Abstract
Actin is an integral component of epithelial apical junctions, yet the interactions of branched actin regulators with apical junction components are still not clear. Biochemical data have shown that α–catenin inhibits Arp2/3-dependent branched actin. These results suggested that branched actin is only needed at earliest stages of apical junction development. We use live imaging in developing C. elegans embryos to test models for how WAVE-induced branched actin collaborates with other apical junction proteins during the essential process of junction formation and maturation. We uncover both early and late essential roles for WAVE in apical junction formation. Early, as the C. elegans intestinal epithelium becomes polarized, we find that WAVE components become enriched concurrently with the Cadherin components and before the DLG-1 apical accumulation. Live imaging of F-actin accumulation in polarizing intestine supports that the Cadherin complex components and branched actin regulators work together for apical actin enrichment. Later in junction development, the apical accumulation of WAVE and Cadherin components is shown to be interdependent: Cadherin complex loss alters WAVE accumulation, and WAVE complex loss increases Cadherin accumulation. To determine why Cadherin levels rise when WVE-1 is depleted, we use FRAP to analyze Cadherin dynamics and find that loss of WAVE as well as of the trafficking protein EHD-1/RME-1 increases Cadherin dynamics. EM studies in adults depleted of branched actin regulators support that WVE-1 maintains established junctions, presumably through its trafficking effect on Cadherin. Thus we propose a developmental model for junction formation where branched actin regulators are tightly interconnected with Cadherin junctions through their previously unappreciated role in Cadherin transport.
INTRODUCTION
Actin is essential for cell polarity. Actin filaments are intrinsically polar polymers that are composed of globular actin (G-actin) monomers that bind and hydrolyze ATP to form polymeric filamentous actin (F-actin) (Li and Gundersen, 2008). F-actin exists in two forms, branched actin that is nucleated by Arp2/3, and linear actin that is nucleated by formins. During the specialized events of morphogenesis and in mature cells, the actin cytoskeleton has the ability to rapidly reorganize in response to modulating signals and conditions by undergoing structural rearrangements including elongation, branching, bundling and depolymerization (Miyoshi and Takai, 2008). The intrinsic polarity of actin promotes polarized events including trafficking along actin filaments in collaboration with actin associated proteins, like myosin motors. The principal indicators of a fully polarized epithelial cell are apical junctions, which promote adhesion and communication between the cells. The Cadherin junction components interact with the actin cytoskeleton, some directly, like α-catenin, and some indirectly through their catenin partners. In mammalian epithelial cells the existence of at least two populations of actin at apical junctions (AJs) have been shown, namely junctional actin and peripheral thin actin, that go on to form the cortical actin ring of mature junctions (Zhang et al., 2005).
It is widely accepted that branched actin contributes to the initial formation of apical junctions. During this process cells use the force of branched actin polymerization to produce membrane protrusions that bring cell membranes together (Mege et al., 2006; Cavey and Lecuit, 2009; Hoelzle and Svitkina, 2012). However, only recently has the role of branched actin and its regulators in junctional maturation been established (Kovacs et al., 2011; Verma et al., 2012; Han et al., 2014) Reviewed in (Michael and Yap, 2013). Earlier studies suggested that once cadherin-based junctions are established, branched actin interferes with further junction formation, and is therefore depleted from junctions (Yamada and Nelson, 2007). In contrast, later studies in multiple systems suggested that branched actin regulators are continuously required at junctions to promote junctional maintenance (Georgiou et al., 2008; Leibfried et al., 2008; Fricke et al., 2009; Giuliani et al., 2009; Silva et al., 2009; Bernadskaya et al., 2011; Tang and Brieher, 2012). These findings have raised questions about the role of branched actin in junctional maturation and how the regulators of branched actin interact with other apical junction proteins (Brüser and Bogdan, 2017).
In previous work we analyzed the contribution of the WAVE/Scar complex to the development of apical junctions using the developing C. elegans intestinal epithelia as an in vivo model. The C. elegans Apical Junction (CeAJ) contains two molecularly and functionally independent complexes that have been well described, the cadherin/catenin complex (CCC), and the DLG-1/AJM-1 complex (DAC) (Costa et al., 1998; Labouesse, 2006; Lynch and Hardin, 2009; Simske, 2013) (Figure 1A). A third junctional complex, that includes the transmembrane protein SAX-7/L1CAM, has also been proposed to be a part of the CeAJ (Pásti and Labouesse, 2014). The C. elegans CCC is similar to the cadherin-catenin complexes found in flies and vertebrates (Costa et al., 1998). It is comprised of homologs of classical E-cadherin (HMR-1) (referred to as Cadherin throughout the paper), α-catenin (HMP-1), β-catenin (HMP-2) (Costa et al., 1998). C. elegans also has a p120 catenin homologue, JAC-1, and that is not essential for junction formation or viability (Pettitt et al., 2003). We found that actin regulators, including the WAVE complex components and ERM-1, an Ezrin, Radixin, Moesin homolog, were needed to assemble apical F-actin. In our previous studies in developing embryos, phalloidin staining showed that apical actin enrichment required the DAC complex and SAX-7/L1CAM, while the role of the CCC seemed more limited. In contrast, phalloidin staining showed that larval and adult animals required both the CCC and DAC apical complexes, and F-actin regulators for apical actin enrichment. These findings suggested that the role of Cadherin complex changes as the junctions mature.
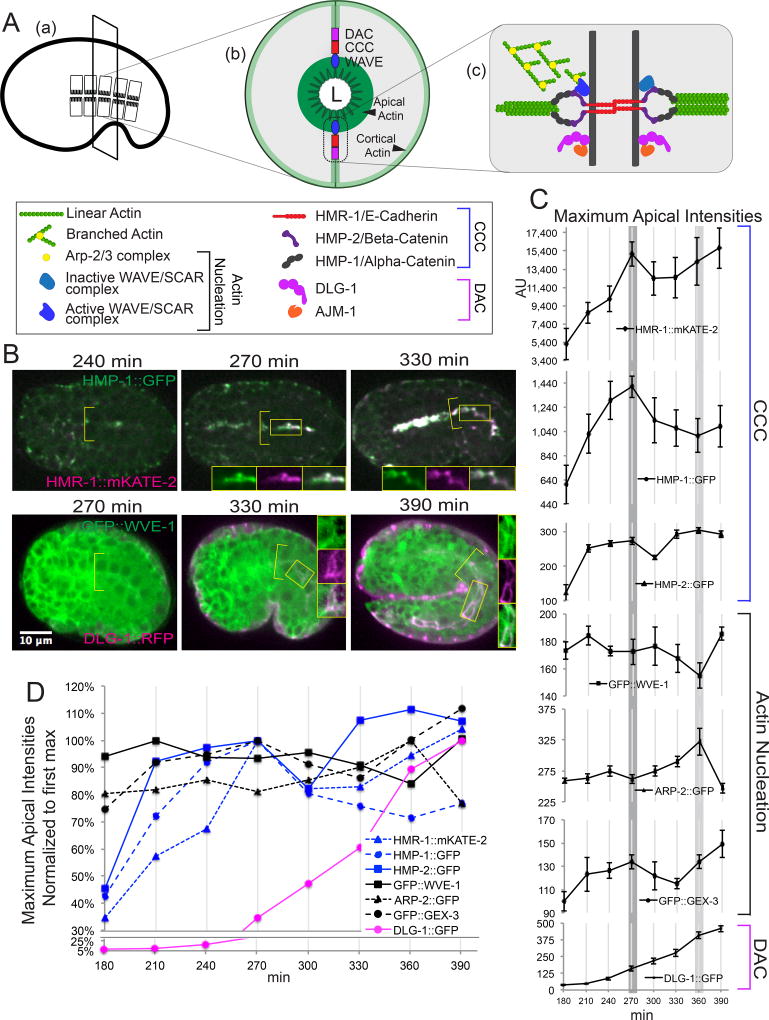
Figure 1. Recruitment timeline of junctional components and branched actin regulators.
(A) Schematic overview of anatomy and molecular architecture of Apical Junction (AJ) in C. elegans. Cross-section (b) of intestinal tube shows location of main AJ components relative to a narrow lumen (L). Zoom-in view (c) of AJ region depicts interactions of branched actin nucleation, Cadherin-Catenin complex (CCC) and DLG-1/AJM-1 complex (DAC) proteins. Active WAVE/SCAR promotes branched actin polymerization by activating Arp2/3 complex. α-catenin/HMP-1 binds to linear actin bundles. (B) Representative images to illustrate the relative peaks of enrichment of Apical Junction components. Top panel: The CCC components, Cadherin/hmr-1::mKate2 and α-catenin/hmp-1::gfp, show a similar peak of apical enrichment at 270 min. after first cleavage. Bottom panel: gfp::wve-1 apical enrichment relative to dlg-1::rfp apical enrichment. Values in (C-D) were obtained from time-lapse sequences taken every 30 minutes of embryos expressing single or double transgenes and CRISPR strains: OX704 hmr-1::mKate-2; hmp-1::gfp (n=3), LP237 hmp-2::gfp (n=8), OX670 gfp^3×FLAG::wve-1 (ML1153) mcIs46 dlg-1::rfp (n=4), arp-2::gfp (n=4), OX663 gfp::gex-3 (n=4), (OX583) dlg-1::gfp (n=4). n, number of embryos included in the analysis. (C) Temporally-aligned graphs of the apical enrichment values for each transgene from Table 1. The shaded lines that cross through all the graphs indicate key events in intestinal development lumen formation (270 minutes), and intestinal elongation as the embryo stretches along the anteroposterior axis (360 minutes). (D) Normalized curves of the apical levels of the transgenes at the apical intestine. The first intensity peak was set to 100%, and other values plotted relative to this value. The x-axis shows the time in minutes after first cleavage. All images in this figure and others, unless stated otherwise, were obtained on a Zeiss Axio Imager Z1 with a Yokogawa CSUX1-5000 spinning disc with a Plan Apo 40×/1.3 Oil lens. All measurements were done on raw data. For all of the images in 1B the display range was equally adjusted to better illustrate the faint intestinal signal. The brighter pharyngeal signal therefore appears saturated at later time points, while the original images were not saturated. All embryos shown in this and other figures are oriented with anterior to the left and dorsal up unless otherwise indicated. For this and other figures error bars show standard error of the mean (SEM).
Once the apical junction forms, its healthy function and maintenance requires trafficking of the Cadherin molecule. A recent study in Drosophila suggested Cadherin is localized to junctions by three mechanisms including Rab11-dependent targeting, endocytosis and targeted recycling, and by lateral flow within the plasma membrane (Woichansky et al., 2016). Studies in C. elegans support a role for Arp2/3-dependent branched actin in the regulation of endocytic recycling dynamics and organization (Shivas and Skop, 2012). Drosophila studies have shown a role for WASp and Arp2/3 in the regulation of Cadherin endocytosis (Leibfried et al., 2008). Our studies of WAVE function in epithelia showed that WAVE and Arp2/3 promote endocytosis of endogenous C. elegans receptors in the intestinal epithelium (Patel and Soto, 2013). Therefore we wanted to investigate if Cadherin trafficking in epithelia requires WAVE function.
In this study we address how branched actin regulators contribute to apical junction maturation. Our live imaging experiments utilize newly developed transgenes, and CRISPR-tagged endogenous versions of the various apical proteins to determine the relative timing of accumulation at the apical junction during development. Live imaging of WVE-1 and Cadherin components allow us to address how the WAVE complex and Cadherin components regulate each other to promote the development of the apical junction. New transgenic strains allow us to image intestinal apical actin enrichment in live embryos undergoing junction formation to better clarify how the two main C. elegans apical junction complexes contribute to apical actin during development. FRAP studies suggest that WAVE contributes to Cadherin trafficking by regulating the dynamics of Cadherin turnover at a developing apical junction. Comparison of the WAVE effects on Cadherin dynamics to known trafficking components identifies a new player in Cadherin trafficking at the apical junction. In addition, we use EM to analyze what happens subcellularly to mature AJs that are depleted of WAVE.
RESULTS
Timeline of accumulation of branched actin regulators and AJ proteins at the apical intestine
To better understand how complex interactions between junctional components (CCC and DAC) and actin regulators contribute to apical junction establishment and thus set up the structure that organizes apical F-actin (Figure 1A), we determined in which order these different components assemble at the junction during the formation of the C. elegans embryonic intestine. We used live imaging to compare the timing of apical enrichment of the apical junction proteins and WAVE-Arp2/3-related actin nucleators. Endogenously tagged CRISPR strains and traditional integrated multicopy array transgenes (details in methods) were used to track the apical enrichment of components of the CCC including hmr-1::gfp (FT250 and LP238), hmp-2::gfp (LP237) and hmp-1::gfp (LP169) (Achilleos et al., 2010; Marston et al., 2016); the DAC, dlg-1::gfp (OX583) and dlg-1::rfp (ML1153), (Diogon et al., 2007; Totong et al., 2007); the WAVE complex, gfp::wve-1 (OX669) (this study) and gfp::gex-3 (LP431) (Marston et al., 2016) and the Arp2/3 complex, arp-2::gfp/AKA arx-2::gfp (TH246) (Sarov et al., 2012). Since some transgenes can cause small developmental delays, we checked how each transgene affected the timing of development relative to wild type embryos with no transgenes, and found only modest delays. DIC imaging showed that the different transgenes reached key developmental events at similar times as control embryos and as each other (Movie S2). The different strains reached “gut alignment” (Leung et al., 1999) when the intestinal nuclei move apically and junctions begin to mature at approximately 240 min at 23°C, and lumen formation, at 270 min at 23°C.
The different components of the apical junction reached a peak of enrichment at a similar time in the process of intestinal development, with some notable differences (Figure 1B,C, Table 1). The temporal data, graphed on Figure 1D, shows that the three components of the CCC begin to rise steadily by 210 minutes after first cleavage, and reach a first peak of apical enrichment at 270 minutes, the time we aligned to lumen formation (Figure 1B, Movie S1). gfp::wve-1 and gfp::gex-3 also rise by 210 min. and reached a peak of enrichment at 300 min., though the levels of apical accumulation are modest, compared to the CCC levels. To compare the range of values, we also plotted the maximum apical intensities normalized to the first peak (Figure 1D). This shows that the actin regulators, like gfp::wve-1, show relatively small changes over time, compared to CCC components that show broader temporal increases. By comparison, dlg-1::gfp (and dlg-1::rfp, which showed similar accumulation, Figure 1B), begins to rise by 270 minutes, and continues to rise at 390 minutes. Therefore, the CCC components accumulate much earlier than the DAC apical complex components, as had been suggested by fixed imaging, and the WAVE complex closely follows the accumulation of the CCC.
Table 1.
Table of apical fluorescent intensities, from 180 min to 390 min, for CCC proteins (OX704 hmr-1::mkate-2; hmp-1::gfp, LP237 hmp-2::gfp), actin nucleation proteins (OX669 gfp::WVE-1, TH246 arp-2::gfp, OX663 gfp::gex-3) and DAC protein OX583 dlg-1::gfp. (Related to Fig. 1).
| CCC | Actin Nucleation | DAC | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| t, min | HMR-1::MKATE-2 | HMP-1::GFP | HMP-2::GFP | GFP::WVE-1 | ARP-2::GFP | GFP::GEX-3 | DLG-1::GFP |
| 180 | 5200 | 601 | 124 | 173 | 259 | 100 | 38 |
| 210 | 8622 | 1019 | 251 | 184 # | 263 | 123 | 48 |
| 240 | 10,154 | 1296 | 265 | 173 | 275 # | 127 | 85 |
| 270 | 15,057# | 1410 # | 273 # | 172 | 262 | 134 # | 159 |
| 300 | 12,368 | 1134 | 225 | 176 | 275 | 122 | 217 |
| 330 | 12,464 | 1069 | 292 | 167 | 290 | 115 | 278 |
| 360 | 14,228 | 1006 | 303 | 155 | 322 | 134 | 409 |
| 390 | 15,708 | 1083 | 292 | 186 | 248 | 149 | 458 |
- First apical intensity peak for each AJ component.
A second notable feature of the enrichment timelines is that some components show a second, later peak, centered around 360 minutes. At this time point, the intestine is elongating along the anteroposterior axis. The apical enrichment of the CCC components hmr-1::mKate2 and hmp-2::gfp rose at this time, as did gfp::wve-1 and gfp::gex-3. This also coincides with the highest levels of apical arp-2::gfp and dlg-1::gfp in the intestine.
The early peak of GFP::WVE-1 (Figure 1D) enrichment slightly ahead of the CCC accumulation suggested that WAVE complex could play a role in CCC components recruitment to the AJ
WAVE/SCAR regulates apical enrichment of CCC complex components
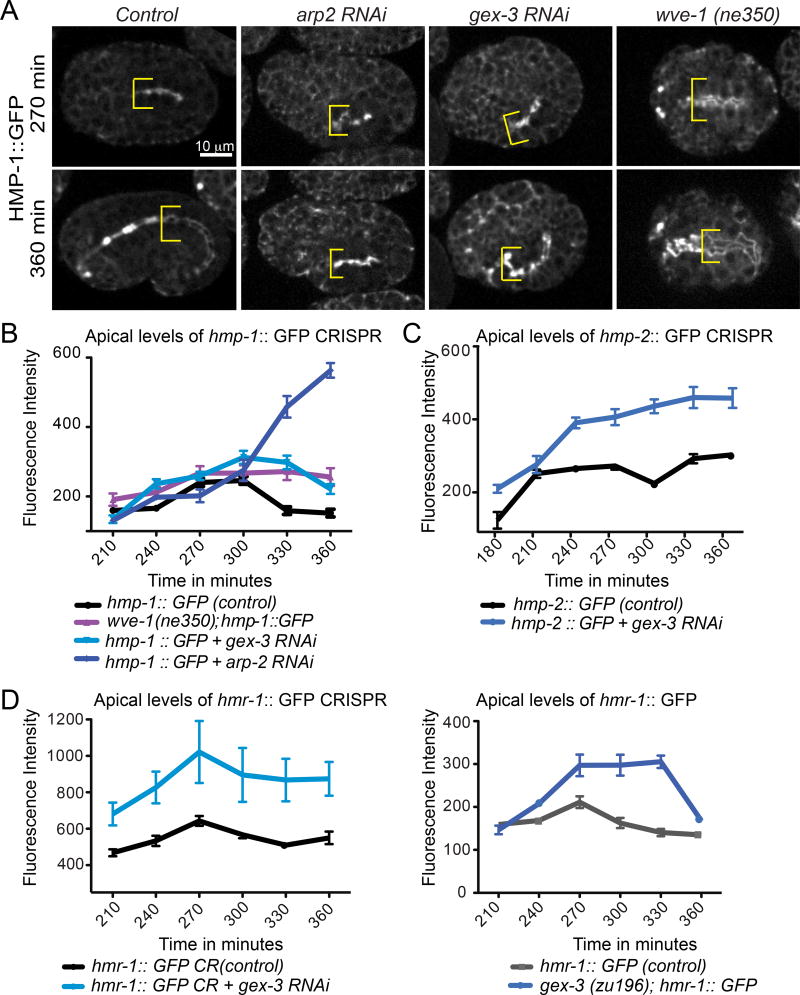
We investigated if WAVE components affect how Cadherin components assemble at the apical intestine. Previous studies showed that loss of the WAVE complex, embryonically or post-embryonically, resulted in reduced accumulation of the DLG-1 component of the DAC. However, loss of WAVE complex, embryonically or postembryonically, did not reduce the levels of Cadherin/HMR-1 component of the CCC, and instead resulted in increased HMR-1 accumulation in embryos (Bernadskaya et al., 2011). In this study we used new strains in which Cadherin components were tagged at their normal chromosomal locations using CRISPR to examine if loss of the WAVE complex affects accumulation of CCC proteins, including α–catenin, the component that binds to actin. The effects of WAVE component depletion were measured using an α–catenin/hmp-1::gfp genetically engineered CRISPR strain (LP169), (Marston et al., 2016). Wild type HMP-1::GFP is enriched apically by 210 min, and apical enrichment peaks at 270 min. Depletion of WAVE components via RNAi or genetic mutation (wve-1(ne350) and gex-3 RNAi) or of arp-2 via RNAi resulted in increased apical levels of HMP-1::GFP beginning around 240 min. (Figure 2A). Therefore, studies with endogenously tagged protein show that loss of WAVE complex increases the level of HMP-1/α–catenin.
Figure 2. The WAVE/SCAR complex regulates the apical enrichment levels of α-catenin/HMP-1::GFP, β-catenin/HMP-2::GFP and E-Cadherin/HMR-1::GFP.
(A) Apical accumulation of CRISPR tagged LP169 hmp-1::gfp (Marston et al., 2016) in the developing intestine when WAVE components (gex-3 RNAi, OX609 wve-1(ne350)/hT2-gfp)) and Arp2/3 (arp-2 RNAi) are reduced. Embryos are shown at two developmental stages, 270 and 360 minutes after first cleavage at 23°C, stages when the intestine polarizes along the apical/basal axis and elongates along the anteroposterior axis (Leung et al., 1999). Yellow brackets are placed at the anterior region of the intestine and indicate its width. The bright signal anterior to the brackets seen in some embryos is in the pharynx. (B, C, D) Graphs of maximal fluorescent intensity, at the apical intestine, over time in WT and mutant embryos. Embryos were measured at each data point at the same region of the intestine (past the foregut). Three measurements were made per embryo per time point, and the highest signal was recorded as apical maximum. B. Measurements on the hmp-1::gfp strain, n=6 embryos. (C) Measurements on the LP237 hmp-2::gfp strain (Marston et al., 2016), n= 8 embryos. (D) Measurements on two different hmr-1 strains: LP172 hmr-1::gfp CRISPR strain (Marston et al., 2016) and FT250 hmr-1::gfp integrated transgenic strain (Achilleos et al., 2010), n= 8 embryos.
CRISPR-tagged strains of Cadherin/HMR-1 (LP172) and β-catenin/HMP-2::GFP (LP237) are also available (Marston et al., 2016). Loss of gex-3 via RNAi led to increased levels of hmp-2::gfp and hmr-1::gfp (Figure 2B, C) beginning at 240 min. A different Cadherin transgene made with an integrated extrachromosomal array, FT250 xnIs96 hmr-1::gfp (Achilleos et al., 2010) that was used in our previous studies also showed increased apical enrichment when gex-3 was reduced, also evident by 240 min (Figure 2C). Therefore, analysis with CRISPR tagged junctional components confirmed our previous results showing increased HMR-1::GFP when WAVE was removed (Bernadskaya et al., 2011). The peak levels of apical enrichment of CCC proteins were at least 50% higher in Gex animals as compared to controls (Figure 2D). The largest effects were seen on the levels of HMR-1/Cadherin and HMP-2/β-catenin. We also noted that the effects of depleting WAVE components on Cadherin complex levels were evident by 240 minutes, soon after the intestinal junction sets up its polarity and before 270 min time point, when CCC components normally display the peak of apical accumulation (Figure 1D). This observation suggests that the early WAVE peak (Figure 1C, D) signifies an event that is important in CCC protein recruitment to the AJ.
WAVE localization to the apical junction depends on the two apical junction complexes
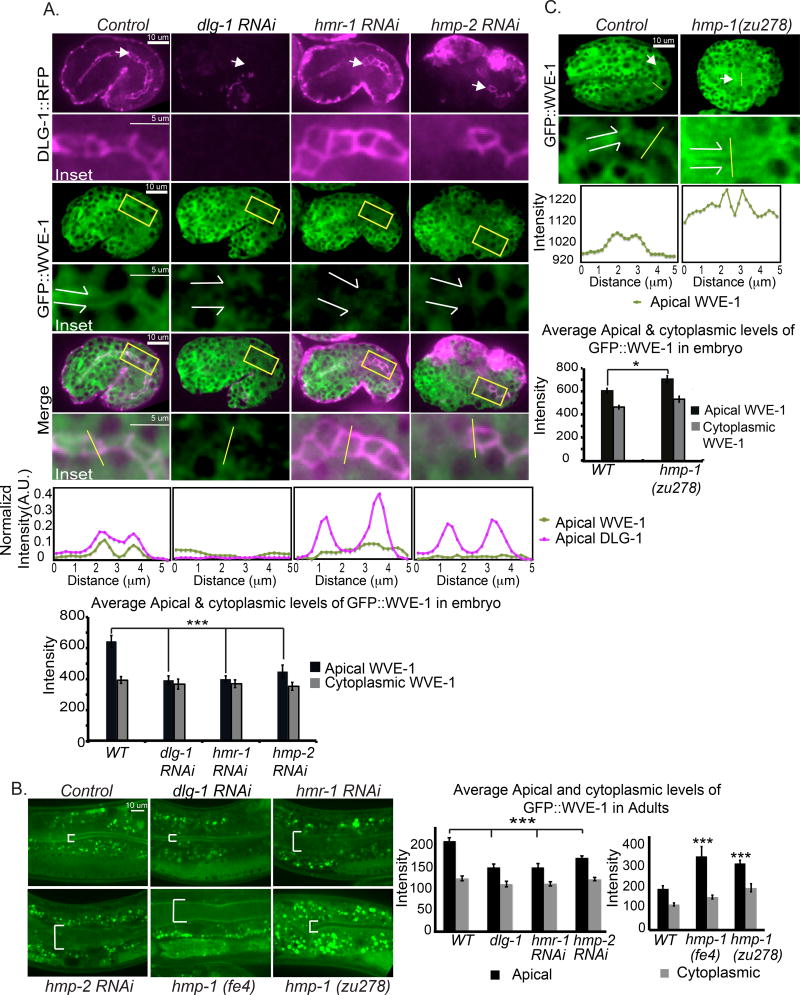
To test if the two apical junction complexes affect embryonic apical enrichment of WAVE at the intestine, we generated a CRISPR-tagged gfp::wve-1 strain, OX669 wve-1(pj64[gfp::3×FLAG::wve-1]). This endogenously tagged wve-1 strain has a low level of embryonic lethality (10% lethal), is at least 50% brighter and shows similar WVE-1 pattern as our previously made rescuing transgene built using a traditional multicopy array. We first crossed gfp::wve-1 (OX669) to mcIs46 dlg-1::rfp (ML1153) (to build strain OX670) so we could image WVE-1 relative to the apical intestinal junctions. Depleting dlg-1 via RNAi led to 96% embryonic lethality with the expected phenotype, and strongly reduced dlg-1::rfp signal, showing dlg-1 RNAi was effective. Depletion of dlg-1 reduced the apical enrichment of gfp::wve-1 at the intestine and overall WVE-1 levels appeared lower (Figure 3A). Similarly, depletion of Cadherin/hmr-1 or β-catenin/hmp-2 reduced apical enrichment of gfp::wve-1 in the developing intestine (Figure 3A).
Figure 3. Effects of the CCC and DAC complexes on GFP::WVE-1 apical enrichment.
(A) OX670 Embryos carrying both CRISPR strain gfp::wve-1 and transgene dlg-1::rfp are shown at 360 minutes after first cleavage at 23°C. White arrows point to the anterior intestine and the apical lumen region of gfp:: wve-1 enrichment. Yellow rectangles indicate the area of gut shown in the insets below. The contrast was enhanced equally on the cropped insets to assist comparison. White split arrows in the insets indicate the intestinal lumen and its width. Representative single line scans through the intestines (yellow lines in the insets), 5 um long with 2.5 um on either side of the AJ, were used to quantify apical enrichment of both strains. The line graphs below show normalized intensity values. Average apical and cytoplasmic levels (n=6) are shown in bar graphs. Statistical significance: *** = p<0.0005. (B) Adults carrying gfp::wve-1 and dlg-1::rfp (OX670) or just gfp::wve-1 (OX669) (for the hmp-1 alleles (OX671) and(OX77) bottom row) were imaged at the young adult stage (one day after L4 at 23°C). White brackets indicate the adult intestinal lumen and its width. The apical and cytoplasmic levels of gfp::wve-1 at the intestine were measured as in A and shown in the graphs on the right. (C) OX671 Embryos carrying the gfp::wve-1 strain were crossed into the hmp-1(zu278) putative null allele and imaged as in A. The representative single line scan was measured as in A and the respective line graph below shows actual intensity values. Average apical and cytoplasmic intensity levels (n=6) are plotted below. Statistical significance: * =p<0.05, *** =p<0.0005.
To test if the two apical complexes similarly affected the post-embryonic apical enrichment of WAVE components we used the same CRISPR-tagged gfp::wve-1 strain. In wild type adult animals this strain shows gfp::wve-1 enrichment that is highly concentrated in the apical intestine. As in embryos, when larval animals were depleted of dlg-1, hmr-1 or hmp-2 via RNAi, the apical enrichment was strongly reduced (Figure 3B). The intestinal lumen also became distended (Figure 3B, white brackets) as we had previously noted (Bernadskaya et al., 2011).
Depleting α-catenin, another component of the CCC, gave different results as compared to the loss of dlg-1, hmr-1 or hmp-2. Loss of α-catenin/HMP-1 using the hmp-1 hypomorphic allele fe4, (OX774) or the putative null allele, zu278, (OX671) resulted in increased gfp::wve-1 enrichment in the embryonic intestine in the cytoplasm and at the apical region (Figure 3C). Just as in embryos, we observed higher apical GFP::WVE-1 in the adult intestine of the gfp::wve-1; hmp-1(fe4) and gfp::wve-1; hmp-1(zu278) animals (Figure 3B).
Therefore both the initial establishment and the maintenance of apically enriched WAVE complex depend on the CCC apical complex, while the DAC contributes to WAVE apical enrichment by 360 minutes, the stage shown in Figure 3A. The dependence of CCC accumulation on WAVE (Figure 2) and the observed effects of junctional proteins on WAVE accumulation (Figure 3) suggested interdependency between branched actin and junctional components during the AJ establishment. Interestingly, the CCC component HMP-1/α-catenin, a well-established actin binding protein, had a different effect on WVE-1 enrichment compared to other Cadherin complex components.
Live imaging shows that WAVE/SCAR, Cadherin and other Apical Junction complexes are required for F-actin accumulation in the developing intestine
The distinct effects of the different Cadherin components on WAVE accumulation led us to re-examine how each Cadherin component affected F-actin enrichment at the intestine. We needed new live imaging reporters to monitor F-actin accumulation in live developing embryos. For technical reasons we were not able to express LifeAct or Utrophin in the embryonic intestine. However, we were able to express an endogenous C. elegans actin binding protein, the ABD of VAB-10, which has been used extensively to characterize F-actin in the embryonic epidermis. This transgene uses the intestine-specific promoter end-1 (Calvo et al., 2001) to drive expression of the actin binding domain of VAB-10/Specktraplakin (Bosher et al., 2003), fused to GFP (Pend-1::vab-10 ABD::gfp).
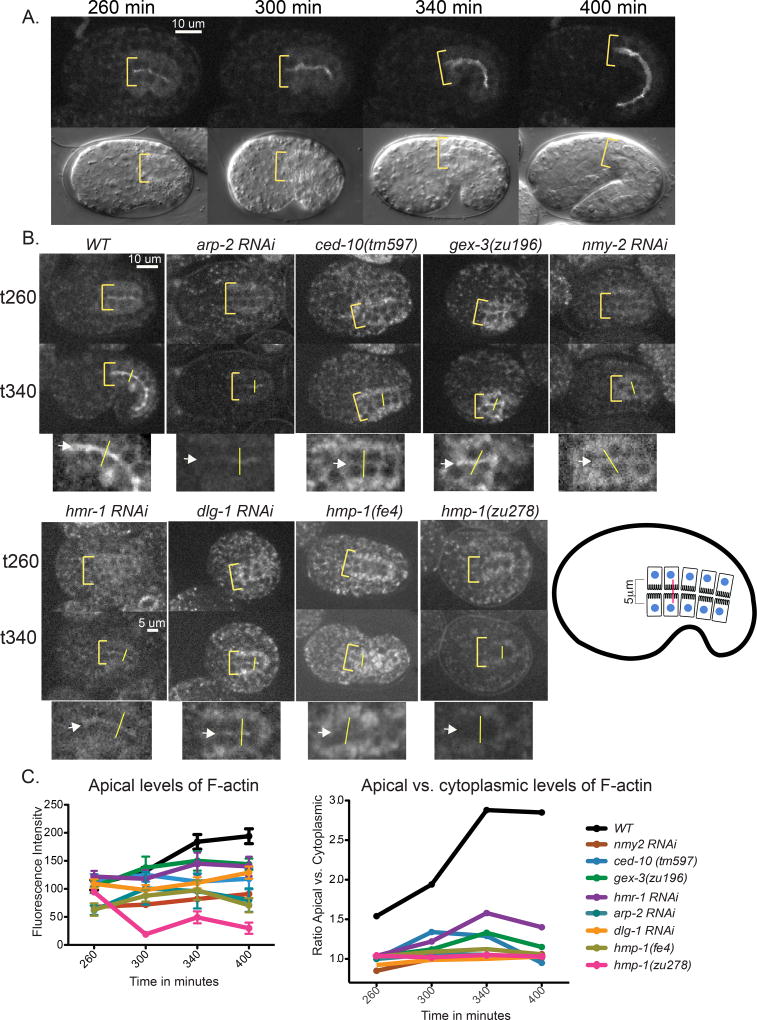
Our previous studies using phalloidin staining on fixed embryos showed that while the WAVE/SCAR complex and the DAC junction components are required for embryonic apical F-actin enrichment in the intestine, CCC components (paradoxically) did not seem to be needed (Bernadskaya et al., 2011). Live imaging of OX616 Pend-1::vab-10 ABD::gfp expressing animals illustrates that F-actin in the embryonic intestine becomes progressively enriched at the apical membrane and reduced in the cytoplasm during the time when apical junctions are forming and maturing (Leung et al., 1999)(Figure 4A,C, Movie S3). The ratio of apical to cytoplasmic F-actin almost doubles from 260 to 340 minutes (Figure 4C). Depletion of actin regulators, including the WAVE components and arp-2, resulted in dramatic decreases in live accumulation of apical F-actin as the embryos developed (Figure 4B). For example, by 340 minutes after first cleavage, apical F-actin levels were reduced over 30% compared to controls in embryos with a deletion null allele of CED-10/Rac1 (OX618) (Figure 4B,C), the GTPase that recruits the WAVE/GEX complex to membranes (Bernadskaya et al., 2012). F-actin apical levels were similarly reduced in gex-3 (zu196) (OX617) embryos, which have a strong loss of function mutation in the GEX-3/Kette component of the WAVE complex. Strong RNAi depletion of non-muscle myosin, nmy-2, arrests embryos before morphogenesis, as NMY-2 is involved in cytokinesis (Guo and Kemphues, 1996). Therefore we used milder nmy-2 RNAi conditions and examined embryos with differentiated epithelia, (as shown by epithelial markers), that reached the morphogenesis stage. These embryos showed a dramatic drop of F-actin accumulation similar to what is seen when arp-2 is removed (Figure 4B). Therefore, the nmy-2 gene has what to our knowledge is a previously undescribed role in apical F-actin regulation in the developing intestine.
Figure 4. Live imaging of F-actin accumulation in the developing intestine depends on actin regulators and components of two C. elegans apical junctions.
(A) Top panels: Control Wild Type (WT) embryo expressing the intestinal-specific F-actin transgene, OX616 Pend-1::vab-10 ABD::gfp, demonstrates the increasing enrichment of F-actin at the developing apical intestine. Bottom panels: DIC images of the same time points. Yellow brackets demarcate the anterior apical intestine and its width, (B) Control Wild Type (WT) embryo and embryos depleted of individual proteins via mutation (ced-10 (tm597) (OX618), gex-3 (zu196) (OX617), hmp-1 (zu278) (OX656), hmp-1 (fe4) (OX655)) or RNAi (arp-2, nmy-2, hmr-1, dlg-1) are shown at 260 and 340 minutes after first cleavage. Yellow brackets indicate the anterior region of the intestine and its width. In the cropped images, magnified views of the intestine at 340 min, white arrows point to the apical region of the intestine, verified in the DIC channel. The apical intestinal signal was calculated by placing a line (shown in yellow in the actual embryos, and in red in the cartoon) across the intestine, and the maximum reading generated using the Line Function of ImageJ was recorded as the apical signal. The cytoplasmic signal was calculated as the average of the signal at the center of the two intestinal cells 2.5 µm on either side of the apical intestine. Embryos were dissected from mothers and timed from the 2-cell stage. Live imaging was done at 23°C. (C) Quantitation of the F-actin enrichment shown as average apical intensity (left) or as a ratio of apical to cytoplasmic levels (right). Multiple embryos were measured this way (n=6) to average apical intensity.
The Pend-1::vab-10 ABD::gfp transgene was used to examine how the proteins that make up the two main C. elegans apical junction complexes contribute to apical F-actin accumulation in the developing intestine of live embryos. Depletion of either CCC (hmr-1 or hmp-1) or DAC (dlg-1) components, by RNAi or by mutation, resulted in decreased apical F-actin enrichment, similar to the effects of depleting WAVE components (Figure 4B). Introducing a hypomorphic mutation in α-catenin that reduces binding to F-actin, hmp-1(fe4) (OX655) (Maiden et al., 2013) resulted in partially reduced accumulation of intestinal apical F-actin, while introducing a putative null mutation, zu278 (OX656), showed even more strongly decreased apical F-actin. Thus, in contrast to fixed staining with phalloidin, live imaging supports the important role of both C. elegans apical junction complexes in F-actin enrichment during embryonic formation of the intestinal epithelia.
Effect of the WAVE/SCAR complex on HMR-1/Cadherin dynamics at the apical junction
Junctions still formed in all the RNAi-treated and mutant embryos examined, and the cells remained adherent (Figure 4). However, it is possible that these junctions are not fully functional, since, for example, the lumen of the intestine is wider than normal (Figures 3,4; (Patel et al., 2008). The decrease in total F-actin accumulation is likely related to greater accumulation of dysfunctional CCC components at the apical junctions (Figure 2), resulting in defective AJs. Formation and size of Cadherin clusters at the junctions has been shown to depend on F-actin (Hong et al., 2013; Chen et al., 2015) and Cadherin receptors in the clusters undergo a dynamic turnover (Erami et al., 2015). We therefore investigated if WAVE-dependent branched actin plays a role in HMR-1/Cadherin turnover and delivery to the AJ region.
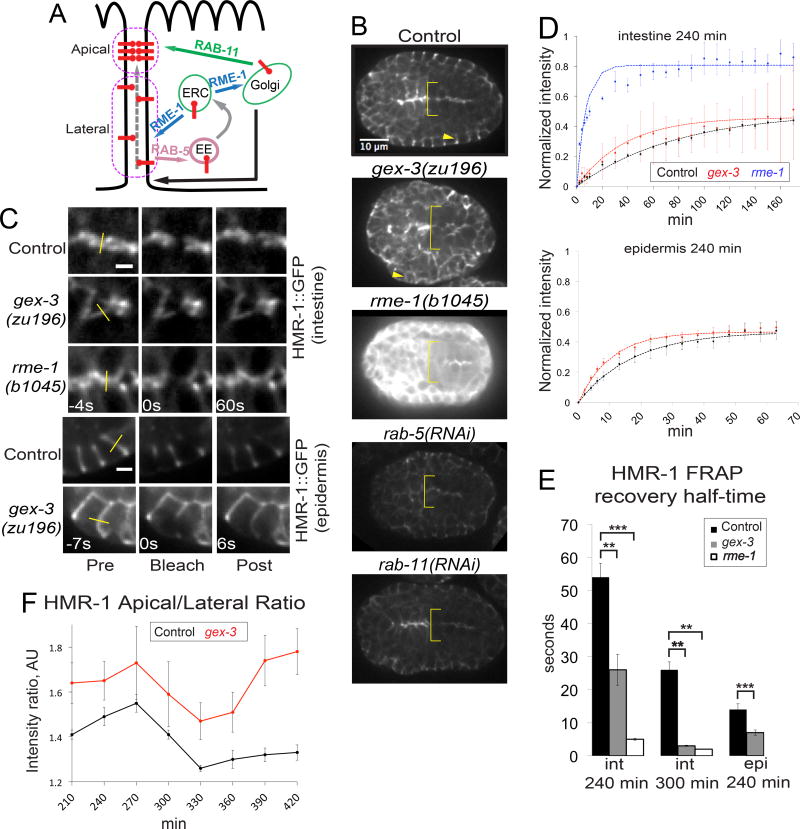
To address if elevated levels of HMR-1/Cadherin seen in WAVE mutants may be due to the role of WAVE in protein trafficking (Patel and Soto, 2013), we compared the changes in steady state levels of FT250 HMR-1::GFP (Achilleos et al., 2010) in WAVE mutants to the loss of regulators of endocytosis (rab-5 RNAi) or endocytic recycling (rab-11 RNAi or rme-1) (Figure 5A). In the embryonic intestine, loss of rab-5 or rab-11 via RNAi led to decreased levels of apical HMR-1::GFP, instead of the elevated level seen in WAVE mutants (Figure 5B). However, loss of rme-1, a homolog of the EHD1 protein, using a homozygous viable null allele (b1045), resulted in highly elevated apical HMR-1::GFP. The RME-1/EHD1 protein has been proposed to conduct multiple trafficking roles, including the promotion of recycling endosome to membrane transport of specific basolateral cargo in the adult C. elegans intestine (Shi et al., 2010). It is also required for recycling endosome to Golgi transport of some cargo including MIG-14/Wls and TGN-38/TGN38 in a pathway that my also involve WAVE-1 (Bai and Grant, 2015). This result suggested the elevated Cadherin/HMR-1 levels in WAVE mutants might be due to changes in protein trafficking dynamics in the intestine.
Figure 5. Loss of WAVE components increases HMR-1/Cadherin dynamics.
(A) Main trafficking steps involved in E-Cadherin delivery to AJ region. ERC – early endocytic compartment, EE – early endosome, dashed grey arrow – apically directed flow of E-Cadherin, black line with arrow – lateral exocytosis. Cartoon is adapted from (Woichansky et al., 2016). (B) Images of FT250 hmr-1::gfp embryos illustrate steady state levels of HMR-1::GFP at the apical embryonic intestine (yellow brackets) and epidermis in control, gex-3, rme-1, rab-5 and rab-11 animals. Arrows indicate epidermal junctions that were bleached. Images are shown with the same display range to directly compare intestinal and epidermal HMR-1::GFP signal in control and mutant animals. (C) Select frames from FRAP time sequences (Movies S4, S5, S6). In the post-bleach frame gex-3 and rme-1 mutants have recovered in contrast to control. Top three panels: FRAP of intestinal apical junctions. Scale bar = 2 µm. Bottom panels: epidermal junctions. Scale bar = 5 µm. FRAP imaging was done with the same conditions. For this panel, gex-3 and rme-1 images are shown with higher display range, because these mutants (specially rme-1) are much brighter than control. Yellow lines – bleached regions. (D) Average normalized FRAP curves (dots) and average fitted curves (dashed lines) for control (black), gex-3 (red) and rme-1 (blue) embryos, generated in FRAP Profiler plugin for ImageJ. See Methods for more details on FRAP acquisition and analysis. (E) Quantification of t½ (recovery half-time) in different tissues and times of development. epi - epidermis, int – intestine. (F) Apical to lateral HMR-1::GFP ratio in control (black) and gex-3 (red) embryos. Apical and lateral regions are defined as in (A) – magenta ovals.
To examine the dynamics of HMR-1::GFP in the embryonic intestine, we used FRAP (fluorescence recovery after photo bleaching). In control animals at 240 min, photobleaching a small region of the intestinal apical junction resulted in a recovery half-time of 56 sec. FRAP studies on slightly older control embryos, at 300 min, just before muscle twitching interfered with the imaging, showed a faster recovery half-time of 25 sec. This suggested increasing dynamics of Cadherin as the intestinal junction matures. FRAP studies on this hmr-1::gfp transgenic strain were then performed on animals with a putative null mutation in the WAVE component gex-3 (zu169) and in the trafficking mutant rme-1(b1045) (strains OX525 and OX494, respectively). gex-3 mutant embryos had significantly faster recovery half-times of 25 sec at 240 min (Movie S4) and 15 sec at 300 min (Figure 5C,D). rme-1 mutant embryos showed even faster recovery half-times, only 4 seconds at 240 min (Movie S5) and 2 sec by 300 min. We did not detect significant changes in the HMR-1::GFP mobile fraction in gex-3 mutants as compared to control embryos, while rme-1 mutants had an increase in the HMR-1::GFP mobile fraction (Figure 5D). Thus, higher apical levels of Cadherin junction proteins in embryos missing WAVE-dependent branched actin coincides with faster recovery of Cadherin/HMR-1 to the apical junction. The increased dynamics in gex-3 mutants is evident both in embryos with maturing junctions (240 min), and more mature junctions (300 min), so branched actin regulators are continuously regulating Cadherin dynamics. Finally, the increased levels and dynamics seen in gex-3 embryos is similar to the result for one trafficking mutant, rme-1, which has defects in both trafficking from endosomes to the plasma membrane, and from endosomes to the Golgi (Shi et al., 2010; Bai and Grant, 2015).
We noticed that Cadherin levels were also elevated in the epidermis in embryos depleted of WAVE components (Figure 5B). Therefore we performed FRAP studies on the epidermal cells. For ease of imaging, we bleached junctions between the epidermal cells in the lateral row of epidermal cells at 240 min. The results in the epidermis mirrored the results with the intestine. However, the HMR-1::GFP dynamics in the migrating epidermal cells are much faster than in the intestine (Table 2). Control embryos showed recovery half-times of 14 sec, while gex-3 embryos had recovery half-times of 7 sec (Figure 5D, Movie S6). Similarly to the results in the intestine, we did not detect a significant change in the HMR-1::GFP mobile fraction in gex-3 mutants at 240 min (Figure 5D,E), although it is possible that subtle changes would be difficult to measure in these tiny developing cells.
Table 2.
Summary of Intestinal and Epidermal Junctional FRAP
| Genotype | Tissue | Time, min | Half-time, s | Significance (half-time) |
Mobile Fraction, % |
n |
|---|---|---|---|---|---|---|
| Control | gut | 240 | 54 | 49 | 4 | |
| gex-3 | gut | 240 | 26 | ** | 46 | 5 |
| rme-1 | gut | 240 | 5 | *** | 80 | 4 |
| Control | gut | 300 | 26 | 34 | 2 | |
| gex-3 | gut | 300 | 3 | ** | 25 | 2 |
| rme-1 | gut | 300 | 2 | ** | 69 | 2 |
| Control | epi | 240 | 14 | 49 | 3 | |
| gex-3 | epi | 240 | 7 | *** | 49 | 3 |
= p<0.005,
=p<0.0005
Cadherin is also delivered to the AJ via lateral transport within the membrane (Woichansky et al., 2016). We thus hypothesized that the apically directed flow of Cadherin might be altered in WAVE-deficient embryos. We measured the intestinal apical-to-lateral ratio of HMR-1::GFP and found that it is higher in gex-3 mutants (Figure 5F). This result suggests that WAVE restricts apically directed flow of Cadherin. Altogether these results suggest WAVE/SCAR affects the apical junctions in at least two ways: by supporting the correct apical accumulation of other apical components responsible for maintaining the apical actin population, and also by regulating dynamics of junctional proteins, which further contributes to the apical/basal polarity establishment and maintenance.
EM evidence that WAVE/SCAR Promotes Junctional maintenance
We have shown that branched actin regulators maintain structures along the apical/basal axis of developing C. elegans intestinal epithelia. For example, animals depleted of WAVE/SCAR or Arp-2/3 components develop an intestinal lumen that is less enriched in apical F-actin, and that lumen becomes distended and wider in late stage embryos and in adults (Patel et al., 2008; Bernadskaya et al., 2011). Therefore we investigated how lack of WAVE-dependent branched actin affects the structure of the adult apical junction.
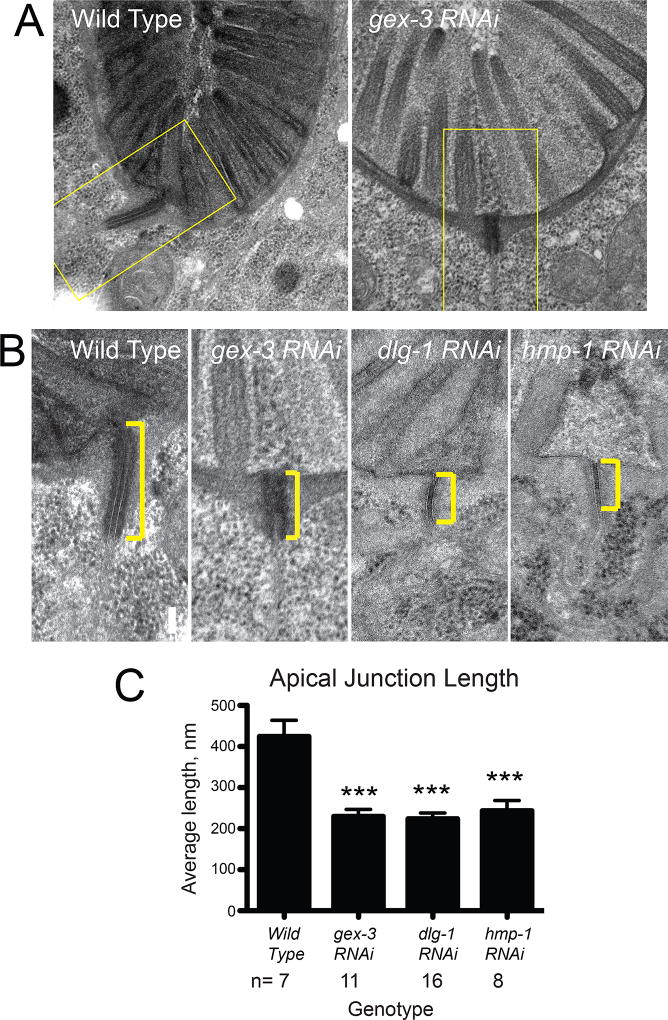
We conducted transmission electron microscopy (TEM) on young adult animals in which WAVE/SCAR components were depleted via RNAi at the beginning of larval stage 1. We down-regulated the GEX-3 component of WAVE, since depleting any component of the five-member WAVE complex affects the stability of the rest of the complex (Patel et al., 2008). To monitor that RNAi was working, we monitored the two Gex phenotypes: Mel, maternal effect lethal and Egl, egg laying defective. We therefore counted the percent of dead embryos obtained from young adults fed the same batch of RNAi bacterial preparation, and we noted the appearance of the egg laying defect to larvae fed the same RNAi bacteria. In wild type adults AJs appear long, thick and electron dense. In contrast, the AJs in GEX-3-depleted animals are significantly shorter and somewhat less electron dense (Figure 6A, B). As we had previously noted, microvilli still form when we deplete regulators of branched actin (Patel et al., 2008) and the same is true when we deplete CCC or DAC components (Fig. 6A). The effect of GEX-3 depletion on AJs was compared to depletion of components of the two C. elegans apical junction complexes. Depletion of DLG-1, part of the DAC complex, led to similar changes in the length, and stronger changes in electron density resulting in small, thin and sometimes fragmented junctions. Depletion of HMP-1/α-catenin also resulted in thinner, shorter AJs (Figure 6B). We compared AJs in at least seven age-matched animals submitted to the same EM conditions, and analyzed the same region of the anterior intestine, just behind the foregut. The analysis (Figure 6C) showed that the average length of the AJs in WAVE animals was significantly shorter than in wild type animals, about as short as in dlg-1 or hmp-1 depleted animals (Figure 6B). Thus, the WAVE/SCAR complex, similarly to the components of the two C. elegans apical junction complexes, is required to maintain the correct length and ensure robustness of the AJs. These findings suggest that WAVE complex continues to be an essential component of mature apical junctions in adult animals.
Figure 6. WAVE/SCAR promotes robust apical junctions in adult C. elegans intestinal epithelia.
TEM (transmission electron microscopy) images of wild type young adult C. elegans, and adults in which gex-3, dlg-1 or hmp-1 were depleted by RNAi. Larval stage (L1) N2 larvae were fed bacteria expressing the control vector L4440 (labeled Wild Type) or vectors encoding double stranded RNA for gex-3, dlg-1 or hmp-1. After 3 days, young adults were fixed and prepared for TEM as described in (Bernadskaya et al., 2011). (A) Cross sections of wild type and gex-3 RNAi-treated representative animals, at the anterior intestinal region just past the foregut, are shown. (B) Zoomed-in view (yellow rectangles in (A)) of AJs in wild type, gex-3, dlg-1 or hmp-1 RNAi-treated animals. Yellow brackets indicate the length of the adherens junction. (C) The graph shows statistical analysis of adherens junction length from the TEMs. n= number of individual animals, with measurements taken at the same region of the anterior intestine, just past the foregut.
DISCUSSION
In this study we analyzed how three complexes, CCC, DAC and the WAVE, assemble and affect each other’s steady-state levels and activity to form functional and stable apical junctions. To address how CCC, DAC and WAVE assemble at the AJ, we compared the timing of enrichment of each complex at the apical junction and their effects on the apical F-actin enrichment in the developing intestine (Figures 1 and 4). Live imaging of F-actin in the embryonic C. elegans intestine showed increase in apical enrichment of F-actin as the intestine develops (Figure 4A). Live imaging of apical junction components showed that as F-actin is accumulating, the levels of all the apical junction components, and the WAVE complex, are also increasing. The CCC levels mostly peak at mid-embryogenesis (240–360 minutes after first cleavage), and then level off or drop slightly. A first peak at 240 min, shown by the Cadherin components and WAVE components, coincides with intestinal lumen formation. A second peak (360 min) shown by Discs Large/DLG-1::GFP and ARP-2::GFP, coincides with the elongation of the intestine along the anteroposterior axis (Figure 1). These peaks in levels may reflect times in development when the apical region is undergoing the most dynamic remodeling.
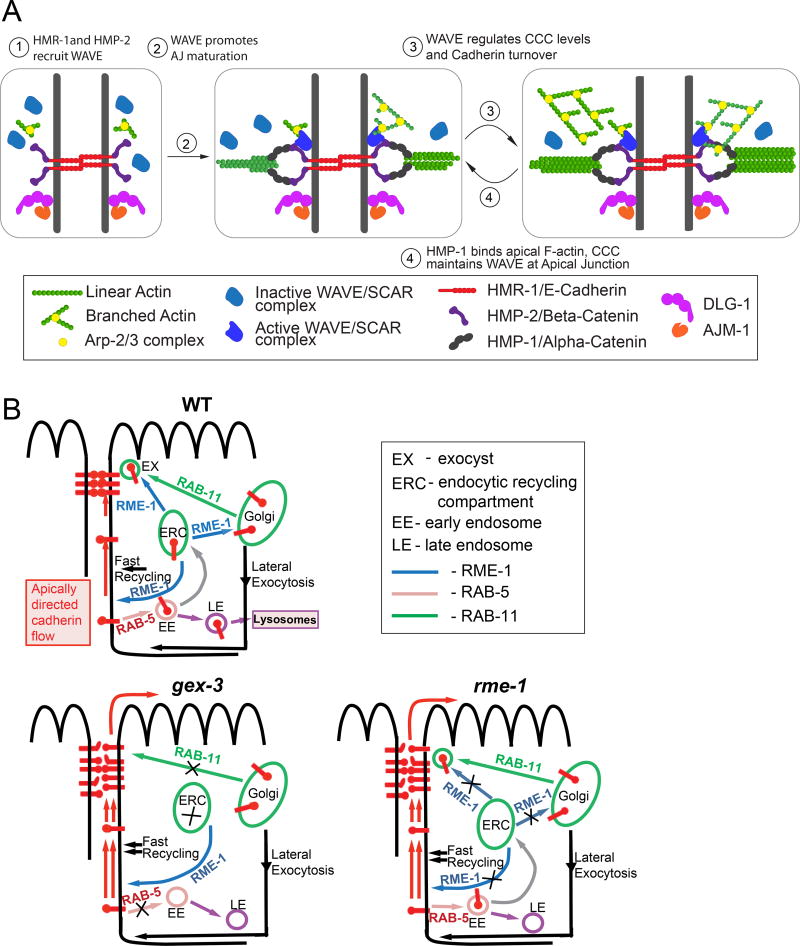
Our investigation of the role of branched actin in formation and maintenance of epithelial apical junctions (AJs) leads us to propose a developmental model (Figure 7A) for junction maturation and maintenance. As cells polarize, branched actin supports the apical recruitment of two apical junction complexes: the Cadherin complex, which directly binds apical actin, and the DAC which promotes maintenance of apical actin levels through an unknown mechanism. As the junctions mature, the movements of embryonic development require dynamic turnover of the Cadherin transmembrane receptor (Figure 7B), and the turnover depends on the WAVE complex. In turn, Cadherin and other CCC components are actively involved in recruiting WAVE complex to the apical junction. Therefore feedback between the activities of WAVE and CCC complexes is a critical aspect of AJ formation.
Figure 7. Models of WAVE effects on junctional maturation and Cadherin transport.
(A) Developmental model: Recruitment of WAVE depends on components of the DAC and CCC, with the exception of HMP-1/ α-catenin, the CCC component that binds to the belt actin. HMR-1, HMP-2 & DLG-1 promote apical WAVE enrichment. However, when HMP-1 is down regulated, apical WAVE enrichment moderately increases. This may indicate that WAVE and α-catenin compete for binding to the apical junction. As the junction matures, WAVE promotes cadherin junction function that leads α-catenin to bind increasing apical F actin. How does WAVE promote Cadherin function? (B) Effects of WAVE on Cadherin transport: Our data shows that cadherin turnover at the junction is affected in WAVE-mutants. Cadherin is targeted to the Apical Junction by at least three mechanisms: RAB-11-dependent delivery to AJ, lateral exocytosis to lateral membrane and targeted recycling (black line with arrow), and apically directed flow at the membrane (Woichansky et al., 2016). Previous work in our lab showed that WAVE regulates RAB-5-dependent endocytosis and RAB-11-dependent recycling of some cargoes in C. elegans (Patel, et. al., 2013). Our results suggest that in WAVE (gex-3) mutants rab-5 and rab-11-dependent trafficking steps are disrupted and apically directed flow of Cadherin in plasma membrane (PM) increases. In rme-1 mutants targeted recycling is disrupted and we propose that fast recycling becomes dominant. Apically directed flow of Cadherin in the PM is also increased as compared to wild type. In summary, we propose that WAVE controls Cadherin levels through specific trafficking steps, and we identify EHD1/RME-1 as a regulator of Cadherin at the AJ.
Our studies advance the understanding of how Cadherin is regulated at developing apical junctions. Three mechanisms have been shown to localize Cadherin to the apical junction (Woichansky et al., 2016): targeted RAB-11-dependent delivery to the apical junction, transport to the lateral membrane followed by endocytosis and targeted recycling, and apically directed flow within the plasma membrane (Figure 5A). Our findings suggest that branched-actin contributes to at least two of these mechanisms. In WAVE-depleted animals increased apically directed Cadherin flow and altered transport into the apical junction may cause the higher steady state levels and faster Cadherin recovery after photobleaching. First, to test if lateral transport was altered, we measured the ratio of apical to lateral Cadherin/HMR-1::GFP in control and gex-3 depleted intestines over time. We detected a mild increase in the ratio of apical to lateral Cadherin levels, from 1.3 in controls to 1.5 in the mutants at 360 min (Figure 5F). This suggests that branched actin restricts diffusion of Cadherin along the apical/basal axis within the membrane. It is possible that in gex-3 mutants more Cadherin trans-membrane receptors are diffusible rather than trapped in clusters. Therefore branched actin may alter Cadherin dynamics not only at the apical junction but also in the lateral membrane, to promote cellular polarity. This may be implemented in collaboration with formin proteins, which also can affect apically directed flow of Cadherin (Nishimura et al., 2016). Second, we considered that the changes in Cadherin levels and dynamics were caused by altered trafficking. Which trafficking steps (Figure 5A, 7B) require branched actin and are affected in the absence of WAVE? We compared Cadherin levels in wve-1 mutants to trafficking mutants (rab-5 or rab-11 RNAi). Increase in Cadherin levels at AJs cannot be simply explained by loss of recycling, since loss of the recycling regulator rab-11 results in less apical Cadherin. Loss of RAB-5 should increase Cadherin retention at the PM since it is a key regulator of endocytosis. Instead rab-5 RNAi-treated embryos showed a drop of apical Cadherin, like we see with loss of rab-11. RAB-5 associates not only with early endosomes but also with the plasma membrane and clathrin-coated vesicles (Chavrier et al., 1990; Bucci et al., 1992). One explanation for decreased Cadherin at the membrane in rab-5-depleted embryos is that Cadherin receptors become trapped in uncoated endocytic vesicles that cannot fuse with the early endosome due to lack of RAB-5 (Bucci et al., 1992). The amount of Cadherin in the subsequent trafficking steps would be diminished and less Cadherin would be delivered back to the AJ region.
We propose a novel role for EHD1/RME-1 as a regulator of Cadherin at the AJ. Surprisingly, loss of the endosomal regulator RME-1/EHD1 led to increased Cadherin levels and increase in the rate of Cadherin recovery. These effects are similar to changes in Cadherin apical retention and dynamics that occur due to removal of WAVE components. In C. elegans RME-1 is known to regulate transport of some cargo from recycling endosomes to membrane (Shi et al., 2010) and transport from recycling endosomes to Golgi via a pathway that may also involve WAVE-1 (Bai and Grant, 2015). RME-1 is the only EHD protein in C. elegans, while humans have four EDHs. It is therefore possible that RME-1/EHD regulates Cadherin trafficking at the various steps regulated by mammalian EHDs which include: recycling of membrane cargo from the ERC (endocytic recycling compartment) to the plasma membrane (EHD1), retrograde transport from EE (early endosomes) to Golgi (EHD3), vesicle transport from EE to the ERC, and EE to the lysosomal degradation pathway (EHD4) (Reviewed in (Zhang et al., 2012)). Therefore we propose that in gex-3 and rme-1 mutants more Cadherin is delivered to the AJ region while not enough is being removed causing an increase in AJ levels. This possibly occurs due to imbalance in targeted Cadherin recycling (ERC to PM), RAB-5 and RAB-11-dependent mechanisms, while fast recycling may not be affected (Figure 7B). The effect of RME-1/EDH on Cadherin transport is an important subject of future research. Our results warrant further analysis of how branched actin regulates Cadherin trafficking.
WAVE levels and recruitment to the apical intestine were also dependent on AJ proteins supporting the idea of interdependent accumulation of WAVE and junctional components. We analyzed WVE-1 accumulation in embryos depleted of AJ proteins (Figure 3). Loss of Cadherin/HMR-1 or β-catenin/HMP-2 led to strongly reduced apical enrichment of GFP::WVE-1. Unexpectedly loss of α-catenin/HMP-1 instead led to increased GFP::WVE-1 in both embryonic and adult apical intestine. Biochemical studies have long supported that α-catenin can inhibit branched actin formation or function (Drees et al., 2005; Pokutta et al., 2008). Preliminary studies in our lab suggest CCC components can immunoprecipitate WAVE, and vice-versa. An exciting explanation for our results would be that WVE-1 and α-catenin compete for binding to β-catenin, or that under some circumstances β-catenin is more likely to bind to WVE-1 rather than to α-catenin. Biochemical studies underway in our lab will test this model. This finding also suggests a mechanism for transitioning from recruitment of branched actin regulators to recruitment of α-catenin. Such a mechanism would further support that the interplay between the CCC and branched actin helps junctions to mature. Why does more WAVE need to be recruited as the junctions keep maturing? Yashiro and colleagues (Yashiro et al., 2014) reported that Rho1 regulates adherens junction remodeling by promoting recycling endosome formation through activation of myosin II. Therefore the Rac1/WAVE/Arp2/3 may be continuously needed at the junction to counteract Rho1/myosin II-dependent Cadherin trafficking. Rac1 has been also been shown to stabilize the VE-cadherin junction by counteracting actomyosin tension at the junction (Daneshjou et al., 2015).
Live imaging of apical F-actin for the most part confirmed what we had shown using phalloidin staining on fixed embryos, with one notable exception. Phalloidin staining gave the puzzling result that Cadherin components were not needed for apical F-actin enrichment (Bernadskaya et al., 2011), while live imaging using the VAB-10 actin binding domain instead showed that the CCC is required for apical F-actin (Figure 4). We had proposed that phalloidin detects belt actin, and perhaps even more strongly detects actin in the microvilli, which do not disappear in gex-3 or arp-2-depleted embryos ((Patel et al., 2008); Figure 6A,B). In contrast, actin binding proteins like VAB-10 may better bind belt actin, compared to microvillar actin. Therefore, the effects of cadherin loss on belt actin are more apparent with the VAB-10 actin binding transgene. This can be tested by creating new transgenes using other actin binding domains, like Lifeact and Utrophin. We are actively pursuing these experiments. A second striking result from this study and our previous study is the strong reduction in apical F-actin when the DAC junction is depleted (Figure 4B, and by phalloidin, (Bernadskaya et al., 2011)), which raises the question of how DLG-1 and AJM-1 connect to the actin cytoskeleton. While DLG-1 and AJM-1 do not appear to have direct actin binding domains, they may interact with other proteins that do. A recent genome-wide search for DLG-1 binding proteins may suggest some candidates (Waaijers et al., 2016).
WAVE complex and WAVE-induced branched actin continue to be essential for AJ structure in adult animals. TEM of C. elegans animals that were depleted of CCC, DAC or WAVE components after hatching showed that all three are needed for junction maintenance of the intestine. Thus, even in animals with previously established junctions, loss of the branched actin regulator GEX-3/Kette/NAP1 resulted in junctions of reduced length (Figure 6C), suggesting a continuing role for branched actin at junctions that have already formed. TEM results emphasize that the junctions in WAVE-depleted animals are disrupted. Therefore higher levels and faster turnover of Cadherin in embryonic AJs result in formation of dysfunctional apical junctions most likely because strength of adhesion is diminished. It is likely that cis- and trans-Cadherin interactions as well as proper size clusters cannot form as efficiently as in wild type embryos.
These studies suggest apical junction protein interactions in live animals are extremely complex and dynamic, even in tissues, like the C. elegans intestinal epithelium, that appear to be undergoing minimal movements or changes. Our future studies will address how the AJ proteins recruit WAVE, and how WAVE regulates Cadherin trafficking and localization to the AJ region. Since Cadherin trafficking changes are found in numerous cancers (Mosesson et al., 2008) insights into Cadherin junction establishment and maintenance are of high interest (Serrels et al., 2009). Understanding how branched actin protects cells from Cadherin dynamics misregulation could lead to new therapeutic approaches for suppressing metastasis.
Materials and Methods
Strains
C. elegans strains were cultured as described in Brenner (1974). The following published strains were used in this analysis: WM43 gex-3(zu196)/DnT1, RT1120 vha-6::ph::gfp, FT48 xnIs16 [dlg-1::gfp]; him-8, FT250 xnIs96 [pJN455: hmr-1p::hmr-1::gfp::unc-54 3’UTR; unc-119(+); unc-119 (ed3)], OX583 dlg-1::gfp ML1153 mcIs46 [dlg-1::RFP; unc-119+]; jcEX44, SU251 hmp-1(zu278); jcEX72 (pRF4, hmp-1::gfp), PE97 hmp-1(fe4), DH1201 rme-1(b1045), TH246 arx-2::TY1::EGFP::3×FLAG::Chr unc-119(+).
New strains built for this paper
OX616 pjIs16[Pend-1::vab-10 ABD::gfp; rol-6(su1006)], OX617 gex-3(zu196)/nT1-GFP; pjIs16[Pend-1::vab-10 ABD::gfp; rol-6(su1006)], OX618 ced-10(tm597)/nT1GFP; pjIs16[Pend-1::vab-10 ABD::gfp; rol-6(su1006)], OX655 hmp-1(fe4)/nT1-gfp; pjIs16 [Pend-1::vab10ABD::gfp; rol-6(si1006), OX656 hmp-1(zu278)/nT1-gfp; pjIs16 [Pend-1::vab10ABD::gfp; rol-6(si1006) OX631 pjIs16[Pend-1::vab-10 ABD::gfp; rol-6(su1006)]; hmp-1(zu278)/nT1-gfp, OX669 wve-1(pj64[gfp::3×FLAG:: wve-1]), OX704 hmr-1::mKate2; hmp-1::gfp, OX670 wve-1(pj64[gfp::3×FLAG::wve-1]); mcIs46 [dlg-1::RFP; unc-119+], OX671 wve-1(pj64[gfp::3×FLAG::wve-1]); hmp-1(zu278)/nT1::gfp, OX608 wve-1(ne350)/hT2-gfp; hmp1::gfp, OX525 gex-3(zu196)/nT1-gfp; xnIs96 [hmr-1::gfp], OX494 rme-1(n1045); xnIs96 [hmr-1::gfp]], OX609 wve-1(ne350)/hT2-gfp; hmp-1(cp20[hmp-1::gfp+ LoxP unc-119(+) LoxP]). OX774 (wve-1(pj64[gfp::3×FLAG::wve-1]; PE97 hmp-1(fe4))
Construction of the integrated transgene OX616 pjIs16 [Pend-1::vab-10 ABD::gfp; rol-6(su1006)]
The end-1 promoter from pJN507 was cloned into HindIII and PstI sites of pMC150, made from pML1572 digested with HindIII to remove the lin-26 promoter. Traditional injections (Mello et al., 1991) with the pRF4 Roller plasmid at 200ng/µl and the new construct at 20ng/µl were done, F3 Rollers were examined for intestinal expression. Several lines were integrated using UV-Tmp (Evans, 2006) All of the results presented used one integrated line, OX631, which has relatively steady expression if Rollers are picked in each generation.
Goldstein lab CRISPR strains
LP169 hmp-1(cp20[hmp-1::gfp + LoxP unc-119(+) LoxP]), LP237, hmp-2(cp56[gfp::hmp-2a]), LP238 hmr-1(cp57[gfp::hmr-1::mKate2]), LP172 hmr-1(cp21[hmr-1::gfp+LoxP], LP431 gex-3(cp163[GFP-C1^3×Flag::gex-3]).
Construction of CRISPR Knock In OX669 wve-1 (pj64 [gfp::3×FLAG::wve-1)]
The “Self-Excising Cassette” (SEC) cloning, tagging, and selection strategy developed by Dickinson et al. 2015 (Dickinson et al., 2015) was used. GFP SEC plasmid (pDD282) from Addgene in ccdB survival bacteria was digested with ClaI and SpeI for N-Terminus tag. An sgRNA directed at the 5’ end of the wve-1 gene [ATGCCTCTAACAAAACGGGCGG – PAM site is shown in bold] was first validated using the dpy-10 coCRISPR strategy(Arribere et al., 2014). F1 and F2 animals displaying Dumpy and Roller phenotypes were lysed and the wve-1 locus was PCR amplified and sequenced with primer pairs MSO1288 gctcgcttttatgaagtttccag and MSO1290 [acctaatttcccacctcaac]. 5’ and 3’ homology arms were amplified by PCR on genomic DNA using iProof (Cat. #127-5300) using the primers shown below. Purified homology arm PCR products and the pDD282 digest were ligated by Gibson Assembly using the NEB HiFi DNA Assembly Mix (E2621S). Plasmids were verified by sequencing. Injections mixes contained Cas9 plasmid (pDD162), wve-1 sgRNA, wve-1 SEC plasmid, and ttx-3::rfp at 50 ng/µL each in injection buffer(Arribere et al., 2014). Plates with rolling F1 progeny had hygromycin, 5 mg/mL, added for two days, and surviving Rollers were singled out. F2 Rollers expressing ttx-3::rfp were discarded since these indicate extrachromosomal, not integrated, SEC expression. N-terminal SEC tags introduce a stop codon, and null mutations of wve-1 are embryonic lethal, so ttx-3::rfp(−) hygromycin-resistant rollers were balanced over hT2-GFP, then heat shocked at 34°C for 4 hours to excise the SEC cassette. Wild type (non-Roller, non-Balancer) progeny of the heat shocked animals were allowed to self fertilize, and progeny were tested for correct excision by sequencing, out-crossed at least 3 times, and the expression was examined. Strain OX669 has 8% embryonic lethality. Primers for homology arms are listed below. Uppercase letters are SEC overlap given by Dickinson et al. 2015, lowercase letters are specific to the wve-1 locus. MSO1362 introduces silent mutations and a restriction site (bolded) into wve-1 PAM site at 5’ end. Designed with watcut [http://watcut.uwaterloo.ca/].
| MSO1360 | ACGTTGTAAAACGACGGCCAGTCGCCGGCAtgaatcacagcgagacactg |
| MSO1361 | TCCAGTGAACAATTCTTCTCCTTTACTCATtgctgaaaatgtgcagaaattgc |
| MSO1362 | CGTGATTACAAGGATGACGATGACAAGAGAatgcctctaacaaaacgtgctgtatc |
| MSO1363 | TCACACAGGAAACAGCTATGACCATGTTATcgcgtgatccactaatctgtcgattcc |
Feeding RNAi
For all feeding RNAi experiments, cDNAs of the genes were inserted into L4440 vector and transformed into HT115 cells. Saturated overnight cultures were diluted 1:250 and grown for 6–7 h until the OD600 was close to 1. Bacteria were resuspended in LB Amp, 100 µg/ml. 1mM IPTG was added to the bacteria and Amp plates before use. C. elegans animals were synchronized by hypochlorite treatment followed by hatching in M9 Buffer. For lysate preparation L1 worms were fed either control HT115 E. Coli, or HT115 containing the L4440 plasmid carrying the gene of interest and were grown at 23°C for 3 days. Lysates were made from the mixed population of adults and eggs. For embryonic analysis embryos were collected on day 3 and imaged. For adult analysis, animals were assayed for several intestinal phenotypes on day 3, except for animals fed arp-2 dsRNA, which were assayed on both day 2 and day 3.
Live imaging of embryos
Two to four cell stage embryos (0–20 minutes after first cleavage) were dissected from adult hermaphrodites and mounted either on a 3% agarose pads or with glass beads (Whitehouse Scientific Monodispersable Particle Standards, 25.60µm, #MS0026) using egg salts. Coverslips of 1.5mm thickness were sealed with Vaseline. The embryos were incubated at 23° C for 180/240 minutes, then imaged every 20 or 30 minutes for the next 240 minutes. Intervals were chosen to reduce photo bleaching of the signal and to avoid damage to the embryo due to the exposure to UV light. Spinning Disc images were acquired on a Zeiss AxioImager Z1 with a Yokogawa CSUX1-5000 spinning disc, using either a Plan Apo 40×/1.3 Oil lens or a Plan Apo 63×/1.4 Oil lens, on a Photometric Evolve 512 EMCCD camera, using Metamorph software and analyzed using ImageJ software.
Images were obtained so that the raw data of the original images was never saturated. Image analysis was done on raw data. Some images were enhanced for presentation purposes only as indicated in the corresponding Figure legends (Figures 1, 3 and 5). Adjusting the display range of these images, to better illustrate the faint intestinal signal sometimes led to saturation of the brighter anterior pharyngeal signal.
Microscopy of adults
Young adult stage animals (one day after L4 stage) were placed on agar pads in M9 solution and immobilized using 10µl of Levamizole(10µM) salts and covered with 1.5um coverslips. Images were taken within 15 minutes of making the pads. Imaging was done on the Zeiss AxioImager Z1 with a Yokogawa CSUX1-5000 spinning disc, using the Plan Apo 40×/1.3 Oil lens. Images were analyzed and measured using ImageJ.
Quantitation of fluorescence
Quantitation of live fluorescence was performed using the line selection and the dynamic profile function of ImageJ to measure fluorescence along lines of equal length that transversed the apical intestine and the cytoplasmic regions of the intestinal cells. The center of the line was placed over the apical intestine and the maximum reading generated from that region was recorded as the apical signal. The cytoplasmic signal of embryos was calculated as the average of the signal at the center of the two intestinal cells 2.5 um on either side of the apical intestine. For all experiments shown, the images were captured at the same exposure settings for wild type and mutants. All quantitation was done on the raw images.
FRAP imaging and data analysis
FRAP experiments were performed on the Zeiss AxioImager Z1 using the ILAS High Speed FRAP system. For FRAP experiments of intestinal AJs, the following multidimensional acquisition (MDA) profile was used: Prebleach 3 × 1s; Bleach with 100% power, 405nm laser: 8ms, line ROI; Postbleach: 5 × 2s; 15 × 10s; 2 × 60s. Images were acquired using 488nm laser, 25%, 500ms. For epidermal junctions: MDA profile was - Pre-bleach 3 × 2s; Bleach with 100% 405nm: 10ms × 8, line ROI; Post-bleach: 5 × 2s; 10 × 5s; 30 × 20s; acquisition with 488nm, 40%, 400ms. Normalized fluorescence intensity (IN(t)) profiles (Figure 5D) were obtained using FRAP profiler plugin in ImageJ. The half-time of recovery of and mobile fraction were calculated by fitting (Excel, Solver add-in) normalized curves to single-exponential equation IN(t) = Fm × (1 − e−τt), where Fm is mobile fraction, τ is time constant and half-time is calculated as t1/2 = −ln0.5/τ. For fitting, 22 (171 sec) post-bleach time points of intestinal and 14 (53 sec) post-bleach time points of epidermal FRAP curves were used. Normalized recovery curves for at least 2 embryos of each genotype, tissue or developmental time point were used to calculate the half-time and mobile fraction. Average normalized ± SEM and fitted curves are shown for 240 min samples in Figure 5C.
Electron Microscopy was performed as in (Bernadskaya et al., 2011).
Statistical Analysis
All graphs show the mean of the data and the error bars represent Standard Error of the Mean (SEM). For Figures 4 and 6, analysis was performed using GraphPad Prism. For grouped data, statistical significance was established by performing a two-way Analysis of Variance (ANOVA) followed by the Bonferroni multiple comparisons post-test. For ungrouped data, a one-way ANOVA was performed followed by the Tukey post-test. For Figures 3 and 5 Excel was used: significance of the mean difference between control and each mutant was determined by two-tailed, unpaired Student T-test. Asterisks (*) denote p values <0.05, (**) denote p values <0.005, (***) denote p values <0.0005.
Supplementary Material
Movie S1. Cadherin (HMR-1) and α-catenin (HMP-1) at epithelial junctions, shown by an hmr-1::mKate-2; hmp-1::gfp endogenously tagged strain. Arrow points to the intestine. Scale bar = 10 um. Related to Figure 1.
Movie S2. Time lapse DIC sequence of control (N2) and mutant strains analyzed in Figure 1. Scale bar = 10 um.
Movie S3. Live imaging of F-actin at the apical junction, shown by Pend-1::vab-10 ABD::gfp. Arrow points to the apical intestine. Scale bar = 10 um. Related to Figure 4.
Movie S4. Cadherin/HMR-1::GFP recovery in WT and gex-3 embryos in the intestine at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.
Movie S5. Cadherin/HMR-1::GFP recovery in WT and rme-1 embryos in the intestine at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.
Movie S6. Cadherin/HMR-1::GFP recovery in WT and gex-3 embryos in epidermis at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.
Highlights.
Accumulation of WAVE and Cadherin complexes is interdependent during AJ formation.
WAVE and Cadherin complexes accumulate concurrently at the AJ, ahead of DLG-1.
Loss of WAVE increases Cadherin accumulation by affecting its turnover at AJ.
WAVE and trafficking protein RME-1/EHD have similar effects on Cadherin turnover.
Acknowledgments
We thank the National Center for Research Resources–funded Caenorhabditis Genetics Center and Barth Grant, for strains, Bob Goldstein and Chris Higgins for the CRISPR CCC strains, Eric Larsen for technical support including generation of the WVE-1 CRISPR strain; the National Institute of Child Health and Human Development–funded University of Iowa Hybridoma Bank for DLG-1, and HMR-1 antibodies; Rajesh Patel and David Hall for help with the TEM. We thank our colleagues Loren Runnels, Barth Grant and lab members for helpful suggestions on the manuscript. S.B. and M.A. were supported by the NIH-funded INSPIRE IRACDA Fellowship (GM093854). This research was funded by a grant from the National Institutes of Health (GM081670) to M.C.S. and used a Spinning Disk Microscope acquired through a Shared Instrument Grant (1S10OD010572) to M.C.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Nonstandard Abbreviations: None
References
- Achilleos A, Wehman AM, Nance J. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development. 2010;137:1833–1842. doi: 10.1242/dev.047647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere JA, Bell RT, Fu BX, Artiles KL, Hartman PS, Fire AZ. Efficient marker-free recovery of custom genetic modifications with CRISPR/Cas9 in Caenorhabditis elegans. Genetics. 2014;198:837–846. doi: 10.1534/genetics.114.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Z, Grant BD. A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proceedings of the National Academy of Sciences. 2015;112:E1443–E1452. doi: 10.1073/pnas.1418651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadskaya YY, Patel FB, Hsu H-T, Soto MC. Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins. Molecular biology of the cell. 2011;22:2886–2899. doi: 10.1091/mbc.E10-10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadskaya YY, Wallace A, Nguyen J, Mohler WA, Soto MC. UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph polarize F-actin during embryonic morphogenesis by regulating the WAVE/SCAR actin nucleation complex. PLoS Genet. 2012;8:e1002863. doi: 10.1371/journal.pgen.1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher JM, Hahn B-S, Legouis R, Sookhareea S, Weimer RM, Gansmuller A, Chisholm AD, Rose AM, Bessereau J-L, Labouesse M. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. The Journal of cell biology. 2003;161:757–768. doi: 10.1083/jcb.200302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüser L, Bogdan S. Adherens Junctions on the Move—Membrane Trafficking of E-Cadherin. Cold Spring Harbor perspectives in biology. 2017;9:a029140. doi: 10.1101/cshperspect.a029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Calvo D, Victor M, Gay F, Sui G, Luke MPS, Dufourcq P, Wen G, Maduro M, Rothman J, Shi Y. A POP 1 repressor complex restricts inappropriate cell type-specific gene transcription during Caenorhabditis elegans embryogenesis. The EMBO journal. 2001;20:7197–7208. doi: 10.1093/emboj/20.24.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Lecuit T. Molecular bases of cell–cell junctions stability and dynamics. Cold Spring Harbor perspectives in biology. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen C-S, Hong S, Indra I, Sergeeva AP, Troyanovsky RB, Shapiro L, Honig B, Troyanovsky SM. α-Catenin–mediated cadherin clustering couples cadherin and actin dynamics. J Cell Biol. 2015;210:647–661. doi: 10.1083/jcb.201412064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin–cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. The Journal of cell biology. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshjou N, Sieracki N, van Nieuw Amerongen GP, Conway DE, Schwartz MA, Komarova YA, Malik AB. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J Cell Biol. 2015;208:23–32. doi: 10.1083/jcb.201409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Pani AM, Heppert JK, Higgins CD, Goldstein B. Streamlined Genome Engineering with a Self-Excising Drug Selection Cassette. Genetics. 2015;200:1035–1049. doi: 10.1534/genetics.115.178335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development. 2007;134:2469–2479. doi: 10.1242/dev.005074. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erami Z, Timpson P, Yao W, Zaidel-Bar R, Anderson KI. There are four dynamically and functionally distinct populations of E-cadherin in cell junctions. Biology open, bio. 2015 doi: 10.1242/bio.014159. 014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC. Transformation and microinjection. WormBook. 2006;10 [Google Scholar]
- Fricke R, Gohl C, Dharmalingam E, Grevelhörster A, Zahedi B, Harden N, Kessels M, Qualmann B, Bogdan S. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Current Biology. 2009;19:1429–1437. doi: 10.1016/j.cub.2009.07.058. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Current Biology. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG, Disanza A, Stradal TB, Cassata G, Confalonieri S, Hardin JD, Soto MC, Grant BD, Scita G. Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet. 2009;5:e1000675. doi: 10.1371/journal.pgen.1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature. 1996;382:455. doi: 10.1038/382455a0. [DOI] [PubMed] [Google Scholar]
- Han SP, Gambin Y, Gomez GA, Verma S, Giles N, Michael M, Wu SK, Guo Z, Johnston W, Sierecki E. Cortactin scaffolds Arp2/3 and WAVE2 at the epithelial zonula adherens. Journal of Biological Chemistry. 2014;289:7764–7775. doi: 10.1074/jbc.M113.544478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzle MK, Svitkina T. The cytoskeletal mechanisms of cell–cell junction formation in endothelial cells. Molecular biology of the cell. 2012;23:310–323. doi: 10.1091/mbc.E11-08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Troyanovsky RB, Troyanovsky SM. Binding to F-actin guides cadherin cluster assembly, stability, and movement. J Cell Biol. 2013;201:131–143. doi: 10.1083/jcb.201211054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Verma S, Ali RG, Ratheesh A, Hamilton NA, Akhmanova A, Yap AS. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nature Cell Biology. 2011;13:934–943. doi: 10.1038/ncb2290. [DOI] [PubMed] [Google Scholar]
- Labouesse M. Epithelial junctions and attachments. WormBook. 2006;13:1–21. doi: 10.1895/wormbook.1.56.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Current Biology. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Leung B, Hermann GJ, Priess JR. Organogenesis of the Caenorhabditis elegans intestine. Developmental biology. 1999;216:114–134. doi: 10.1006/dbio.1999.9471. [DOI] [PubMed] [Google Scholar]
- Li R, Gundersen GG. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nature reviews. Molecular cell biology. 2008;9:860. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Hardin J. The assembly and maintenance of epithelial junctions in C. elegans. Frontiers in bioscience: a journal and virtual library. 2009;14:1414. doi: 10.2741/3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden SL, Harrison N, Keegan J, Cain B, Lynch AM, Pettitt J, Hardin J. Specific conserved C-terminal amino acids of Caenorhabditis elegans HMP-1/α-catenin modulate F-actin binding independently of vinculin. Journal of Biological Chemistry. 2013;288:5694–5706. doi: 10.1074/jbc.M112.438093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston DJ, Higgins CD, Peters KA, Cupp TD, Dickinson DJ, Pani AM, Moore RP, Cox AH, Kiehart DP, Goldstein B. MRCK-1 Drives Apical Constriction in C. elegans by Linking Developmental Patterning to Force Generation. Current Biology. 2016;26:2079–2089. doi: 10.1016/j.cub.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege R-M, Gavard J, Lambert M. Regulation of cell–cell junctions by the cytoskeleton. Current opinion in cell biology. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. The EMBO journal. 1991;10:3959. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M, Yap AS. The regulation and functional impact of actin assembly at cadherin cell–cell adhesions. Seminars in cell & developmental biology. 2013;24:298–307. doi: 10.1016/j.semcdb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2008;1778:670–691. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ito S, Saito H, Hiver S, Shigetomi K, Ikenouchi J, Takeichi M. DAAM1 stabilizes epithelial junctions by restraining WAVE complex–dependent lateral membrane motility. J Cell Biol. 2016;215:559–573. doi: 10.1083/jcb.201603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pásti G, Labouesse M. Epithelial junctions, cytoskeleton, and polarity. WormBook: the online review of C. elegans biology. 2014:1–35. doi: 10.1895/wormbook.1.56.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel FB, Bernadskaya YY, Chen E, Jobanputra A, Pooladi Z, Freeman KL, Gally C, Mohler WA, Soto MC. The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev Biol. 2008;324:297–309. doi: 10.1016/j.ydbio.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel FB, Soto MC. WAVE/SCAR promotes endocytosis and early endosome morphology in polarized C. elegans epithelia. Developmental biology. 2013;377:319–332. doi: 10.1016/j.ydbio.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin–catenin function during epidermal morphogenesis. The Journal of cell biology. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem Soc Trans. 2008;36:141–147. doi: 10.1042/BST0360141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M, Murray JI, Schanze K, Pozniakovski A, Niu W, Angermann K, Hasse S, Rupprecht M, Vinis E, Tinney M. A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell. 2012;150:855–866. doi: 10.1016/j.cell.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrels A, Timpson P, Canel M, Schwarz JP, Carragher NO, Frame MC, Brunton VG, Anderson KI. Real-time study of E-cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer research. 2009;69:2714–2719. doi: 10.1158/0008-5472.CAN-08-4308. [DOI] [PubMed] [Google Scholar]
- Shi A, Chen CC-H, Banerjee R, Glodowski D, Audhya A, Rongo C, Grant BD. EHBP-1 functions with RAB-10 during endocytic recycling in Caenorhabditis elegans. Molecular biology of the cell. 2010;21:2930–2943. doi: 10.1091/mbc.E10-02-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas JM, Morrison HA, Bilder D, Skop AR. Polarity and endocytosis: reciprocal regulation. Trends in cell biology. 2010;20:445–452. doi: 10.1016/j.tcb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas JM, Skop AR. Arp2/3 mediates early endosome dynamics necessary for the maintenance of PAR asymmetry in Caenorhabditis elegans. Molecular biology of the cell. 2012;23:1917–1927. doi: 10.1091/mbc.E12-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JM, Ezhkova E, Silva J, Heart S, Castillo M, Campos Y, Castro V, Bonilla F, Cordon-Cardo C, Muthuswamy SK. Cyfip1 is a putative invasion suppressor in epithelial cancers. Cell. 2009;137:1047–1061. doi: 10.1016/j.cell.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simske JS. Claudins reign: The claudin/EMP/PMP22/γ channel protein family in C. elegans. Tissue barriers. 2013;1:e25502. doi: 10.4161/tisb.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang VW, Brieher WM. α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol. 2012;196:115–130. doi: 10.1083/jcb.201103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totong R, Achilleos A, Nance J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development. 2007;134:1259–1268. doi: 10.1242/dev.02833. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schötz E-M, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech P-H, Heisenberg C-P. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Developmental cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG. A WAVE2–Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Molecular biology of the cell. 2012;23:4601–4610. doi: 10.1091/mbc.E12-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijers S, Muñoz J, Berends C, Ramalho JJ, Goerdayal SS, Low TY, Zoumaro-Djayoon AD, Hoffmann M, Koorman T, Tas RP. A tissue-specific protein purification approach in Caenorhabditis elegans identifies novel interaction partners of DLG-1/Discs large. BMC biology. 2016;14:66. doi: 10.1186/s12915-016-0286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woichansky I, Beretta CA, Berns N, Riechmann V. Three mechanisms control E-cadherin localization to the zonula adherens. Nature communications. 2016;7 doi: 10.1038/ncomms10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. The Journal of cell biology. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro H, Loza AJ, Skeath JB, Longmore GD. Rho1 regulates adherens junction remodeling by promoting recycling endosome formation through activation of myosin II. Molecular biology of the cell. 2014;25:2956–2969. doi: 10.1091/mbc.E14-04-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM. Actin at cell-cell junctions is composed of two dynamic and functional populations. Journal of cell science. 2005;118:5549–5562. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]
- Zhang J, Naslavsky N, Caplan S. Rabs and EHDs: alternate modes for traffic control. Bioscience reports. 2012;32:17–23. doi: 10.1042/BSR20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Cadherin (HMR-1) and α-catenin (HMP-1) at epithelial junctions, shown by an hmr-1::mKate-2; hmp-1::gfp endogenously tagged strain. Arrow points to the intestine. Scale bar = 10 um. Related to Figure 1.
Movie S2. Time lapse DIC sequence of control (N2) and mutant strains analyzed in Figure 1. Scale bar = 10 um.
Movie S3. Live imaging of F-actin at the apical junction, shown by Pend-1::vab-10 ABD::gfp. Arrow points to the apical intestine. Scale bar = 10 um. Related to Figure 4.
Movie S4. Cadherin/HMR-1::GFP recovery in WT and gex-3 embryos in the intestine at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.
Movie S5. Cadherin/HMR-1::GFP recovery in WT and rme-1 embryos in the intestine at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.
Movie S6. Cadherin/HMR-1::GFP recovery in WT and gex-3 embryos in epidermis at 240 min. Arrows point to the bleached ROIs. Related to Figure 5.