal mast cells were unable to kill the ingested fungi and were actually killed by them with massive MCPT-1 release. In contrast, stromal mast cells killed the ingested yeasts. Importantly, while the expression of inflammatory genes was not different between the mast cell types, mast cells discriminated between the fungal morphotypes in terms of transforming growth factor (TGF)-β and IL-10 production, in which the stromal mast cells released higher levels of these cytokines than mucosal mast cells in response to fungal hyphae. Mucosal mast cells contributed to barrier function loss, fungal dissemination, and inflammation in experimental leaky gut models, while stromal mast cells, by inducing regulatory cytokines and indoleamine 2,3-dioxygenase, contributed to mucosal immune tolerance. Of great interest, mast cells also affected the local microbial composition in Candida-infected mice. Much like antibiotics, intestinal inflammation may perturb the resident bacterial community, creating conditions that favor both high levels of Candida colonization and inflammation. Firmicutes and Proteobacteria were particularly expanded in the gut of infected-KitW/W-v mice, suggesting a unique ability of mast cells to affect the microbial composition via inflammation-driven dysbiosis. These results suggest that the activity of mast cells upon C.albicans exposure may go beyond host immunity to include regulation of the microbiota in the gut.
Figure 4.
Proposed model for the role of mast cells in promoting inflammation and tolerance during Candida albicans (A) colonization in the gut or Aspergillus fumigatus (B) exposure in the lung (see text for explanation).
ECs, Epithelial cells; IDO1,Indoleamine 2,3-dioxygenase 1; ILC2, Group 2 Innate lymphoid cells; MC, mast cells; MCPT1, Mast cell protease 1; TGF-b, transforming growth factor beta
Pathogenic role of mast cells in respiratory fungal allergy
Mast cells and their activation contribute to lung health via innate and adaptive immune responses to respiratory pathogens. However, there is evidence for activation of mast cells contributing to the pathophysiology of lung diseases and cancer.(88) Mast cell-deficient mice demonstrate significantly attenuated fibrosis and inflammation after environmental injury.(89) Tryptase-positive and chymase-positive mast cells are expanded in the lungs of asthmatic (90) and cystic fibrosis patients.(91) We have recently found that chymase-positive and tryptase-positive mast cells were expanded in the lungs of mice with transmembrane conductance regulator (CFTR) deficiency (Cftr−/− mice), a murine model of cystic fibrosis in which defective fungal clearance is associated with an overzealous inflammatory response.(92) Both acute inflammation and airway remodeling were reduced in KitW/W-v mice as well as in Cftr−/− mice upon treatment with the tyrosine kinase inhibitor, imatinib, a finding pointing to the pathogenic role of mast cells in airway fungal allergy. Consistent with the finding that mast cells express a functional CFTR that may impact mediator release,(93) lung mast cells from these mice poorly responded to IgE but released IL-2 and TGF-β in response to IL-9, which was autocrinally produced. IL-2 production by mast cells expanded CD25+ innate lymphoid type 2 cells, which activated CD4+Th9 cells. By producing IL-9, Th9 cells in turn amplified allergic inflammation, in which the pro-fibrotic cytokine, TGF-β, plausibly contributed.(92) This study suggests that clinical targeting of the IL-9-mast cell axis could alleviate respiratory allergy and inflammation (Fig. 4B). However, Aspergillus growth and inflammation were apparently increased early in infection in KitW/W-v mice or after imatinib treatment, a finding suggesting that c-Kit+ cells may contribute to antifungal resistance through regulation of fungal burden and inflammation. This may explain the immunosuppressive activity of fungal metabolites that block mast cell activation and contribute to the establishment of infections.(94)
CONCLUDING REMARKS
By taking advantage of c-Kit dependent mast cell deficient mice, c-Kit independent mast cell specific conditional mice, and mice with restricted mast cell mediator deficiencies, such as proteases,(18) the non-redundant roles for mast cells in the host response against pathogens are being elucidated. These studies clearly demonstrate that mast cells can either promote host resistance to infection or contribute to a dysregulated immune response that can increase host morbidity and mortality.
The contribution of mast cells to innate immune responses against bacterial infections depends in part on the infection model. However, the complexity of this picture is accentuated when we consider that some of these studies were performed with c-Kit mutant mice that exhibit alterations in neutrophil numbers in addition to mast cell-deficiency.(50, 95, 96) Although mast cell engraftment was performed in these studies to confirm mast cell-dependency, we cannot rule out the possibility that mast cells cannot compensate for the severe neutropenia observed in the KitW/W-v mice or add to the pathogenic role of neutrophils in the KitWsh/Wsh mice, which exhibit neutrophilia in their naïve condition. In fact, the alterations in neutrophil numbers was deemed as one of the main confounding factors in the contradictory results obtained with c-Kit mutant mice in inflammatory disorders, such as rheumatoid arthritis.(97–99) C-kit independent mast cell-deficient mice will be an invaluable tool to confirm and/or rectify some of the findings obtained with c-Kit mutant mice. However, it is important to consider that the discrepancies amongst the mast cell-deficient mouse strains were observed in recent studies when the features of the model used did not replicate those previously reported.(100) This may be particularly important for certain models of bacterial infection, such as CLP, because of the high variability in the severity of the model observed amongst research groups. Ideally, the same laboratory will have access to multiple strains of mast cell-deficient mice (c-Kit mutant and c-Kit independent) to perform CLP with the same degree of severities used in previous studies. By using this approach, Reber LL et al. confirmed previous findings in c-Kit mutant mice that indicated that mast cells and mast cell-derived IL-10 can limit inflammation and other pathologies at sites of severe hapten-induced contact hypersensitivity reactions.(100)
What are the factors or mechanisms that determine the contribution of mast cells to immune defense? Recent transcriptional and proteomics analyses responding to our urgent need for a better and more comprehensive understanding of mast cell biology may provide critical information to answer this question.(101–103) For example, by using a proteomics analysis approach of mast cell secretomes upon IgE-dependent activation, we recently found that mast cells can produce large amounts of coagulation factor XIIIA,(102) a coagulation factor that may prevent pathogen dissemination by contributing to pathogen entrapment into clots.(104) Although useful information can be retrieved from these studies, it should be pointed out that mast cell local and systemic responses can exhibit different features depending on the nature of the stimulus.(105) Therefore, we think that it will be necessary to perform similar comprehensive analyses of mast cells in response to stimuli relevant to a particular infection or pathogen.
Are the studies summarized here translatable to what we know about mast cell biology and infections with similar pathogens in humans? In vitro studies with human primary mast cells may be indicative of mast cell responsiveness to a certain pathogen during infection in humans. However, discrepancies between results obtained in vitro vs. in vivo exposure of mast cells to the same pathogen have been reported(106) indicating that critical factors or mechanisms triggered by the host during an infection can influence how mast cells will respond to pathogens. There is no comparable human model to mast cell-deficient mice to study how mast cells contribute to infection outcomes in humans. However, there is a possibility that we will obtain some insight into this question with the use of therapies that target mast cells in humans. For example, blocking the effects of c-kit in mast cells by using the tyrosine kinase inhibitor, imatinib, produces a profound decrease in mast cells in patients with chronic myeloid leukemia (CML)(107) and severe asthma (108). Thus far, a small study with twenty three CML patients showed no increase in the frequency of severe bacterial or fungal infections after treatment with imatinib. However, we should point out that the degree of mast cell reduction in tissues is unknown, as only serum tryptase levels were measured as an indicator of systemic mast cell depletion.(107) Therefore, it is possible that the small number of mast cells remaining in tissues after treatment with imatinib is enough to protect the patient from potential bacterial and/or fungal infections.
Overall, we can conclude that recent findings in the field of mast cells and infection suggest that mast cells can influence infection outcomes by multiple mechanisms. Several challenging questions remain and some of them were posed in this section of the review. Although some of these questions are difficult to answer specially, such as those pertaining to mast cells and infections in humans, we think that strong collaborations between mast cell biologists and microbiologists will provide with a better understanding of how mast cells and pathogens interact to influence host innate immunity and infection outcomes.
Acknowledgments
This work was supported by funding from the National Institutes of Health; Grant R01HL113351 to A.P., from the Italian Cystic Fibrosis Research Foundation, (Research Project number FFC#22/2014) and the Specific Targeted Research Project FunMeta (ERC-2011-AdG-293714) to LR.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
References
- 1.Wells PD. Mast cell, eosinophil and histamine levels in Nippostrongylus brasiliensis infected rats. Exp Parasitol. 1962;12:82–101. doi: 10.1016/s0014-4894(62)80002-2. [DOI] [PubMed] [Google Scholar]
- 2.Briggs NT. Immunological Injury of Mast Cells in Mice Actively and Passively Sensitized to Antigens from Trichinella Spiralis. J Infect Dis. 1963;113:22–32. doi: 10.1093/infdis/113.1.22. [DOI] [PubMed] [Google Scholar]
- 3.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 4.Crowle PK. Mucosal mast cell reconstitution and Nippostrongylus brasiliensis rejection by W/Wv mice. J Parasitol. 1983;69:66–69. [PubMed] [Google Scholar]
- 5.Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/WV mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti-infection. Parasite Immunol. 1987;9:31–38. doi: 10.1111/j.1365-3024.1987.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 6.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, Nakanishi K. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med. 2005;202:607–616. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan AI, Horii Y, Nawa Y. Defective mucosal immunity and normal systemic immunity of Mongolian gerbils, Meriones unguiculatus, to reinfection with Strongyloides venezuelensis. Parasite Immunol. 1993;15:565–571. doi: 10.1111/pim.1993.15.10.565. [DOI] [PubMed] [Google Scholar]
- 9.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oku Y, Itayama H, Kamiya M. Expulsion of Trichinella spiralis from the intestine of W/Wv mice reconstituted with haematopoietic and lymphopoietic cells and origin of mucosal mast cells. Immunology. 1984;53:337–344. [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama K, Ito Y. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunol. 2000;22:13–20. doi: 10.1046/j.1365-3024.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- 12.Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, Hartmann S. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A. 2012;109:6644–6649. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 14.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groschwitz KR, Ahrens R, Osterfeld H, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106:22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides ratti infection. Parasite Immunol. 1987;9:477–485. doi: 10.1111/j.1365-3024.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 17.Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient Kit(W)/Kit(W-v) mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab Invest. 2005;85:388–396. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
- 18.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukai K, Karasuyama H, Kabashima K, Kubo M, Galli SJ. Differences in the Importance of Mast Cells, Basophils, IgE, and IgG versus That of CD4+ T Cells and ILC2 Cells in Primary and Secondary Immunity to Strongyloides venezuelensis. Infect Immun. 2017;85 doi: 10.1128/IAI.00053-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katakura K, Saito S, Hamada A, Matsuda H, Watanabe N. Cutaneous leishmaniasis in mast cell-deficient W/Wv mice. Infect Immun. 1993;61:2242–2244. doi: 10.1128/iai.61.5.2242-2244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wershil BK, Theodos CM, Galli SJ, Titus RG. Mast cells augment lesion size and persistence during experimental Leishmania major infection in the mouse. J Immunol. 1994;152:4563–4571. [PubMed] [Google Scholar]
- 22.Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, von Stebut E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 23.Paul C, Wolff S, Zapf T, et al. Mast cells have no impact on cutaneous leishmaniasis severity and related Th2 differentiation in resistant and susceptible mice. Eur J Immunol. 2016;46:114–121. doi: 10.1002/eji.201545613. [DOI] [PubMed] [Google Scholar]
- 24.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 25.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 26.Wei OL, Hilliard A, Kalman D, Sherman M. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect Immun. 2005;73:1978–1985. doi: 10.1128/IAI.73.4.1978-1985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siebenhaar F, Syska W, Weller K, Mageri M, Zuberbier T, Metz M, Maurer M. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–1916. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelson BT, Li Z, Pappan LK, Zutter MM. Mast cell-mediated inflammatory responses require the alpha 2 beta 1 integrin. Blood. 2004;103:2214–2220. doi: 10.1182/blood-2003-08-2978. [DOI] [PubMed] [Google Scholar]
- 29.Matsui H, Sekiya Y, Takahashi T, et al. Dermal mast cells reduce progressive tissue necrosis caused by subcutaneous infection with Streptococcus pyogenes in mice. J Med Microbiol. 2011;60:128–134. doi: 10.1099/jmm.0.020495-0. [DOI] [PubMed] [Google Scholar]
- 30.Velin D, Bachmann D, Bouzourene H, Michetti P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology. 2005;129:142–155. doi: 10.1053/j.gastro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Ketavarapu JM, Rodriguez AR, Yu JJ, et al. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc Natl Acad Sci U S A. 2008;105:9313–9318. doi: 10.1073/pnas.0707636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Zhang D, Zhang H, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012;3:185. doi: 10.3389/fimmu.2012.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 35.Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeley EJ, Sutherland RE, Kim SS, Wolters PJ. Systemic mast cell degranulation increases mortality during polymicrobial septic peritonitis in mice. J Leukoc Biol. 2011;90:591–597. doi: 10.1189/jlb.0910531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piliponsky AM, Chen CC, Nishimura T, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14:392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piliponsky AM, Chen CC, Rios EJ, et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol. 2012;181:875–886. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Filippo K, Dudeck A, Hasenberg M, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 40.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 41.Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 42.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orinska Z, Maurer M, Mirghomizadeh F, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 44.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 45.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565–7573. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malaviya R, Ikeda T, Abraham SN. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett. 2004;91:103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piliponsky AM, Chen CC, Grimbaldeston MA, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Echtenacher B, Falk W, Mannel DN, Krammer PH. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 52.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64:3231–3235. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 54.Pejler G, Ronnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115:4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 55.Metz M, Piliponsky AM, Chen CC, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 56.Reimer JM, Enoksson M, Samollow PB, Hellman L. Extended substrate specificity of opossum chymase--implications for the origin of mast cell chymases. Mol Immunol. 2008;45:2116–2125. doi: 10.1016/j.molimm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Reimer JM, Samollow PB, Hellman L. High degree of conservation of the multigene tryptase locus over the past 150–200 million years of mammalian evolution. Immunogenetics. 2010;62:369–382. doi: 10.1007/s00251-010-0443-2. [DOI] [PubMed] [Google Scholar]
- 58.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi HW, Bowen SE, Miao Y, et al. Loss of Bladder Epithelium Induced by Cytolytic Mast Cell Granules. Immunity. 2016;45:1258–1269. doi: 10.1016/j.immuni.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gendrin C, Vornhagen J, Ngo L, et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 2015;1:e1400225. doi: 10.1126/sciadv.1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gendrin C, Shubin NJ, Boldenow E, et al. Mast Cell Chymase Decreases The Severity Of Group B Streptococcus Infections. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 64.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–143. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 65.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Coletta C, Modis K, Olah G, et al. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18:511. doi: 10.1186/s13054-014-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lilla JN, Chen CC, Mukai K, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grivennikov SI, Tumanov AV, Liepinsh DJ, et al. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38:349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 71.Sakurai A, Yamaguchi N, Sonoyama K. Cell Wall Polysaccharides of Candida albicans Induce Mast Cell Degranulation in the Gut. Biosci Microbiota Food Health. 2012;31:67–70. doi: 10.12938/bmfh.31.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi N, Sugita R, Miki A, Takemura N, Kawabata J, Watanabe J, Sonoyama K. Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. Gut. 2006;55:954–960. doi: 10.1136/gut.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamaki K, Yoshino S. Aspergillus oryzae lectin induces anaphylactoid oedema and mast cell activation through its interaction with fucose of mast cell-bound non-specific IgE. Scand J Immunol. 2011;74:445–453. doi: 10.1111/j.1365-3083.2011.02598.x. [DOI] [PubMed] [Google Scholar]
- 74.Mathias CB, Freyschmidt EJ, Caplan B, et al. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182:2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urb M, Pouliot P, Gravelat FN, Olivier M, Sheppard DC. Aspergillus fumigatus induces immunoglobulin E-independent mast cell degranulation. J Infect Dis. 2009;200:464–472. doi: 10.1086/600070. [DOI] [PubMed] [Google Scholar]
- 76.Lopes JP, Stylianou M, Nilsson G, Urban CF. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci Rep. 2015;5:12287. doi: 10.1038/srep12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlapbach C, Gehad A, Yang C, et al. Human TH9 cells are skin-tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6:219ra218. doi: 10.1126/scitranslmed.3007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinke KH, Lima HG, Cunha FQ, Lara VS. Mast cells phagocyte Candida albicans and produce nitric oxide by mechanisms involving TLR2 and Dectin-1. Immunobiology. 2016;221:220–227. doi: 10.1016/j.imbio.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Trevisan E, Vita F, Medic N, Soranzo MR, Zabucchi G, Borelli V. Mast cells kill Candida albicans in the extracellular environment but spare ingested fungi from death. Inflammation. 2014;37:2174–2189. doi: 10.1007/s10753-014-9951-9. [DOI] [PubMed] [Google Scholar]
- 80.Moretti S, Bellocchio S, Bonifazi P, Bozza S, Zelante T, Bistoni F, Romani L. The contribution of PARs to inflammation and immunity to fungi. Mucosal Immunol. 2008;1:156–168. doi: 10.1038/mi.2007.13. [DOI] [PubMed] [Google Scholar]
- 81.Frossi B, Mion F, Tripodo C, Colombo MP, Pucillo CE. Rheostatic Functions of Mast Cells in the Control of Innate and Adaptive Immune Responses. Trends Immunol. 2017;38:648–656. doi: 10.1016/j.it.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Gerard R, Sendid B, Colombel JF, Poulain D, Jouault T. An immunological link between Candida albicans colonization and Crohn's disease. Crit Rev Microbiol. 2015;41:135–139. doi: 10.3109/1040841X.2013.810587. [DOI] [PubMed] [Google Scholar]
- 83.Bonifazi P, Zelante T, D'Angelo C, et al. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal Immunol. 2009;2:362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 84.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–6308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 85.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 86.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015;8:444–463. doi: 10.1038/mi.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Virk H, Arthur G, Bradding P. Mast cells and their activation in lung disease. Transl Res. 2016;174:60–76. doi: 10.1016/j.trsl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Kleeberger SR, Seiden JE, Levitt RC, Zhang LY. Mast cells modulate acute ozone-induced inflammation of the murine lung. Am Rev Respir Dis. 1993;148:1284–1291. doi: 10.1164/ajrccm/148.5.1284. [DOI] [PubMed] [Google Scholar]
- 90.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 91.Andersson CK, et al. Activated MCTC mast cells infiltrate diseased lung areas in cystic fibrosis and idiopathic pulmonary fibrosis. Respir Res. 2011;12:139. doi: 10.1186/1465-9921-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moretti S, Renga G, Oikonomou V, et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun. 2017;8:14017. doi: 10.1038/ncomms14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulka M, Gilchrist M, Duszyk M, Befus AD. Expression and functional characterization of CFTR in mast cells. J Leukoc Biol. 2002;71:54–64. [PubMed] [Google Scholar]
- 94.Niide O, Suzuki Y, Yoshimaru T, Inoue T, Takayama T, Ra C. Fungal metabolite gliotoxin blocks mast cell activation by a calcium- and superoxide-dependent mechanism: implications for immunosuppressive activities. Clin Immunol. 2006;118:108–116. doi: 10.1016/j.clim.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Nigrovic PA, Gray DH, Jones T, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michel A, Schuler A, Friedrich P, et al. Mast cell-deficient Kit(W-sh) "Sash" mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid-derived suppressor cells. J Immunol. 2013;190:5534–5544. doi: 10.4049/jimmunol.1203355. [DOI] [PubMed] [Google Scholar]
- 97.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 98.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feyerabend TB, Weiser A, Tietz A, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 100.Reber LL, Sibilano R, Starkl P, et al. Imaging protective mast cells in living mice during severe contact hypersensitivity. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dwyer DF, Barrett NA, Austen KF. Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 2016;17:878–887. doi: 10.1038/ni.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shubin NJ, Glukhova VA, Clauson M, et al. Proteome analysis of mast cell releasates reveals a role for chymase in the regulation of coagulation factor XIIIA levels via proteolytic degradation. J Allergy Clin Immunol. 2017;139:323–334. doi: 10.1016/j.jaci.2016.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chhiba KD, Hsu CL, Berdnikovs S, Bryce PJ. Transcriptional Heterogeneity of Mast Cells and Basophils upon Activation. J Immunol. 2017;198:4868–4878. doi: 10.4049/jimmunol.1601825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loof TG, Morgelin M, Johansson L, et al. Coagulation, an ancestral serine protease cascade, exerts a novel function in early immune defense. Blood. 2011;118:2589–2598. doi: 10.1182/blood-2011-02-337568. [DOI] [PubMed] [Google Scholar]
- 105.Gaudenzio N, Sibilano R, Marichal T, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126:3981–3998. doi: 10.1172/JCI85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ronnberg E, Johnzon CF, Calounova G, et al. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology. 2014;143:155–163. doi: 10.1111/imm.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cerny-Reiterer S, Rabenhorst A, Stefanzl G, et al. Long-term treatment with imatinib results in profound mast cell deficiency in Ph+ chronic myeloid leukemia. Oncotarget. 2015;6:3071–3084. doi: 10.18632/oncotarget.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cahill KN, Katz HR, Cui J, et al. KIT Inhibition by Imatinib in Patients with Severe Refractory Asthma. N Engl J Med. 2017;376:1911–1920. doi: 10.1056/NEJMoa1613125. [DOI] [PMC free article] [PubMed] [Google Scholar]