Abstract
Five species of parasite cause malaria in humans with the most severe disease caused by Plasmodium falciparum. Many of the proteins encoded in the P. falciparum genome are unusually enriched in repetitive low-complexity sequences containing a limited repertoire of amino acids. These repetitive sequences expand and contract dynamically and are among the most rapidly changing sequences in the genome. The simplest repetitive sequences consist of single amino acid repeats such as poly-asparagine tracts that are found in approximately 25% of P. falciparum proteins. More complex repeats of two or more amino acids are also common in diverse parasite protein families. There is no universal explanation for the occurrence of repetitive sequences and it is possible that many confer no function to the encoded protein and no selective advantage or disadvantage to the parasite. However, there are increasing numbers of examples where repetitive sequences are important for parasite protein function. We discuss the diverse roles of low-complexity repetitive sequences throughout the parasite life cycle, from mediating protein–protein interactions to enabling the parasite to evade the host immune system.
Keywords: malaria, Plasmodium falciparum, protein repeats, low complexity, host-pathogen interaction, protein evolution
Repetitive sequences are widespread in Plasmodium falciparum proteins; the importance of these sequences is increasingly apparent.
INTRODUCTION
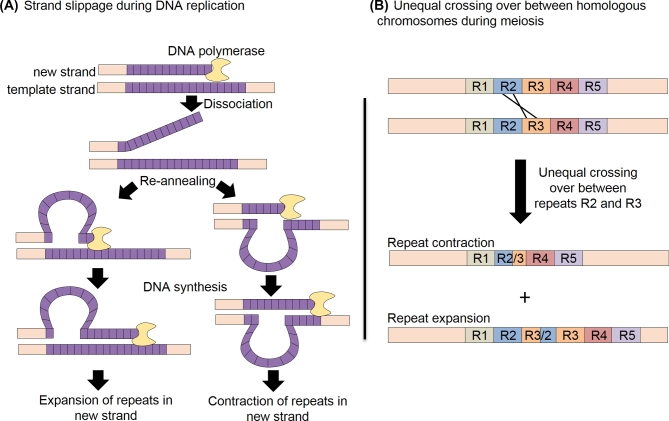
Repetitive nucleotide sequences expand and contract via strand slippage during DNA replication and unequal crossing over during meiosis (Fig. 1; Gemayel et al.2010). Within the genome of the Plasmodium falciparum strain 3D7, repetitive sequences are found on average once every 467 nucleotides (Tan et al.2010). These repeat encoding sequences tend to be highly dynamic; the rate of insertion and deletion mutations in genomes of parasites propagated in the asexual blood stage is approximately 10-fold greater than the rate of single base pair substitution (Hamilton et al.2016) and within coding sequences, the majority of insertion and deletion mutations occur in short tandemly repeated sequences (Hamilton et al.2016; Miles et al.2016). The variability of these sequences is also highlighted by the observation that 9% of genes contain repetitive sequences that vary in length between P. falciparum strains 3D7, Dd2 and HB3 (Tan et al.2010). Perfect repeats are predicted to have expanded more recently than degenerate repeats and are particularly common in Plasmodium species (Mendes et al.2013).
Figure 1.
Repeat expansion and contraction during DNA replication and unequal crossing over in meiosis.
Many short tandemly repeated sequences within P. falciparum proteins are predicted to be intrinsically disordered (Feng et al.2006; Guy et al.2015). In isolation, such sequences are likely dynamic and do not fold into stably folded structures but exist as an ensemble of conformations (Oldfield and Dunker 2014). In many cases, these sequences may not have a function per se but simply form disordered loops or termini protruding from folded domains. However, repeat sequences can also significantly change the properties of proteins and over evolutionary time expansion and contraction of repeats may fine-tune protein activity or even generate sequences de novo that can confer novel function. Indeed, low-complexity sequences, which include many repetitive sequences, are highly diverse. Whilst many are evolving neutrally, others are likely under selective pressure (Zilversmit et al.2010; Battistuzzi et al.2016).
Diverse functions for repetitive protein sequences in diverse organisms
Functional repetitive proteins from many species have been extensively characterized and illustrate many of the concepts that will also apply to this class of polypeptides from Plasmodium. Stretches of single amino acid repeats are often prone to self-association, leading to the formation of protein aggregates or fibres (Oma et al.2004). The detrimental effects of poly-glutamine (Gln) and poly-alanine sequence aggregation are well understood as they are associated with many human diseases (Gatchel and Zoghbi 2005; Shoubridge et al.2007). Expansion of these repeat-encoding sequences increases their aggregation propensity and contributes to disease onset. Expansion of single amino acid repeats is also important in non-pathological contexts; the activity of many yeast transcription factors can be fine-tuned over evolutionary time by expansion or contraction of poly-Gln sequences (Gemayel et al.2015). Artificial homo-polymeric sequences composed of different amino acids can direct proteins to different cellular compartments in mammalian cells (Oma et al.2004) and poly-histidine repeats in several human DNA-binding proteins can confer localization to nuclear speckles (Salichs et al.2009). Plasmodium falciparum contains a striking number of asparagine (Asn)-rich tracts; similar sequences have been shown to form fibres in yeast prion proteins that are believed to regulate protein activity and allow the cell to respond to environmental stimuli (Peters and Huang 2007; Halfmann, Alberti and Lindquist 2010; Halfmann et al.2011; Chernova, Wilkinson and Chernoff 2014).
Notably, there are several examples in which repeat length can influence the phenotype of an organism. For example, experimentally changing the number of repeats in the Saccharomyces cerevisiae cell surface proteins Flo1p and Flo11p proportionally changes the adhesion and hydrophobicity of the cells, respectively (Verstrepen et al.2005; Fidalgo et al.2006). In the case of Flo1p, a central repeat sequence forms a linker that allows an N-terminal adhesion domain to extend away from the cell surface; expansion of this sequence increases the efficiency of cell adherence. The repetitive sequence in Flo11p is hydrophobic and repeat expansion therefore increases the overall hydrophobicity of the yeast cell surface, allowing cells expressing Flo11p with longer repeats to float on the surface of a liquid medium. Repeat sequences can also contribute to altered phenotypes in more complex organisms; changes in repeat sequences in morphogen proteins are likely responsible for some of the different morphological features that have been selected for in domesticated dogs (Fondon and Garner 2004; Fondon and Garner 2007).
These examples establish several key concepts: firstly, repeat sequences can be important for protein function per se; secondly, expansion or contraction of repetitive sequences can cause partial or complete loss or gain of a protein function; thirdly, changes in protein activity arising from changes to repeat length can cause phenotypic changes, the magnitude of which can be proportional to the repeat length and finally, expansion and contraction of repeat sequences can potentially facilitate adaption of an organism over evolutionary time.
Protein interactions and structures of repetitive protein sequences
Although relatively few repetitive sequences from Plasmodium proteins have been extensively characterized, many of the applicable concepts relating to their structure and function have been well established in the context of repetitive proteins from other organisms (Andrade, Perez-Iratxeta and Ponting 2001; Kajava 2012). In isolation, many repetitive sequences are intrinsically disordered but upon interaction with a binding partner will assume a structured conformation. The structural plasticity and repeating modular architecture of repetitive low-complexity sequences result in binding interactions that can differ from those typically described for globular proteins.
Repetitive protein sequences can potentially recruit multiple copies of an interacting partner. For example, multiple copies of transcriptional terminator proteins can interact cooperatively with neighbouring repeats in the C-terminal domain (CTD) of yeast RNA polymerase II (Lunde et al.2010). Interaction with multiple copies of a binding partner can also increase interaction avidity. For example, the Staphylococcus aureus protein FnBPA contains short repeated motifs that interact with tandemly repeated β-sheet domains in human fibronectin. Although in isolation, FnBPA repeats are intrinsically disordered, each repeat within a single FnBPA protein interacts with up to four consecutive fibronectin domains by contributing an additional β-strand to each domain (Bingham et al.2008). As FnBPA contains multiple fibronectin-binding repeats, it can interact with multiple fibronectin proteins that are important for adhesion to and invasion of endothelial cells (Edwards et al.2010).
Alternatively, multiple repeats within a sequence can simultaneously interact with a single non-repetitive protein as illustrated by the interaction of the RNA polymerase II CTD with the mediator complex; here, the repeats interact with four structurally non-identical binding sites with each identical repeat assuming a different conformation (Robinson et al.2012). In addition, the CTD of RNA polymerase II interacts with many other proteins in an orchestrated manner that is regulated by its post-translational modification. This illustrates the key paradigm that repetitive sequences can function as regulated protein interaction hubs (Cumberworth et al.2013). In this context, the role of the P. falciparum RNA polymerase II CTD is discussed in a later section.
Although many repetitive sequences acquire structure only upon binding to a structured binding protein, some form structured domains in isolation. For example, the repeated three amino acid motifs in collagen assemble to form a right-handed triple helix (Shoulders and Raines 2009). Recent data also indicate that some repetitive sequences have a propensity to self-associate and undergo phase separation and thus partition into membraneless organelles within the cell (Kato et al.2012). Although in most cases the functions and interactions of repetitive sequences in Plasmodium proteins are not well understood, it is likely that these same principles will be relevant to many proteins that are expressed throughout the parasite lifecycle.
THE MALARIA PARASITE LIFECYCLE
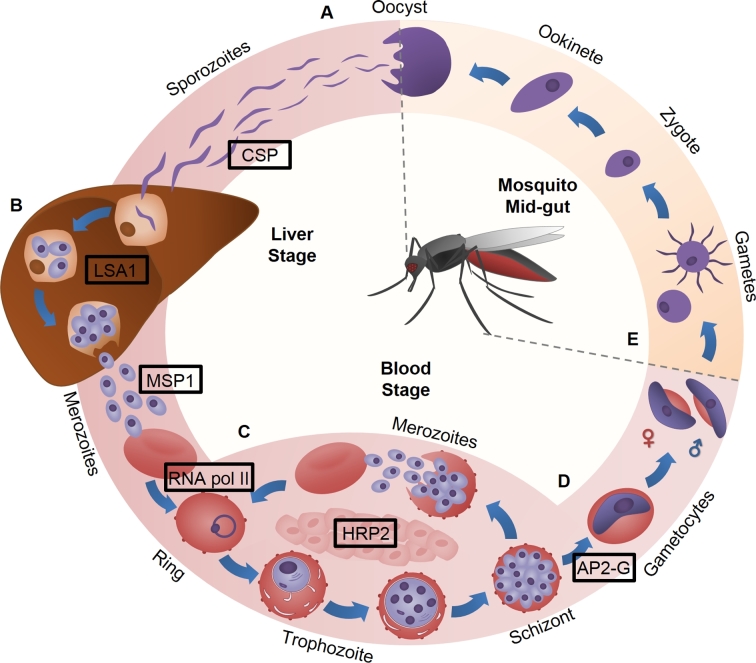
Repetitive proteins are expressed throughout the malaria parasite lifecycle during which the parasite undergoes several differentiation steps within both insect and vertebrate hosts (Fig. 2). The repetitive proteins discussed in this review, their repeat sequences, the variability of repeats between different P. falciparum strains, and their localization are summarized in Table 1.
Figure 2.
The P. falciparum life cycle. (A) Sporozoites are injected from the salivary glands of the Anopheles mosquito and migrate to the host liver. (B) Upon invasion of hepatocytes, thousands of merozoites are produced and subsequently released into the bloodstream. (C) Merozoites invade host erythrocytes and cycle through the ring, trophozoite and schizont stages before daughter merozoites egress from the host erythrocyte and invade new cells. (D) A small proportion of parasites differentiate into male and female gametocytes. (E) Gametocytes are ingested into the mosquito's midgut as it takes a blood meal where they form gametes and fuse to form a diploid zygote. The zygote differentiates into an ookinete which develops into an oocyst within the midgut wall. Upon oocyst rupture, sporozoites are released into the hemolymph and travel to the mosquito salivary gland. Proteins containing repetitive/low-complexity sequences are labelled at the approximate life stage where they are utilised.
Table 1.
Repetitive sequences in P. falciparum proteins. The repeat consensus for each protein was determined from P. falciparum 3D7 strain sequences using the programme XSTREAM, with gaps removed for clarity. The phases of some repeats were adjusted to those described in the literature. Where the repeating motif differs between isolates the alternative repeating motifs are shown in parentheses after the 3D7 consensus sequence. Variation in repeat numbers seen in 16 different P. falciparum strains (3D7, IT, HB3, DD2, 7G8, CD1, GA1, GB4, GN1, KE1, KH1, KH2, ML1, SD1, SN1, TG1, where two-letter codes represent the country of origin for the 11 field isolates) were determined manually using Jalview with TCOFFEE used to align the sequences. Several proteins, such as AP2-G, also contain homopolymeric amino acid repeats; these are not indicated in the table. Long-read PACBIO genome sequencing data was obtained from the Pf3K consortium.
| Gene identifier | Alias | Consensus motif in 3D7 | Number of repeats | Localisation |
|---|---|---|---|---|
| PF3D7_0930300 | MSP1 | GASAQS (GSGGSVASG/SGGSVT) | 2–6 (3–5/4) | Merozoite surface |
| PF3D7_0206800 | MSP2 | AGGS (TTTESNSPSPPI/PAGAGASGNP/AGAGASGS/GASGSG/SGSAGG/GAGAS/SGSAG) | 7–11 (4/6/8/13/6/9/3) | Merozoite surface |
| PF3D7_0207600 | SERA5 | QGSTGASP | 3–12 | PV + Merozoite surface |
| PF3D7_1035300 | GLURP | EILPEDKNEKVQHEIVEVE | 4–14 | Merozoite surface |
| SEKSVSEPAEHVEIV | 3 | |||
| PF3D7_1036400 | LSA1 | DLEQERLAKEKLQEQQS | 32–93 | Liver Stage |
| PF3D7_0318200 | RNA polymerase 2 | YSPTSPK | 6–11 | Nucleus |
| PF3D7_0831800 | HRP2 | AHHAAD | 38–46 | RBC cytoplasm |
| PF3D7_1222600 | AP2-G | KNN | 7–9 | Nucleus |
| DTYN | 16–18 | |||
| PF3D7_1370300 | MAHRP1 | HDHD | 3–5 | Maurer's clefts |
| DHG | 6–12 | |||
| PF3D7_0304600 | CSP | NANP | 40–46 | Sporozoite Surface |
| PF3D7_0935900 | REX1 | KPQAEKDASKLTTTYDQTKEV | 3–6 | Maurer's clefts |
| NKETKPQNDKYTL | 2 | |||
| PF3D7_0501300 | SBP1 | ASGIGNLVGDA | 5–6 | Maurer's clefts |
| QNAQ | 7–14 | |||
| PF3D7_1149000 | Pf332 | PVEEKNVSEEI | 6–11 | Maurer's clefts |
| FVTGELPEEDIINEKVQEEEE | 3–5 | |||
| EESASEEIVEDEGSV | 5–7 | |||
| ENVEEKKTMDEEIVDQGSVV | 3–4 | |||
| TEEVVEEEGSV | 5–10 | |||
| EEVVEEGSAT | 2–11 | |||
| EEIVEEEESSS | 11–12 | |||
| EEIVEEEGSVV | 10–24 | |||
| SVTEELVDEG | 2–3 | |||
| TEEIVED-EGSF | 6–7 | |||
| NEEILEEEGSY | 6–11 | |||
| GSATDYFVGQGSDNEEIIEE | 2–4 | |||
| IKEEQLDSEE | 6–20 | |||
| EVEEVSVDD | 2–5 | |||
| EEIEEIESVT | 2–4 | |||
| TEDVEEVSS | 4–8 | |||
| PF3D7_0401800 | PFD80 | STA | 9–18 | Maurer's clefts |
| RSASAASTT | 8–12 | |||
| STSTTQSPST | 3–6 | |||
| PF3D7_0102200 | RESA | EEPTVADEHV | 6–8 | RBC periphery |
| EENV | 41–47 | |||
| PF3D7_0113000 | GARP | EKK | 12–17 | RBC periphery |
| EKEKKKQ | 7 | |||
| EEHKE | 8–9 | |||
| KGKKD | 5 | |||
| EEDEDDA | 8–10 | |||
| PF3D7_0201900 | PfEMP3 | LEEYNETDLAKGKEVTNKAHEN | 17–19 | RBC periphery |
| KNKELQNKGSEGLKENAEL | 9–12 | |||
| NKDISNKDMKNKELL | 2–3 | |||
| QQNTGLKNTPSEG | 54–87 | |||
| PF3D7_0202000 | KAHRP | SKKHKDHDGEKKK | 4 | RBC periphery |
| ATKEASTSKE | 4–7 | |||
| PF3D7_0402000 | PHISTa | KQGGKKEEV | 9–14 | RBC periphery |
| PF3D7_0500800 | MESA | GESKET | 11–22 | RBC periphery |
| EKNDEKKDKVLGEGDKEDVK | 4 | |||
| KEKEEV | 7–11 | |||
| KEKEEV | 3–9 | |||
| ESEE | 17–26 | |||
| PF3D7_0532400 | LYMP | NKKVRGA | 5 | RBC periphery |
| ENKKAGT | 5–7 | |||
| PF3D7_1102300 | N/A | ERKEREEREKK | 9 | RBC periphery |
| EREKREKKEKE | 13–14 | |||
| PF3D7_1148700 | GEXP12 | KECVPNECMK | 8 | RBC periphery |
| PF3D7_1201000 | PHISTb/c | EKDEK | 18–36 | RBC periphery |
| DDDDEDDED | 7–8 | |||
| PF3D7_1476200 | PHISTb | KEQEKEKERKRKE | 4 | RBC periphery |
| PF3D7_1301400 | HYP12 | KKKEKQE | 8 | RBC cytoplasm |
| NEDE | 12 |
An infection is initiated when haploid sporozoites are injected into the human host as a female Anopheles mosquito takes a blood meal. Having migrated via the bloodstream to the liver, sporozoites invade hepatocytes and multiply into thousands of haploid merozoite forms before being released back into the blood (reviewed in Lindner, Miller and Kappe (2012)). The released merozoites invade erythrocytes and replicate intracellularly (reviewed in (Cowman et al.2016)). After replication within the erythrocyte, daughter merozoites egress and after a brief extracellular period invade new erythrocytes, undergoing further rounds of replication, egress and reinvasion. During the blood stage, a fraction of the parasite population becomes committed to differentiate into gametocytes, the sexual stage of the parasite lifecycle. Gametocytes can be taken up by a mosquito during a blood meal (reviewed in Bennink, Kiesow and Pradel (2016)). Within the mosquito, midgut male gametocytes undergo exflagellation to form motile microgametes and female gametocytes form macrogametes. Gamete fusion leads to the formation of a diploid zygote that differentiates into a motile ookinete that penetrates the mosquito midgut and forms an oocyst. Haploid sporozoites develop within the oocyst and are eventually released into the mosquito hemolymph before travelling to the salivary glands from where they can be injected into the next host (reviewed in Aly, Vaughan and Kappe (2009)). After gamete fusion, the parasite is briefly diploid but following a meiotic division the parasite returns to a haploid state and replicates mitotically for the remainder of the lifecycle. It is likely that expansion and contraction of gene sequences encoding repetitive proteins occur in both meiotic and mitotic phases of the lifecycle.
FUNCTIONS OF REPEAT PROTEINS ON THE PARASITE SURFACE, WITHIN THE PARASITE AND IN THE INTERACTION WITH HOST CELLS
Whilst many proteins expressed throughout the lifecycle contain repetitive sequences, the roles of these sequences are in most cases poorly understood. In the following sections, we will discuss how repetitive proteins interact with the host immune system as well as specific proteins and groups of proteins where the function or importance of repeat sequences has been established.
Repetitive Plasmodium proteins and the immune response
Within the human host, many repetitive parasite proteins are recognised by the immune system. A total of 79% of tandem repeats in parasite proteins fall within protein sequences predicted to be intrinsically disordered (Guy et al.2015). These sequences, in comparison to those from globular folded proteins, have a biased amino acid composition that results in fewer predicted MHCI and II peptides but a greater number of predicted B-cell epitopes (Verra and Hughes 1999; Hughes 2004; Guy et al.2015). Notably, antibody interactions with disordered epitopes are predicted to be more sensitive to epitope sequence changes than interactions with structured epitopes. Given the propensity of disordered sequences to tolerate sequence polymorphism, this may allow effective evasion of antibody responses against these sequences (MacRaild et al.2016).
Although sera from individuals living in endemic regions contain high levels of antibodies against repetitive proteins, it is unclear whether these confer protection against disease. It has been speculated that repetitive proteins can even be detrimental to an effective immune response by crosslinking B-cell receptors and inducing an inferior T-cell independent immune response (Schofield 1991). Although the antibody responses to most Plasmodium repetitive antigens have not been characterized, in mice there is a T-cell independent B-cell response to a repetitive sequence in the circumsporozoite protein (CSP; discussed later) but the bulk of the B-cell response is T-cell dependent (Fisher et al.2017). It has also been proposed that repetitive antigens can generate an ‘antigenic smokescreen’ in which numerous cross-reactive repetitive antigens interfere with the affinity maturation of antibodies against protective antigens (Kemp, Coppel and Anders 1987).
Nonetheless, many repetitive sequences are recognised by the immune system, and in particular those located on the parasite surface potentially represent important vaccine components and targets of host immunity. For the majority of the 48-h asexual replication cycle in the blood stage, the parasite resides within the host erythrocyte. However, after egress merozoites are extracellular for up to several minutes before they invade new erythrocytes. During this period, many repetitive merozoite surface proteins including MSP1, MSP2, GLURP and SERA5 (SERA5 is a parasitophorous vacuole protein important for egress (Collins et al.2017) but remains associated with the merozoite surface (Li et al.2002)) and others are exposed to the host immune system (Beeson et al.2016). SERA5 antibodies isolated from serum of Ugandan adults predominantly recognise the repetitive sequences within the protein and also show parasite growth inhibition in vitro in the presence of monocytes (Yagi et al.2014). Similarly, antibodies that recognise the repetitive sequences in MSP1 and GLURP also show parasite growth inhibition (Theisen et al.1998; Galamo et al.2009). Alleles of the repetitive sequence in MSP1 appear to be under balancing selection that is consistent with this sequence being under immune selection (Conway et al.2000). The repeating motifs of both MSP1 and MSP2 vary between isolates (Table 1), suggesting that there is not a strong functional constraint on these sequences and that they might easily change in response to immune pressure.
CSP is the major protein on the surface of the sporozoite that is also extracellular in the human host (Sinnis and Nardin 2002). Both T-cell and antibody responses are elicited against CSP during infection. The RTS,S vaccine, which is currently the most successful malaria vaccine, provides partial protection against disease (Hoffman et al.2015; Rts 2015) and is based on a fragment of the CSP comprising tandemly repeated NANP motifs and a C-terminal domain. The central repetitive sequence contains immunodominant antibody epitopes (Zavala et al.1985). Significantly, the repeat sequence in CSP is variable between parasite species. The repeating sequence motif in Plasmodium berghei CSP differs from those found in the P. falciparum and Plasmodium vivax proteins but despite this the repeat sequence from P. berghei CSP can be replaced with the repeat sequence from the P. vivax or P. falciparum protein without compromising function (Persson et al.2002; Espinosa et al.2013). Also, the number of repeats varies from 37 to 44 in different strains of P. falciparum (Neafsey et al.2015). Although variation in the number of repeat units in CSP does not significantly affect vaccine efficacy (Neafsey et al.2015), significant sequence variation can clearly be tolerated in the repeat sequences and their mutation may allow the parasite to evade B-cell responses without disrupting the function of CSP.
CSP—a repetitive sequence essential for sporozoite development
CSP, which is essential for parasite survival (Ferguson et al.2014), is linked via a C-terminal glycophosphatidylinositol anchor to the sporozoite plasma membrane. It consists of an N-terminal domain that binds heparin sulphate proteoglycans, a repeating central sequence and a C-terminal Thrombospondin repeat domain (Sinnis and Nardin 2002). Deletion of the central repeating sequence in the P. berghei CSP gene leads to a defect in sporozoite development in the mosquito. Although oocyst formation appears to be normal, the development of sporozoites is defective and sporozoites fail to appear in either the hemolymph or salivary glands of the mosquito (Ferguson et al.2014).
While many repetitive sequences are predicted to be entirely disordered, biophysical analyses are consistent with the CSP repeats adopting an elongated but flexible cylinder-like structure that is about 18 nm in length and 1.5 nm in diameter and composed of β-turn structures (Plassmeyer et al.2009). The model is based on the propensity of short NANP peptides to adopt a β-turn conformation (Nanzer et al.1997; Ghasparian et al.2006). NMR and circular dichroism studies suggest that CSP repeat peptides are in equilibrium between folded and extended states (Dyson et al.1990; Fisher et al.2017). Consistent with this, experiments examining the force required to unfold the CSP repeat region support a model in which CSP repeats can exist in a state that is partially structured, requiring relatively low force to unfold, but also in an unstructured state that requires almost no force to unfold (Patra, Sharma and Ainavarapu 2017). Antibody accessibility studies also support a model in which CSP is conformationally dynamic undergoing significant conformational changes in the oocyst, mosquito salivary gland and human liver (Coppi et al.2011; Herrera et al.2015). The CSP repeat structure may serve to extend the N-terminal domain away from the plasma membrane of the parasite. It is possible that deletion of the repeat sequence (Ferguson et al.2014) results in a shorter rod-like structure or alternatively it may prevent the entire protein from folding correctly. Such differences in an important protein on the surface of the sporozoite may be detrimental to development in the mosquito.
Liver stage antigen-1—a network of covalently crosslinked repeats
Liver stage antigen-1 (LSA-1) is localised in the parasitophorous vacuole during the liver stage of the lifecycle and is required for the progression of P. falciparum through this stage (Fidock et al.1994; Mikolajczak et al.2011). The repeats in LSA-1 are rich in Gln and Lys residues and are an in vitro substrate for transglutaminase that catalyses the covalent crosslinking of polypeptides. Although transglutaminase-crosslinked peptides can be detected in liver stage parasites by microscopy, it is unclear whether LSA-1 itself is crosslinked in vivo (Nicoll et al.2011), and whether this is important for liver-stage development.
RNA polymerase—a hub for regulated recruitment of binding partners
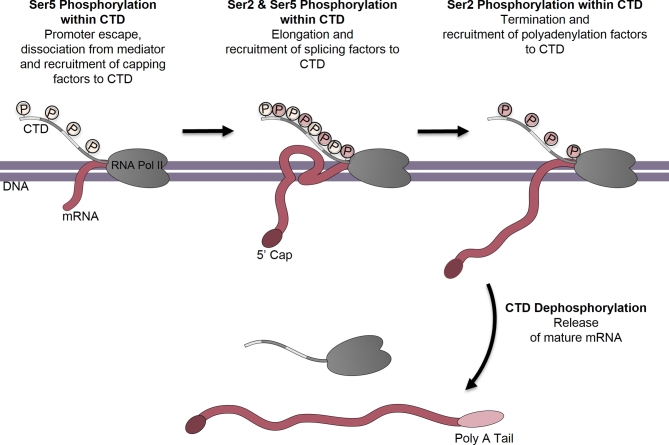
In eukaryotes, three different RNA polymerases (RNA Pol) are required to transcribe the nuclear genome; RNA Pol I transcribes ribosomal RNA (rRNA), RNA Pol II transcribes messenger RNA (mRNA) and RNA Pol III transcribes 5S rRNA and transfer RNA (tRNA; Vannini and Cramer 2012). As discussed previously, the C-terminus of the largest subunit (RbpI) of RNA Pol II contains a repetitive sequence known as the CTD (Corden et al.1985). This domain consists of a tandemly repeated Tyr-Ser-Pro-Thr-Ser-Pro-Ser/Lys sequence that undergoes reversible phosphorylation during the transcription cycle. Changes in the phosphorylation pattern of repeats within the CTD are thought to orchestrate the recruitment of various nuclear factors that couple processing of the nascent mRNA to transcription (Howe 2002; Fig. 3).
Figure 3.
RNA polymerase II CTD—phosphorylation and recruitment of proteins by the RNA pol II CTD during transcription
The CTD is essential for cell viability in yeast, drosophila and mice (Nonet et al.1987; Zehring et al.1988; Litingtung et al.1999). The overall length or number of the heptad repeats is also important as decreasing the number of repeats in the CTD of yeast from 26 to below 8 causes loss of viability (West and Corden 1995). Although small truncations of the yeast CTD are tolerated, these result in reduced transcript levels of certain genes. Truncation of the CTD in yeast to 17, 14, 13 and 11 repeats leads to the production of 58%, 8%, 5% and 2% of normal transcript levels of specific genes, respectively, signifying the importance of the repetitive nature of the CTD (Liao et al.1991).
Although the consensus sequence and number of heptad repeats in RNA pol II vary from one organism to another, it is strictly conserved along evolutionary lineages; for example, all mammalian CTDs possess 52 heptapeptide repeats with little variation in the consensus (Chapman et al.2008). Strikingly, there is considerable variation in the number of repeats found across the Plasmodium genus. Rodent and bird-infecting species, including P. berghei and Plasmodium gallinaceum, respectively, possess eight repeats, while the CTD of some primate-infecting species has expanded resulting in 13–15 repeats. This expansion appears to have arisen twice during Plasmodium evolution; once in the lineage that has given rise to P. falciparum and once in the lineage that has given rise to P. vivax (Kishore et al.2009). Primate infecting parasites appear to have preferentially expanded a heptad repeat containing a lysine (Lys) residue in the seventh position (Kishore et al.2009). Furthermore, this plasticity extends not only to different Plasmodium species, but also to different isolates within a given species, making Plasmodium one of the few organisms that show intra-species variation in the number of heptad repeats in its RNA pol II CTD (Kishore et al.2009). Although the significance of the repeat expansions is unclear, it has been suggested that repeat expansion may confer additional functions to the CTD (Ukaegbu and Deitsch 2015).
In higher eukaryotes, it is known that the CTD recruits proteins with diverse roles in transcription and gene expression. These include factors such as histone-modifying enzymes (Yoh, Lucas and Jones 2008). The CTD of P. falciparum RNA pol II interacts with PfSET2 (also referred to as PfSETvs), a histone methyl transferase that tri-methylates histone H3 at Lys 36 (H3K36me3) (Cui et al.2008; Jiang et al.2013; Ukaegbu et al.2014). This histone modification is found in telomeric and subtelomeric chromatin and associated with genomic regions encoding the PfEMP1 var gene family (Jiang et al.2013; Ukaegbu et al.2014). Although there are more than 60 var genes in the P. falciparum genome, mRNA from only a single var gene is produced at any one time and consequently a single PfEMP1 protein is displayed on the surface of an infected erythrocyte. By switching var gene expression, the parasite is able to evade an immune response directed against a particular PfEMP1 protein. Although mRNA from only a single var gene is transcribed at any one time, RNA pol II also transcribes non-coding RNAs from var genes. It is likely that RNA pol II recruits PfSET2 to var genes during the latter process. Although the precise mechanism by which PfSET2 and H3K36 tri-methylation regulate var gene silencing is unclear, deletion of PfSET2 or expression of a dominant-negative version of PfSET2 that interacts with the RNA pol II CTD results in dis-regulation of var gene expression, strongly indicating a role for the CTD and its interacting partner PfSET2 in this process (Jiang et al.2013; Ukaegbu et al.2014).
Homopolymeric Asn-rich repeats and protein aggregation
One of the best-studied examples of repetitive protein sequences modulating protein properties comes from the study of poly-Gln tracts. Expansion of these homopolymeric tracts leads to protein aggregation and is associated with ‘poly-Gln diseases’ such as Huntington's disease in humans (Gatchel and Zoghbi 2005). However, it has also been proposed that such poly-Gln repetitive sequences modulate protein function and that expansion and contraction of repeats may fine-tune protein function and phenotype over evolutionary time (Gemayel et al.2015).
Single amino acid repeats of the amino acid Asn are prevalent in the P. falciparum genome. Like poly-Gln sequences, poly-Asn sequences are also prone to aggregation particularly at elevated temperatures (Halfmann et al.2011) that the parasite would experience during fever. Given that protein aggregation is often toxic to cells, it is remarkable that the parasite proteome maintains so many potentially toxic Asn-rich repeats; more than a quarter of its proteins contain such sequences. Expression of a GFP-tagged version of the Asn-rich protein from P. falciparum, PF3D7_0923500, in human cells leads to accumulation of fluorescent protein aggregates as does expression of the N-terminal prion forming domain of S. cereviasiae Asn/Gln-rich Sup35 (Muralidharan et al.2012). Co-expression of the human Hsp110 or Plasmodium homologue PfHsp110c inhibits aggregate formation; PfHsp110c does so approximately 10–15-fold more efficiently than the human chaperone (Muralidharan et al.2012). This suggests that Plasmodium chaperones may be particularly efficient at suppressing the aggregation of Asn-rich repeat sequences. Although PfHsp110c is essential for survival, a reduction of expression leads to increased aggregation of Asn-rich proteins and parasites cannot recover from short periods of heat shock (Muralidharan et al.2012). These data are consistent with a model in which PfHsp110c plays a key role in maintaining the solubility of Asn-rich proteins under heat shock conditions (Muralidharan et al.2012).
The function of Asn-rich repeats in the parasite is unclear and in many cases these sequences have likely evolved without cost or benefit. Notably, deletion of the Asn-rich repeat sequence in the proteasome protein Rpn6 does not compromise its function (Muralidharan et al.2011). However, several transcription factors in Saccharomyces cerevisiae contain poly-Gln repeats and variation of the repeat length can fine-tune transcription of target genes and resulting phenotypes (Gemayel et al.2015). This is at least partially due to changes in protein solubility and interaction partners. Notably, the majority of AP-2 transcription factors encoded in the parasite genome have very high asparagine content of between 20% and 30%. The P. falciparum protein AP2-g, which triggers the transition to gametocytogenesis (Kafsack et al.2014; Sinha et al.2014), comprises 2432 residues, 29.9% of which are Asn. Asn residues are found both in homopolymeric repeats, the longest of which is 33 amino acids in length, and in short tandemly repeated sequences. It is unclear if variations in the AP2-g Asn-rich repeats seen in different strains (Campino et al.2016) fine-tune its solubility/activity and hence the propensity of parasites to form gametocytes. Changes in poly-Asn sequences in other AP2 transcription factors might also modulate their activity leading to small changes in progression through the lifecycle; even small delays in the asexual cycle of the parasite could lead to a selective advantage. Additionally, given the propensity of Asn-rich repeats to aggregate, particularly under heat shock, it is possible that solubility/activity of transcription factors might change during fever.
Asn repeats and protein translation
The rate of translation of a protein is determined by the concentration of available aminoacylated tRNAs. Given the unusually high occurrence of Asn residues in the P. falciparum genome, particularly in long low-complexity repetitive sequences, the parasite might require large amounts of Asn:tRNA to efficiently translate Asn-rich proteins. In many organisms, there is redundancy in the genes encoding tRNAs for each amino acid. For example, in Caenorhabditis elegans, there are 46 genes encoding tRNAs that recognise the four glycine codons (Duret 2000). Given the abundance of Asn in encoded proteins, it might be expected that the P. falciparum genome would encode multiple tRNAs that recognize Asn codons thus allowing the parasite to make high levels of Asn:tRNA. However, only a single gene encodes Asn:tRNA (Gardner et al.2002) and levels of Asn:tRNA are not proportionally more abundant. This has led to a model in which translation is slowed down when the ribosome encounters long low-complexity Asn-repeat sequences due to the relatively lower concentrations of Asn:tRNA (Frugier et al.2010; Filisetti et al.2013). This could slow the rate of polypeptide emergence from the ribosome tunnel and allow N-terminal domains that have already been synthesised more time to fold before emergence of subsequent domains. This may increase the overall efficiency of protein folding.
MODIFICATION OF THE HOST ERYTHROCYTE BY REPETITIVE EXPORTED PROTEINS
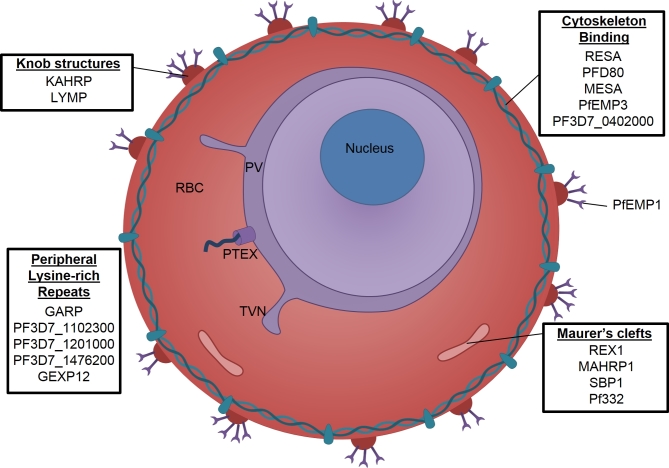
The human erythrocyte is a relatively simple terminally differentiated cell that lacks internal organelles. During the blood stage of infection, the parasite profoundly remodels its host cell, establishing new permeability pathways for acquiring nutrients, modifying the erythrocyte cytoskeleton to increase rigidity and generating knob-like structures on the surface of the host cell that allow infected cells to cytoadhere to endothelial cells in the microvasculature (Boddey and Cowman 2013; Spielmann and Gilberger 2015; Spillman, Beck and Goldberg 2015; de Koning-Ward et al.2016; Przyborski, Nyboer and Lanzer 2016). The parasite brings about these changes by exporting over 400 proteins into the host cell; these proteins can be localised to the erythrocyte cytoplasm, plasma membrane, the underlying spectrin cytoskeleton or to Maurer's clefts, which are membranous structures assembled by the parasite (Fig. 4). Many exported proteins are characterized by the presence of an N-terminal signal sequence followed by a short motif referred to as a protein export element (PEXEL) or host targeting (HT) motif (Hiller et al.2004; Marti et al.2004; Boddey et al.2013; Schulze et al.2015). Other exported proteins lack PEXEL sequences and are referred to as PEXEL negative exported proteins (PNEPs; Heiber et al.2013). Notably, many exported proteins contain repetitive sequences that play key roles in diverse processes including cytoadhesion, formation of Maurer's clefts, protein localisation and detoxification of heme (Fig. 4).
Figure 4.
Repetitive proteins exported into the host cell. Many proteins are exported into the host erythrocyte by P. falciparum. These are involved in creating new structures within the host cell: membranous structures called Maurer's clefts (MC) appear in the host cell cytoplasm, a ‘tubovesicular network’ (TVN) extends from the parasitophorous vacuole (PV) and ‘knob’ protrusions form on the cell surface which present the cytoadhesive surface protein PfEMP1. Many repetitive proteins interact with the spectrin cytoskeleton of the erythrocyte resulting in increased cell rigidity.
Lys-rich repeats in exported proteins—changes in repeat length can influence protein localization
There are several examples of repetitive proteins, such as the yeast proteins Flo1p and Flo11p (discussed in previous section), where the function of the protein can be proportionally modulated by repeat length (Verstrepen et al.2005; Fidalgo et al.2006). It is conceptually clear in such cases how relatively small changes in the number of tandemly repeated sequences could be important in facilitating adaption of an organism to its environment. In Plasmodium, many proteins including exported proteins contain low-complexity Lys-rich repetitive sequences. In some cases, these sequences are adjacent to a folded domain but they can also be found in proteins that are predicted to be entirely intrinsically disordered. In several proteins that are exported into the host cell by a PEXEL motif, Lys-rich sequences can target the protein to the periphery of the infected erythrocyte (Davies, Thalassinos and Osborne 2016). Comprising relatively simple tandemly repeated motifs of as few as three residues (e.g. Lys-Lys-Glu), these sequences are likely to interact with either lipids or proteins in the erythrocyte plasma membrane or the underlying erythrocyte spectrin cytoskeleton.
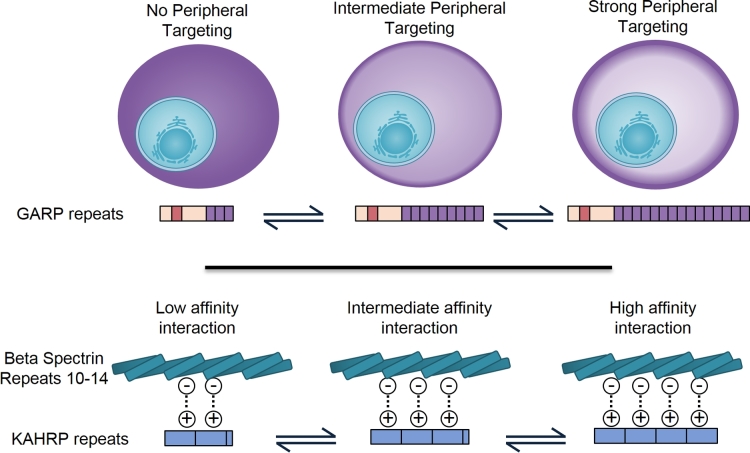
The exported P. falciparum protein glutamic acid-rich protein (GARP) contains multiple Lys-rich sequences that function in this manner. Significantly, the targeting propensity of a repetitive Lys-rich sequence from GARP is proportional to its length—increasing the number of tandemly repeated sequences proportionally shifts the protein from being diffusely localised in the erythrocyte cytoplasm to being highly concentrated adjacent to the plasma membrane of the infected cell (Fig. 5; Davies, Thalassinos and Osborne 2016). At least nine exported parasite proteins contain Lys-rich sequences that independently target to the periphery of the erythrocyte. The lengths of repeating Lys-rich sequences from many of these exported proteins vary between parasite strains (Davies, Thalassinos and Osborne 2016). Additionally, in the protein MESA, a block of sequence made up of tandemly repeated Lys-rich motifs has itself been duplicated with two to four copies of the sequence in different parasite strains (Davies, Thalassinos and Osborne 2016). Although the phenotypic consequences of repeat expansion and duplication have not been determined experimentally, several of these proteins are known to modulate rigidity and adhesion of infected cells. Given this, expansion and contraction of Lys-rich sequences may proportionally change the concentration of proteins localised to the periphery of the host cell, fine-tuning processes such as cell rigidity or adhesion, and facilitating adaption of the parasite to selective pressure over evolutionary time.
Figure 5.
Expansion and contraction of Lys-rich repetitive sequences and changes in protein localization or protein-binding affinity. The top panel illustrates the correlation between the length of a Lys-rich sequence from the protein GARP and the efficiency with which it is targeted to the erythrocyte periphery. The lower panel illustrates the changes in binding affinity between β-spectrin and KAHRP repeats as the number of repeats in KAHRP is altered.
A comparison of the protein sequence of GARP from three different species, P. falciparum, Plasmodium reichenowi, and Plasmodium gaboni, indicates that repeat expansion can also lead to de novo formation of a targeting domain (Davies, Thalassinos and Osborne 2016). There are three stretches of Lys-rich repetitive sequence within P. falciparum GARP. The first sequence in the P. falciparum protein is able to target a GFP reporter to the periphery of the host cell. However, the equivalent Lys-rich sequence in the P. reichenowi protein is shorter and does not efficiently target to the periphery of the host cell. The second Lys-rich sequence is conserved and is likely to be functional in all three species. The third Lys-rich sequence, which comprises two different repeated motifs in P. falciparum GARP, can target a protein to the erythrocyte periphery but the equivalent sequence in P. gaboni comprises only one or two repeated Lys-rich motifs and cannot target GFP to the periphery of the infected erythrocyte. These observations show not only that repeat expansion of Lys-rich sequences can modulate protein localization, but also that functional targeting sequences can be generated de novo by repeat expansion over evolutionary time.
The localisation of Hyp12 (PF3D7_1301400), which modulates the rigidity of the infected cell (Maier et al.2008), appears to be defined by two conflicting sequences: a repetitive Lys-rich sequence and a repetitive acidic sequence (Davies, Thalassinos and Osborne 2016). Hyp12 is unusual in that the Lys-rich repeat sequence alone is able to target GFP to the periphery of the infected cell but the GFP-tagged full-length protein is localised in the erythrocyte cytoplasm (Petersen, Matuschewski and Ingmundson 2015; Davies, Thalassinos and Osborne 2016). Removal of the acidic sequence results in the protein being localised to the red cell periphery suggesting that the acidic sequence inhibits the targeting function of the Lys-rich repeat sequence. It is possible that a specific stimulus might unmask the sequence during an infection. Over evolutionary time, it is likely that either expansion of the Lys-rich region or contraction of the acidic sequence could shift the protein from the erythrocyte cytoplasm to the periphery of the erythrocyte.
Knob-associated histidine-rich protein—repeat protein interactions and parasite virulence
Knob-associated histidine-rich protein (KAHRP) is one of the best-characterised repeat-containing proteins. It is a key component of structured spiral-like ‘knob’ protrusions that the parasite assembles at the surface of the infected erythrocyte (Crabb et al.1997; Watermeyer et al.2016). Knobs are involved in displaying PfEMP1 proteins on the surface of the infected cell where they mediate adhesion to specific ligands allowing parasites to become sequestered in tissues thus avoiding passage through the spleen (Crabb et al.1997). KAHRP interacts with the host cytoskeletal components spectrin (Kilejian et al.1991; Pei et al.2005) and ankyrin (Weng et al.2014) and deletion of the gene causes a loss of knob structures, decreases adhesion under flow conditions (Crabb et al.1997) and reduces erythrocyte rigidity (Glenister et al.2002). KAHRP contains an N-terminal repetitive poly-histidine stretch that will target GFP to the periphery of the infected erythrocyte (Rug et al.2006). It also has two unique Lys-rich repetitive regions, known as the 5' and 3' repeats; only the 5' repeat sequence is sufficient to independently target GFP to the erythrocyte periphery (Davies, Thalassinos and Osborne 2016). Progressive truncation of the C-terminus of KAHRP indicates that the 5' repeats are important for the role of KAHRP in cytoadhesion (Rug et al.2006). Several studies indicate that these repeats interact with spectrin (Kilejian et al.1991; Pei et al.2005; Cutts et al.2017). Most recently, it has been demonstrated in vitro that the repeats interact with β-spectrin domains 10–14 and more weakly with α-spectrin domains 12–16. Significantly, progressive removal of repeats from the KAHRP repeat sequence leads to a corresponding reduction of binding affinity for β-spectrin domains 10–14 i.e. the binding affinity is proportional to the number of KAHRP repeats (Fig. 5; Cutts et al.2017). This again highlights the potential importance of repeat expansion and contraction in modulating protein function. Modelling and molecular dynamics simulations support a model whereby the KAHRP repeats adopt an extended conformation of the surface of spectrin, interacting with charge complementary surfaces on multiple β-spectrin domains (Cutts et al.2017).
Although knob structures are unique to P. falciparum, KAHRP-like proteins are found in all primate-infecting Plasmodium species (Sargeant et al.2006; Davies, Thalassinos and Osborne 2016). These proteins share an N-terminal EKAL domain (EMP3 KAHRP-like domain) followed by Lys-rich repeating sequences but the function of the proteins in other species is not known. The repeated Lys-rich motifs in the P. falciparum and Plasmodium knowlesi proteins both contain consecutive Lys residues but otherwise differ significantly. However, when expressed in P. falciparum, both can target a protein to the periphery of the infected cell (Davies, Thalassinos and Osborne 2016). It is likely that both these repetitive Lys-rich sequences interact with a negatively charged surface on spectrin. Presumably the binding interface can tolerate somewhat diverse conformations of positively charged interacting peptides. Notably, there does not appear to be a strong consensus sequence among all of the different Lys-rich targeting sequences identified thus far. Identification of the binding partners, which could be proteins or lipids, for each protein and further structural studies will be required to understand the functions of these diverse repetitive sequences.
Although KAHRP contains a conserved EKAL domain of unknown function at its N-terminus (Sargeant et al.2006; Davies, Thalassinos and Osborne 2016), the remainder of the protein is predicted to be intrinsically disordered and much of the sequence is repetitive. Likewise, PfEMP3 contains a conserved N-terminal EKAL domain and several different repetitive sequences at its C-terminus but the repetitive sequences are very different from those of KAHRP. It is likely that the PfEMP3 gene arose as a duplication of the KAHRP gene or vice versa yet gene knockout and functional studies show that the two proteins are not functionally equivalent (Crabb et al.1997; Waterkeyn et al.2000; Glenister et al.2002). Likely, after gene duplication, the dynamic repetitive sequences have diverged dramatically and consequently the function of the two proteins has likewise also diverged. The dynamic nature of repetitive sequences together with the telomeric location of many of the genes encoding exported proteins suggests that gene duplication and rapid diversification have occurred frequently during the evolution of the parasite; repeat expansion likely plays an important role in the latter process.
Plasmodium helical interspersed subtelomeric/Plasmodium RESA N-terminal domain proteins
A large group of functionally diverse exported parasite proteins belong to the Plasmodium helical interspersed subtelomeric (PHIST) or Plasmodium RESA N-terminal (PRESAN) domain family (Sargeant et al.2006; Oakley et al.2007; Warncke, Vakonakis and Beck 2016). The proteins are characterized by the presence of a conserved alpha helical domain (Mayer et al.2012) and are exported into the infected erythrocyte during the asexual erythrocyte stages and during gametocytogenesis (Sargeant et al.2006; Oakley et al.2007; Silvestrini et al.2010; Tiburcio et al.2015). One of the best characterised proteins in this family is LyMP (Lys-rich membrane-associated PHISTb), which modulates erythrocyte cytoadhesion by linking PfEMP1 to the erythrocyte cytoskeleton (Oberli et al.2014; Proellocks et al.2014; Tarr et al.2014; Oberli et al.2016). LyMP contains an N-terminal PHIST/PRESAN domain, which interacts with the cytoplasmic tail of PfEMP1 (Oberli et al.2014), and a C-terminal sequence that interacts with the erythrocyte cytoskeletal protein Band 3 (Oberli et al.2016). The extreme C-terminus of LyMP contains a Lys-rich repetitive sequence that when GFP tagged and exported into the erythrocyte is sufficient to localise to the erythrocyte periphery (Davies, Thalassinos and Osborne 2016). Although the fragment of LyMP that has been shown to interact with Band 3 in vitro contains the Lys-rich sequence, it is unclear whether this sequence interacts with Band 3 or another component of the erythrocyte cytoskeleton.
LyMP is an example of a gene that encodes a folded domain (PHIST/PRESAN domain) but has expanded a repetitive Lys-rich sequence that likely allows the encoded protein to interact with an additional protein. Similarly, GEXP12, PF3D7_1201000, PF3D7_1476200 and PF3D7_0402000 contain a PHIST/PRESAN domain but also contain Lys-rich sequences that alone localise to the periphery of the infected erythrocyte (Davies, Thalassinos and Osborne 2016). The PHIST/PRESAN domain of PF3D7_0402000 interacts with band 4.1 and ankyrin that are components of the erythrocyte cytoskeleton (Vignali et al.2008; Parish et al.2013; Shakya et al.2017), but the protein also contains a C-terminal Lys-rich repetitive targeting sequence (Davies, Thalassinos and Osborne 2016). Other PHIST/PRESAN proteins, such as PFD80 (Tarr et al.2014) and RESA (ring-expressed surface antigen) (Brown et al.1985), localize to the erythrocyte cytoskeleton and have expanded repetitive sequences that are not Lys-rich and do not independently confer a cytoskeletal localization. RESA modulates the rigidity of the infected cell and deletion of the gene does not affect cytoadhesion (Maier et al.2008). Although the role of PFD80 is unclear, it is likely essential as attempts to delete the gene have been unsuccessful (Maier et al.2008). The localization and likely functions of PHIST proteins are diverse and in most cases the functions of their repetitive sequences are unclear. However, as with KAHRP and PfEMP3, it is possible that the functions of the protein family members have been diversified by duplication of genes encoding a protein with a conserved domain followed by expansion of different repetitive sequences that may contribute to modulating or changing the function of the protein.
Repetitive Maurer's cleft proteins
There are numerous exported proteins with repetitive sequences that are not Lys rich. In some cases, the function of these sequences is well understood. Ring exported protein-1 (REX1) is a PNEP that has a repetitive sequence that is essential for protein function (McHugh et al.2015). The protein is peripherally associated with Maurer's clefts (Hawthorne et al.2004) which are flattened membrane vesicles in the infected erythrocyte and are essential for the trafficking of PfEMP1 to the surface of the infected erythrocyte. Deletion of genes encoding several Maurer's cleft proteins disrupts PfEMP1 trafficking (Cooke et al.2006; Maier et al.2007; Spycher et al.2008; Rug et al.2014).
Deletion of the repetitive region of REX1 (residues 371–579) reduces the number of Maurer's clefts detected in the erythrocyte. On average, 13 punctae corresponding to Maurer's clefts are seen in erythrocytes infected with wildtype parasites but only 2 large punctae are seen in cells infected with parasites expressing REX1 that is missing the repetitive sequence (McHugh et al.2015). Electron microscopy reveals that these Maurer's clefts have an unusual stacking phenotype with an average of six clefts stacked on top of each other (McHugh et al.2015). Although PfEMP1 is trafficked to the abnormal stacked Maurer's clefts, transfer to the erythrocyte plasma membrane is less efficient when REX1 is either knocked down or the repetitive region is deleted (McHugh et al.2015). Removal of the repetitive region also reduces the adhesion of infected erythrocytes to CD36 cells under flow conditions (McHugh et al.2015). This indicates that the repetitive region of REX1 is functionally important for both Maurer's cleft architecture and efficient trafficking of PfEMP1. It is unlikely that the repeat region is simply forming a linker sequence between the N- and C-termini of the protein, as the C-terminal residues are not required for protein function and can be deleted. This suggests that the repeat sequences play an active role in protein function, possibly forming a key structural element or recruiting other proteins to the Maurer's cleft membrane. Notably, the sequence and length of the repetitive domain vary between different parasites; this could contribute to the formation of stacked cleft structures in some strains (Wickert and Krohne 2007) and may modulate the propensity of parasites to cytoadhere (McHugh et al.2015).
Skeleton-binding protein-1 (SBP1) is an exported PNEP that contains a single transmembrane segment that is integrated within the Maurer's cleft membrane. Deletion of the gene results in reduced trafficking of PfEMP1 to the erythrocyte surface and reduced cytoadhesion (Cooke et al.2006; Maier et al.2007; Chan et al.2016). SBP1 contains repetitive sequences located N- and C-terminally on either side of the transmembrane segment. The N-terminal repeats can self-associate (Saridaki et al.2009), while the Gln-rich repeats at its C-terminus associate with the cytoskeletal components spectrin and 4.1 in vitro (Kats et al.2015). Although these interactions likely contribute to the tethering of Maurer's clefts to the erythrocyte cytoskeleton, the C-terminus of SBP1 can be replaced by an unrelated protein sequence without altering the location of Maurer's clefts or cytoadhesion of infected cells. However, as it is known that there are multiple mechanisms for tethering of Maurer's clefts, there may be redundancy in this function of SBP1 (Hanssen, Mcmillan and Tilley 2010; Pachlatko et al.2010; Cyrklaff et al.2011; Rug et al.2014).
Pf332 is a large exported protein that localises to Maurer's clefts and is mainly composed of several different glutamate-rich repeats. Pf332 deletion and truncation of its repetitive C-terminus have been shown to have varying effects on the rigidity and cytoadhesion of infected cells; differences between studies may be a result of using different parasite strains and the particular deletion strategies (Glenister et al.2009; Hodder et al.2009).
Exported Repetitive histidine-rich proteins
Histidine-rich protein 2 (HRP2) is a P. falciparum protein comprised almost entirely of repetitive histidine-rich sequences. HRP2 is exported into the erythrocyte cytoplasm during the blood stage of infection and released into the blood during parasite egress (Howard et al.1986) where it can accumulate to high levels (Ramutton et al.2012). Several functions for HRP2 have been proposed including the detoxification of heme. The parasite internalizes haemoglobin from the cytoplasm of the host erythrocyte and digests it within the food vacuole; a fraction of HRP2 is also localised in the food vacuole (Sullivan, Gluzman and Goldberg 1996; Papalexis et al.2001). Within the food vacuole, the toxic heme moiety, a by-product of haemoglobin degradation, is converted into inert hemozoin. In vitro, HRP2 is able to catalyse the conversion of heme to hemozoin as is a similar histidine-rich repetitive protein HRP3 (Sullivan, Gluzman and Goldberg 1996). HRP2 is also able to promote the degradation of heme (Papalexis et al.2001). However, P. falciparum parasites lacking genes encoding both HRP2 and HRP3 are able to produce hemozoin suggesting that there are multiple mechanisms by which hemozoin is generated in vivo or that HRP2 plays a relatively minor role in this process. Indeed, other protein-based (Sullivan, Gluzman and Goldberg 1996; Jani et al.2008) and non-protein-based mechanisms have been proposed (reviewed in (Egan 2008)).
HRP2 has also been implicated in regulation of thrombin inhibition (Ndonwi et al.2011). In vitro, anti-thrombin inhibits the activity of thrombin and factor 10a and the inhibition is potentiated by glycosaminoglycans such as heparin. The potentiation can be antagonised by the interaction of HRP2 with glycosaminoglycans (Ndonwi et al.2011). Thus, HRP2 may behave as a pro-coagulant during infection.
More recent studies indicate that HRP2 may play a role in cerebral malaria by disrupting the integrity of brain endothelial cell barriers. Using a system that comprises an in vitro cultured monolayer of human endothelial cells that mimics the blood brain barrier, it has been shown that HRP2 can disrupt the integrity of an endothelial barrier (Pal et al.2016). Addition of parasites that produce HRP2 (but not parasites that do not make HRP2) or recombinant HRP2 protein to the monolayer of endothelial cells results in redistribution of tight junction and adherens junction proteins and disruption of the barrier formed by the cell monolayer. Blocking of key signalling factors in the host cell prevents the disruption caused by HRP2 supporting a model of inflammasome-mediated activation of the innate immune system in this process. Exposure to HRP2 also increases the number of VCAM-1 and ICAM-1 receptors on the surface of endothelical cells. Both receptors can be used by infected erythrocytes to adhere to endothelial cells suggesting that activation of an inflammatory response may enhance cytoadherence and sequestration of parasites (Pal et al.2016). Although mouse malaria parasites lack an HRP2 homolog, injection of HRP2 protein reduces survival of infected mice (Pal et al.2017). It remains to be determined whether the incidence of cerebral malaria in humans is lower in infections caused by HRP2-negative parasites.
Maurer's cleft-associated histidine-rich protein 1 (MAHRP-1) is an exported protein localized to Maurer's clefts. The protein contains a single transmembrane segment and a repetitive histidine-rich C-terminus and is involved in protein trafficking within the infected erythrocyte (Spycher et al.2008). Like HRP2, the repetitive histidine-rich sequence of MAHRP is implicated in heme detoxification (Papalexis et al.2001). The repetitive sequence is able to bind ferric heme, which is toxic to cells, and accelerate its degradation mediated by hydrogen peroxide. This suggests that the histidine-rich sequence of MAHRP-1 may play a role in scavenging ferric heme and detoxification of reactive oxygen species within the infected erythrocyte (Spycher et al.2003). Notably, the binding stoichiometry between ferric heme and MAHRP-1 is proportional to the number of repeats within the protein. This suggests that the potential protective function of MAHRP-1 could be influenced by repeat expansion (Spycher et al.2003).
Histidine-rich repetitive proteins and diagnosis of malaria
The World Health Organisation now recommends diagnostic testing of people with suspected malaria before treatment (WHO 2016). Rapid diagnostic tests (RDTs) represent an effective tool in this context. Most RDTs utilize antibodies to detect the HRP2 protein in blood. The mature protein, after cleavage of the PEXEL/HT motif (Chang et al.2008), comprises histidine-rich repeated sequences that form the epitopes recognized in RDTs (Wellems and Howard 1986). More than 20 sequence variants of these repeat sequences have been identified (Baker et al.2010; Deme et al.2014). There is significant polymorphism in overall length, sequence composition of the repeated motifs and the number of repeated motifs, between different P. falciparum strains (Baker et al.2005; Baker et al.2010; Ramutton et al.2012; Deme et al.2014) but there is currently no indication that sequence polymorphism can cause false negative RDT tests (Baker et al.2010; Ramutton et al.2012). However, increasing RDT use may place a selective pressure on the HRP2 gene and a contraction or mutation of repeats recognised by antibodies in RDTs may result in reduced test sensitivity or failure. Parasites lacking the HRP2 gene, initially identified in Peru (Gamboa et al.2010) and subsequently other regions, do yield negative HRP2-based RDT tests (Houze et al.2011; Koita et al.2012; Maltha et al.2012; Kumar et al.2013; Wurtz et al.2013; Cheng et al.2014; Abdallah et al.2015; Akinyi Okoth et al.2015; Baldeviano et al.2015; Li et al.2015; Murillo Solano et al.2015; Amoah, Abankwa and Oppong 2016; Bharti et al.2016; Dorado et al.2016).
CONCLUSION
In summary, repetitive sequences are widespread in Plasmodium genomes (Mendes et al.2013) and are particularly abundant in P. falciparum genes. These dynamic sequences can arise, expand or contract rapidly. Due to the assumption that they represent non-functional ‘junk DNA’ and are challenging to accurately assemble from short-read sequence data, they are mostly excluded from genomic analyses. Indeed, it is reasonable to assume in many cases that repeats simply expand and contract rapidly due to frequent errors in DNA replication, and evolve neutrally. Although it is difficult to provide a universal explanation for the presence of these sequences in the parasite genome, it appears that relatively simple homopolymeric repeats tend to evolve neutrally but more complex sequences appear to be under selection suggesting that they are functional (Zilversmit et al.2010; Battistuzzi et al.2016).
The fraction of Plasmodium repetitive protein sequences that are functionally important remains unclear and most remain uncharacterized. It is evident that some sequences within open reading frames encode repetitive polypeptide sequences that can modulate protein activity or are integral to protein function forming key structural elements, binding sites for other proteins and ligands and localisation signals. Repeat expansion and contraction can likely modulate and diversify protein function resulting in gain or loss of function phenotypes that allow the parasite to adapt under selective pressure. Despite the diverse functions of repetitive sequences, it is conceptually clear in many cases how changes in repeat length might influence protein function and parasite phenotype. The development of new genetic tools will now allow the importance of these sequences to be tested. In the simplest cases, relatively small changes in length of diverse repeat types may alter protein folding efficiency, stability or aggregation leading to selective advantage for a parasite. In cases such as the CTD of RNA polymerase II, where repeat sequences have very specific functions in the regulated recruitment of protein-binding partners, changes in repeat number may fine-tune protein function in a manner proportional to the number of repeats. The abundance of repetitive sequences that are recognized by the immune system and the occurrence of functional repetitive sequences in proteins such as KAHRP and REX1 that are central players in the pathology of severe malaria highlight why an understanding of these sequences is important to our understanding of severe disease, its treatment and prevention.
FUNDING
This work was supported by the Wellcome Trust [099764 Z/12/Z] the Biotechnological and Biological Sciences research council [BB/M009513/1].
Conflict of interest. None declared.
REFERENCES
- Abdallah JF, Okoth SA, Fontecha GA et al. . Prevalence of pfhrp2 and pfhrp3 gene deletions in Puerto Lempira, Honduras. Malaria J 2015;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyi Okoth S, Abdallah JF, Ceron N et al. . Variation in Plasmodium falciparum histidine-rich protein 2 (Pfhrp2) and Plasmodium falciparum histidine-rich protein 3 (Pfhrp3) gene deletions in Guyana and Suriname. PLoS One 2015;10:e0126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol 2009;63:195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah LE, Abankwa J, Oppong A. Plasmodium falciparum histidine rich protein-2 diversity and the implications for PfHRP 2: based malaria rapid diagnostic tests in Ghana. Malaria J 2016;15:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: structures, functions, and evolution. J Struct Biol 2001;134:117–31. [DOI] [PubMed] [Google Scholar]
- Baker J, Ho MF, Pelecanos A et al. . Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malaria J 2010;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Mccarthy J, Gatton M et al. . Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis 2005;192:870–7. [DOI] [PubMed] [Google Scholar]
- Baldeviano GC, Okoth SA, Arrospide N et al. . Molecular epidemiology of Plasmodium falciparum malaria outbreak, Tumbes, Peru, 2010–2012. Emerg Infect Dis 2015;21:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistuzzi FU, Schneider KA, Spencer MK et al. . Profiles of low complexity regions in Apicomplexa. BMC Evol Biol 2016;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson JG, Drew DR, Boyle MJ et al. . Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 2016;40:343–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol 2016;18:905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti PK, Chandel HS, Ahmad A et al. . Prevalence of pfhrp2 and/or pfhrp3 gene deletion in Plasmodium falciparum population in eight highly endemic states in India. PLoS One 2016;11:e0157949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham RJ, Rudino-Pinera E, Meenan NA et al. . Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. P Natl Acad Sci USA 2008;105:12254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey JA, Carvalho TG, Hodder AN et al. . Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 2013;14:532–50. [DOI] [PubMed] [Google Scholar]
- Boddey JA, Cowman AF. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu Rev Microbiol 2013;67:243–69. [DOI] [PubMed] [Google Scholar]
- Brown GV, Culvenor JG, Crewther PE et al. . Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med 1985;162:774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campino S, Benavente ED, Assefa S et al. . Genomic variation in two gametocyte non-producing Plasmodium falciparum clonal lines. Malaria J 2016;15:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JA, Howell KB, Langer C et al. . A single point in protein trafficking by Plasmodium falciparum determines the expression of major antigens on the surface of infected erythrocytes targeted by human antibodies. Cell Mol Life Sci 2016;73:4141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Falick AM, Carlton PM et al. . N-terminal processing of proteins exported by malaria parasites. Mol Biochem Parasit 2008;160:107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RD, Heidemann M, Hintermair C et al. . Molecular evolution of the RNA polymerase II CTD. Trends Genet 2008;24:289–96. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Gatton ML, Barnwell J et al. . Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malaria J 2014;13:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova TA, Wilkinson KD, Chernoff YO. Physiological and environmental control of yeast prions. FEMS Microbiol Rev 2014;38:326–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CR, Hackett F, Atid J et al. . The Plasmodium falciparum pseudoprotease SERA5 regulates the kinetics and efficiency of malaria parasite egress from host erythrocytes. PLoS Pathog 2017;13:e1006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DJ, Cavanagh DR, Tanabe K et al. . A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med 2000;6:689–92. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Buckingham DW, Glenister FK et al. . A Maurer's cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J Cell Biol 2006;172:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi A, Natarajan R, Pradel G et al. . The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med 2011;208:341–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden JL, Cadena DL, Ahearn JM Jr et al. . A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. P Natl Acad Sci USA 1985;82:7934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Healer J, Marapana D et al. . Malaria: biology and disease. Cell 2016;167:610–24. [DOI] [PubMed] [Google Scholar]
- Crabb BS, Cooke BM, Reeder JC et al. . Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 1997;89:287–96. [DOI] [PubMed] [Google Scholar]
- Cui L, Fan Q, Cui L et al. . Histone lysine methyltransferases and demethylases in Plasmodium falciparum. Int J Parasitol 2008;38:1083–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberworth A, Lamour G, Babu MM et al. . Promiscuity as a functional trait: intrinsically disordered regions as central players of interactomes. Biochem J 2013;454:361–9. [DOI] [PubMed] [Google Scholar]
- Cutts EE, Laasch N, Reiter DM et al. . Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog 2017;13:e1006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrklaff M, Sanchez CP, Kilian N et al. . Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 2011;334:1283–6. [DOI] [PubMed] [Google Scholar]
- Davies HM, Thalassinos K, Osborne AR. Expansion of lysine-rich repeats in Plasmodium proteins generates novel localization sequences that target the periphery of the host erythrocyte. J Biol Chem 2016;291:26188–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning-Ward TF, Dixon MW, Tilley L et al. . Plasmodium species: master renovators of their host cells. Nat Rev Microbiol 2016;14:494–507. [DOI] [PubMed] [Google Scholar]
- Deme AB, Park DJ, Bei AK et al. . Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malaria J 2014;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado EJ, Okoth SA, Montenegro LM et al. . Genetic characterisation of Plasmodium falciparum isolates with deletion of the pfhrp2 and/or pfhrp3 genes in Colombia: the Amazon region, a challenge for malaria diagnosis and control. PLoS One 2016;11:e0163137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L. tRNA gene number and codon usage in the C. elegans genome are co-adapted for optimal translation of highly expressed genes. Trends Genet 2000;16:287–9. [DOI] [PubMed] [Google Scholar]
- Dyson HJ, Satterthwait AC, Lerner RA et al. . Conformational preferences of synthetic peptides derived from the immunodominant site of the circumsporozoite protein of Plasmodium falciparum by 1H NMR. Biochemistry 1990;29:7828–37. [DOI] [PubMed] [Google Scholar]
- Edwards AM, Potts JR, Josefsson E et al. . Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog 2010;6:e1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TJ. Haemozoin formation. Mol Biochem Parasit 2008;157:127–36. [DOI] [PubMed] [Google Scholar]
- Espinosa DA, Yadava A, Angov E et al. . Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect Immun 2013;81:2882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZP, Zhang X, Han P et al. . Abundance of intrinsically unstructured proteins in P. falciparum and other apicomplexan parasite proteomes. Mol Biochem Parasit 2006;150:256–67. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Balaban AE, Patzewitz EM et al. . The repeat region of the circumsporozoite protein is critical for sporozoite formation and maturation in Plasmodium. PLoS One 2014;9:e113923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidalgo M, Barrales RR, Ibeas JI et al. . Adaptive evolution by mutations in the FLO11 gene. P Natl Acad Sci USA 2006;103:11228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock DA, Gras-Masse H, Lepers JP et al. . Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J Immunol 1994;153:190–204. [PubMed] [Google Scholar]
- Filisetti D, Theobald-Dietrich A, Mahmoudi N et al. . Aminoacylation of Plasmodium falciparum tRNA(Asn) and insights in the synthesis of asparagine repeats. J Biol Chem 2013;288:36361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Sutton HJ, Kaczmarski JA et al. . T-dependent B cell responses to Plasmodium induce antibodies that form a high-avidity multivalent complex with the circumsporozoite protein. PLoS Pathog 2017;13:e1006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondon JW 3rd, Garner HR. Molecular origins of rapid and continuous morphological evolution. P Natl Acad Sci USA 2004;101:18058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondon JW 3rd, Garner HR. Detection of length-dependent effects of tandem repeat alleles by 3-D geometric decomposition of craniofacial variation. Dev Genes Evol 2007;217:79–85. [DOI] [PubMed] [Google Scholar]
- Frugier M, Bour T, Ayach M et al. . Low Complexity regions behave as tRNA sponges to help co-translational folding of plasmodial proteins. FEBS Lett 2010;584:448–54. [DOI] [PubMed] [Google Scholar]
- Galamo CD, Jafarshad A, Blanc C et al. . Anti-MSP1 block 2 antibodies are effective at parasite killing in an allele-specific manner by monocyte-mediated antibody-dependent cellular inhibition. J Infect Dis 2009;199:1151–4. [DOI] [PubMed] [Google Scholar]
- Gamboa D, Ho MF, Bendezu J et al. . A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoS One 2010;5:e8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E et al. . Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002;419:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet 2005;6:743–55. [DOI] [PubMed] [Google Scholar]
- Gemayel R, Chavali S, Pougach K et al. . Variable glutamine-rich repeats modulate transcription factor activity. Mol Cell 2015;59:615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemayel R, Vinces MD, Legendre M et al. . Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet 2010;44:445–77. [DOI] [PubMed] [Google Scholar]
- Ghasparian A, Moehle K, Linden A et al. . Crystal structure of an NPNA-repeat motif from the circumsporozoite protein of the malaria parasite Plasmodium falciparum. Chem Commun 2006, 174–6. [DOI] [PubMed] [Google Scholar]
- Glenister FK, Coppel RL, Cowman AF et al. . Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 2002;99:1060–3. [DOI] [PubMed] [Google Scholar]
- Glenister FK, Fernandez KM, Kats LM et al. . Functional alteration of red blood cells by a megadalton protein of Plasmodium falciparum. Blood 2009;113:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy AJ, Irani V, Macraild CA et al. . Insights into the immunological properties of intrinsically disordered malaria proteins using proteome scale predictions. PLoS One 2015;10:e0141729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Alberti S, Krishnan R et al. . Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol Cell 2011;43:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Alberti S, Lindquist S. Prions, protein homeostasis, and phenotypic diversity. Trends Cell Biol 2010;20:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WL, Claessens A, Otto TD et al. . Extreme mutation bias and high AT content in Plasmodium falciparum. Nucleic Acids Res.2017;45:1889–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen E, Mcmillan PJ, Tilley L. Cellular architecture of Plasmodium falciparum-infected erythrocytes. Int J Parasitol 2010;40:1127–35. [DOI] [PubMed] [Google Scholar]
- Hawthorne PL, Trenholme KR, Skinner-Adams TS et al. . A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer's clefts. Mol Biochem Parasit 2004;136:181–9. [DOI] [PubMed] [Google Scholar]
- Heiber A, Kruse F, Pick C et al. . Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog 2013;9:e1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R, Anderson C, Kumar K et al. . Reversible conformational change in the Plasmodium falciparum circumsporozoite protein masks its adhesion domains. Infect Immun 2015;83:3771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller NL, Bhattacharjee S, Van OOIJ et al. . A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 2004;306:1934–7. [DOI] [PubMed] [Google Scholar]
- Hodder AN, Maier AG, Rug M et al. . Analysis of structure and function of the giant protein Pf332 in Plasmodium falciparum. Mol Microbiol 2009;71:48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman SL, Vekemans J, Richie TL et al. . The March Toward Malaria Vaccines. Am J Prev Med 2015;49:S319–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houze S, Hubert V, Le Pessec G et al. . Combined deletions of pfhrp2 and pfhrp3 genes result in Plasmodium falciparum malaria false-negative rapid diagnostic test. J Clin Microbiol 2011;49:2694–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Uni S, Aikawa M et al. . Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J Cell Biol 1986;103:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KJ. RNA polymerase II conducts a symphony of pre-mRNA processing activities. Biochim Biophys Acta 2002;1577:308–24. [DOI] [PubMed] [Google Scholar]
- Hughes AL. The evolution of amino acid repeat arrays in Plasmodium and other organisms. J Mol Evol 2004;59:528–35. [DOI] [PubMed] [Google Scholar]
- Jani D, Nagarkatti R, Beatty W et al. . HDP-a novel heme detoxification protein from the malaria parasite. PLoS Pathog 2008;4:e1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mu J, Zhang Q et al. . PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature 2013;499:223–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafsack BF, Rovira-Graells N, Clark TG et al. . A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014;507:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajava AV. Tandem repeats in proteins: from sequence to structure. J Struct Biol 2012;179:279–88. [DOI] [PubMed] [Google Scholar]
- Kato M, Han TW, Xie S et al. . Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012;149:753–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kats LM, Proellocks NI, Buckingham DW et al. . Interactions between Plasmodium falciparum skeleton-binding protein 1 and the membrane skeleton of malaria-infected red blood cells. Biochim Biophys Acta 2015;1848:1619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DJ, Coppel RL, Anders RF. Repetitive proteins and genes of malaria. Annu Rev Microbiol 1987;41:181–208. [DOI] [PubMed] [Google Scholar]
- Kilejian A, Rashid MA, Aikawa M et al. . Selective association of a fragment of the knob protein with spectrin, actin and the red cell membrane. Mol Biochem Parasit 1991;44:175–81. [DOI] [PubMed] [Google Scholar]
- Kishore SP, Perkins SL, Templeton TJ et al. . An unusual recent expansion of the C-terminal domain of RNA polymerase II in primate malaria parasites features a motif otherwise found only in mammalian polymerases. J Mol Evol 2009;68:706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koita OA, Doumbo OK, Ouattara A et al. . False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg 2012;86:194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Pande V, Bhatt RM et al. . Genetic deletion of HRP2 and HRP3 in Indian Plasmodium falciparum population and false negative malaria rapid diagnostic test. Acta Trop 2013;125:119–21. [DOI] [PubMed] [Google Scholar]
- Li J, Mitamura T, Fox BA et al. . Differential localization of processed fragments of Plasmodium falciparum serine repeat antigen and further processing of its N-terminal 47 kDa fragment. Parasitol Int 2002;51:343–52. [DOI] [PubMed] [Google Scholar]
- Li P, Xing H, Zhao Z et al. . Genetic diversity of Plasmodium falciparum histidine-rich protein 2 in the China-Myanmar border area. Acta Trop 2015;152:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Taylor IC, Kingston RE et al. . RNA polymerase II carboxy-terminal domain contributes to the response to multiple acidic activators in vitro. Genes Dev 1991;5:2431–40. [DOI] [PubMed] [Google Scholar]
- Lindner SE, Miller JL, Kappe SH. Malaria parasite pre-erythrocytic infection: preparation meets opportunity. Cell Microbiol 2012;14:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Lawler AM, Sebald SM et al. . Growth retardation and neonatal lethality in mice with a homozygous deletion in the C-terminal domain of RNA polymerase II. Mol Gen Genet 1999;261:100–5. [DOI] [PubMed] [Google Scholar]
- Lunde BM, Reichow SL, Kim M et al. . Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nat Struct Mol Biol 2010;17:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macraild CA, Richards JS, Anders RF et al. . Antibody recognition of disordered antigens. Structure 2016;24:148–57. [DOI] [PubMed] [Google Scholar]
- Maier AG, Rug M, O’neill MT et al. . Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood 2007;109:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier AG, Rug M, O’neill MT et al. . Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 2008;134:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltha J, Gamboa D, Bendezu J et al. . Rapid diagnostic tests for malaria diagnosis in the Peruvian Amazon: impact of pfhrp2 gene deletions and cross-reactions. PLoS One 2012;7:e43094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M, Good RT, Rug M et al. . Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 2004;306:1930–3. [DOI] [PubMed] [Google Scholar]
- Mayer C, Slater L, Erat MC et al. . Structural analysis of the Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) intracellular domain reveals a conserved interaction epitope. J Biol Chem 2012;287:7182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mchugh E, Batinovic S, Hanssen E et al. . A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machinery architecture and virulence protein trafficking. Mol Microbiol 2015;98:1101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes TA, Lobo FP, Rodrigues TS et al. . Repeat-enriched proteins are related to host cell invasion and immune evasion in parasitic protozoa. Mol Biol Evol 2013;30:951–63. [DOI] [PubMed] [Google Scholar]
- Mikolajczak SA, Sacci JB Jr, De La Vega P et al. . Disruption of the Plasmodium falciparum liver-stage antigen-1 locus causes a differentiation defect in late liver-stage parasites. Cell Microbiol 2011;13:1250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A, Iqbal Z, Vauterin P et al. . Indels, structural variation, and recombination drive genomic diversity in Plasmodium falciparum. Genome Res 2016;26:1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan V, Oksman A, Iwamoto M et al. . Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. P Natl Acad Sci USA 2011;108:4411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan V, Oksman A, Pal P et al. . Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat Commun 2012;3:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo Solano C, Akinyi Okoth S, Abdallah JF et al. . Deletion of Plasmodium falciparum histidine-rich protein 2 (pfhrp2) and histidine-rich protein 3 (pfhrp3) genes in colombian parasites. PLoS One 2015;10:e0131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanzer AP, Torda AE, Bisang C et al. . Dynamical studies of peptide motifs in the Plasmodium falciparum circumsporozoite surface protein by restrained and unrestrained MD simulations. J Mol Biol 1997;267:1012–25. [DOI] [PubMed] [Google Scholar]
- Ndonwi M, Burlingame OO, Miller AS et al. . Inhibition of antithrombin by Plasmodium falciparum histidine-rich protein II. Blood 2011;117:6347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey DE, Juraska M, Bedford T et al. . Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med 2015;373:2025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll WS, Sacci JB, Rodolfo C et al. . Plasmodium falciparum liver stage antigen-1 is cross-linked by tissue transglutaminase. Malaria J 2011;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M, Scafe C, Sexton J et al. . Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol 1987;7:1602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley MS, Kumar S, Anantharaman V et al. . Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect Immun 2007;75:2012–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberli A, Slater LM, Cutts E et al. . A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface. FASEB J 2014;28:4420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberli A, Zurbrugg L, Rusch S et al. . Plasmodium falciparum Plasmodium helical interspersed subtelomeric proteins contribute to cytoadherence and anchor P. falciparum erythrocyte membrane protein 1 to the host cell cytoskeleton. Cell Microbiol 2016;18:1415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem 2014;83:553–84. [DOI] [PubMed] [Google Scholar]
- Oma Y, Kino Y, Sasagawa N et al. . Intracellular localization of homopolymeric amino acid-containing proteins expressed in mammalian cells. J Biol Chem 2004;279:21217–22. [DOI] [PubMed] [Google Scholar]
- Pachlatko E, Rusch S, Muller A et al. . MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol Microbiol 2010;77:1136–52. [DOI] [PubMed] [Google Scholar]
- Pal P, Balaban AE, Diamond MS et al. . Plasmodium falciparum histidine-rich protein II causes vascular leakage and exacerbates experimental cerebral malaria in mice. PLoS One 2017;12:e0177142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P, Daniels BP, Oskman A et al. . Plasmodium falciparum histidine-rich protein ii compromises brain endothelial barriers and may promote cerebral malaria pathogenesis. MBio 2016;7:pii: e00617–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalexis V, Siomos MA, Campanale N et al. . Histidine-rich protein 2 of the malaria parasite, Plasmodium falciparum, is involved in detoxification of the by-products of haemoglobin degradation. Mol Biochem Parasit 2001;115:77–86. [DOI] [PubMed] [Google Scholar]
- Parish LA, Mai DW, Jones ML et al. . A member of the Plasmodium falciparum PHIST family binds to the erythrocyte cytoskeleton component band 4.1. Malaria J 2013;12:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra AP, Sharma S, Ainavarapu SR. Force spectroscopy of the Plasmodium falciparum vaccine candidate circumsporozoite protein suggests a mechanically pliable repeat region. J Biol Chem 2017;292:2110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X, An X, Guo X et al. . Structural and functional studies of interaction between Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and erythrocyte spectrin. J Biol Chem 2005;280:31166–71. [DOI] [PubMed] [Google Scholar]
- Persson C, Oliveira GA, Sultan AA et al. . Cutting edge: a new tool to evaluate human pre-erythrocytic malaria vaccines: rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol 2002;169:6681–5. [DOI] [PubMed] [Google Scholar]
- Peters TW, Huang M. Protein aggregation and polyasparagine-mediated cellular toxicity in Saccharomyces cerevisiae. Prion 2007;1:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen W, Matuschewski K, Ingmundson A. Trafficking of the signature protein of intra-erythrocytic Plasmodium berghei-induced structures, IBIS1, to P. falciparum Maurer's clefts. Mol Biochem Parasit 2015;200:25–9. [DOI] [PubMed] [Google Scholar]
- Plassmeyer ML, Reiter K, Shimp RL Jr et al. . Structure of the Plasmodium falciparum circumsporozoite protein, a leading malaria vaccine candidate. J Biol Chem 2009;284:26951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proellocks NI, Herrmann S, Buckingham DW et al. . A lysine-rich membrane-associated PHISTb protein involved in alteration of the cytoadhesive properties of Plasmodium falciparum-infected red blood cells. FASEB J 2014;28:3103–13. [DOI] [PubMed] [Google Scholar]
- Przyborski JM, Nyboer B, Lanzer M. Ticket to ride: export of proteins to the Plasmodium falciparum-infected erythrocyte. Mol Microbiol 2016;101:1–11. [DOI] [PubMed] [Google Scholar]
- Ramutton T, Hendriksen IC, Mwanga-Amumpaire J et al. . Sequence variation does not confound the measurement of plasma PfHRP2 concentration in African children presenting with severe malaria. Malaria J 2012;11:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Bushnell DA, Trnka MJ et al. . Structure of the mediator head module bound to the carboxy-terminal domain of RNA polymerase II. P Natl Acad Sci USA 2012;109:17931–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rts SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015;386:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rug M, Cyrklaff M, Mikkonen A et al. . Export of virulence proteins by malaria-infected erythrocytes involves remodeling of host actin cytoskeleton. Blood 2014;124:3459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rug M, Prescott SW, Fernandez KM et al. . The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes. Blood 2006;108:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salichs E, Ledda A, Mularoni L et al. . Genome-wide analysis of histidine repeats reveals their role in the localization of human proteins to the nuclear speckles compartment. PLoS Genet 2009;5:e1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant TJ, Marti M, Caler E et al. . Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol 2006;7:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saridaki T, Frohlich KS, Braun-Breton C et al. . Export of PfSBP1 to the Plasmodium falciparum Maurer's clefts. Traffic 2009;10:137–52. [DOI] [PubMed] [Google Scholar]
- Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today 1991;7:99–105. [DOI] [PubMed] [Google Scholar]
- Schulze J, Kwiatkowski M, Borner J et al. . The Plasmodium falciparum exportome contains non-canonical PEXEL/HT proteins. Mol Microbiol 2015;97:301–14. [DOI] [PubMed] [Google Scholar]
- Shakya B, Penn WD, Nakayasu ES et al. . The Plasmodium falciparum exported protein PF3D7_0402000 binds to erythrocyte ankyrin and band 4.1. Mol Biochem Parasit 2017;216:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoubridge C, Cloosterman D, Parkinson-Lawerence E et al. . Molecular pathology of expanded polyalanine tract mutations in the aristaless-related homeobox gene. Genomics 2007;90:59–71. [DOI] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem 2009;78:929–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini F, Lasonder E, Olivieri A et al. . Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics 2010;9:1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, Hughes KR, Modrzynska KK et al. . A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 2014;507:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P, Nardin E. Sporozoite antigens: biology and immunology of the circumsporozoite protein and thrombospondin-related anonymous protein. Chem Immunol 2002;80:70–96. [DOI] [PubMed] [Google Scholar]
- Spielmann T, Gilberger TW. Critical steps in protein export of Plasmodium falciparum blood stages. Trends Parasitol 2015;31:514–25. [DOI] [PubMed] [Google Scholar]
- Spillman NJ, Beck JR, Goldberg DE. Protein export into malaria parasite-infected erythrocytes: mechanisms and functional consequences. Annu Rev Biochem. 2015;84:813–41. [DOI] [PubMed] [Google Scholar]
- Spycher C, Klonis N, Spielmann T et al. . MAHRP-1, a novel Plasmodium falciparum histidine-rich protein, binds ferriprotoporphyrin IX and localizes to the Maurer's clefts. J Biol Chem 2003;278:35373–83. [DOI] [PubMed] [Google Scholar]
- Spycher C, Rug M, Pachlatko E et al. . The Maurer's cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol Microbiol 2008;68:1300–14. [DOI] [PubMed] [Google Scholar]
- Sullivan DJ Jr, Gluzman IY, Goldberg DE. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 1996;271:219–22. [DOI] [PubMed] [Google Scholar]
- Tan JC, Tan A, Checkley L et al. . Variable numbers of tandem repeats in Plasmodium falciparum genes. J Mol Evol 2010;71:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr SJ, Moon RW, Hardege I et al. . A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol Biochem Parasit 2014;196:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M, Soe S, Oeuvray C et al. . The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun 1998;66:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio M, Dixon MW, Looker O et al. . Specific expression and export of the Plasmodium falciparum Gametocyte EXported Protein-5 marks the gametocyte ring stage. Malaria J 2015;14:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukaegbu UE, Deitsch KW. The emerging role for rna polymerase II in regulating virulence gene expression in malaria parasites. PLoS Pathog 2015;11:e1004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukaegbu UE, Kishore SP, Kwiatkowski DL et al. . Recruitment of PfSET2 by RNA polymerase II to variant antigen encoding loci contributes to antigenic variation in P. falciparum. PLoS Pathog 2014;10:e1003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini A, Cramer P. Conservation between the RNA polymerase I, II, and III transcription initiation machineries. Mol Cell 2012;45:439–46. [DOI] [PubMed] [Google Scholar]
- Verra F, Hughes AL. Biased amino acid composition in repeat regions of Plasmodium antigens. Mol Biol Evol 1999;16:627–33. [DOI] [PubMed] [Google Scholar]
- Verstrepen KJ, Jansen A, Lewitter F et al. . Intragenic tandem repeats generate functional variability. Nat Genet 2005;37:986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali M, Mckinlay A, Lacount DJ et al. . Interaction of an atypical Plasmodium falciparum ETRAMP with human apolipoproteins. Malaria J 2008;7:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warncke JD, Vakonakis I, Beck HP. Plasmodium helical interspersed subtelomeric (PHIST) proteins, at the Center of Host Cell Remodeling. Microbiol Mol Biol R 2016;80:905–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterkeyn JG, Wickham ME, Davern KM et al. . Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J 2000;19:2813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermeyer JM, Hale VL, Hackett F et al. . A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood 2016;127:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems TE, Howard RJ. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. P Natl Acad Sci USA 1986;83:6065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng H, Guo X, Papoin J et al. . Interaction of Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) with erythrocyte ankyrin R is required for its attachment to the erythrocyte membrane. Biochim Biophys Acta 2014;1838:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ML, Corden JL. Construction and analysis of yeast RNA polymerase II CTD deletion and substitution mutations. Genetics 1995;140:1223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- Wickert H, Krohne G. The complex morphology of Maurer's clefts: from discovery to three-dimensional reconstructions. Trends Parasitol 2007;23:502–9. [DOI] [PubMed] [Google Scholar]
- Wurtz N, Fall B, Bui K et al. . Pfhrp2 and pfhrp3 polymorphisms in Plasmodium falciparum isolates from Dakar, Senegal: impact on rapid malaria diagnostic tests. Malaria J 2013;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Bang G, Tougan T et al. . Protective epitopes of the Plasmodium falciparum SERA5 malaria vaccine reside in intrinsically unstructured N-terminal repetitive sequences. PLoS One 2014;9:e98460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh SM, Lucas JS, Jones KA. The Iws1:Spt6:CTD complex controls cotranscriptional mRNA biosynthesis and HYPB/Setd2-mediated histone H3K36 methylation. Gene Dev 2008;22:3422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F, Tam JP, Hollingdale MR et al. . Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science 1985;228:1436–40. [DOI] [PubMed] [Google Scholar]
- Zehring WA, Lee JM, Weeks JR et al. . The C-terminal repeat domain of RNA polymerase II largest subunit is essential in vivo but is not required for accurate transcription initiation in vitro. P Natl Acad Sci USA 1988;85:3698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilversmit MM, Volkman SK, Depristo MA et al. . Low-complexity regions in Plasmodium falciparum: missing links in the evolution of an extreme genome. Mol Biol Evol 2010;27:2198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]