Abstract
Background
The aim of this trial was to evaluate the safety and efficacy of oral hydration as a substitute for intravenous hydration after cisplatin (CDDP) administration.
Methods
The major eligibility criteria included patients with lung cancer, indications for a CDDP-based regimen at a dose of 60 mg/m2 or higher, an age of between 20 and 74 years and adequate renal function. Antiemetic prophylaxis consisted of an appropriate dose of palonosetron, aprepitant, dexamethasone and magnesium sulfate (8 mEq). Five hundred millilitres of commercially available oral hydration solution (OS-1: Otsuka Pharmaceutical Factory, Tokushima, Japan) was used as a substitute for intravenous posthydration. The planned sample size was 46 to reject a proportion of 70% under an expectation of 88% with a power of 90% and an alpha error of 5%.
Results
Between May and November 2013, 31 men and 15 women with a median (range) age of 65 (33–74) years were enrolled from three institutions. Of these, five received adjuvant chemotherapy, 17 received definitive chemoradiotherapy and 24 received chemotherapy for advanced diseases. The median (range) number of chemotherapy cycles was 4 (1–5). After the first cycle of CDDP administration, none of the patients experienced a creatinine elevation of grade 2 or higher, thereby meeting the primary endpoint. Of the 46 patients, 45 (97.8%, 95% CI 88.2 to 99.9) completed the CDDP-based chemotherapy without grade 2 or higher renal dysfunction.
Conclusion
Oral hydration can be used as a safe and convenient substitute for intravenous posthydration for CDDP administration at the standard dose.
Trial registration number
UMIN000010201.
Keywords: oral hydration, cisplatin, OS-1, short hydration, nephrotoxicity
Key questions.
What is already known about this subject?
In order to reduce renal dysfunction after cisplatin (CDDP) administration, preintravenous and postintravenous fluid infusion is necessary. The long and high volume fluid infusion was developed in the era before modern antiemetics such as aprepitant and palonosetron.
What does this study adds?
In this multicenter prospective trial, we demonstrated that OS-1 oral post-hydration was successfully conducted without resulting in a grade 2 or higher creatinine elevation in 97.8% of the trial participants and was an adequate substitute for conventional intravenous posthydration. By combining short term and lower volume hydration with oral posthydration, CDDP-based chemotherapy could be administered in less than 3 hours of intravenous infusion. Oral posthydration did not appear to have significant effect on either the treatment delivery (median four cycles) or the response (45% (95% CI 24.4 to 67.8) in patients with non-small cell lung cancer with target lesions).
How might this impact on clinical practice?
By replacing post-cisplatin intravenous fluid infusion, patients and healthcare providers might not only save significant time after chemotherapy but also save renal function against cisplatin nephrotoxicity.
Introduction
Cisplatin is a key drug in the treatment of lung cancer in a wide range of treatment setting.1–4 Conventional long term and large volume hydration is widely performed because of the nephrotoxicity of CDDP, which prevents the optimal use of this platinum agent.5 However, several reports have suggested the absence of any correlation between CDDP nephrotoxicity and the amount of hydration.6 The development of novel antiemetics such as aprepitant and palonosetron has significantly reduced the incidence of nausea and vomiting and has improved oral intake. Furthermore, several strategies including magnesium supplementation and forced diuresis have been reported to prevent CDDP nephrotoxicity.7–10 We previously conducted a prospective trial examining the feasibility of short-term and lower volume hydration in patients receiving CDDP.11 In that trial, 97.8% of the participants completed the CDDP-based chemotherapy without experiencing grade 2 or higher elevation in their creatinine (Cr) levels.
OS-1 is a commercially available oral hydration solution containing sodium (50 mEq/L), chloride (50 mEq/L), potassium (20 mEq/L), magnesium sulfate (2 mEq/L), lactate (31 mEq/L) and glucose (18 g/L) with added citrus flavour. OS-1 was developed based on WHO formula of oral rehydration solution. In a rat model,12 13 OS-1 exhibited a protective effect against CDDP nephrotoxicity in terms of the serum Cr level, blood urea nitrogen level, Cr clearance and histopathological change in the kidney. To evaluate the feasibility of oral hydration using OS-1, we conducted a first multicenter prospective trial in patients with lung cancer receiving chemotherapy containing CDDP using state-of-the-art protective strategies against renal and gastrointestinal toxicities.
Patients and methods
Patients from two cancer centre hospital and one university hospital provided their written informed consent and participated in this trial. The eligibility criteria were as follows: an age between 20 and 74 years; an Eastern Cooperative Oncology Group (ECOG) performance status13 of 0 or 1; histologically or cytologically proven lung cancer; candidate for platinum-based chemotherapy or chemoradiotherapy with CDDP (≥60 mg/m2); adequate bone marrow function (white cell count (WCC) ≥3.0×109/L, neutrophil count ≥1.5×109/L, haemoglobin ≥9.0 g/dL and platelet count ≥100×109/L), liver function (total bilirubin ≤1.5 mg/dL and transaminase ≤100 IU/L) and renal function (serum Cr less than or equal to the upper limit of the normal value and Cr clearance ≥60 mL/min) and a peripheral capillary oxygen saturation of 95% or more. Patients were excluded if they had dysphagia caused by recurrent nerve paralysis or large mediastinal masses, uncontrolled malignant pleural or pericardial effusion or concomitant serious illness (such as angina pectoris, myocardial infarction within the previous 6 months, heart failure, infection or other diseases contraindicating chemotherapy or radiotherapy). All the patients provided their written informed consent.
Treatment
Patients received CDDP-based chemotherapy with a CDDP dose ≥60 mg/m2 every 3–4 weeks. As a common antiemetic premedication, palonosetron (0.75 mg) and dexamethasone (9.9 mg) were dissolved in 50 mL of normal saline solution and infused, and oral aprepitant (125 mg on day 1, 80 mg on days 2–3) and dexamethasone (8 mg, days 2–4) were administered before and after chemotherapy. An hour-long infusion of CDDP dissolved in 250 mL of normal saline solution was inserted between the prehydration (potassium chloride (10 mEq) and magnesium sulfate (8 mEq) dissolved in 500 mL of saline-based solution) and the oral posthydration (500 mL of OS-1). Mannitol was infused just before the CDDP administration as an enforced diuresis. The patients received one other cytotoxic agent including pemetrexed (500 mg/m2, figure 1), tegafur–gimeracil–oteracil potassium (S-1, 80–120 mg/body, see figure 1 in the online Supplementary file 1), docetaxel (60 mg/m2, see figure 2 in the online Supplementary file 2), vinorelbine (20 or 25 mg/m2, see figure 3 in the online Supplementary file 3), gemcitabine (1000 mg/m2, see figure 4 in the online Supplementary file 4), irinotecan (60 mg/m2, see figure 5 in the online Supplementary file 5) or etoposide (100 mg/m2, see figure 6 in the online Supplementary file 6) with appropriate premedication in combination with CDDP.
Figure 1.
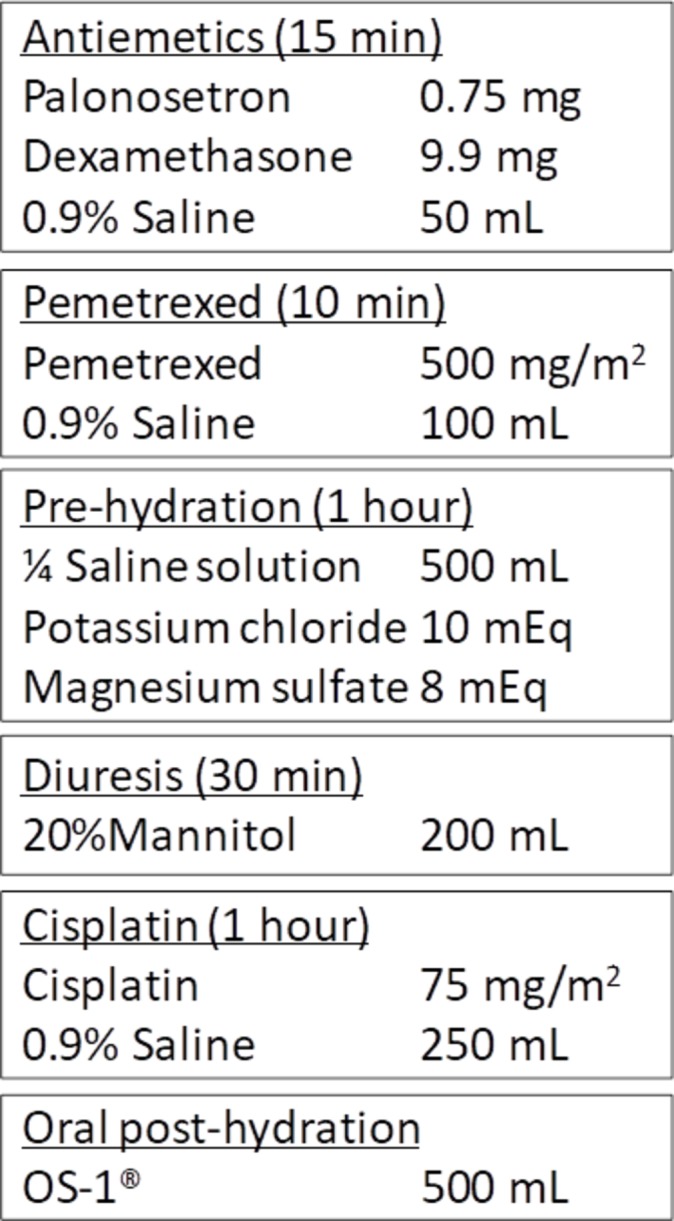
Example of hydration method (cisplatin plus pemetrexed).
esmoopen-2017-000288supp001.jpg (80.6KB, jpg)
esmoopen-2017-000288supp002.jpg (115.4KB, jpg)
esmoopen-2017-000288supp003.jpg (95KB, jpg)
esmoopen-2017-000288supp004.jpg (98.2KB, jpg)
esmoopen-2017-000288supp005.jpg (98.3KB, jpg)
esmoopen-2017-000288supp006.jpg (108.3KB, jpg)
Assessment of toxicities and treatment modification
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), V.4.0, issued in 2009. The CTCAE V.4.0 contains two criteria pertaining to Cr; we adopted the classical upper limit of normal-based method to evaluate Cr elevation as primary analysis endpoint. Additionally, we evaluated change of renal function based on baseline Cr value. Complete blood cell and differential counts and routine chemistry determinants were performed on day 8 of the first cycle and on day 1 of every cycle thereafter. Subsequent cycles of CDDP-based chemotherapy were delayed if any of the following toxicities were noted on day 1: WCC <3.0×109/L, neutrophil count <1.5×109/L, platelet count <100×109/L, serum Cr level >1.4 mg/dL, elevated hepatic transaminase level >100 IU/L or a performance status of two or over. The dose of CDDP was reduced by 25% in all subsequent cycles if the serum Cr level increased to a grade 2 or higher.
Statistical analysis
The primary endpoint was the proportion of patients without renal dysfunction, defined as the proportion of patients without a grade 2 or higher elevation in Cr from the baseline value after the first cycle of CDDP. The sample size was estimated using a Fleming single stage design to test the null hypothesis for a proportion of patients without renal dysfunction of ≤70% versus an alternative hypothesis of a proportion of patient ≥88% at a power of 90%. Under the assumption of a type I error rate of 0.05, with the stated statistical hypothesis, a total of 45 patients were required for the study.14 It was considered that the study would have fulfilled the primary endpoint if 37 out of a total of 45 patients were able to complete the first cycle CDDP without a grade 2 or higher elevation in Cr. The overall proportion of patients without renal dysfunction and the 95% CI were calculated for the final analysis using data from all the patients who received the study treatment. Secondary endpoints included the number of cycles of chemotherapy, adverse events and the overall response in patients who had measurable lesions according to the Response Evaluation Criteria In Solid Tumours (RECIST) criteria (V.1.1).15 The STATA V.13 for Windows software package was used for the statistical analyses.
Results
Patient characteristics
Forty-six patients were enrolled between May and December of 2013. The participant’s characteristics were as follows: male/female 31/15; median age (range) 65 (33–74) years and ECOG performance status of 0/1 24/22. Of these, five received adjuvant chemotherapy, 17 received chemoradiotherapy and 24 received chemotherapy for advanced diseases. Most patients had lung adenocarcinoma (n=22), but eight had non-small cell lung cancer (NSCLC) not otherwise specified, six had squamous cell carcinoma, eight small cell carcinoma and two had large cell neuroendocrine carcinoma. The agents that were combined with CDDP were as follows: pemetrexed, n=13; vinorelbine, n=11; S-1, n=10; etoposide, n=7; irinotecan, n=3; docetaxel, n=1 and gemcitabine, n=1. The results of the pretreatment renal function tests (median (range)) were as follows: serum Cr 0.7 (0.42–0.99) mg/dL and estimated Cr clearance 89 (57–173) mL/min (table 1).
Table 1.
Patient characteristics
| n=46 | % or range | |
| Median age (years, range) | 65 | 33–74 |
| Sex (female/male) | 15/31 | 33/67 |
| Performance status (0/1) | 24/22 | 52/48 |
| Treatment setting | ||
| Adjuvant therapy | 5 | 11 |
| Chemoradiotherapy | 17 | 37 |
| Postsurgical recurrence | 2 | 4 |
| Advanced disease | 22 | 48 |
| Treatment regimen | ||
| CDDP+pemetrexed | 13 | 28 |
| CDDP+vinorelbine | 11 | 24 |
| CDDP+S-1 | 10 | 22 |
| CDDP+etoposide | 7 | 15 |
| CDDP+irinotecan | 3 | 7 |
| CDDP+docetaxel | 1 | 2 |
| CDDP+gemcitabine | 1 | 2 |
| Histology | ||
| Adenocarcinoma | 22 | 48 |
| Squamous cell carcinoma | 6 | 13 |
| NSCLC* | 8 | 17 |
| Small cell carcinoma | 8 | 17 |
| Large cell neuroendocrine carcinoma | 2 | 4 |
| Comorbidities | ||
| Hypertension | 14 | 30 |
| Diabetes mellitus | 4 | 9 |
| Cardiac disease | 2 | 4 |
| Pulmonary disease | 9 | 20 |
| Coadministered medications | ||
| Non-steroidal anti-inflammatory drugs | 10 | 22 |
| Contrast agent | 14 | 30 |
| Serum Cr (mg/dL, median) | 0.7 | 0.42–0.99 |
| Estimated Cr clearance (mL/min, median)† | 89 | 57–173 |
| Calculated eGFR‡ (mL/min 1.73 m2, median) | 80 | 56–138 |
*Non-small cell lung cancer, not otherwise specified.
†Calculated Cr clearance using the Cockcroft-Gault equation.
‡Estimated glomerular filtration rate calculated using Japanese equations.
CDDP, cisplatin; Cr, creatinine; eGFR, estimated glomerular filtrate; S-1, tegafur–gimeracil–oteracil potassium.
Post-treatment renal function and other toxicities
The proportion of patients without a grade 2 or higher Cr elevation after the first cycle of study treatment was 97.8% (95% CI 88.5 to 99.9). Therefore, this trial successfully met the primary endpoint. One patient experienced a grade 2 elevation in Cr (maximum value, 1.97 mg/dL) after the administration of CDDP plus S-1. The patient experienced grade 3 diarrhoea because of chemotherapy and exhibited a prompt improvement in the Cr level to 1.11 mg/dL after the resolution of the diarrhoea. The results of the post-treatment renal function tests (median (range)) were as follows: serum Cr 0.69 (0.44–1.31) mg/dL and estimated glomerular filtration rate 84 (53–155) mL/min (table 2). Additional evaluation using change from baseline Cr method showed post-cisplatin renal function as follows: patients with same or better Cr value, 43.5%; patients with grade 1 Cr elevation, 54.3%; one patient (2.2%) with grade 2 Cr elevation (table 2 and figure 2). The profiles for toxicities other than renal dysfunction are summarised in table 3. Subgroup analysis based on cisplatin dosage (60 mg/m2 or 75–80 mg/m2) showed higher incidence (60.6% vs 38.5%) of grade 1 post-cisplatin Cr elevation in comparison with baseline Cr value in patients with higher dose cisplatin.
Table 2.
Renal function after the first cycle of cisplatin administration
| n=46 | % or range | |
| Cr elevation by CTCAE | ||
| Without G2 or more elevation by ULN Cr | 45 | 97.8 |
| Normal | 44 | 95.6 |
| G1 | 1 | 2.2 |
| G2* Cr Elevation | 1 | 2.2 |
| Without G2 or more elevation by baseline Cr | 45 | 97.8 |
| Normal | 20 | 43.5 |
| G1 | 25 | 54.3 |
| G2* Cr elevation | 1 | 2.2 |
| Serum Cr value | ||
| Median (mg/dL, range) | 0.69 | 0.44–1.31 |
| Estimated Cr clearance (mL/min, median) | 84 | 53–155 |
| Calculated eGFR (mL/min, median) | 79 | 42–114 |
*The patient who experienced a G2 elevation in Cr (maximum value, 1.97 mg/dL) experienced G3 chemotherapy-induced diarrhoea and exhibited a prompt improvement in the Cr level to 1.11 mg/dL after the resolution of the diarrhoea.
Cr, creatinine; CTCAE, Common Terminology Criteria for Adverse Events; eGFR, estimated glomerular filtration rate; ULN, upper limit of normal value.
Figure 2.
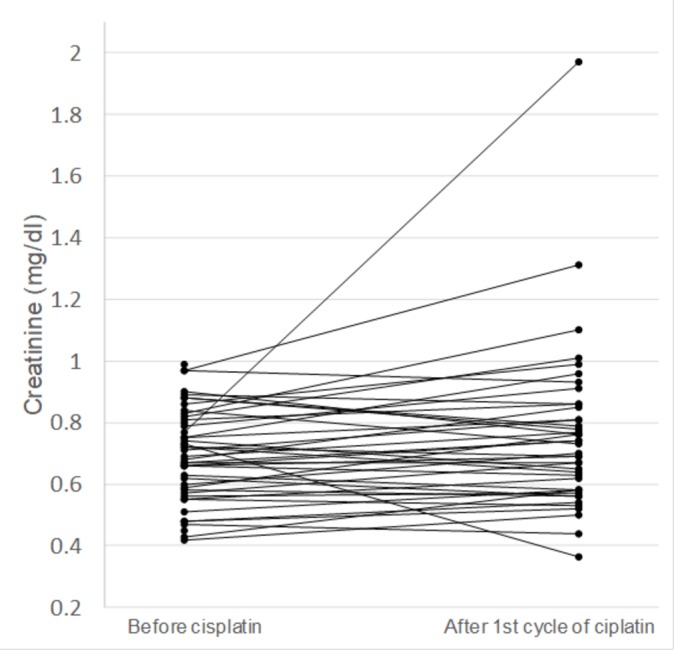
Change in creatinine value in individual participants.
Table 3.
Adverse events other than renal toxicities according to Common Terminology Criteria for Adverse Events V.4
| n=46 | G1 (%) | G2 (%) | G3 (%) | Total (%) |
| Any adverse event | ||||
| Fever | 10 (22) | 0 | 0 | 10 (22) |
| Fatigue | 14 (30) | 3 (7) | 0 | 17 (37) |
| Body weight loss | 6 (13) | 0 | 0 | 6 (13) |
| Anorexia | 18 (39) | 12 (26) | 2 (4) | 32 (70) |
| Nausea | 17 (37) | 12 (26) | 1 (2) | 30 (65) |
| Vomiting | 5 (11) | 1 (2) | 0 | 6 (13) |
| Constipation | 23 (50) | 1 (2) | 0 | 24 (52) |
| Diarrhoea | 5 (11) | 1 (2) | 2 (4) | 8 (17) |
| Stomatitis | 2 (4) | 0 | 0 | 2 (4) |
| Febrile neutropaenia | 0 | 0 | 3 (7) | 3 (7) |
| Alopecia | 6 (13) | 4 (9) | 0 | 10 (22) |
No G4 or severe adverse events were observed.
Treatment delivery and efficacy
Twenty patients received CDDP combined with pemetrexed as the most frequent regimen. The majority of patients (39 patients, 85%) completed the preplanned 3–5 cycles of CDDP-based chemotherapy. The reasons for the early termination of chemotherapy in the remaining patients were as follows: seven (16%) developed progressive disease and one (2%) patient refused to continue treatment because of gastrointestinal toxicities. During the study treatment, seven patients received intravenous hydration and six patients required a dose reduction of CDDP mainly because of gastrointestinal toxicities except for one patient with a grade 2 elevation of Cr (table 4). The objective response was 45.0% (95% CI 24.4 to 67.8) among patients with postsurgical recurrences and advanced NSCLC who had measurable lesions according to the RECIST criteria (V.1.1, table 5).
Table 4.
Treatment summary
| n=46 | Percentage | |
| Number of chemotherapy cycles (number, %) | ||
| 1 cycle | 3 | 7 |
| 2 cycles | 4 | 9 |
| 3 cycles | 6 | 13 |
| 4 cycles | 32 | 69 |
| 5 cycles | 1 | 2 |
| Additional intravenous hydration* | ||
| Number of patients | 7 | 15 |
| Total number of days (median, range) | 2 | 1–19 |
*Intravenous hydration on days other than those on which cisplatin was administered.
Table 5.
Response
| n=22 | Percentage | |
| Objective response* † | 10 | 45 |
| Complete response | 0 | 0 |
| Partial response | 10 | 45 |
| Stable disease | 6 | 27 |
| Progressive disease | 5 | 23 |
| Not evaluable | 1 | 5 |
*Responses were evaluated in patients with postsurgical recurrence and advanced non-small cell lung cancer with target lesions.
†95% CI 24.4% to 67.8%.
Discussion
In this first multicenter prospective trial evaluating OS-1 as an adequate substitute for conventional intravenous posthydration, we demonstrated that oral hydration was successfully conducted without resulting in a grade 2 or higher Cr elevation in 97.8% of the trial participants. By combining short-term and lower volume hydration with oral posthydration, CDDP-based chemotherapy could be administered in less than 3 hours of intravenous infusion. Oral posthydration did not appear to have significant effect on either the treatment delivery (median four cycles) or the response (45% (95% CI 24.4 to 67.8) in patients with NSCLC with target lesions).
The pathophysiological mechanism for renal injury is not fully understood; however, high-volume hydration and forced diuresis are usually employed to prevent CDDP-related nephrotoxicity.7 8 Basic and clinical research suggest that both mannitol and furosemide are equally effective for the prevention of renal dysfunction.16–18 These strategies are intended to lower the concentration and to shorten the period of direct CDDP exposure. Hypomagnesaemia has also been considered as a cause of renal dysfunction and as a target of intervention. Magnesium is associated with the active transport mechanism in the tubular cells of the kidney. Sobrero et al suggested that hypomagnesaemia during CDDP administration may lead to an elevated concentration of CDDP in tubular cells, thereby damaging the proximal tubules and resulting in subsequent renal dysfunction.19 Several studies, including a randomised trial, have demonstrated a favourable effect to prevent renal dysfunction during CDDP-based chemotherapies.9 10 20 21
Based on these findings, the National Comprehensive Cancer Network (NCCN) has provided chemotherapy order templates to improve the safe use of drugs and biologics in cancer care. The CDDP template of the NCCN recommends hyperdiuresis using mannitol and magnesium supplementation, which were included in the protocol treatment for the current trial. Several trials have been conducted to evaluate the efficacy and safety of short-term low-volume hydration using contemporary antiemetics and magnesium supplementation. Hotta et al reported that 0% (evaluation based on upper limit of the normal range) and 4% (evaluation based on baseline Cr value in each patient) of the participants in their series experienced grade 2 or greater Cr elevation and concluded that short-term low-volume hydration was feasible in a phase II (OLCSG1002) trial.22 Horinouchi et al demonstrated that 43 out of 46 participants (97.8%) completed chemotherapy containing CDDP (75 mg/m2 or over) without experiencing grade 2 or greater elevation in creatinine in a phase II trial.11
Oral rehydration solution has been widely used as an effective method of fluid resuscitation in many medical situations, especially for patients with acute infectious diarrhoea.23 24 In several clinical trial, oral hydration was evaluated as an appropriate substitute for intravenous hydration to prevent drug-induced nephrotoxicities.25 The composition of OS-1 is based on common oral rehydration solution formula recommended by WHO. Dana et al reported a small randomised trial comparing the oral and intravenous hydration in patients receiving CDDP-based chemotherapies. The participants (65 patients) were randomised into three arms of prehydration before CDDP: a control arm (conventional intravenous administration of 3000 mL of normal saline hydration followed by hyperdiuresis with mannitol), oral hydration with mannitol (3000 mL of oral fluids followed by mannitol) and oral hydration with furosemide (3000 mL of oral fluid followed by furosemide). As a result, the participants experienced a similar frequency of kidney dysfunction when evaluated using the blood urea value, and the authors concluded that oral hydration was feasible for patients receiving CDDP.26 In the present trial, we demonstrated that oral posthydration using OS-1 could shorten the time required for intravenous hydration by about 1 hour without increasing renal or other toxicities. A 1-hour time savings would benefit both patients and medical staff and would free up equipment required for drug administration, especially in outpatient settings. Substitution of post-cisplatin hydration by oral hydration could be widely adapted using other oral rehydration solution which have similar composition as OS-1.
In this multicenter phase II trial, we confirmed the safety of oral posthydration with regard to renal function in almost all (98%) of the participants who received CDDP-based chemotherapy. Oral hydration can be used as a safe and convenient substitute for intravenous posthydration for cisplatin administration at the standard dose.
Footnotes
Contributors: All authors contributed this study equally.
Funding: This work was supported in part by the National Cancer Center Research and Development Fund (23-A-30).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study was conducted with the approval of the Institutional Review Board at each site.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692–9. 10.1200/JCO.1999.17.9.2692 [DOI] [PubMed] [Google Scholar]
- 2. Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589–97. 10.1056/NEJMoa043623 [DOI] [PubMed] [Google Scholar]
- 3. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 4. Horinouchi H, Sekine I, Sumi M, et al. Long-term results of concurrent chemoradiotherapy using cisplatin and vinorelbine for stage III non-small-cell lung cancer. Cancer Sci 2013;104:93–7. 10.1111/cas.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamada K, Yoshida T, Zaizen Y, et al. Clinical practice in management of hydration for lung cancer patients receiving cisplatin-based chemotherapy in Japan: a questionnaire survey. Jpn J Clin Oncol 2011;41:1308–11. 10.1093/jjco/hyr145 [DOI] [PubMed] [Google Scholar]
- 6. Stewart DJ, Dulberg CS, Mikhael NZ, et al. Association of cisplatin nephrotoxicity with patient characteristics and cisplatin administration methods. Cancer Chemother Pharmacol 1997;40:293–308. 10.1007/s002800050661 [DOI] [PubMed] [Google Scholar]
- 7. Cvitkovic E, Spaulding J, Bethune V, et al. Improvement of cis-dichlorodiammineplatinum (NSC 119875): therapeutic index in an animal model. Cancer 1977;39:1357–61. [DOI] [PubMed] [Google Scholar]
- 8. Hayes DM, Cvitkovic E, Golbey RB, et al. High dose cis-platinum diammine dichloride: amelioration of renal toxicity by mannitol diuresis. Cancer 1977;39:1372–81. [DOI] [PubMed] [Google Scholar]
- 9. Willox JC, McAllister EJ, Sangster G, et al. Effects of magnesium supplementation in testicular cancer patients receiving cis-platin: a randomised trial. Br J Cancer 1986;54:19–23. 10.1038/bjc.1986.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bodnar L, Wcislo G, Gasowska-Bodnar A, et al. Renal protection with magnesium subcarbonate and magnesium sulphate in patients with epithelial ovarian cancer after cisplatin and paclitaxel chemotherapy: a randomised phase II study. Eur J Cancer 2008;44:2608–14. 10.1016/j.ejca.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 11. Horinouchi H, Kubota K, Itani H, et al. Short hydration in chemotherapy containing cisplatin (≥75 mg/m2) for patients with lung cancer: a prospective study. Jpn J Clin Oncol 2013;43:1105–9. 10.1093/jjco/hyt122 [DOI] [PubMed] [Google Scholar]
- 12. Sato J, Kudo K, Hino K, et al. [Usefulness of oral rehydration solution for hydration in cancer chemotherapy: toward the application of outpatient chemotherapy with high-dose Cisplatin therapy-]. Gan To Kagaku Ryoho 2011;38:1471–6. [PubMed] [Google Scholar]
- 13. Sato J, Takahashi K, Hino K, et al. A study of the protective effect of oral rehydration solution on cisplatin-induced nephrotoxicity in rats. Iryo Yakugaku 2014;40:17–27. 10.5649/jjphcs.40.17 [DOI] [Google Scholar]
- 14. Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982;38:143–51. 10.2307/2530297 [DOI] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16. Pera MF, Zook BC, Harder HC. Effects of mannitol or furosemide diuresis on the nephrotoxicity and physiological disposition of cis-dichlorodiammineplatinum-(II) in rats. Cancer Res 1979;39:1269–78. [PubMed] [Google Scholar]
- 17. Ostrow S, Egorin MJ, Hahn D, et al. High-dose cisplatin therapy using mannitol versus furosemide diuresis: comparative pharmacokinetics and toxicity. Cancer Treat Rep 1981;65:73–8. [PubMed] [Google Scholar]
- 18. Santoso JT, Lucci JA, Coleman RL, et al. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol 2003;52:13–18. 10.1007/s00280-003-0620-1 [DOI] [PubMed] [Google Scholar]
- 19. Sobrero A, Guglielmi A, Aschele C, et al. Current strategies to reduce cisplatin toxicity. J Chemother 1990;2:3–7. 10.1080/1120009X.1990.11738971 [DOI] [PubMed] [Google Scholar]
- 20. Hodgkinson E, Neville-Webbe HL, Coleman RE. Magnesium depletion in patients receiving cisplatin-based chemotherapy. Clin Oncol 2006;18:710–8. 10.1016/j.clon.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 21. Tiseo M, Martelli O, Mancuso A, et al. Short hydration regimen and nephrotoxicity of intermediate to high-dose cisplatin-based chemotherapy for outpatient treatment in lung cancer and mesothelioma. Tumori 2007;93:138–44. [DOI] [PubMed] [Google Scholar]
- 22. Hotta K, Takigawa N, Hisamoto-Sato A, et al. Reappraisal of short-term low-volume hydration in cisplatin-based chemotherapy: results of a prospective feasibility study in advanced lung cancer in the Okayama Lung Cancer Study Group Trial 1002. Jpn J Clin Oncol 2013;43:1115–23. 10.1093/jjco/hyt128 [DOI] [PubMed] [Google Scholar]
- 23. Santosham M, Daum RS, Dillman L, et al. Oral rehydration therapy of infantile diarrhea: a controlled study of well-nourished children hospitalized in the United States and Panama. N Engl J Med 1982;306:1070–6. 10.1056/NEJM198205063061802 [DOI] [PubMed] [Google Scholar]
- 24. Avery ME, Snyder JD. Oral therapy for acute diarrhea. The underused simple solution. N Engl J Med 1990;323:891–4. 10.1056/NEJM199009273231307 [DOI] [PubMed] [Google Scholar]
- 25. Martin-Moreno PL, Varo N, Martínez-Ansó E, et al. Comparison of intravenous and oral hydration in the prevention of contrast-induced acute kidney injury in low-risk patients: a randomized trial. Nephron 2015;131:51–8. 10.1159/000438907 [DOI] [PubMed] [Google Scholar]
- 26. Dana R, Kachhwaha VS. Comparison of oral and intravenous hydration and diuretic, choice for protecting cisplatin induced nephrotoxicity. Indian J Cancer 1996;33:168–70. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000288supp001.jpg (80.6KB, jpg)
esmoopen-2017-000288supp002.jpg (115.4KB, jpg)
esmoopen-2017-000288supp003.jpg (95KB, jpg)
esmoopen-2017-000288supp004.jpg (98.2KB, jpg)
esmoopen-2017-000288supp005.jpg (98.3KB, jpg)
esmoopen-2017-000288supp006.jpg (108.3KB, jpg)


