Abstract
Herpes simplex virus (HSV) adenitis is a rare but important cause of morbidity in immunocompromised patients. Chronic lymphocytic leukaemia (CLL)/Small lymphocytic lymphoma (SLL) is an indolent disease which impairs the cellular and humoral immunity, predisposing patients to a myriad of infections. Clinically, herpetic adenitis can mimic large cell (Richter’s) transformation in patients with CLL. To date, less than 30 cases of HSV adenitis have been reported in the literature. We report a case of a patient with CLL with no prior history of HSV infection, who presented with rapidly enlarging lymph nodes after initial response to idelalisib raising the suspicion of Richter’s transformation. However, excisional biopsy of a lymph node revealed HSV adenitis along with CLL, which was confirmed by immunohistochemical staining.
Keywords: haematology (drugs and medicines), infections, malignant and benign haematology, cancer intervention
Background
Chronic lymphocytic lymphoma (CLL)/small lymphocytic lymphoma (SLL) is a chronic indolent disease which impairs the cellular and humoral immunity due to quantitative and qualitative defects in the immune effector cells. The incidence of fatal infections increases with advancing stage and treatment duration. Hypogammaglobinemia, neutropenia and hypocomplementemia are important predisposing factors for infections in these patients. Treatment-related factors such as the use of conventional alkylating agents, purine analogues and monoclonal antibodies such as rituximab and alemtuzumab further increase the risk of bacterial, fungal, viral and opportunistic infections.1
Herpesviridae is a large family of DNA viruses. Eight of more than 100 known herpes viruses are known to be pathogenic in humans. These include herpes simplex virus (HSV) types 1 and 2, varicella-zoster virus, cytomegalovirus (CMV), Epstein-Barr virus, human herpesvirus 6 (variants A and B), human herpesvirus 7 and Kaposi’s sarcoma virus or human herpesvirus 8. All herpes viruses can establish latent infection and later reoccur as manifestations ranging from cutaneous outbreaks to life-threatening central nervous system infections. Though Richter’s transformation is the prime concern in a patient of CLL/SLL with rapidly enlarging lymph nodes, necrotising lymphadenitis should be considered as one of the differential diagnoses. There is evidence that herpes simplex and zoster infections can occur several months after treatment completion in more than 75% of the immunocompromised patients. It is still an unanswered question if the treating oncologist should consider lifelong antiviral prophylaxis in patients treated with purine analogues.
Case presentation
A 64-year-old man was diagnosed with stage IV CLL/SLL in 2008 after initially presenting with generalised lymphadenopathy involving the bilateral cervical, axillary and para-aortic lymph nodes. Physical examination revealed multiple firm, non-tender, bilateral cervical (largest measuring 3×3 cm in size), axillary (largest measuring 2×2 cm in size on the right) and inguinal lymph nodes. The patient denied any significant medical or surgical history. Patient denied history of chronic alcohol use, tobacco use or recreational drug use. Laboratory data were significant for absolute lymphocytosis of 8700 cell/mm3 (normal: 1000–4800 cells/mm), normocytic anaemia with haemoglobin of 12.9 g/dL and mean corpuscular volume of 88 fL. He tested negative for hepatitis A, B and C and HIV. He was started on treatment with six cycles of fludarabine and rituximab with complete response as evident on positron emission tomography CT imaging. This was followed by maintenance rituximab. He remained disease free until February 2010 when the patient had return of bulky left cervical lymphadenopathy. He was then started on treatment with bendamustine, bortezomib and rituximab for six cycles followed by maintenance rituximab. The second recurrence was noted in January 2015 which was treated with six cycles of rituximab, cyclophosphamide, adriamycin, vincristine and prednisone. The patient obtained partial response and was kept under close observation. Idelalisib was initiated in December 2015 after disease progression as evident by new pelvic, inguinal, abdominal and axillary lymphadenopathy. Favourable response to idelalisib was noted as all the nodes regressed in size (figures 1A,B, 2A,B and 3A,B). After 11 months of idelalisib, there was rapid enlargement of inguinal lymph nodes along with fever, night sweats and weight loss (20 lbs in over 2 months), which raised suspicion for large cell Richter’s transformation (figure 4A,B). An excisional biopsy of an inguinal lymph node was performed. Flow cytometry revealed a population of lambda light chain restricted B cells with coexpression of CD5 and CD23. Morphologically, the majority of the lymph node was replaced with necrotising granulomatous inflammation with a small area of residual SLL. (figure 5A,B). Immunohistochemistry for HSV returned strongly positive in the cells with glassy, smudged nuclei/nuclear inclusions (figure 5C). The immunohistochemistry stains for CMV, acid fast and fungal organisms were negative. Hence, the diagnosis of herpetic adenitis was established. The patient was then started on therapeutic valacyclovir which led to complete resolution of lymphadenopathy. He continues to be on maintenance valacyclovir. He was switched to obinutuzumab (monoclonal CD20 antibody) 1000 mg/m2 intravenously once every 28 days and chlorambucil 24 mg orally once every 15 days along with supportive medications, as per the National Comprehensive Cancer Care Guidelines guidelines, for a total of six cycles.
Figure 1.
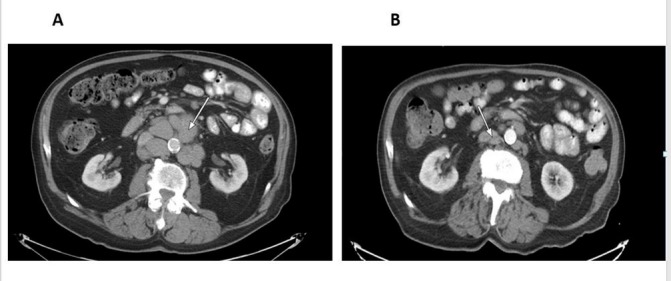
Contrast-enhanced CT scan of the abdomen with enlarged retroperitoneal lymphadenopathy, before (A) and after (B) idelalisib, showing regression in size.
Figure 2.
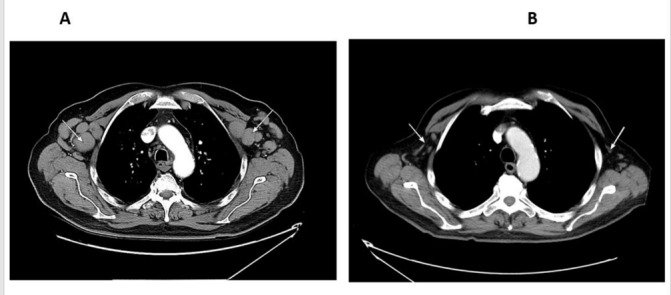
Contrast-enhanced CT scan of the chest with enlarged axillary lymph nodes before (A) and after (B) idelalisib, showing regression in size.
Figure 3.
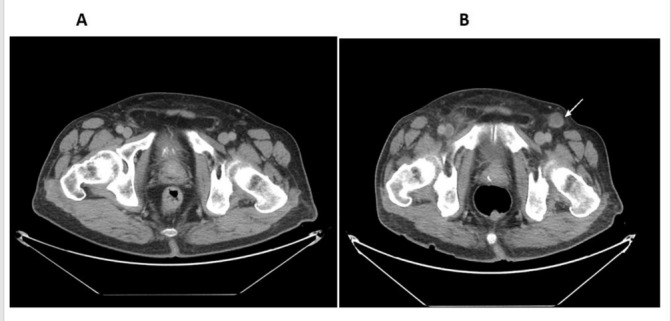
Contrast-enhanced CT scan of the pelvis with enlarged pelvic lymph nodes before (A) and after (B) idelalisib, showing regression in size.
Figure 4.
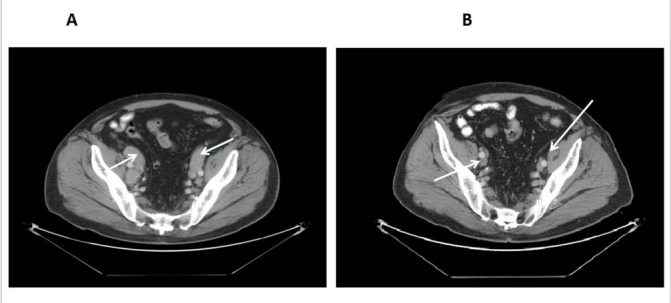
Contrast-enhanced CT scan of the pelvis with newly enlarged inguinal lymph node on idelalisib (B).
Figure 5.
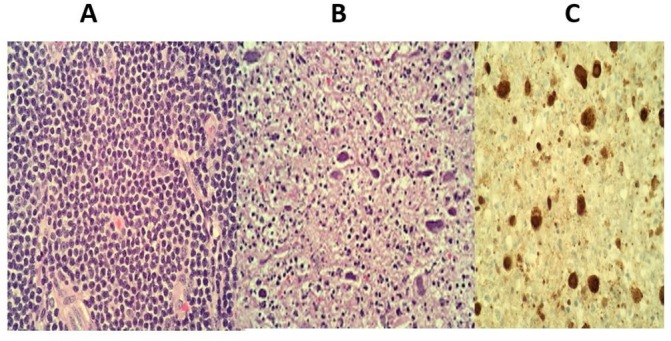
H&E of inguinal lymph node at 40× magnification in area of small lymphocytic lymphoma (A), necrotising acute inflammation and intranuclear viral inclusions (B), herpes simplex virus immunohistochemistry, 40× magnification demonstrating positive staining in the intranuclear Inclusions (C).
Outcome and follow-up
Patient tolerated the treatment well except for mild persistent fatigue. There was complete resolution of lymphadenopathy and he remains independent in his activities of daily living.
Discussion
Generalised lymphadenopathy in a patient with CLL/SLL can be secondary to disease relapse, coexisting conditions such as bacterial, viral, fungal, parasitic and spirochetal infections, immunological disorders or reactivation of autoimmune conditions like systemic lupus erythematosus, rheumatoid arthritis or Still’s disease.
Herpes adenitis is a rare manifestation of HSV and is seen most commonly in immunocompromised patients. It can present as a mucocutaneous or disseminated infection with generalised or localised lymphadenopathy. However, mucocutaneous infections remain the most common manifestation in both immunocompetent and immunocompromised hosts. The histopathological changes seen in the lymph nodes affected by herpes infection are characterised by discrete focal areas of necrosis in paracortical regions. HSV-1 and -2 viruses can be detected by immunohistochemistry and fluorescence in situ hybridisation studies in the affected tissue.
Higgins and Warnke from Stanford University emphasised on the rarity of the disease. Authors reported the largest series of five patients in the literature with CLL and herpes adenitis. The lymph nodes biopsied had histological and immunophenotypic features of HSV infection concurrent with CLL.2 One patient had history of cutaneous infection preceding the nodal enlargement. Four patients received no therapy for herpes at any time, whereas one was treated with intravenous and oral acyclovir. None of the patients developed a fulminant course of the disease. The authors observed that the clinical course of herpes adenitis may not be fulminant and may not always merit treatment.
Few other cases of herpes lymphadenitis have been reported in patients with haematological malignancies.3–6 Of these patients, three had CLL or SLL.3–5 Other associated malignancies were monocytoid B-cell lymphoma,4 acute myeloid leukaemia undergoing bone marrow transplantation and chronic myelogenous leukaemia.4 A case of concurrent herpes simplex lymphadenitis manifesting as rapidly enlarging bilateral cervical lymphadenopathy in a patient with mantle cell lymphoma has also been described.7
Studies have also described the mechanism for which the HSV-1 virus is allowed entry into the cell. HveA and HveC are cell surface proteins expressed on normal cells that are known to function as receptors that facilitate HSV-1 virus entry into the cells. HveA is a type I transmembrane receptor that belongs to the superfamily of tumour necrosis factor. The HSV-1 glycoprotein D has been shown to bind directly to HveA and HveC cell surface receptors.8 CLL B-cells have been found to express high levels of herpes virus entry mediator HveA, which facilitates the virus entry into the cells.9 Due to this mechanism, it is postulated that the HSV-1-derived vectors could be excellent vehicles for gene transfer into CLL B-cells and therein lies a potential use of gene therapy for this disease.8
Besides host-related factors, the use of purine analogues and monoclonal antibodies such as rituximab and alemtuzumab also predispose patients with CLL to several opportunistic infections. Bortezomib, a commonly used proteasome inhibitor for treatment of multiple myeloma and lymphoma, can also increase the risk of herpes zoster reactivation (6% to 11%), herpes simplex infection (1% to 3%), herpes zoster infection (1% to 2%), aspergillosis, bacteraemia, listeria and toxoplasmosis. Patients on bortezomib require antiviral prophylaxis while on treatment.
Byrd et al did a prospective study to assess the frequency of opportunistic infections in low-grade lymphoproliferative disorders treated with fludarabine. Study demonstrated that varicella zoster virus or HSV infections occur in approximately 57% of patients following treatment with fludarabine. The median time from starting fludarabine to the onset of infection was 8 (range 1–17) months and occurred following completion of therapy in 75% of patients.10 In the intergroup trial, the rate of infection was compared in patients on fludarabine and chlorambucil therapy. Fludarabine therapy was associated with an increased number of major infections and higher incidence of herpes virus infections compared with chlorambucil (P=0.008 and P=0.004, respectively).11 Patients on fludarabine-based chemotherapy are usually given antiviral prophylaxis, though the routine use of antiviral prophylaxis in fludarabine-treated patients needs to be studied prospectively. Multiple studies have recommended antiviral prophylaxis in the setting of low CD4 count in elderly patients treated with fludarabine.12 Idelalisib is a first-in-class selective oral, phosphatidylinositol 3-kinase delta (PI3Kδ) inhibitor approved for the treatment of several haematological malignancies including CLL/SLL.13 PI3Kδ is a protein that plays a role in the activation, proliferation and viability of B cells, a critical component of the immune system. PI3Kδ signalling is active in many B-cell leukaemia and lymphomas. By inhibiting this protein, idelalisib blocks several cellular signalling pathways that drive B-cell viability.14 Common adverse reactions reported are pneumonia, sepsis, febrile neutropenia, diarrhoea, nausea, fatigue, cough, dyspnoea and rash. Treatment-related laboratory abnormalities of this drug include elevations in alanine aminotransferase, aspartate aminotransferase, gamma-glutamyltransferase, absolute lymphocytes and triglycerides.13 Serious cases (some fatal) of Pneumocystis jirovecii pneumonia (PCP) and CMV have also been reported and PCP prophylaxis throughout the duration of idelalisib treatment is recommended. However, no clear association with HSV infection has been reported in the literature.
Our patient was treated with multiple lines of chemotherapy including alkylating agents and monoclonal antibodies. There was good clinical response to idelalisib with gross reduction in measurable disease at all sites. Enlarging pelvic and inguinal nodes noted on follow-up imaging prompted the excisional biopsy of the inguinal node. Since herpes adenitis was noted after initiation of idelalisib, we suspect this was the triggering agent. The patient was treated with valacyclovir and is currently doing well. Idelalisib was discontinued and the patient was transitioned to obinutuzumab (monoclonal CD20 antibody) 1000 mg/m2 intravenously once every 28 days and chlorambucil 24 mg orally once every 15 days along with supportive medications, for six cycles. To the best of our knowledge, there are no case reports of herpetic adenitis secondary to idelalisib reported in the literature thus far.
Learning points.
Lymph node involvement by herpes virus is usually seen in widespread fatal infections. It can rarely present as localised lymphadenitis in an immunocompromised host.
Though Richter’s transformation is the prime concern with rapidly enlarging lymph nodes in a patient of chronic lymphocytic leukaemia /small lymphocytic lymphoma, necrotising lymphadenitis should be considered in the differential diagnosis.
There are no clear guidelines in the literature to suggest that patients on long-term purine analogues should remain on lifelong antiviral therapy. There is evidence that herpes simplex and zoster infections can occur several months after treatment completion in more than 75% of the patients. Oncologists should consider lifelong antiviral prophylaxis in such patients.
Footnotes
Contributors: RS: Participated in patient care, prepared the initial draft of the case report, revised the manuscript critically, approved the version to be published and agreed to be accountable for all aspects of the work. DG: Examined the slides and made the pathological diagnosis, provided the key images, revised the manuscript critically, approved the version to be published and agreed to be accountable for all aspects of the work. MOJ: Primary oncologist, participated in patient care, revised and amended the manuscript critically, approved the version to be published and agreed to be accountable for all aspects of the work.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol 2006;33:240–9. 10.1053/j.seminoncol.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 2.Higgins JP, Warnke RA. Herpes lymphadenitis in association with chronic lymphocytic leukemia. Cancer 1999;86:1210–5. [DOI] [PubMed] [Google Scholar]
- 3.Pierre C, Jaubert D, Carloz E, et al. [Massive necrotizing adenitis complicating a disseminated herpes simplex virus 2 infection in chronic lymphoid leukemia]. Ann Pathol 1991;11:31. [PubMed] [Google Scholar]
- 4.Gaffey MJ, Ben-Ezra JM, Weiss LM. Herpes simplex lymphadenitis. Am J Clin Pathol 1991;95:709–14. 10.1093/ajcp/95.5.709 [DOI] [PubMed] [Google Scholar]
- 5.Epstein JI, Ambinder RF, Kuhajda FP, et al. Localized herpes simplex lymphadenitis. Am J Clin Pathol 1986;86:444–8. 10.1093/ajcp/86.4.444 [DOI] [PubMed] [Google Scholar]
- 6. Anon. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 45-1994. A 47-year-old man with inguinal lymphadenopathy and fever during preparation for a bone marrow transplant. N Engl J Med 1994;331:1703–9. 10.1056/NEJM199412223312508 [DOI] [PubMed] [Google Scholar]
- 7.Pilichowska ME, Smouse JH, Dorfman DM. Concurrent herpes simplex viral lymphadenitis and mantle cell lymphoma: a case report and review of the literature. Arch Pathol Lab Med 2006;130:536–9.doi:10.1043/1543-2165(2006)130[536:CHSVLA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 8.Eling DJ, Johnson PA, Sharma S, et al. Chronic lymphocytic leukemia B cells are highly sensitive to infection by herpes simplex virus-1 via herpesvirus-entry-mediator A. Gene Ther 2000;7:1210–6. 10.1038/sj.gt.3301241 [DOI] [PubMed] [Google Scholar]
- 9.Krummenacher C, Rux AH, Whitbeck JC, et al. The first immunoglobulin-like domain of HveC is sufficient to bind herpes simplex virus gD with full affinity, while the third domain is involved in oligomerization of HveC. J Virol 1999;73:8127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, McGrail LH, Hospenthal DR, et al. Herpes virus infections occur frequently following treatment with fludarabine: results of a prospective natural history study. Br J Haematol 1999;105:445–7. 10.1111/j.1365-2141.1999.01364.x [DOI] [PubMed] [Google Scholar]
- 11.Morrison VA, Rai KR, Peterson BL, et al. Impact of therapy with chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. J Clin Oncol 2001;19:3611–21. 10.1200/JCO.2001.19.16.3611 [DOI] [PubMed] [Google Scholar]
- 12.Shvidel L, Shtalrid M, Bairey O, et al. Conventional dose fludarabine-based regimens are effective but have excessive toxicity in elderly patients with refractory chronic lymphocytic leukemia. Leuk Lymphoma 2003;44:1947–50. 10.1080/1042819031000110991 [DOI] [PubMed] [Google Scholar]
- 13.Miller BW, Przepiorka D, de Claro RA, et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res 2015;21:1525–9. 10.1158/1078-0432.CCR-14-2522 [DOI] [PubMed] [Google Scholar]
- 14.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014;370:1008–18. 10.1056/NEJMoa1314583 [DOI] [PMC free article] [PubMed] [Google Scholar]


