Abstract
Increased vascular endothelial growth factor (VEGF) expression in osteosarcoma correlates with a poor outcome. We conducted a phase II trial to evaluate the feasibility and efficacy of combining bevacizumab, a monoclonal antibody against VEGF, with methotrexate, doxorubicin and cisplatin (MAP) in patients with localized osteosarcoma. Eligible patients received 2 courses of MAP chemotherapy before definitive surgery at week 10. Bevacizumab (15 mg/kg) was administered 3 days before starting chemotherapy then on day 1 of weeks 3 and 5 of chemotherapy. After surgery, patients received MAP for a total of 29 weeks; bevacizumab was added every 2 or 3 weeks on day 1 of chemotherapy at least 5 weeks after surgery. Group sequential monitoring rules were used to monitor for unacceptable bevacizumab-related targeted toxicity (grade 4 hypertension, proteinuria, or bleeding, grade 3 or 4 thrombosis/embolism, and grade 2–4 major wound complications). Thirty-one patients (median age 12.8 years) with localized osteosarcoma were enrolled. No unacceptable targeted toxicities were observed except for wound complications (9 minor and 6 major), which occurred in 15 patients; none required removal of prosthetic hardware or amputation. The estimated 4-year event-free survival (EFS) rate and overall survival rate were 57.5%±10.0% and 83.4%±7.8%, respectively. Eight (28%) of 29 evaluable patients had good histologic response (< 5% viable tumor) to preoperative chemotherapy. The addition of bevacizumab to MAP for localized osteosarcoma is feasible but frequent wound complications are encountered. The observed histologic response and EFS do not support further evaluation of bevacizumab in osteosarcoma.
Keywords: osteosarcoma, bevacizumab, chemotherapy, wound healing, phase II, survival
INTRODUCTION
Osteosarcoma is the most common malignant bone tumor in children and adolescents. Approximately 20% of patients will have clinically detectable metastatic disease, mostly to lung and bone, at the time of diagnosis.1 The survival for patients with newly-diagnosed localized osteosarcoma is approximately 70 percent compared to 30 percent for patients with metastatic disease.2–4 Therapy consists of aggressive surgery and multi-agent chemotherapy, which usually includes a standard regimen of high-dose methotrexate, doxorubicin, and cisplatin (MAP).4–7 The use of other drugs such as ifosfamide and interferon or the intensification of active drugs (e.g., doxorubicin) has not improved patient outcomes since the 1990’s.4,7–9 Thus, the outcome of patients with localized osteosarcoma appears to have reached a plateau over the past three decades with no added benefit from intensifying or adding new cytotoxic chemotherapy, underscoring the need for novel therapeutic strategies.
Vascular endothelial growth factor (VEGF) plays a key role in angiogenesis, which is essential for tumor growth and metastasis.10,11 Preclinical xenograft models demonstrate that targeting VEGF in osteosarcoma results in growth inhibition.12,13 Further, increased serum levels and protein expression of VEGF in tumor specimens have been correlated with worse outcome in patients with osteosarcoma, suggesting that VEGF is a potential therapeutic target in this tumor type.14–16 Bevacizumab, a monoclonal antibody against VEGF, combined with cytotoxic chemotherapy has significantly improved the outcomes in adults with a variety of cancers.17–19 The addition of bevacizumab not only inhibits angiogenesis, but also is thought to improve the delivery of chemotherapy by altering or “normalizing” the tumor vasculature and decreasing elevated interstitial pressure in tumors.20 Adverse events commonly related to bevacizumab as a single agent or in combination with chemotherapy have been limited to mild to moderate hypertension, proteinuria, bleeding, and thrombosis.21 Because neovascularization plays a critical role in wound healing, the potential for bevacizumab to affect post-operative healing is a concern that has not been systematically studied in patients undergoing resection of bone tumors.
Based on the rationale above, we wished to investigate whether targeting angiogenesis may be a novel therapeutic strategy for osteosarcoma. Therefore, we conducted a single arm, multi-institutional, open-label phase II trial (NCT00667342) of combining bevacizumab with MAP chemotherapy in patients with localized, resectable osteosarcoma. The MAP chemotherapy regimen was based on the standard of care treatment arm (Regimen A) of a large randomized study conducted through the Children’s Oncology Group, INT01337 in a similar patient population with newly diagnosed localized osteosarcoma. The study had two primary objectives. The first was to evaluate the feasibility of adding bevacizumab to the MAP chemotherapy. Feasibility was defined by the number of observed unacceptable toxicities related to bevacizumab, including hypertension, proteinuria, bleeding, and thrombosis and wound complications following definitive surgery. The second primary objective of the study was to compare the event-free survival (EFS) of patients with localized osteosarcoma treated with the combination of bevacizumab and MAP chemotherapy to the reported 3-year EFS of 71% for the 172 patients with localized osteosarcoma treated with MAP chemotherapy (Regimen A) on INT0133. Secondary analysis in this study included exploring the relationship between methotrexate clearance and bevacizumab exposure, correlation between imaging response and histologic tumor response and comparison of percent good histologic tumor (<5% viable tumor as defined in INT0031) in patients who received bevacizumab with MAP chemotherapy vs. MAP chemotherapy alone.
PATIENTS AND METHODS
Patients
Eligibility criteria included: age ≤30 years, newly-diagnosed histologically confirmed, high-grade, localized, resectable osteosarcoma, Karnofsky/Lansky performance score ≥50 (not disabled, requiring special care or assistance) or WHO/ECOG ≤2 (ambulatory and capable of all self-care; out of bed more than 50% of waking hours) and no previous chemotherapy or radiation therapy. Other organ-specific and prior therapy inclusion/exclusion criteria are provided in Appendix.
Written informed consent was obtained from patients, parents, or legal guardians, with assent as appropriate. The protocol was approved by the Institutional Review Board of each of the four institutions who participated in the study: St. Jude Children’s Research Hospital, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Rady Children’s Hospital, and MD Anderson Cancer Center. Clinical trials registration number: NCT0066734.
Treatment
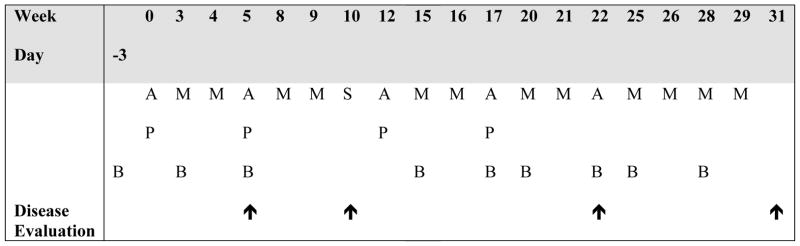
The treatment schema is shown in Figure 1. Bevacizumab was administered 3 days pior to the first dose of chemotherapy at week 0 in order to characterize the single-dose single agent pharmacokinetics of the drug in children22 and to assess the effect of bevacizumab alone on the tumor vasculature using dynamic contrast-enhanced MRI23, as previously reported. All subsequent doses of bevacizumab were given on day 1 of chemotherapy. Two weeks after definitive surgery for primary tumor removal, chemotherapy was resumed once adequate wound healing was observed. Due to concerns about potential effects of bevacizumab on wound healing, the study was designed such that bevacizumab was not administered for at least 5 weeks before and for at least 5 weeks after definitive surgery. Granulocyte colony-stimulating factor (either filgrastim or pegfilgrastim) was administered after each cycle of chemotherapy that included doxorubicin or cisplatin. At the time of definitive surgery, the bone resection margins were determined to be a minimum of 1.5 cm proximal (or distal) to the known extent of intraosseous (intracortical) involvement by the tumor as defined by the scanograms and MRI at the time of initial diagnosis.
Figure 1.
Treatment schema. Abbreviations: A, doxorubicin (25 mg/m2/day continuous infusion over 72 hours on days 1 through 3); P, cisplatin (60 mg/m2/day IV over 4 hours on day 1 and day 2); M, methotrexate (12 grams/m2 IV over 4 hours on day 1 followed by leucovorin rescue); B, bevacizumab (15 mg/kg IV on day -3 with first dose, then on day 1 prior to chemotherapy with subsequent doses; initial dose administered over 90 min, and subsequently, if tolerated, over 60 minutes and then over 30 minutes); S, definitive surgery;
 , disease evaluation
, disease evaluation
Evaluations, Toxicity Grading and Response Criteria
Evaluations at baseline, during and after completion of therapy are outlined in Appendix. All adverse events except for wound complications were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.24 Given the limitations of the CTCAE criteria for grading wound complications, we developed more comprehensive criteria for grading these adverse events. We categorized wound complications as minor or major based on whether the wound was superficial (above the fascia) or deep (below the fascia), respectively. Minor wound complications were graded as follows: Grade 1, epidermolysis or marginal wound necrosis requiring only routine wound care; Grade 2, wound dehiscence requiring advanced wound care including vacuum-assisted closure; Grade 3, wound infection/dehiscence treated conservatively including antibiotics; Grade 4, wound dehiscence requiring surgical closure; and Grade 5, wound infection/dehiscence requiring debridement and primary or secondary wound closure. Major wound complications were graded as follows: Grade 1, culture proven infection treated with antibiotics without surgical intervention; Grade 2, infection/dehiscence requiring debridement and primary or secondary wound closure; Grade 3, infection requiring removal of hardware with limb salvage; and Grade 4, infection requiring amputation.
Radiologic response of the primary tumor to neoadjuvant therapy (at weeks 5 and 10) was evaluated using three-dimensional (3D) volumetric measurements using an elliptical model (0.5 times the product of the three largest perpendicular diameters). Disease assessment was classified as: complete response (CR), complete disappearance of tumor; partial response (PR), at least 50% decrease in volume compared to the measurement obtained at study entry; progressive disease (PD), at least 25% increase in tumor volume compared to the smallest volume obtained since the beginning of therapy; and stable disease (SD), neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD taking as a reference the smallest disease volume since treatment started.
Tumor necrosis following preoperative chemotherapy was classified using a modified Huvos grading system7,25 as follows: Grade I, no response to therapy; Grade IIA, >50% viable tumor; Grade IIB, 5–50% viable tumor; Grade III, < 5% viable tumor; and Grade IV, no viable tumor.
Pharmacokinetics
Bevacizumab pharmacokinetic studies were performed at weeks 0, 3 and 5. The sampling strategy, sample analysis, and development of the population pharmacokinetic model for bevacizumab have been previously described.22 Bevacizumab concentration-time data were fit to a two-compartment model with first order elimination from the central compartment. Bevacizumab exposure [area under curve (AUC)] from the first dose (week 0) to time of methotrexate administration was estimated for each patient using a nonlinear mixed effects model in NONMEM (version 7.3; ICON solutions, Hanover, MD), a software package utilized for population pharmacokinetic modeling. Methotrexate pharmacokinetic studies were performed in all patients for clinical monitoring purposes with samples routinely collected at the end of infusion (~4 hr) and 24, 48, and 72 hours post infusion. A two-compartment model with first order elimination using ADAPT II (Biomedical Simulations Resource, University of Southern California) was fit to plasma methotrexate concentrations, which were analyzed by a fluorescence polarization immunoassay (TDx System; Abbott Laboratories, Abbott Park, IL). Methotrexate systemic clearance (CLt) was calculated based on the model-predicted elimination rate constant (ke) and volume of distribution of the central compartment (Vc).
Study Design and Statistical Methods
A historical control design was adopted to assess the effect EFS of adding bevacizumab to MAP chemotherapy, where the MAP chemotherapy arm of the INT0133 study7 served as the historical control. The sample size was determined to increase 3-year EFS from 70% to 85% based on the method of Dixon and Simon26 with one-sided significance level 5% and power of 80%. EFS was defined as the interval from the date on study to the date of first event (relapse or progressive disease, second malignancy, death from any cause) or last follow-up. Overall survival (OS) was defined as the interval from the date on study to the date of death from any cause or last follow-up. EFS and OS were estimated using the method of Kaplan and Meier. Group sequential stopping rule was used for monitoring unacceptable toxicities related to the addition of bevacizumab to MAP chemotherapy. Unacceptable toxicities were defined as grade 4 hypertension, proteinuria, or bleeding, grade 3 or 4 thrombosis/embolism (excluding catheter-related thrombosis) and grade 2, 3 or 4 major wound complications. The study closed early due to slow accrual.
Fisher’s exact test was used to assess association between imaging response and histologic response. The Wilcoxon rank sum test was used to compare therapy completion times between patients with and without wound complications. Logistic regression was used to examine the association between the presence or absence of a wound complication with time to complete therapy. For the purpose of this analysis, the time to complete therapy was the date of the last dose of chemotherapy. Cox regression was used to assess associations between outcome, development of wound complication, and time to complete therapy. To investigate the effect of bevacizumab on methotrexate disposition, the cumulative bevacizumab exposure prior to methotrexate administration for each course was estimated and analyzed with respect to the methotrexate CLt.
RESULTS
Patient Characteristics
Between June 2008 and May 2012, 31 patients were enrolled. Patient characteristics are summarized in Table 1 and Appendix (Table 1). Surgical resection of the primary tumor was performed after planned chemotherapy in 29 patients. The type of surgery performed included limb-salvage surgery (i.e., en-bloc resection followed by reconstruction with endoprosthesis, allograft, allograft prosthetic composite) (N=20), amputation (N=8), and hemimandibulectomy (N=1). Two patients did not undergo surgery at week 10 because of progressive disease at week 5 in one patient and patient refusal in the other.
Table 1.
Summary of characteristics of 31 patients with localized osteosarcoma enrolled on the study
| Characteristic | No. of Patients |
|---|---|
| Age at Study Enrollment (years) | |
| Median | 12.8 |
| Range | 6.8 – 20.3 |
| Sex | |
| Female | 15 |
| Male | 16 |
| Race | |
| White | 17 |
| Black | 11 |
| Other | 2 |
| Unknown | 1 |
| Primary Tumor Site | |
| Femur | 15 |
| Tibia | 9 |
| Humerus | 3 |
| Fibula | 1 |
| Radius | 1 |
| Pelvis | 1 |
| Mandible | 1 |
Toxicity
The most common grade 3 or 4 toxicities were those typically observed with MAP chemotherapy, namely elevated liver transaminases and myelosuppression (Table 2). No unacceptable non-wound related targeted toxicities [i.e., grade 4 hypertension, proteinuria, or bleeding, and grade 3 or 4 thrombosis/embolism (excluding catheter-related thrombosis)] related to bevacizumab were observed. Common grade 1 or 2 toxicities during therapy that may be attributed to bevacizumab included epistaxis (74% of patients), proteinuria (68%), hypertension (39%), and left ventricular dysfunction (13%). With the exception of fever in one patient during the first infusion of bevacizumab, no other infusion-related side effects were observed. None of the interim stopping boundaries for toxicity were met, and there were no deaths due to toxicity. Growth plate abnormalities as assessed by radiograph of the unaffected knee were not observed.
Table 2.
Grade 3 and 4 adverse eventsa possibly, probably and likely related to therapy observed in >1% of 462 cycles of chemotherapy cycles administered, excluding wound complications
| Adverse Event | Grade 3 | Grade 4 | ||
|---|---|---|---|---|
| No. of Cycles (% of Total Cycles) | No. of Patients With Toxicity | No. of Cycles (% of Total Cycles) | No. of Patients With Toxicity | |
| Hematologic | ||||
| Anemia | 77 (20) | 26 | - | - |
| Leukopenia | 70 (16) | 25 | 34 (8) | 17 |
| Neutropenia | 66 (15) | 24 | 106 (23) | 30 |
| Thrombocytopenia | 54 (13) | 24 | 61 (15) | 24 |
| Metabolic | ||||
| Elevated AST | 120 (29) | 26 | 49 (11) | 19 |
| Elevated ALT | 123 (29) | 29 | 63 (14) | 20 |
| Hypokalemia | 27 (7) | 14 | - | - |
| Hypophosphatemia | 25 (6) | 17 | - | - |
| Elevated GGT | 11 (2) | 2 | - | - |
| Hyponatremia | 8 (2) | 6 | - | - |
| Hyperglycemia | 7 (1) | 6 | - | - |
| Constitutional | ||||
| Weight loss | 8 (2) | 3 | - | - |
| Gastrointestinal | ||||
| Vomiting | 17 (4) | 12 | - | - |
| Anorexia | 16 (3.5) | 10 | - | - |
| Mucositis/stomatitis | 31 (7) | 15 | - | - |
| Nausea | 13 (3) | 7 | - | - |
| Dehydration | 5 (1) | 5 | - | - |
| Infection | ||||
| Febrile neutropenia | 35 (7) | 20 | - | - |
Adverse events grading according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase.
The planned interval to resume chemotherapy and bevacizumab after surgery (week 10) as set by protocol was 14 days and 35 days, respectively (Figure 1). The actual median interval to resume chemotherapy (N=27) and bevacizumab (N=22) after surgery was 16 days (range, 13–51 days) and 40 days (range, 34–151 days), respectively. Fifteen patients (15/29, 52%) had wound complications, 9 minor and 6 major, following definitive surgery (Table 3). No patient experienced significant bleeding or thrombosis nor did any patient require revision of the prosthesis or amputation during the postoperative period. Although protocol specified stopping rules for wound complications were not met, poor wound healing prohibited resuming bevacizumab in 5 patients and required holding at least one of the six planned doses in 10 patients after definitive surgery. Wound complications/delayed wound healing was the primary reason for omitting 52 (21.3%) of the 244 planned doses of bevacizumab. Other reasons for omitting bevacizumab doses included thoracotomy (2 doses) and bone fracture (1 dose).
Table 3.
Minor and major wound complicationsa following definitive surgery (limb sparing, amputation or hemimandibulectomy) among 29 patients
| No. of Patients with Complicationb | ||||
|---|---|---|---|---|
| Complication Severity | All Surgery Types (29 Patients) | Limb Sparing (20 Patients) | Amputation (8 Patients) | Other (1 Patientb) |
| Minor | ||||
| Grade 1 | - | - | - | - |
| Grade 2 | 2 | 1 | 1 | - |
| Grade 3 | 3 | 2 | 1 | - |
| Grade 4 | - | - | - | - |
| Grade 5 | 4 | 3 | - | 1 |
| All Grades | 9 (31%) | 6 (30%) | 2 (25%) | 1 |
| Major | ||||
| Grade 1 | - | - | - | - |
| Grade 2 | 6 | 4 | 2 | |
| Grade 3 | - | - | - | - |
| Grade 4 | - | - | - | - |
| All Grades | 6 (21%) | 4 (20%) | 2 (25%) | - |
Highest grade per patient
Hemimandibulectomy
Response and Outcome
Two patients were excluded from the assessment of histologic response: the patient who refused surgery at the protocol-specified time point and another patient who had a cystic lesion that was fluid filled in the center and had viable tumor cells at the rim. Assessment of histologic response in the latter patient was felt to be inaccurate. Of the remaining 29 patients, 8 (28%) had a good histologic response (Huvos Grade III, < 5% viable tumor) to preoperative chemotherapy.
Of the 28 patients who had an MRI of the primary tumor to assess tumor imaging response at week 5, 27 had SD and 1 had PD. Of the 30 patients who underwent an MRI at week 10, 4 had PR, 25 had SD, and 1 was not evaluable because of a pathologic fracture that had developed on therapy. The association between histologic response and imaging response at week 10 approached but did not reach statistical significance (p = 0.0646).
The median duration of follow-up from the time of study enrollment was 4.8 years (range, 1.7 years–7.0 years) for the 24 survivors. All survivors were seen or contacted within 18.4 months from the time of analysis. Thirteen patients have experienced recurrence or progression of disease as a first event (1 local, 11 distant, and 1 local and distant); 6 of these patients died of disease. One additional patient developed a second malignancy (acute myeloid leukemia) and subsequently died. Six patients were removed from protocol therapy for reasons other than recurrence or progressive disease; three because the treating physician determined that it was in the best interest of the patient, one for non-adherence to protocol therapy (refused surgery at week 10), one because of family/patient request, and one due to development of second malignancy. The 4-year estimates for EFS and OS for all patients were 57.5%±10.0% and 83.4%±7.8%, respectively.
We assessed whether wound complications after definitive surgery resulted in delay in therapy and whether wound complications (minor, major or both) had an impact on EFS and OS. Using logistic regression analysis, any wound complication (major and minor) after surgery was significantly associated with longer time to complete therapy [median 274 days (range, 235– 331 days) with wound complications vs. median 240 days (range, 84–278 days) without wound complication (P=0.0118)]. However, the development of wound complications after definitive surgery did not have a significant effect on EFS (P=0.11, hazard ratio=2.66). Hazard ratio for OS could not be determined because no deaths were observed in patients who developed wound complications. The 4-year EFS estimates for patients without and with wound complications were 47.6%±14.1% and 73.3%±12.0%, respectively (P=0.0964, log-rank test). The 4-year OS estimates for patients without and with wound complications were 69.6%±12.8% and 100%±0.0%, respectively (P=0.0042, log rank test).
Pharmacokinetics
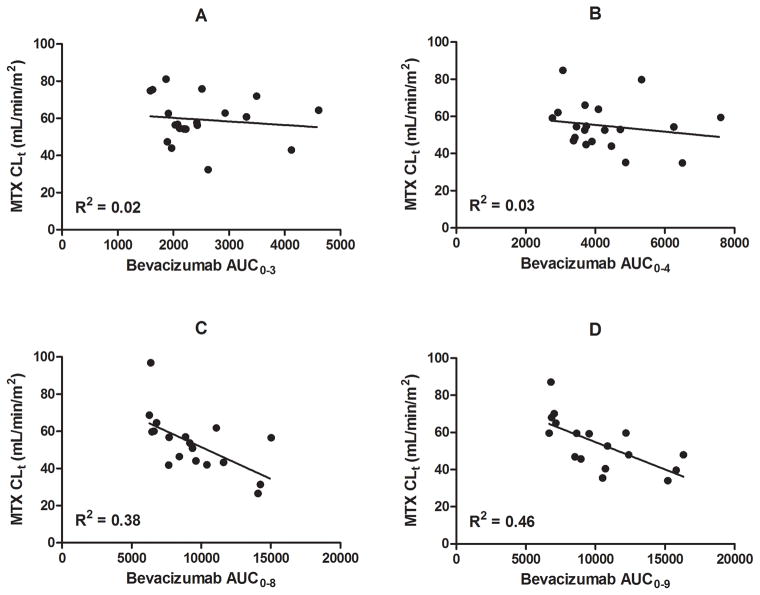
Serial bevacizumab and methotrexate pharmacokinetic studies were performed in 20 patients. A summary of the bevacizumab exposures and methotrexate clearance values by treatment week is provided in Table 4. Cumulative bevacizumab exposures at weeks 3 and 4 were not significantly correlated with methotrexate CLt at weeks 3 and 4 (Figure 2A and 2B). However, cumulative bevacizumab exposures at weeks 8 and 9 were negatively correlated with methotrexate CLt at weeks 8 (R2=0.38) and 9 (R2=0.46) (Figure 2C and 2D).
Table 4.
Summary of bevacizumab exposure (AUC) and methotrexate clearance (MTX CLt) by treatment week
| Bevacizumab AUC (μg/mL*day) | MTX CLt (mL/min/m2) | |||
|---|---|---|---|---|
| Week | Median | Range | Median | Range |
| 3 | 2225 | 1586 – 4609 | 57.2 | 32.4 – 81.1 |
| 4 | 3821 | 2764 – 7601 | 53.7 | 34.9 – 84.8 |
| 8 | 9030 | 6274 – 15026 | 55.1 | 26.5 – 96.9 |
| 9 | 9550 | 6667 – 16330 | 50.4 | 34.0 – 87.1 |
Figure 2.
Methotrexate CLt vs. bevacizumab exposure (AUC) at (A) week 3, (B) week 4, (C) week 8, and (D) week 9. The solid line represents a linear regression and the R2 value is listed in the bottom left of each plot. There was no significant correlation between bevacizumab cumulative exposures and methotrexate CLt at weeks 3 (A) and 4 (B); however, at weeks 8 (C) and 9 (D), there was a significant negative correlation between methotrexate CLt and bevacizumab systemic exposure.
DISCUSSION
This clinical trial investigated the feasibility and safety of bevacizumab in combination with standard MAP chemotherapy in newly diagnosed patients with localized, resectable high- grade osteosarcoma. The observed bevacizumab-related systemic toxicities were relatively mild in severity, but major and minor wound healing complications were seen in approximately half of the patients following definitive surgery and resulted in discontinuation or omission of 21% of the planned doses of bevacizumab. No grade 4 hypertension, proteinuria, or bleeding, nor grade 3 or 4 thrombosis/embolism potentially related to the addition of bevacizumab were observed.
Wound healing is a complex process27 that includes a neovascularization phase similar to the one that supports tumor growth with VEGF and VEGF receptors as key regulators.28–30 Issues related to wound healing have been a concern in patients receiving bevacizumab for several tumor types with variable recommendations for patients undergoing surgery.31–34 However, the most prominent effect of bevacizumab on wound healing seems to occur when it is administered during or prior to surgery. This is the basis for the recommendation in the prescribing label of bevacizumab to discontinue bevacizumab at least 28 days prior to elective surgery and to resume bevacizumab at least 28 days post surgery, once the surgical wound is fully healed. A novel finding from the present study that we previously reported is that bevacizumab exposure (i.e., area under the concentration-time curve, AUC) prior to surgery was associated with increased risk of major wound healing complications after surgery.22
Our study is the first to explore the incidence of wound healing complications after limb salvage or amputation in patients receiving bevacizumab and chemotherapy. In planning the study, we realized that the CTC grading criteria were inadequate to properly define wound complications. Furthermore, the rate of wound complications in patients undergoing these procedures who are receiving chemotherapy alone is difficult to discern from the literature. Therefore, we established a comprehensive grading system and retrospectively applied this grading system to our preceding clinical trial (OS99).35 This allowed us to establish an estimate of an expected rate of wound healing complications for developing the stopping rules as outlined. The stopping rules were not met; however, there are limitations to this approach. First, the prevalence of wound healing complications in OS99 was determined retrospectively. Second, the chemotherapy regimen used in the OS99 trial (ifosfamide, doxorubicin, and carboplatin) was different from the MAP chemotherapy used in the present trial and could potentially affect the incidence of wound healing. Third, our stopping rules did not take into consideration the impact of delayed wound healing on the dose intensity of bevacizumab and ability to deliver chemotherapy on time. In our study, patients with wound healing complications had a longer time to complete therapy than those without wound complications; although, their outcomes were not significantly worse than those without wound complications. In fact, our data actually shows a trend toward a better outcome in patients with wound complications. This latter finding is intriguing but should be interpreted with caution due to the small number of patients.
The addition of bevacizumab did not improve the percentage of patients with good histologic response to pre-operative chemotherapy, a strong predictor of outcome in osteosarcoma.7,36,37 The observed good histologic response (< 5% viable tumor) to two courses of MAP chemotherapy alone was 43% in the INT0133 study,7 vs. 27% to two courses of MAP with bevacizumab in our trial. Further, compared to outcomes reported for localized osteosarcoma, no apparent improvement in EFS or OS was observed with the addition of bevacizumab to MAP chemotherapy in our small cohort of patients.4,6,35 Other efforts to improve patient outcomes have been largely unsuccessful.7–9,38 It remains to be seen whether novel therapeutic approaches such as immunotherapy will be beneficial in this population.38
Consistent with prior reports, we found no significant correlation between 3D anatomic measurements of primary tumor on MRI and histologic response 39–41. This is because the extent of MR signal abnormality in the affected bone typically represents a combination of viable tumor, partially treated tumor, necrosis and bland edema. Furthermore the signal abnormality has irregular contours that cannot be accurately measured by simple 1D, 2D or 3D measurements. This finding underscores the urgent need for more robust methods of assessing bone tumor response to neoadjuvant therapy. Metabolic imaging modalities such as dynamic enhanced MRI and positron emission tomography may provide more accurate measurements of tumor response.23
The primary mechanism of elimination of methotrexate is by the kidney. Because VEGF targeting agents may cause renal vascular endothelial dysfunction and glomerular epithelial cell dysregulation,42 we sought to investigate the effect of bevacizumab on renal clearance of methotrexate (CLt) by analyzing the effect of cumulative bevacizumab exposure effect on methotrexate pharmacokinetics. We found no correlation between bevacizumab cumulative exposures and methotrexate CLt at weeks 3 and 4 (Figures 2A and 2B). This suggests no immediate or acute effect of bevacizumab on methotrexate disposition since bevacizumab is given immediately prior to methotrexate on week 3. Moreover, since we did not observe an effect on week 4 methotrexate clearance, it is unlikely that a delayed effect will be observed after a dose of bevacizumab. However, after the third bevacizumab dose, the methotrexate clearances decreased in relation to the bevacizumab systemic exposure (Figures 2C and 2D). This suggests patients with greater overall bevacizumab exposures are at risk for lower methotrexate CLt. However, after the third dose of bevacizumab, our patients received additional doses of nephrotoxic chemotherapy (i.e, cisplatin), which has been previously shown to decrease methotrexate CLt.43 Therefore, the observed decrease in methotrexate CLt at weeks 8 and 9 may be attributed to a combination of factors including bevacizumab exposures suggesting additional care should be taken when administering bevacizumab in these clinical situations.
In summary, the addition of bevacizumab to MAP chemotherapy was tolerated with no additional toxicity except for frequent wound complications requiring debridement and primary or secondary wound closure. In addition, this combination did not appear to improve histologic response or outcome for patients with localized osteosarcoma. Although limited by the small number of patients, our findings do not suggest that further evaluation of bevacizumab in patients with osteosarcoma is warranted. Our study highlights that careful monitoring of wound healing complications is needed when considering other antiangiogenic agents (e.g., tyrosine kinase inhibitors such as sorafenib44) for osteosarcoma treatment.
Novelty and Impact.
The outcome of patients with osteosarcoma has reached a plateau. Based on the rationale that the vascular endothelial growth factor (VEGF) is expressed in osteosarcoma and increased VEGF levels in patients is associated with a poor outcome, we added bevacizumab to standard osteosarcoma chemotherapy. Bevacizumab-related toxicities were relatively mild in severity; however, wound-healing complications were encountered frequently. Importantly, the addition of bevacizumab did not improve histologic tumor response or survival outcomes. The 4-year estimates for EFS and OS for this study for all patients were 57.5%±10.0% and 83.4%±7.8%, respectively. However, the study provides important data that informs future studies of antiangiogenic agents where an integral part of the treatment requires surgical resection of a primary bone tumor.
Acknowledgments
This study was supported in part by Cancer Center Support CORE Grant P30 CA 21765 from the National Cancer Institute, the American Lebanese Syrian Associated Charities, and Genentech, Inc.
The authors would like to thank all the patients who participated in this study and their families and the research staff who assisted with the conduct of this study.
Abbreviations
- AUC
area under the curve
- CLt
systemic clearance
- CR
complete response
- CTCAE
common terminology criteria for adverse events
- EFS
event-free survival
- Ke
elimination rate constant
- MAP
methotrexate, doxorubicin, cisplatin
- MRI
magnetic resonance imaging
- PD
progressive disease
- PR
partial response
- SD
stable disease
- OS
overall survival
- Vc
volume of distribution central compartment
- VEGF
vascular endothelial growth factor
- WHO/ECOG
World Health Organization/Eastern Cooperative Oncology Group
- 3D
three-dimensional
Appendix
Eligibility Criteria
Laboratory criteria for enrollment included an absolute neutrophil count (ANC) ≥ 1000/m3, a platelet count ≥ 100,000/m3, total bilirubin < 1.5 × upper limit of normal (ULN) for age, PT/PTT ≤ 1.2 × ULN, GFR ≥ 70 ml/min/1.73 m2 or a normal serum creatinine for age, and urine protein less than 2+ or urine protein:creatinine ratio ≤ 1. Normal cardiac function by echocardiography or by radionuclide study, and good control of hypertension (if present) for at least 2 weeks were required for study entry. Female participants of childbearing age had to have a negative pregnancy test and not be breast-feeding. Exclusion criteria included osteosarcoma or MFH of bone as a second malignancy; a major surgical procedure or significant traumatic injury within 28 days of study entry; a history of deep venous or arterial thrombosis within 6 months of study entry; history of arterial thromboembolic events, myocardial infarction, severe or unstable angina, severe peripheral vascular disease, hypertensive crisis, or hypertensive encephalopathy; known bleeding diathesis, platelet disorder, thrombophilic condition, or coagulopathy; known hypersensitivity to Chinese hamster ovary cell products or other recombinant human antibodies; a serious non-healing wound, ulcer, or bone fracture (other than pathologic fracture); a history of abdominal fistula, gastrointestinal perforation or intra-abdominal abscess within 6 months prior to study entry; and known central nervous system disease.
Pretreatment, On-study and Follow-up Evaluations
Standard laboratory tests to assess toxicity, including complete blood counts, serum chemistries, and urinalysis, were obtained at baseline and at regular intervals during and after completion of therapy. Coagulation screening with PT, PTT, fibrinogen and D-dimer assay were obtained at baseline. Growth plates of patients without skeletal maturity were assessed with a radiograph of the unaffected knee at baseline, week 17, end of therapy, and yearly thereafter. An audiogram was obtained prior to each cycle of cisplatin and an ECHO/EKG prior to each cycle of doxorubicin. The initial staging workup comprised a magnetic resonance imaging (MRI) and plain radiographs of the primary tumor, technetium 99 methylenediphosphonate bone scan, computed tomography (CT) of the chest, and [18F]fluorodeoxyglucose positron emission tomography CT. These studies were repeated at week 5 and week 10 (before definitive surgery) and without MRI at end of therapy. CT chest and radiographs of the primary site were obtained at week 22. After completion of therapy, the patients were monitored with CT chest, bone scan, and plain radiographs of the primary site for disease recurrence at protocol-specified time points for 5 years.
Appendix Table 1.
Patient Characteristics and Outcome
| Patient Number |
Gender/ Race |
Age at Diagnosis (Years) |
Primary Site |
Surgery Type | Percent Tumor Necrosis |
Category and Grade Wound Complication after Surgery |
Months from diagnosis to Relapse or Other Event |
Outcome, mo. from diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | M/Mx | 13.8 | Tibia | Limb Salvage | 90 | Minor 2 | 11.6, Relapse | AWR, 84 |
| 2 | M/W | 10.8 | Humerus | Amputation | >90 | – | 15.2, Relapse | DOD, 54 |
| 3 | F/B | 6.8 | Humerus | Amputation | 50 | – | 17.1, Relapse | DOD, 34 |
| 4 | M/B | 14.4 | Femur | Limb Salvage | 73 | – | – | A, 82 |
| 5 | M/W | 10.9 | Tibia | Amputation | >95 | – | – | A, 77 |
| 6 | F/B | 8.2 | Humerus | Amputation | 61 | – | 2.8, Event1 | DOD, 17 |
| 7 | F/W | 14.2 | Femur | Limb Salvage | >95 | Minor 5 | – | A, 73 |
| 8 | F/W | 8.2 | Femur | Limb Salvage | 90 | Minor 5 | – | A, 69 |
| 9 | M/B | 10.6 | Fibula | Resection | >99 | Major 2 | – | A, 70 |
| 10 | M/B | 12.2 | Femur | Limb Salvage | 92 | Major 3 | – | A, 69 |
| 11 | M/W | 7.3 | Femur | Limb Salvage | 85 | – | 50.2, Event2 | DND, 65 |
| 12 | F/W | 14.3 | Tibia | Limb Salvage | 96 | Major 3 | – | A, 61 |
| 13 | F/W | 16.3 | Tibia | Limb Salvage | >95 | Minor 3 | – | A, 68 |
| 14 | F/B | 10.0 | Femur | NE | 0 | – | 3.9, Event3 | DOD, 27 |
| 15 | M/W | 15.1 | Tibia | Limb Salvage | 50 | – | 3.8, Event1 | A, 61 |
| 16 | F/W | 11.0 | Femur | Limb Salvage | 70 | – | – | A, 60 |
| 17 | F/Mx | 14.7 | Femur | Limb Salvage | 65 | – | 22.6, relapse | AWR, 61 |
| 18 | F/W | 12.2 | Femur | Limb Salvage | 85 | Minor 5 | 16.7, relapse | AWR, 54 |
| 19 | F/W | 10.0 | Tibia | Amputation | 95 | Minor 2 | – | A, 51 |
| 20 | F/B | 12.8 | Mandible | Hemimandibulectomy | 10 | Minor 5 | – | A, 49 |
| 21 | M/B | 17.3 | Tibia | Limb Salvage | 36 | – | 8.6, relapse | DOD, 21 |
| 22 | M/W | 17.0 | Femur | Limb Salvage | >96 | – | – | A, 46 |
| 23 | M/B | 9.1 | Femur | Limb sparing | 50 | – | 1.4, PD | AWR, 41 |
| 24 | M/W | 15.7 | Radius | Amputation | Ind | Minor 4 | – | A, 40 |
| 25 | F/B | 14.2 | Femur | Limb Salvage | >95 | Major 2 | 25, relapse | DOD, 51 |
| 26 | F/B | 11.1 | Tibia | Limb Salvage | 90 | Minor 3 | – | A, 46 |
| 27 | M/W | 20.2 | Pelvis | Amputation, Hemipelvectomy | 10 | Major 2 | 20, relapse | AWR, 46 |
| 28 | M/W | 16.4 | Femur | Limb Salvage | 75 | – | 21, relapse | AWR, 58 |
| 29 | M/W | 13.6 | Femur | Limb Salvage | 35 | – | 8.4, Event4 | DOD, 37 |
| 30 | M/W | 9.6 | Tibia | Amputation | >95 | Major 2 | – | A, 57 |
| 31 | F/U | 17.4 | Femur | Limb Salvage | 75 | – | 5.1, Event1 | A, 20 |
Abbreviations: M, male; F, female; W, while; B, black; U, unknown; Mx, mixed; DOD, dead of disease; A, alive with no evidence of disease; AWR, Alive with relapse; DND, dead with no disease; Ind, indeterminate; PD, progressive disease.
Off therapy because physician determined best interest of patient
Off study for secondary malignancy (acute myelogenous leukemia)
Off therapy due to non-adherence to protocol
Off therapy per parent request
Footnotes
DISCLOSURES:
The authors have declared no conflict of interest.
References
- 1.Gurney JG, Swensen AR, Bulterys M. Malignant Bone Tumors. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: 1999. pp. 99–110. [Google Scholar]
- 2.Daw NC, Billups CA, Rodriguez-Galindo C, McCarville MB, Rao BN, Cain AM, Jenkins JJ, Neel MD, Meyer WH. Metastatic osteosarcoma. Cancer. 2006;106:403–12. doi: 10.1002/cncr.21626. [DOI] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jurgens H, Gadner H, Bielack SS Cooperative German-Austrian-Swiss Osteosarcoma Study G. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–8. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 4.Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM, Harris M, Kleinerman E, Link MP, Nadel H, Nieder M, Siegal GP, Weiner MA, Wells RJ, Womer RB, Grier HE. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, Graf N, Heise U, Jurgens H, Kotz R, Salzer-Kuntschik M, Weinel P, Werner M, Winkler K. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–9. doi: 10.1023/a:1008391103132. [DOI] [PubMed] [Google Scholar]
- 6.Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, Helman LJ, Grier HE, Link MP. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–80. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 7.Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W, Gebhardt M, Goorin AM, Harris MB, Healey J, Huvos A, Link M, Montebello J, Nadel H, Nieder M, Sato J, Siegal G, Weiner M, Wells R, Wold L, Womer R, Grier H. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23:2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz CL, Wexler LH, Krailo MD, Teot LA, Devidas M, Steinherz LJ, Goorin AM, Gebhardt MC, Healey JH, Sato JK, Meyers PA, Grier HE, Bernstein ML, Lipshultz SE. Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children’s Oncology Group. Pediatr Blood Cancer. 2016;63:54–61. doi: 10.1002/pbc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Papai Z, Meyer J, Nadel H, Randall RL, Deffenbaugh C, Nagarajan R, Brennan B, Letson GD, Teot LA, Goorin A, Baumhoer D, Kager L, Werner M, Lau CC, Sundby Hall K, Gelderblom H, Meyers P, Gorlick R, Windhager R, Helmke K, Eriksson M, Hoogerbrugge PM, Schomberg P, Tunn PU, Kuhne T, Jurgens H, van den Berg H, Bohling T, Picton S, Renard M, Reichardt P, Gerss J, Butterfass-Bahloul T, Morris C, Hogendoorn PC, Seddon B, Calaminus G, Michelagnoli M, Dhooge C, Sydes MR, Bernstein M investigators E. Methotrexate, Doxorubicin, and Cisplatin (MAP) Plus Maintenance Pegylated Interferon Alfa-2b Versus MAP Alone in Patients With Resectable High-Grade Osteosarcoma and Good Histologic Response to Preoperative MAP: First Results of the EURAMOS-1 Good Response Randomized Controlled Trial. J Clin Oncol. 2015;33:2279–87. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 11.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 12.Scharf VF, Farese JP, Coomer AR, Milner RJ, Taylor DP, Salute ME, Chang MN, Neal D, Siemann DW. Effect of bevacizumab on angiogenesis and growth of canine osteosarcoma cells xenografted in athymic mice. Am J Vet Res. 2013;74:771–8. doi: 10.2460/ajvr.74.5.771. [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZX, Li X, Liu WD, Liu XZ, Wu SJ, Hu XH. Inhibition of Growth and Metastasis of Tumor in Nude Mice after Intraperitoneal Injection of Bevacizumab. Orthop Surg. 2016;8:234–40. doi: 10.1111/os.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaya M, Wada T, Kawaguchi S, Nagoya S, Yamashita T, Abe Y, Hiraga H, Isu K, Shindoh M, Higashino F, Okada F, Tada M, Yamawaki S, Ishii S. Increased pre-therapeutic serum vascular endothelial growth factor in patients with early clinical relapse of osteosarcoma. Br J Cancer. 2002;86:864–9. doi: 10.1038/sj.bjc.6600201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YH, Tokunaga T, Oshika Y, Suto R, Yanagisawa K, Tomisawa M, Fukuda H, Nakano H, Abe S, Tateishi A, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y, Nakamura M. Cell-retained isoforms of vascular endothelial growth factor (VEGF) are correlated with poor prognosis in osteosarcoma. Eur J Cancer. 1999;35:1089–93. doi: 10.1016/s0959-8049(99)00073-8. [DOI] [PubMed] [Google Scholar]
- 16.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–60. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 17.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–12. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 18.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–8. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 21.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(Suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 22.Turner DC, Navid F, Daw NC, Mao S, Wu J, Santana VM, Neel M, Rao B, Willert JR, Loeb DM, Harstead KE, Throm SL, Freeman BB, 3rd, Stewart CF. Population pharmacokinetics of bevacizumab in children with osteosarcoma: implications for dosing. Clin Cancer Res. 2014;20:2783–92. doi: 10.1158/1078-0432.CCR-13-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Glass JO, McCarville MB, Shulkin BL, Daryani VM, Stewart CF, Wu J, Mao S, Dwek JR, Fayad LM, Madewell JE, Navid F, Daw NC, Reddick WE. Assessing vascular effects of adding bevacizumab to neoadjuvant chemotherapy in osteosarcoma using DCE-MRI. Br J Cancer. 2015;113:1282–8. doi: 10.1038/bjc.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf
- 25.Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 26.Dixon DO, Simon R. Sample size considerations for studies comparing survival curves using historical controls. J Clin Epidemiol. 1988;41:1209–13. doi: 10.1016/0895-4356(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 27.Ko J, Ross J, Awad H, Hurwitz H, Klitzman B. The effects of ZD6474, an inhibitor of VEGF signaling, on cutaneous wound healing in mice. J Surg Res. 2005;129:251–9. doi: 10.1016/j.jss.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–9. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar I, Staton CA, Cross SS, Reed MW, Brown NJ. Angiogenesis, vascular endothelial growth factor and its receptors in human surgical wounds. Br J Surg. 2009;96:1484–91. doi: 10.1002/bjs.6778. [DOI] [PubMed] [Google Scholar]
- 30.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–52. [PMC free article] [PubMed] [Google Scholar]
- 31.D’Angelica M, Kornprat P, Gonen M, Chung KY, Jarnagin WR, DeMatteo RP, Fong Y, Kemeny N, Blumgart LH, Saltz LB. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: a matched case-control study. Ann Surg Oncol. 2007;14:759–65. doi: 10.1245/s10434-006-9074-0. [DOI] [PubMed] [Google Scholar]
- 32.Gordon CR, Rojavin Y, Patel M, Zins JE, Grana G, Kann B, Simons R, Atabek U. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg. 2009;62:707–9. doi: 10.1097/SAP.0b013e3181828141. [DOI] [PubMed] [Google Scholar]
- 33.Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar S, Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–80. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 34.Hompes D, Ruers T. Review: incidence and clinical significance of Bevacizumab-related non-surgical and surgical serious adverse events in metastatic colorectal cancer. Eur J Surg Oncol. 2011;37:737–46. doi: 10.1016/j.ejso.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Daw NC, Neel MD, Rao BN, Billups CA, Wu J, Jenkins JJ, Quintana J, Luchtman-Jones L, Villarroel M, Santana VM. Frontline treatment of localized osteosarcoma without methotrexate: results of the St. Jude Children’s Research Hospital OS99 trial. Cancer. 2011;117:2770–8. doi: 10.1002/cncr.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacci G, Forni C, Ferrari S, Longhi A, Bertoni F, Mercuri M, Donati D, Capanna R, Bernini G, Briccoli A, Setola E, Versari M. Neoadjuvant chemotherapy for osteosarcoma of the extremity: intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome. J Pediatr Hematol Oncol. 2003;25:845–53. doi: 10.1097/00043426-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Bielack S, Jurgens H, Jundt G, Kevric M, Kuhne T, Reichardt P, Zoubek A, Werner M, Winkelmann W, Kotz R. Osteosarcoma: the COSS experience. Cancer Treat Res. 2009;152:289–308. doi: 10.1007/978-1-4419-0284-9_15. [DOI] [PubMed] [Google Scholar]
- 38.Kager L, Whelan J, Dirksen U, Hassan B, Anninga J, Bennister L, Bovee JV, Brennan B, Broto JM, Brugieres L, Cleton-Jansen AM, Copland C, Dutour A, Fagioli F, Ferrari S, Fiocco M, Fleuren E, Gaspar N, Gelderblom H, Gerrand C, Gerss J, Gonzato O, van der Graaf W, Hecker-Nolting S, Herrero-Martin D, Klco-Brosius S, Kovar H, Ladenstein R, Lancia C, LeDeley MC, McCabe MG, Metzler M, Myklebost O, Nathrath M, Picci P, Potratz J, Redini F, Richter GH, Reinke D, Rutkowski P, Scotlandi K, Strauss S, Thomas D, Tirado OM, Tirode F, Vassal G, Bielack SS. Workshop Report. Clin Sarcoma Res; The ENCCA-WP7/EuroSarc/EEC/PROVABES/EURAMOS 3rd European Bone Sarcoma Networking Meeting/Joint Workshop of EU Bone Sarcoma Translational Research Networks; Vienna, Austria. September 24–25, 2015; 2016. p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, Ritter J, Roessner A. Musculoskeletal neoplasms: static and dynamic Gd-DTPA--enhanced MR imaging. Radiology. 1989;171:767–73. doi: 10.1148/radiology.171.3.2717749. [DOI] [PubMed] [Google Scholar]
- 40.Holscher HC, Bloem JL, van der Woude HJ, Hermans J, Nooy MA, Taminiau AH, Hogendoorn PC. Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin Radiol. 1995;50:384–90. doi: 10.1016/s0009-9260(05)83135-6. [DOI] [PubMed] [Google Scholar]
- 41.Ongolo-Zogo P, Thiesse P, Sau J, Desuzinges C, Blay JY, Bonmartin A, Bochu M, Philip T. Assessment of osteosarcoma response to neoadjuvant chemotherapy: comparative usefulness of dynamic gadolinium-enhanced spin-echo magnetic resonance imaging and technetium-99m skeletal angioscintigraphy. Eur Radiol. 1999;9:907–14. doi: 10.1007/s003300050765. [DOI] [PubMed] [Google Scholar]
- 42.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep. 2012;14:285–94. doi: 10.1007/s11912-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crews KR, Liu T, Rodriguez-Galindo C, Tan M, Meyer WH, Panetta JC, Link MP, Daw NC. High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer. 2004;100:1724–33. doi: 10.1002/cncr.20152. [DOI] [PubMed] [Google Scholar]
- 44.Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F, Casali PG, Ferrari S, Aglietta M. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol. 2012;23:508–16. doi: 10.1093/annonc/mdr151. [DOI] [PubMed] [Google Scholar]