Abstract
We report the case of a 45-year-old Caucasian woman with a history of psoriasis, admitted to our Medical intensive care unit following the acute onset of diffuse rash and progressive dyspnoea and hypoxaemia requiring escalating respiratory support (continuous positive airway pressure of 10 cm H2O). Her chest X-ray was consistent with findings of non-cardiogenic pulmonary oedema. Echocardiogram was normal. Dermatology considered her skin lesions to be consistent with psoriasis vulgaris with pustular flare. In the absence of an identifiable cause for her respiratory failure, she was diagnosed with acute respiratory distress syndrome due to her psoriatic flare. Treatment with cyclosporine was initiated together with low-dose systemic corticosteroids, intravenous vitamin C and thiamine. The patient made a dramatic recovery being weaned to nasal cannulae within 24 hours after the initiation of this treatment protocol and was discharged home a few days later.
Keywords: respiratory system, dermatology
Background
Pulmonary involvement during a pustular psoriasis flare is a rare and serious complication, initially reported in 1972.1 The pustular subclass of psoriatic dermatoses has been associated with the development of an unusual non-cardiogenic and non-infectious acute respiratory distress syndrome (ARDS) also known as ‘psoriasis-associated aseptic pneumonitis’.2 As a serious, often fatal, complication of pustular and erythrodermic psoriasis, it requires clinical awareness to differentiate it from other causes of respiratory compromise, and for early implementation of corticosteroid therapy. 2.
Case presentation
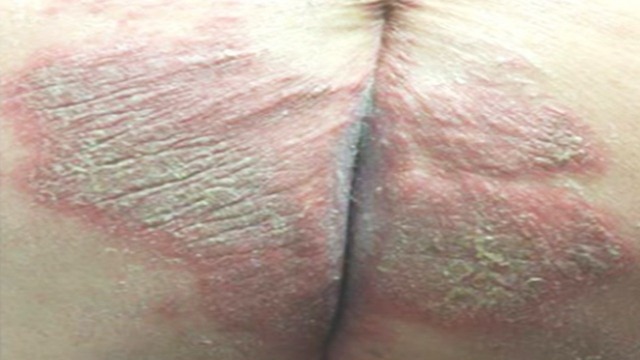
A 45-year-old Caucasian woman with a history of morbid obesity (body mass index (BMI) 60.5 kg/m2), postremote gastric bypass surgery, hypertension, obstructive sleep apnoea (OSA) and pustular psoriasis was admitted to an outside hospital with the initial diagnosis of ‘diffuse rash with cellulitis.’ She had seen her dermatologist the previous day for a flare of pustular psoriasis on her abdomen, and had received a single dose of ustekinumab (Stelara, Janssen Biotech). Within hours of her first injection, her localised flare had developed into several maculopapular pustules with onset of exquisite pain and diffuse pruritus. She was started on piperacillin–tazobactam for the treatment of presumed cellulitis. On hospital day 1, her rash was noted to be coalescing into large macerated plaques along the inframammary chest, axillary vaults, inguinal folds and of the labia majora, buttocks and sacrum (figure 1). At this time, she was transferred to our hospital for concern of possible Stevens-Johnson syndrome and for further management. She was evaluated by our dermatologist who felt her symptoms, and presentation were consistent with Psoriasis vulgaris with pustular flare. She was started on a cyclosporine taper with the goal of limiting further progression of her psoriatic flare. At the time of admission to the medicine service, the patient had denied dyspnoea or cough and she was additionally noted to be saturating well on room air (arterial saturation >94%). In the 24 hours following admission, the patient developed dyspnoea with hypoxaemia, requiring progressive increases in supplementary oxygenation, and subsequently progressed to acute respiratory failure (estimated PaO2/FiO2<100). She was transferred to the intensive care unit (ICU) for further management and started on a continuous positive airway pressure (CPAP) mask at 10 cmH20.
Figure 1.
Large, macerated psoriatic plaques on medial buttocks.
Investigations
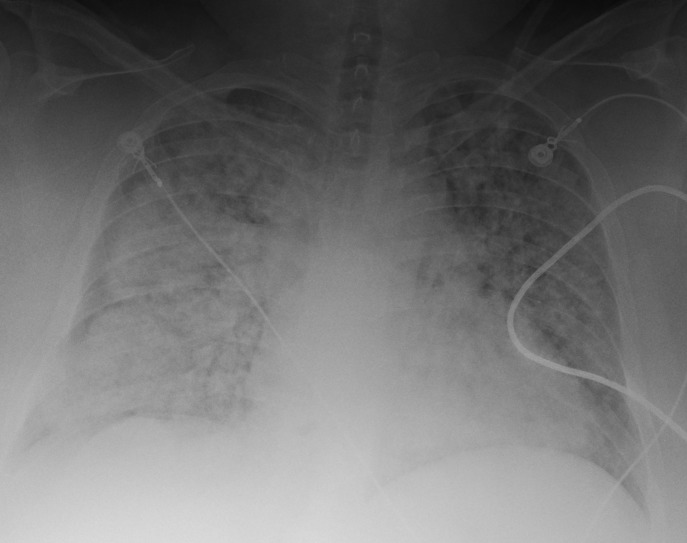
Chest X-ray on admission to the ICU demonstrated bilateral alveolar infiltrates consistent with acute pulmonary oedema (figure 2), and a CT of the chest showed diffuse alveolar infiltrates with subpleural sparing (figure 3). An echocardiogram at this time was unchanged from the previous study with normal ventricular function (ejection fraction of 65%) and no valvular abnormalities.
Figure 2.
Portable chest radiograph on admission to the intensive care unit.
Figure 3.
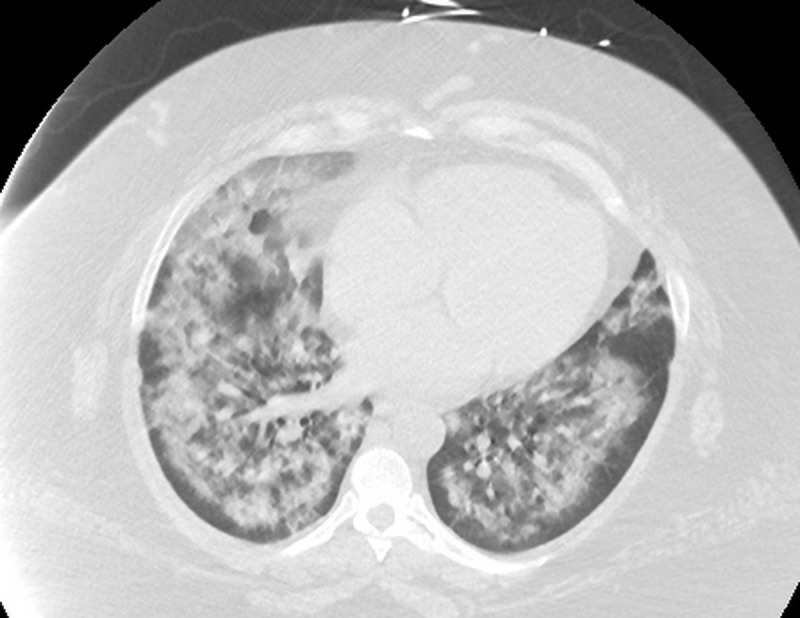
Chest CT scan performed on admission to the intensive care unit showing diffuse alveolar infiltrates.
Differential diagnosis
The patient was diagnosed with ARDS,3 the cause being unclear at the time of admission to the ICU.
Treatment
Treatment with intravenous vitamin C 1500 mg every 6 hours, intravenous hydrocortisone 50 mg every 6 hours and intravenous thiamine 200 mg every 12 hours was initiated.4 Although there was no evidence of infection, antibiotics were continued.
Outcome and follow-up
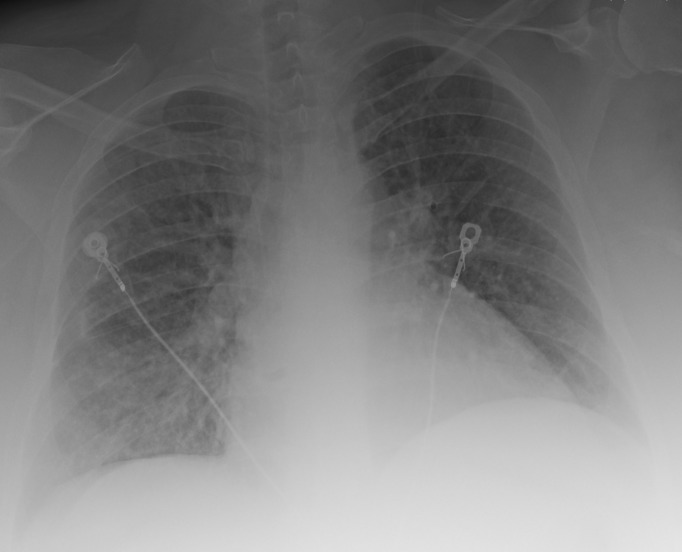
Within hours of starting the ‘metabolic resuscitation protocol’ her dyspnoea and hypoxaemia improved, and she was weaned to a high-flow nasal cannula (flow rate 50 L/min). The following day, she was transitioned to nasal cannula (4 L/min) with nocturnal CPAP (for OSA). By 48 hours, the chest radiograph showed almost complete resolution of the infiltrates (figure 4). On day 3, the metabolic resuscitation protocol and antibiotics were stopped (all microbiological cultures were negative). She was started on oral prednisolone 60 mg daily, to be tapered over the next 14 days. The patient was transferred to the general medicine floor, where she continued to show clinical improvement and was ultimately weaned entirely from supplemental oxygen prior to discharge to home. The final diagnosis was psoriatic-associated ARDS. Cardiogenic pulmonary oedema was unlikely due to the normal echocardiogram and the fact she did not specifically receive heart failure treatment. While the patient was treated with ustekinumab, this drug has not been associated with the development of acute lung injury. Finally, the negative culture data and the dramatic response to the ‘metabolic resuscitation protocol’ make an infective cause unlikely. The patient’s pustular psoriasis improved with the treatment regimen.
Figure 4.
Portable chest radiograph 48 hours after treatment with intravenous vitamin C, low-dose hydrocortisone and thiamine.
Discussion
Psoriasis is generally a chronic and benign condition with pulmonary involvement being a rare complication. In 2011, Kluger and colleagues reported a case of ARDS complicating generalised pustular arthritis in a 14-year-old girl who was successfully treated with CPAP and pulsed corticosteroid (500 mg methylprednisolone for 3 days), followed by oral prednisone 50 mg daily.2 Within 72 hours of initiating corticosteroid therapy, this patient’s respiratory symptoms had improved and the CPAP was stopped. These authors reviewed the literature on ‘psoriasis-related sterile pneumonitis’, and recorded an additional 11 cases. Subsequently, two additional cases have been reported.5 6 In the series by Kluger et al, four patients died (33% mortality), none of whom had been treated with systemic corticosteroids. The patients in this series showed rapid respiratory deterioration with dyspnoea and hypoxaemia occurring after skin fare-up. None of the patients had responded to diuretics or antibiotics, but showed rapid improvement following high-dose systemic corticosteroids. Drug-induced hypersensitivity pneumonitis was considered in the differential diagnosis of these patients; this diagnosis was not likely in our patient. Given that the most common cause of mortality in patients with generalised pustular psoriasis flares has been reported as cardiopulmonary,7–9 it seems reasonable to suspect that this clinical phenomenon may be under-recognised in the inpatient and intensive care settings. Consequently, this condition should be considered in the differential diagnosis of patients with acute respiratory failure in the setting of generalised pustular psoriasis.
ARDS complicating generalised pustular psoriasis is considered a ‘sterile pneumonitis’,2 8 and considered to be caused by the release of cell-derived inflammatory mediators from T helper cell 1 (Th-1) lymphocytes during the psoriatic flare.7 9 In animal models, it has been shown that interleukin-1 (IL-1), IL-2 and tumour necrosis factor-α profoundly increase capillary permeability when bound to the pulmonary vasculature.7 10
Our patient demonstrated a dramatic response to the combination of low-dose systemic corticosteroid, intravenous vitamin C and thiamine. While a retrospective before–after study demonstrated the benefit of this intervention in patients with sepsis, this treatment strategy is not the standard of care.4 We believe this combination has synergetic and potent anti-inflammatory properties with minimal (if any) adverse effects.4 11 Furthermore, this combination is likely to reduce the morbidity and mortality of patients with ARDS regardless of cause, suggesting that corticosteroids should not be used as monotherapy for this syndrome.12 We suggest that the potent anti-inflammatory properties of this combination of medications was responsible for the patient’s dramatic improvement. Prospective randomised trials are currently underway to explore the potential benefit of this treatment strategy in patients with sepsis. While the patient was morbidly obese (BMI 60 kg/m2) she was treated with standard doses of hydrocortisone. It is not clear whether such patients should be dosed with corticosteroids at fixed dose or a dose based on actual body weight. The most recent consensus guidelines make no recommendations regarding dose adjustment according to body weight.13
Learning points.
Acute respiratory distress syndrome (ARDS) is an uncommon but likely underdiagnosed complication of pustular psoriasis.
ARDS should be considered in the differential diagnosis of a patient with a pustular psoriasis flair who develops respiratory symptoms.
Early treatment with corticosteroids is essential in patients with ARDS associated with pustular psoriasis.
The combination of intravenous vitamin C, low-dose systemic corticosteroids and thiamine may prove to be beneficial in patients with ARDS due to a dysregulated inflammatory response.
Footnotes
Contributors: PEM was the specialist involved in the management of the patient and made the decision to treat the patient with the ‘metabolic resuscitation protocol’; was involved in the acquisition and interpretation of the patient data; reviewed the literature of ARDS complicating pustular psoriasis; and reviewed and edited the drafts of the manuscript. AL was responsible for the acquisition of the patient data; was responsible for collating the patient information including the photograph of the skin lesions; reviewed the literature on this topic; was responsible for writing the first draft of the manuscript; was responsible for follow-up discussion with the patient and obtaining consent to publish the case report; and approved the final version of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Landry M, Muller SA. Generalized pustular psoriasis. Observations on the course of the disease in a familial occurrence. Arch Dermatol 1972;105:711–6. [DOI] [PubMed] [Google Scholar]
- 2.Kluger N, Bessis D, Guillot B, et al. Acute respiratory distress syndrome complicating generalized pustular psoriasis (psoriasis-associated aseptic pneumonitis). J Am Acad Dermatol 2011;64:1154–8. 10.1016/j.jaad.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 3.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229–38. 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 5.Maehara LS, Mariano MM, Góis AF, et al. Acute respiratory distress syndrome as a complication of generalized pustular psoriasis. An Bras Dermatol 2011;86:579–81. [DOI] [PubMed] [Google Scholar]
- 6.Al-Niaimi F, Lyon CC. Erythrodermic psoriasis complicated by acute respiratory distress syndrome. Eur J Dermatol 2011;21:429–30. 10.1684/ejd.2011.1303 [DOI] [PubMed] [Google Scholar]
- 7.McGregor JM, Barker JN, MacDonald DM. Pulmonary capillary leak syndrome complicating generalized pustular psoriasis: possible role of cytokines. Br J Dermatol 1991;125:472–4. 10.1111/j.1365-2133.1991.tb14777.x [DOI] [PubMed] [Google Scholar]
- 8.Abou-Samra T, Constantin JM, Amarger S, et al. Generalized pustular psoriasis complicated by acute respiratory distress syndrome. Br J Dermatol 2004;150:353–6. 10.1111/j.1365-2133.2004.05777.x [DOI] [PubMed] [Google Scholar]
- 9.Griffiths MR, Porter W, Fergusson-Wood LA, et al. Generalized pustular psoriasis complicated by acute respiratory distress syndrome. Br J Dermatol 2006;155:496–7. 10.1111/j.1365-2133.2006.07364.x [DOI] [PubMed] [Google Scholar]
- 10.Mizutani H, Yamanaka K, Konishi H, et al. Animal models of psoriasis and pustular psoriasis. Arch Dermatol Res 2003;295 Suppl 1(Suppl 1):S67–S68. 10.1007/s00403-002-0374-3 [DOI] [PubMed] [Google Scholar]
- 11.Barabutis N, Khangoora V, Marik PE, et al. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. In Press 2017;152:954–62. 10.1016/j.chest.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marik PE. Glucocorticosteroids as adjunctive therapy for acute respiratory distress syndrome and sepsis? Yes, but not as monotherapy. Crit Care Med 2017;45:910–1. 10.1097/CCM.0000000000002346 [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Pastores SM, Rochwerg B, et al. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med 2017;45:2078–88. 10.1097/CCM.0000000000002737 [DOI] [PubMed] [Google Scholar]