Abstract
Triacylglycerol (TAG) and glycogen are the two major metabolites for carbon storage in most eukaryotic organisms. We investigated the glycogen metabolism of the oleaginous Yarrowia lipolytica and found that this yeast accumulates up to 16% glycogen in its biomass. Assuming that elimination of glycogen synthesis would result in an improvement of lipid accumulation, we characterized and deleted the single gene coding for glycogen synthase, YlGSY1. The mutant was grown under lipogenic conditions with glucose and glycerol as substrates and we obtained up to 60% improvement in TAG accumulation compared to the wild-type strain. Additionally, YlGSY1 was deleted in a background that was already engineered for high lipid accumulation. In this obese background, TAG accumulation was also further increased. The highest lipid content of 52% was found after 3 days of cultivation in nitrogen-limited glycerol medium. Furthermore, we constructed mutants of Y. lipolytica and Saccharomyces cerevisiae that are deleted for both glycogen and TAG synthesis, demonstrating that the ability to store carbon is not essential. Overall, this work showed that glycogen synthesis is a competing pathway for TAG accumulation in oleaginous yeasts and that deletion of the glycogen synthase has beneficial effects on neutral lipid storage.
Keywords: oleaginous yeast, triacylglycerol, glycogen synthase, storage metabolism
Glycogen and neutral lipid synthesis are competing pathways and the elimination of glycogen storage results in improved lipid synthesis in the yeast Yarrowia lipolytica.
INTRODUCTION
Yarrowia lipolytica is a respiratory oleaginous yeast mainly found in dairy and meat products, as well as in polluted sea water. It has been widely studied for its ability to degrade hydrocarbons and for production of citric acid and single cell proteins (Nicaud 2012). In addition, its natural capability to store high amounts of neutral lipids (NLs) in the form of triacylglycerol (TAG) is investigated because it can serve as a sustainable feedstock for biodiesel production or for the biosynthesis of fatty acids (FA) (Ledesma-Amaro and Nicaud 2016) and fine chemicals (Abghari and Chen 2014). Besides TAG, glycogen serves as a second storage form for excess carbon. Microorganisms continuously sense the nutritional status of their environment and adapt their growth and metabolism to changing conditions. The accumulation of carbon stores in the form of glycogen and/or TAG is regarded as a strategy to deal with extended periods of starvation or other unfavorable conditions. Glycogen metabolism is highly conserved from yeast to humans. In baker's yeast, glycogen accumulates at the onset of stationary phase and can be strongly induced by stress conditions such as a limitation for nitrogen, sulfur or phosphorous when glucose is available (François and Parrou 2001). A similar behavior is found in bacteria (Preiss and Romeo 1994). Furthermore, Parrou et al. (1999) found changes in glycogen and trehalose accumulation as a response to the gradual depletion of nutrients from carbon or nitrogen-limited media. Under glucose limitation, they observed glycogen accumulation after the late logarithmic phase, whereas under nitrogen limitation, accumulation of glycogen was only observed after complete depletion of the nitrogen source. For oleaginous yeasts such as Apiotrichum curvatum and Rhodosporidium toruloides, it was shown that under nitrogen limitation glycogen can account for 10%–45% of the total biomass (Evans and Ratledge 1984; Park, Murphy and Glatz 1990). Similar results were found in a recent study that investigated the potential of several yeasts for FA production (Lamers et al.2016).
Like glycogen accumulation, NL synthesis and storage is greatly induced in response to nitrogen limitation. Indeed, a high ratio of carbon to nitrogen is the condition that is typically used for oleaginous yeasts to obtain high yields of TAG. However, there is only limited knowledge about factors that co-regulate the accumulation or mobilization of both glycogen and NL pools. Upon glucose depletion, the AMPK homolog Snf1p promotes cellular processes such as respiration, glycogen accumulation, peroxisome biogenesis and aging, and downregulates anabolic pathways such as amino acid and de novo FA synthesis (Conrad et al.2014). Under glucose repression, the transcriptional repressor Mig1p binds to the promoter of GSY2, the gene coding for the major glycogen synthase activity, and inactivates glycogen synthesis, whereas Snf1p phosphorylation of Mig1p releases this repression and promotes glycogen accumulation (Hardy, Huang and Roach 1994; Wang et al.2001). The anabolic process of FA synthesis is also subject to regulation through Snf1p, which catalyzes the phosphorylation of the acetyl-CoA carboxylase Acc1p, resulting in the inactivation of the first committed step in FA synthesis (Woods et al.1994). However, it has to be assumed that Snf1p is not the only regulator of storage metabolism because its deletion results in opposite effects for the two storage pools, a decrease in glycogen and an increase in NLs (Wilson, Wang and Roach 2002; Bozaquel-Morais et al. 2010).
Glycogen metabolism in Y. lipolytica has not been investigated in detail yet. Queiroz-Claret et al. (2000) reported a 76-kDa monomeric protein as a putative glycogen synthase. Contrary to Saccharomyces cerevisiae, Y. lipolytica was reported to show glycogen synthase activity already during the exponential phase of growth. This activity was increasing synchronously with the increase in activities of protein phosphatase 2A (PP2A), whereas, with the onset of stationary phase, protein kinase CK2 activity increases and phosphorylation of glycogen synthase results in depletion of glycogen pools. Recently, Dulermo et al. (2015) also showed that Y. lipolytica accumulates 9% glycogen in the biomass under nitrogen-limiting conditions.
In this work, we characterized the glycogen synthase of Y. lipolytica and investigated the effects of a deletion of the encoding gene, YALI0F18502g. We show that this deletion results in a significant increase in NL accumulation, suggesting that the cellular carbon flux is redirected from glycogen to TAG synthesis. This effect was also observed in a strain that has already been genetically engineered for high lipid accumulation. Finally, since storage metabolism is assumed to support viability in stationary-phase cells, we studied the impact on chronological life span for a strain deleted for glycogen synthesis as well as for a strain that is deficient in both glycogen and TAG synthesis.
MATERIALS AND METHODS
Strains, media and cultivation conditions
All strains used in this study are listed in Table 1. Media and growth conditions for Escherichia coli and Yarrowia lipolytica have been described by Sambrook and Russell (2001) and Barth and Gaillardin (1996), respectively. Yeast cultures were grown in minimal media, consisting of the following components: 5 g L−1 (carbon limited) or 0.4 g L−1 (nitrogen limited) (NH4)2SO4; 3 g L−1 KH2PO4; 0.5 g L−1 MgSO4.7H2O; buffered at pH 5.7 with 2-(N-morpholino)ethanesulfonic acid (MES). The carbon sources, glucose or glycerol, were autoclaved separately and 1 mL L−1 sterile-filtered trace metal and 1 mL L−1 vitamin solution as described by Hong and Nielsen (2013) were added after autoclaving. Depending on the nitrogen concentration, we will refer to shake flask cultivations as carbon limited (C-lim: 5 g L−1 glucose or glycerol and 5 g L−1 ammonium sulfate) or nitrogen limited (N-lim: 20 g L−1 glucose or glycerol and 0.4 g L−1 ammonium sulfate). For cultivation of Saccharomyces cerevisiae strains, the C-lim and N-lim media contained 20 g L−1 glucose as carbon source. In C-lim cultivations, this glucose concentration results in approximately the same final biomass for baker's yeast as the concentration of 5 g L−1 for Y. lipolytica. Media for the growth of the NL-deficient mutants were supplemented with amino acids.
Table 1.
Strains used in this study.
| Strains | Genotype | Source |
|---|---|---|
| Yarrowia lipolytica | ||
| YlWT (H222) | MATa wild-type | Barth and Gaillardin (1996) |
| JMY322 | MATa ura3-41 | Mauersberger et al. (2001) |
| Ylgsy1Δ | MATa ura3-41 gsy1Δ::URA3 | This work |
| YlTEFGSY1 | MATa ura3-41 TEFP-GSY1 | This work |
| obese (JMY6210) | MATa ura3-302 leu2-270 xpr2-322 tgl4Δ TEFP-GPD1 TEFP-DGA2-LEU2 URA3 | This work |
| obese gsy1Δ (JMY6212) | MATa ura3-302 leu2-270 xpr2-322 tgl4Δ TEFP-GPD1 TEFP-DGA2-LEU2 gsy1Δ::URA3 | This work |
| YlQM (JMY1877) | MATa ura3-302 leu2-270 dga1Δ lro1Δ are1Δ dga2Δ | Beopoulos et al. (2012) |
| YlPM | MATa ura3-302 leu2-270 dga1Δ lro1Δ are1Δ dga2Δ gsy1Δ | This work |
| JMY195 | PO1d strain - MATa ura3-302 leu2-270 xpr2-322 | Barth and Gaillardin (1996) |
| JMYgsy1Δ | PO1d strain - MATa ura3-302 leu2-270 xpr2-322 gsy1Δ | This work |
| Saccharomyces cerevisiae | ||
| ScWT (CEN.PK113-5D) | MATa SUC2 MAL2-8c ura3Δ | Entian and Koetter (2007) |
| gsy1Δgsy2Δ | MATa SUC2 MAL2-8c gsy1Δ::loxP gsy2Δ::loxP | This work |
| gsy1Δgsy2Δura3Δ | MATa SUC2 MAL2-8c gsy1Δ::loxP gsy2Δ::loxP ura3Δ::loxP | This work |
| ScWT (BY4742) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Brachmann et al. (1998) |
| ScQM (YJP1078) | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ycr048wΔ::KanMX4 ynr019wΔ::KanMX4 yor245cΔ::KanMX4 ynr008wΔ::KanMX4 | Petschnigg et al. (2009) |
| ScHM | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ycr048wΔ::KanMX4 ynr019wΔ::KanMX4 yor245cΔ::KanMX4 ynr008wΔ::KanMX4 | This work |
| gsy1Δ gsy2Δ | ||
Precultures were prepared in 50 mL of the same medium as used for main cultures and cultivated at 28°C on a rotary shaker at 180 rpm for 18–24 h. Prior to inoculation into the cultivation flask, the preculture was washed twice with deionized water to remove any residual media components from the culture. Shake flask cultivations were performed in round bottom 1 L flasks with 200 mL of medium. The shake flasks were inoculated from precultures to a starting OD600 of 0.05 and incubated at 28°C on a rotary shaker at 180 rpm for 72 h.
For the determination of extracellular metabolites, biomass, glycogen and lipid content samples from C-lim media were harvested in exponential phase and in stationary phase, and from N-lim media after 24, 48 and 72 h of cultivation.
For the determination of survival in stationary phase, cultures were grown in C-lim medium containing 5 g L−1 glucose. Diluted aliquots were plated onto YPD plates (1% yeast extract, 2% peptone, 2% glucose, 2% agar) to determine viability of the cultures. The data for the analysis of chronological lifespan are derived from two independent experiments.
Molecular and genetic work
All PCR reactions were performed using Herculase II Fusion DNA Polymerase (Agilent Technologies Österreich GmbH, Vienna, Austria) or Pyrobest (Takara Bio Europe, Saint-Germain-en-Laye, France) for cloning and amplification of sequencing templates, and with Solis BioDyne FIREPOL DNA Polymerase (Medibena, Vienna, Austria) or GoTaq (Promega, Madison, WI, USA) for confirmation of chromosomal integration of the transformation cassettes. The restriction enzymes used in this study were obtained from Roche (Roche Austria GmbH, Vienna, Austria) or OZYME (Ozyme, Montigny-le-Bretonneux, France). The DNA fragments from PCR and restriction digestion were recovered from agarose gels using GeneJET kits (Thermo Fisher Scientific) or QIAgen Purification Kit (Qiagen, Hilden, Germany). Standard protocols for lithium acetate transformations were used for both S. cerevisiae (Gietz and Woods 2002) and Y. lipolytica (Le Dall, Nicaud and Gaillardin 1994). All primers are listed in Table S1 (Supporting Information).
Overexpression of YALI0F18502g
Expression in a glycogen-deficient strain of S. cerevisiae, a loxP flanked KanMX4 cassette from pYGFPgN (Prein, Natter and Kohlwein 2000), was amplified with the primers gsy1_del_f and gsy1_del_r, bearing overhangs for homologous recombination at the GSY1 locus and resulting in the replacement of the GSY1 ORF with the KanMX4 cassette after transformation of the wild-type strain CEN.PK 113–7D. To excise the cassette after confirmation of the deletion of GSY1, the URA3 marker of pSH47 (Güldener et al.1996) was exchanged with the nourseothricin resistance gene. The resulting plasmid, pSH47-NAT, was used to transform the gsy1 deletion strain. After incubation of the transformants in galactose medium for expression of the Cre recombinase, the colonies that had lost the G418 resistance were selected and the excision of the KanMX4 cassette was confirmed by control PCR. The same procedure was used to delete GSY2 and URA3, resulting in a uracil auxotrophic gsy1::loxP gsy2::loxP mutant.
For the strong constitutive expression of YALI0F18502g in S. cerevisiae, the plasmid pYES2 (Thermo Scientific) was digested with SwaI and SphI to excise the f1 origin and the GAL1 promoter. The TEF1 promoter, amplified with the primers TEF-fwd and TEF-rev from S. cerevisiae genomic DNA, was digested with SmaI and SphI and ligated to the linearized vector, resulting in the plasmid pHEY-1. YALI0F18502g was amplified from genomic DNA of Y. lipolytica using the primers Yl_GSY_F/ Yl_GSY_R. The PCR product was digested with EcoRI and XbaI and ligated with the EcoRI/XbaI digested pHEY-1, resulting in pHEY-1/YlGSY1. Successful cloning was confirmed by sequencing, and the vector was used to transform the glycogen-deficient S. cerevisiae strain.
Overexpression in Y. lipolytica: for the strong constitutive expression of YALI0F18502g in Y. lipolytica, the YlTEF1 promoter was amplified by PCR from Y. lipolytica genomic DNA with the primers YlTEF1_GSYoe_F/ YlTEF1_GSYoe_R and circularized with the ClaI digested plasmid pGMKGSY_12 (see below) by Gibson assembly (Gibson et al.2009). This intermediate plasmid was again linearized with ClaI enzyme. The reading frame of the gene YALI0F18502g was amplified with the primers GSYoe_F/ GSYoe_R, and the linearized plasmid and the PCR product were assembled by Gibson assembly to pGMKTEFGSY_02, which was used for transformation of the Y. lipolytica strain deleted for YALI0F18502g (see below).
Deletion of the glycogen synthase gene
The plasmid pFA6a-GFP(S65T)-KanMX6 (Wach et al.1997) was digested with NotI to excise the GFP and KanMX6 coding regions. To generate a URA3 cassette flanked by loxP sites, URA3 together with its promoter and terminator was amplified from Y. lipolytica genomic DNA with the primers Yl_URA3_F1/Yl_URA3_R1, bearing loxP sites. The product was amplified with the primers Yl_URA3_F2/Yl_URA3_R2 for the addition of NotI sites upstream and downstream of the loxP-URA3 cassette. The PCR product was digested with NotI and ligated with the plasmid backbone, resulting in plasmid pFA6aURA3-09.
The deletion cassette for glycogen synthase was constructed according to the procedure described by Fickers et al. (2003). In brief, 1 kb of the promoter (YlGSY1P) and terminator (YlGSY1T) regions of the YALI0F18502g ORF were amplified with primer pairs Yl_GSYP_F/Yl_GSYP_R and Yl_GSYT_F/Yl_GSYT_R, respectively, and sequentially cloned into the plasmid pFA6aURA3-09, resulting in pGMKGSY_12. The correct assembly of the episomal YlGSY1P-loxP-URA3-loxP- YlGSY1T cassette was confirmed by sequencing. This cassette was excised from the plasmid by NotI digestion, gel-purified and used for transformation of JMY322. The integration of the cassette at the correct locus and loss of the YALI0F18502g ORF was confirmed by control primer PCR and sequencing.
The same strategy was used to delete YALI0F18502g in the TAG synthesis-deficient strain but we failed to obtain deletion strains in several attempts. Therefore, we used a CRISPR/Cas9-based approach as described by Schwartz et al. (2016) with the sequence from 27–46 bp in the putative GSY reading frame as guide sequence. The insertion of the guide sequence into the plasmid pCRISPRyl was performed according to Schwartz et al. (2016). In brief, an equimolar mixture of the primers CRSPyl_gsy_f and CRSPyl_gsy_r was heated up to 95°C and allowed to cool down to room temperature at a rate of 1°C/min, to obtain a double-stranded DNA bearing the guide sequence and flanking sequences for Gibson assembly with the linearized plasmid pCRISPRyl. A total of 1.5 μg of the resulting plasmid, pCRISPRyl/GSY, was used to transform the TAG-deficient mutant strain according to Le Dall, Nicaud and Gaillardin (1994). After transformation, the cells were inoculated into selection medium (-leu) and incubated for 48 h. Aliquots were plated onto YPD plates to obtain single colonies, which were stained with Lugol's solution (1% KI, 0.5% I2) to identify glycogen-deficient mutants. YALI0F18502g was amplified from genomic DNA of these colonies with the primers Yl_GSY_F/Yl_GSY_R and sequenced with the primer Yl_GSYT_Ctr_R to confirm a frame shift in the target region of the gene.
Construction of the obese mutants
The previously described plasmids (Lazar et al.2014), JME1364 (tgl4Δ), JME1822 (pTEF-DGA2), JME1128 (pTEF-GPD1) and URA3ex, were NotI digested for excision of the desired DNA fragments. In addition, for the construction of the obese strain deleted in YlGSY1, the plasmid pGMKGSY_12 was used as described above. The linearized DNA fragments were used to transform Y. lipolytica and transformants were selected on YNBUra, YNBLeu or YNB, depending on their genotype. Positive transformants were identified by PCR and confirmed by sequencing. The recycling of the selection markers was carried out by using the LoxP-Cre system as described by Fickers et al. (2003).
Analytical methods
Biomass determination: cells densities were measured with a Casy® TTC cell counter equipped with a 60 μm capillary (Roche Diagnostics GmbH, Roche Applied Science, Penzberg, Germany). The cell dry weights were determined by filtration through 0.45 μm nitrocellulose filters (Sartorius Stedim, Goettingen, Germany) and subsequent drying at 97°C overnight.
Analysis of metabolites: media analyses for determination of extracellular metabolites (glucose, glycerol, mannitol, citrate, succinate, acetic acid and ethanol) were performed with an HP 1100 series HPLC system equipped with an Aminex HPX-87H column (Biorad, Richmond, CA, USA), a UV detector (Agilent Technologies Österreich GmbH, Vienna, Austria) and a Knauer Differential refractometer as explained by Hanscho et al. (2012). During cultivations, 1 mL of culture was harvested, centrifuged at 16 000 g at 4°C for 1 min and the supernatant was directly injected into HPLC or stored at –80°C until analysis.
Glycogen and trehalose analyses and lipid extractions were performed according to Hanscho et al. (2012). Fatty acid methyl esters for GC-MS or GC-FID were prepared from 200 μL of lipid extract according to Kavšček et al. (2015). Heptadecanoid (C17:0) acid was used as an internal standard.
All averaged data and their standard deviations are derived from a minimum of three biologically independent experiments. If it was necessary to increase the number of independent experiments to confirm significance of differences, this is mentioned in the Results section.
Flux balance analysis
The Y. lipolytica iMK735 genome scale metabolic model (Kavšček et al.2015) was used to predict changes in fluxes for a strain deleted for glycogen synthesis. An optimization was done in the MATLAB environment using the COBRA toolbox (Schellenberger et al.2011). The glycogen production rate and glycerol uptake rates were calculated from the experimental data and used as constraints in optimization for lipid production in the wild-type model. For the calculation of the effects of gsy deletion, the glycogen production rate was set to zero and the same glycerol uptake rate as in the wild-type model was used. A dynamic FBA calculation with 3 g L−1 cell dry weight was made to calculate the final TAG content.
RESULTS AND DISCUSSION
Glycogen contributes up to 16% to the biomass of Yarrowia lipolytica
Yarrowia lipolytica is well known and intensively investigated for its ability to accumulate high amounts of storage lipid under conditions where the carbon source is available in excess but another nutrient, typically the nitrogen source, is limiting. However, the behavior of this yeast regarding accumulation of the second important storage metabolite, glycogen, has not been investigated in detail. To assess the effects of different nutrient limitations on glycogen accumulation in Y. lipolytica, we performed shake flask cultivations under carbon as well as under nitrogen-limited conditions. For the carbon-limited cultivations, 5 g L−1 glucose or glycerol were used to avoid physiological stress due to a decrease of the pH when cells are growing to high densities, as it is the case in media with 20 g L −1 carbon source. For nitrogen limitation, 20 g L−1 of the carbon source were used, but the ammonium sulfate concentration was reduced from 5 to 0.4 g L−1, resulting in depletion of the nitrogen source when <50% of the carbon source are consumed (Kavšček et al.2015).
The wild-type strain accumulated 44 ± 2 and 23 ± 2 mg glycogen per gCDW (CDW, cell dry weight) during the exponential growth phase in glucose and glycerol containing media, respectively. These stores were depleted during stationary phase. Interestingly, the opposite behavior was observed in Saccharomyces cerevisiae, where glycogen accumulates only in the stationary phase (Fig. 1A). Under nitrogen-limited conditions, a significant increase in glycogen content was observed for both yeast species, with a maximum of 165 mg gCDW−1 (Fig. 1B) in Y. lipolytica. Hence, in addition to its oleaginous phenotype, Y. lipolytica is able to accumulate high amounts of carbohydrate stores. Remarkably, the dynamics of glycogen storage are similar to those of lipid storage, with low levels during exponential growth and a strong increase under nitrogen-limited, i.e. lipogenic, conditions.
Figure 1.
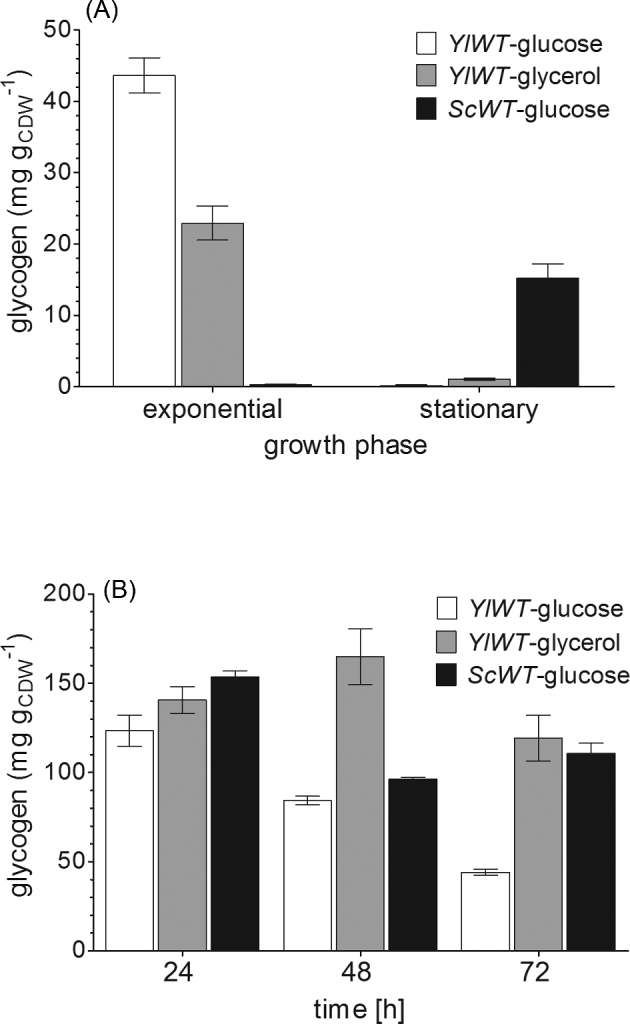
Glycogen accumulation in wild-type strains of Y. lipolytica and S. cerevisiae. (A) Glycogen content in the biomass of Y. lipolytica and S. cerevisiae during cultivation in carbon-limited media. Yarrowia lipolytica (YlWT) accumulated only moderate amounts of glycogen in the exponential phase of growth. These storage pools were depleted in the early stationary phase. In contrast, the glycogen levels in S. cerevisiae were low during exponential growth, but increased in the stationary phase. (B) Glycogen content of Y. lipolytica and S. cerevisiae during cultivation in nitrogen-limited media. Under lipogenic conditions, both yeasts accumulated high amounts of glycogen.
Yarrowia lipolytica is mainly investigated for its oleaginous phenotype. A multitude of different genetic engineering and fermentation strategies have been applied to increase the lipid content of this yeast (Blazeck et al.2014; Dulermo et al.2015; Kavšček et al.2015; Rakicka et al.2015; Friedlander et al.2016). Since Y. lipolytica accumulates more than 16% glycogen in its biomass under the conditions that are typically used for lipid accumulation (Fig. 1B), we wanted to know whether suppression of glycogen storage might result in an increase of the TAG content, due to a redirection of the carbon flux from glycogen to lipid synthesis. We used flux balance analysis (FBA) with a recently published network reconstruction of Y. lipolytica (Kavšček et al.2015) to investigate the theoretically possible effect of the elimination of glycogen storage on a computational level. Such a modeling approach allows for the optimization of a user-defined function. In our case, we calculated the optimal solution for the maximization of lipid synthesis. In a ‘wild-type model’, growing on glycerol as carbon source, we obtained a TAG synthesis rate of 15.9 μmol g−1 h−1 (corresponding to ca. 0.3 g TAG per gCDW in 24 h). If glycogen synthesis was eliminated by setting the glycogen content in the biomass to zero, the flux into TAG increased to 17.0 μmol g−1 h−1, thus predicting an improvement of the synthesis rate by 7.1%.
In practice, FBA is often not able to predict the exact quantitative responses to the change of model parameters because regulatory mechanisms, such changes on the transcriptional or post-translational level, are not implemented in the network reconstruction. Nevertheless, such predictions can serve as guidelines for the optimization of strains by genetic modifications. Therefore, we concluded that the deletion of glycogen synthesis might be a promising strategy to increase NL synthesis in Y. lipolytica.
Glycogen synthase activity is encoded by a single non-essential gene in Yarrowia lipolytica
The genome of Y. lipolytica bears one CDS that was annotated as glycogen synthase, due to its similarities with Gsy1 and Gsy2 of S. cerevisiae (Dujon et al.2004). In a pairwise sequence alignment (Smith and Waterman 1981), the encoded protein, YALI0F18502p, is 67% identical with the two glycogen synthases of baker's yeast. Therefore, we will use the name YlGsy1p for this protein in the following sections. To confirm its glycogen synthase activity by complementation of an S. cerevisiae glycogen synthase-deficient strain, we amplified YlGSY1 from Y. lipolytica and cloned it into an S. cerevisiae expression vector under the control of a strong constitutive promoter. We deleted GSY1 and GSY2 in an S. cerevisiae wild-type strain and transformed this double mutant, which does not accumulate any glycogen, with the vector bearing YlGSY1. As shown in Fig. 2A, heterologous expression of this gene resulted in reconstitution of glycogen storage in the gsy1Δ gsy2Δ double mutant.
Figure 2.
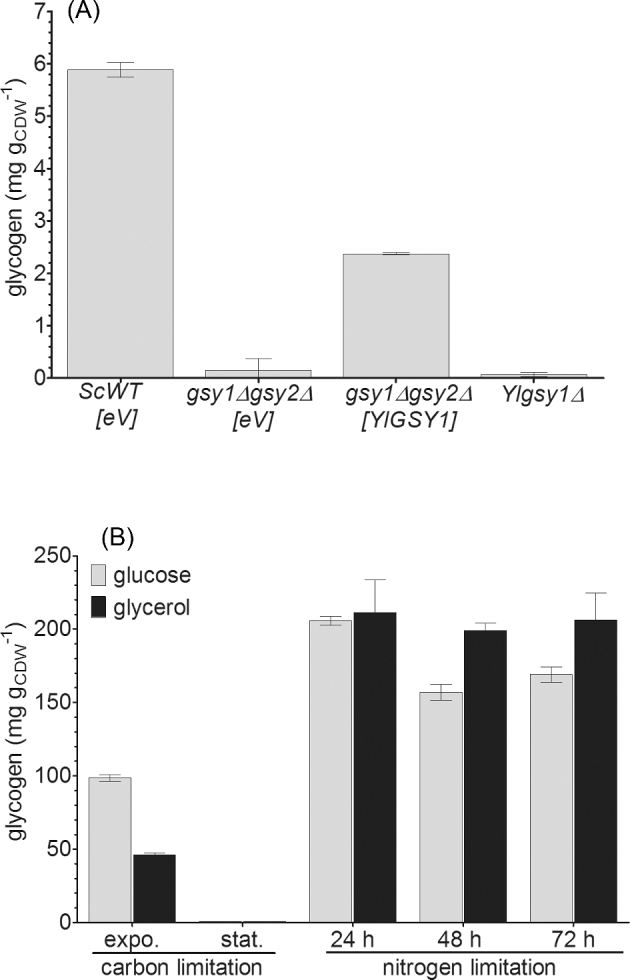
YALI0F18502g encodes the only glycogen synthase in Y. lipolytica. (A) The episomal expression of YALI0F18502g in a S. cerevisiae mutant deleted for GSY1 and GSY2 resulted in the reconstitution of glycogen storage, confirming that YALI0F18502g is indeed a glycogen synthase. The deletion of this reading frame in Y. lipolytica resulted in a complete loss of glycogen synthesis under both carbon and nitrogen limitation. Data are shown for cells grown in carbon-limited media with glucose as carbon source. [eV] empty vector, [YlGSY1] episomal expression of YALI0F18502g, Ylgsy1Δ Y. lipolytica bearing a deletion of the glycogen synthase. (B) In a Y. lipolytica strain overexpressing the glycogen synthase gene, glycogen storage is significantly increased during cultivation in nitrogen-limited media. The degradation of glycogen, as it was observed in the wild-type (Fig. 1B), was strongly delayed or compensated for by de novo synthesis.
Furthermore, we deleted YlGSY1 in JMY322. Our analysis showed that the resulting Ylgsy1Δ mutant strain does not accumulate detectable amounts of glycogen (Fig. 2A), neither in carbon-limited media during exponential growth nor in nitrogen-limited media, where glycogen accounts for up to 16% of the biomass. Taken together, these results confirm that YlGSY1 is the only gene coding for a glycogen synthase in Y. lipolytica.
Finally, we overexpressed YlGSY1 and analyzed the resulting mutant for its properties regarding glycogen accumulation during growth in both carbon- and nitrogen-limited media (Fig. 2B). During exponential growth in carbon-limited media, the glycogen content was two times higher than in the wild-type but the overexpression of YlGSY1 had no influence on the complete degradation of glycogen during stationary phase, as it was also observed for the wild-type (shown in Fig. 1A). Under nitrogen limitation, however, the decrease in glycogen content, which was more pronounced in glucose than in glycerol media, was delayed in the YlGSY1 overexpressing strain, resulting in an up to 4-fold glycogen content compared to the wild-type.
Deletion of glycogen synthesis in Yarrowia lipolytica results in increased TAG accumulation
To experimentally test the predictions obtained with FBA, we cultivated the Y. lipolytica wild type and the Ylgsy1Δ mutant strain under nitrogen-limited conditions, to induce lipid accumulation. For both glucose and glycerol as carbon sources, we observed a significant improvement in lipid accumulation in the mutant, as compared to the wild-type strain. After 24 h of cultivation, the reference strain accumulated 110 ± 18 and 104 ± 28 mg TAG per gCDW on glucose and glycerol as carbon source, respectively. Under the same conditions, we obtained TAG values of 179 ± 21 and 167 ± 31 mg per gCDW in the Ylgsy1Δ mutant, corresponding to a more than 60% increase in TAG synthesis for both carbon sources (Fig. 3). Similar differences were observed at later time points, although they were more pronounced in glycerol than in glucose. The highest TAG content of 26% was obtained with the Ylgsy1Δ mutant cultivated in glycerol medium for 48 h.
Figure 3.
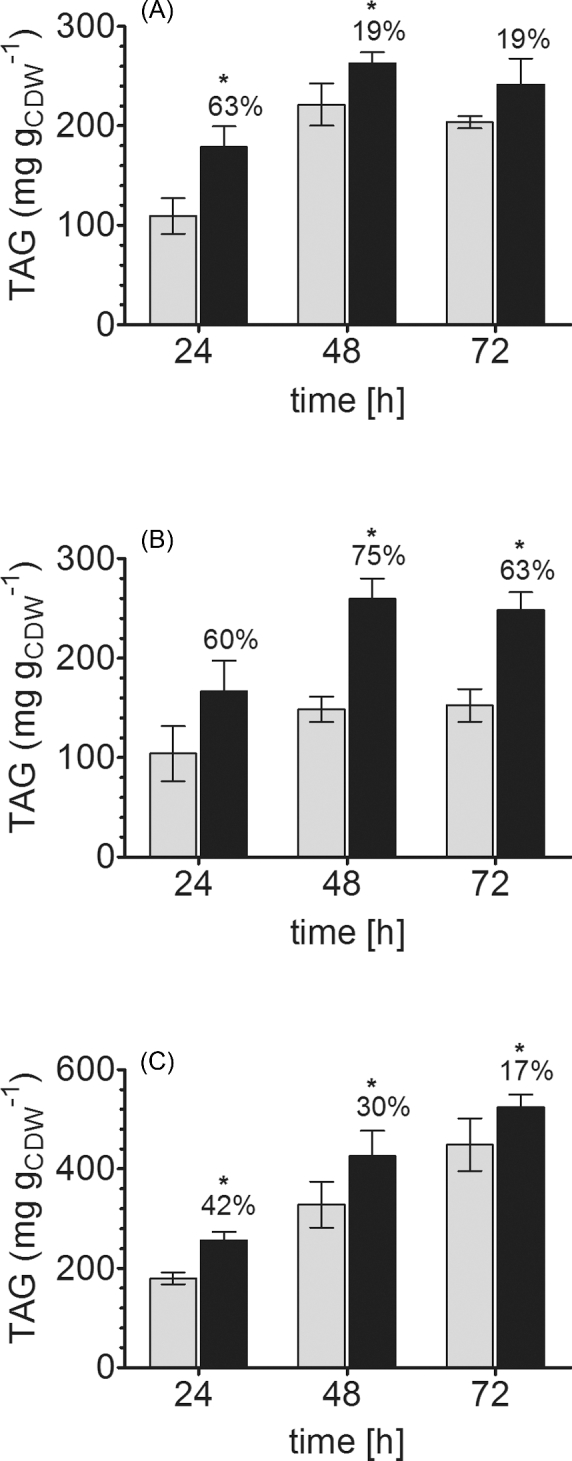
Deletion of glycogen synthase results in improved TAG synthesis. (A and B) Lipid content of Y. lipolytica wild-type and gsy1Δ mutant grown in nitrogen-limited glucose (A) and glycerol (B) media. Under all conditions tested, the deletion of GSY1 resulted in an improvement of lipid accumulation, with the highest increase (+75%) after cultivation in glycerol for 48 h. (C) The deletion of YlGSY1 in the obese background resulted in the same trend, although with lower differences than in the wild type, with the maximal difference (+42%) after 24 h. The highest lipid content of 524 mg TAG per gCDW was obtained after 72 h. * P ≤ 0.05 in a two-tailed t-test. Gray bars: wild-type for panels A, B and ‘obese’ for panel C, black bars: respective gsy1 deletion strains.
The effect of the Ylgsy1 deletion on TAG accumulation was not observed when the cells were grown in carbon-limited medium, where the oleaginous phenotype is not induced. In fact, the TAG content in stationary phase was even reduced in the Ylgsy1Δ mutant, as compared to the wild-type (see Table S2, Supporting Information). Furthermore, lipid analysis of S. cerevisiae strains grown in nitrogen-limited medium showed that the deletion of GSY1 and GSY2 resulted in less pronounced or even no changes in lipid content of this species (see Table S3, Supporting Information). We speculate that this difference can be attributed to the fact that in the Crabtree-positive S. cerevisiae, most of the catabolized glucose is diverted to ethanol for regeneration of NAD+, whereas in the strictly respiratory Y. lipolytica glucose is converted to biomass—in this case TAG—more efficiently.
The increase in the TAG content of the Y. lipolytica gsy1Δ mutant was mainly due to an increase of desaturated C18 FAs. Indeed, the level of C16:0 remained almost unchanged, whereas C18:1 levels increased up to 2-fold (see Table S4, Supporting Information). Hence, the deletion of glycogen synthesis seems to cause an increase of FA de novo synthesis and of FA desaturation activity.
Interestingly, the improvement in TAG accumulation in the deletion mutant was higher than it would have been expected from a simple redirection of the carbon flux from glycogen to FA synthesis. For example, the 119 mg glycogen that are stored in the wild-type after 72 h of cultivation in glycerol (Table 2) would allow for the synthesis of 36.5 mg TAG if this carbon flux were redirected to TAG with the maximum theoretical yield (Ratledge 2014). However, the glycogen-deficient mutant produces ca. 100 mg more TAG than the wild-type at the same time point. Although this difference is lower in glucose-grown cells than in a glycerol medium, it was observed under all conditions. In S. cerevisiae, the glucose dimer trehalose plays an important role in storage of carbohydrates. To investigate whether trehalose might contribute to the changes in TAG content of the glycogen-deficient mutant of Y. lipolytica, we quantified this metabolite in the wild-type and in the mutant under the same conditions as they were used for TAG analysis. These experiments showed that the trehalose content always remains below 1 mg gCDW−1 (Table 2). Although it is further reduced to almost zero in the Ylgsy1Δ mutant, this reduction cannot explain the observed increase of the TAG content in the mutant, indicating that the deletion of YlGSY1 has an additional—unknown—effect on lipid synthesis, beyond the mere redirection of the carbon flux from glycogen to TAG.
Table 2.
Physiological data for wild-type and deletion mutant in nitrogen-limited media.
| YlWT | Ylgsy1Δ | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| I. Glucose | ||||||
| Biomass (g L−1) | ||||||
| CDW | 2.4 ± 0.2 | 3.4 ± 0.2 | 3.5 ± 0.1 | 2.0 ± 0.1 | 3.2 ± 0.2 | 3.4 ± 0.1 |
| Extracellular metabolites (g L−1) | ||||||
| Glucose | 14.7 ± 0.6 | 9.4 ± 0.4 | 6.2 ± 0.3 | 15.4 ± 0.3 | 10.1 ± 0.1 | 6.9 ± 0.1 |
| Citrate | n.d. | 0.4 ± 0.1 | 1.3 ± 0.2 | n.d. | 0.1 ± 0.0 | 1.1 ± 0.1 |
| Succinate | 0.2 ± 0.0 | 0.2 ± 0.0 | n.d. | 0.2 ± 0.0 | 0.2 ± 0.0 | n.d. |
| Mannitol | 0.5 ± 0.2 | 1.6 ± 0.3 | 2.2 ± 0.3 | 0.4 ± 0.1 | 1.3 ± 0.1 | 1.7 ± 0.1 |
| Intracellular metabolites (mg gCDW−1) | ||||||
| Trehalose | 0.33 ± 0.14 | 0.09 ± 0.05 | 0.10 ± 0.02 | 0.06 ± 0.04 | n.d. | 0.02 ± 0.00 |
| Glycogen | 123 ± 9 | 84 ± 2 | 44 ± 2 | n.d. | n.d. | n.d. |
| TAG | 110 ± 18 | 221 ± 21 | 204 ± 6 | 179 ± 21* | 263 ± 11* | 242 ± 26 |
| II. Glycerol | ||||||
| Biomass (g L−1) | ||||||
| CDW | 2.6 ± 0.1 | 3.8 ± 0.0 | 4.0 ± 0.1 | 2.2 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 |
| Extracellular metabolites (g L−1) | ||||||
| Glycerol | 14.7 ± 0.8 | 7.7 ± 0.4 | 2.5 ± 0.3 | 14.9 ± 0.7 | 6.2 ± 0.4 | n.d. |
| Citrate | n.d. | 0.9 ± 0.1 | 2.1 ± 0.4 | n.d. | 1.3 ± 0.1 | 3.2 ± 0.7 |
| Succinate | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 | n.d. |
| Mannitol | 0.4 ± 0.0 | 2.9 ± 0.5 | 5.1 ± 0.9 | 0.7 ± 0.1 | 3.7 ± 0.5 | 6.2 ± 0.5 |
| Intracellular metabolites (mg gCDW−1) | ||||||
| Trehalose | 0.60 ± 0.11 | 0.29 ± 0.02 | 0.18 ± 0.03 | 0.13 ± 0.06 | 0.03 ± 0.00 | 0.01 ± 0.01 |
| Glycogen | 141 ± 8 | 165 ± 16 | 119 ± 13 | n.d. | n.d. | n.d. |
| TAG | 104 ± 28 | 149 ± 13 | 152 ± 16 | 167 ± 31 | 260 ± 20* | 248 ± 18* |
n.d., not detected
P ≤ 0.05 in unpaired two-tailed t-tests
Based on the assumption that yeast is able to compensate for changes in one storage pathway by adapting the flux through the remaining one, we analyzed the lipid content of YlTEFGSY1, the strain overexpressing YlGSY1. Contrary to our expectations, this strain, which accumulates high amounts of glycogen, does not store less NL. In fact, the TAG content of the mutant is the same as in the wild-type during cultivation in N-lim media and even higher than in wild-type in both exponential and stationary phase of growth in C-lim media (see Table 3 and Table S2). Likewise, the deletion of NL synthesis in Y. lipolytica did not result in an increase of glycogen storage, as it would have been expected assuming a compensation mechanism (Table S5, Supporting Information). Indeed, the glycogen content was reduced in the NL-deficient quadruple mutant (JMY1877, Table 1). On the other hand, the deletion of NL synthesis in baker's yeast resulted in the expected effect. This strain accumulated several-fold more glycogen and trehalose than the wild-type parent strain, although such a significant difference was only observed during exponential growth and not under N-lim conditions.
Table 3.
Glycogen and TAG accumulation (mg gCDW−1) in the mutant overexpressing YlGSY.
| Metabolite | Glycogen | TAG | |||
|---|---|---|---|---|---|
| Carbon source | Glucose | Glycerol | Glucose | Glycerol | |
| C-lim | Exponential | 99 ± 2 | 46 ± 1 | 61 ± 5 | 68 ± 4 |
| stationary | 1 ± 0 | 1 ± 0 | 51 ± 7 | 51 ± 4 | |
| N-lim | 24 h | 206 ± 3 | 211 ± 22 | 135 ± 5 | 120 ± 10 |
| 48 h | 157 ± 5 | 199 ± 5 | 226 ± 3 | 135 ± 30 | |
| 72 h | 169 ± 5 | 206 ± 18 | 203 ± 11 | 155 ± 7 | |
Hence, alterations in one of the storage pathways result in changes in the second one in almost all cases and it seems clear that the two processes are connected to each other. However, a simple model assuming a compensatory strategy of the cell is not sufficient to explain our observations. Furthermore, the different responses of S. cerevisiae and Y. lipolytica to modifications in the pathways for TAG and glycogen synthesis indicate that storage metabolism is regulated by different mechanisms in these two yeasts.
Elimination of glycogen storage in an obese mutant results in further improvement of lipid accumulation
From a biotechnological perspective, the reduction or, if possible, deletion of competing pathways is a standard approach to improve product yields. We have shown that glycogen synthesis is indeed a competing process with respect to TAG synthesis, despite the distance of the two pathways in the cellular metabolic network. Therefore, we investigated the impact of the deletion of YlGSY1 in a strain background that is already optimized for TAG production with known effectors of this pathway. This strain was obtained by deletion of the gene coding for TAG lipase, TGL4, and overexpression of DGA2 and GPD1, the genes encoding diacylglycerol transferase and glycerol-3-phosphate dehydrogenase, respectively (Dulermo and Nicaud 2011; Beopoulos et al.2012; Lazar et al.2014). When cultivated in nitrogen-limited media, this strain, named ‘obese’, stores up to 449 ± 53 mg TAG and up to 181 ± 8 mg glycogen per gCDW (Table 4). We deleted YlGSY1 in this strain background and confirmed the loss of its ability to store glycogen. Cultivation in N-lim media and subsequent lipid analysis showed that this deletion has similar effects in the obese background as in the wild-type. After 72 h, the biomass of obese gsy1Δ contained 524 ± 25 mg TAG, corresponding to a 17% improvement in comparison to the already obese parent strain (Fig. 3C and Table 4). As opposed to the wild-type background, the deletion of YlGSY1 in the obese background did not result in an increase of unsaturated FA only, but in an equal improvement of all species, keeping the degree of saturation approximately constant (see Table S4).
Table 4.
Physiological data for obese and obese gsy1Δ in nitrogen-limited glycerol medium.
| obese | obese gsy1Δ | |||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Biomass (g L−1) | ||||||
| CDW* | 2.6 ± 0.3 | 4.5 ± 0.2 | 5.5 ± 0.4 | 2.0 ± 0.2 | 3.6 ± 0.2 | 5.0 ± 0.4 |
| Extracellular metabolites (g L−1) | ||||||
| Glycerol | 14.4 ± 0.3 | 8.2 ± 0.1 | 3.0 ± 0.2 | 15.1 ± 0.3 | 8.2 ± 0.1 | 2.1 ± 0.1 |
| Citrate | n.d. | n.d. | n.d. | n.d. | n.d. | 0.1 ± 0.0 |
| Succinate | 0.3 ± 0.0 | 0.4 ± 0.0 | 1.7 ± 2.2 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 |
| Mannitol | 0.2 ± 0.0 | 1.1 ± 0.1 | 1.9 ± 0.1 | 0.4 ± 0.0 | 1.6 ± 0.0 | 2.5 ± 0.0 |
| Intracellular metabolites (mg gCDW−1) | ||||||
| Trehlaose | 1.64 ± 0.25 | 0.93 ± 0.01 | 0.62 ± 0.06 | 0.09 ± 0.01 | 0.09 ± 0.07 | n.d. |
| Glycogen* | 181 ± 8 | 124 ± 24 | 85 ± 19 | n.d. | n.d. | n.d. |
| TAG* | 180 ± 12 | 328 ± 46 | 449 ± 53 | 256 ± 17** | 426 ± 51** | 524 ± 25** |
n.d., not detected
Values from five independent biological replicates.
P ≤ 0.05 in unpaired two-tailed t-tests.
The chronological lifespan of Yarrowia lipolytica is affected by a loss of storage metabolism
The mutant strains of Y. lipolytica overexpressing glycogen synthase or deleted for this gene did not show any measurable differences to the wild-type with regard to growth rate, biomass yield or cell morphology. Furthermore, like in the wild-type, we did not observe any detectable amounts of metabolites excreted into the medium during growth in carbon-limited media (data not shown).
Because storage metabolism is often assumed to play a role in survival during stationary phase, we also assessed the chronological lifespan of the deletion mutant. These experiments indicated that the inability to store glycogen does not affect viability of Y. lipolytica over 20 days of starvation (Fig. 4). A similar behavior was demonstrated by Osório et al. (2005) for S. cerevisiae, and we confirmed these results for our strain background and growth conditions (Fig. S1, Supporting Information). Viability has also been shown for NL-deficient mutants, deleted for both TAG and steryl ester (SE) synthesis (Sandager et al.2002; Beopoulos et al.2012), but the effect of a complete deletion of storage metabolism has not yet been investigated. Therefore, we deleted glycogen synthesis in the NL-deficient mutant backgrounds of both Y. lipolytica and S. cerevisiae. We obtained viable strains for both species and confirmed that both glycogen and NL levels were below the limit of detection. Subsequent chronological life span experiments showed that the deletion of storage lipid synthesis in Y. lipolytica has a strong negative effect on viability during stationary phase, with already more than 80% dead cells after only 4 days of starvation. For the mutant deleted for both NL and glycogen synthesis, we found a similar behavior during this first period. In the following days, the viability of the NL-deficient strain remained constant, whereas the survival rate of the completely storage-deficient strain further declined, with only ca. 1% surviving cells after 20 days (Fig. 4). In contrast, no differences between wild-type and mutants were observed for S. cerevisiae (Fig. S1).
Figure 4.
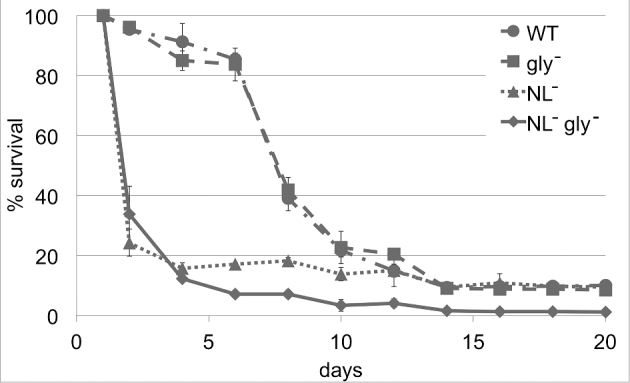
Chronological lifespan is affected by the loss of storage metabolism. The Y. lipolytica wild-type strain (WT) and its derivatives deleted for glycogen (gly−) or neutral lipid (NL−) synthesis or both (NL− gly−) were cultivated until depletion of glucose (C-lim minimal medium), and the survival rate was determined by plating diluted samples. The viability of the NL-deficient strain was strongly reduced in comparison to its parent and the additional deletion of YlGSY1 in this mutant, resulting in a strain without the ability to store carbon, caused a further reduction of the survival rate.
As for the Y. lipolytica strain that was deleted only for glycogen synthesis, the pentuple mutant (YlPM, Table 1) did not compensate for the loss of both NL and glycogen synthesis by accumulating trehalose, neither during growth in C-lim nor in N-lim media (Table S5). Hence, this strain is completely devoid of any form of carbon storage, confirming that storage metabolism is not essential. It has to be noted, however, that already the deletion of TAG and SE synthesis causes a severe growth defect during exponential growth in C-lim media (maximum specific growth rate μ = 0.27 h−1 for wild-type and 0.08 h−1 for the NL-deficient mutant), a phentoype that was also observed for the NL-deficient S. cerevisiae mutant. This phenotype was not further aggravated by the additional deletion of glycogen synthesis, suggesting that the storage of NLs plays a more important role than glycogen. This finding might reflect the fact that the lipids stored in the ‘inert’ lipid droplet play a crucial role in homeostasis and turnover of membrane lipids in yeasts, rather than just serving as energy reserve.
CONCLUSION
Yarrowia lipolytica accumulates large amounts of glycogen during cultivation under conditions that are typically used for lipid accumulation, and the same might be assumed for other oleaginous yeasts. Hence, although the two pathways for TAG and glycogen synthesis are not closely connected to each other, glycogen synthesis is a competing reaction during TAG accumulation and should be suppressed to maximize the yield, even in strain backgrounds that have already been genetically engineered for high lipid yields. In addition, it might be speculated that not only FA synthesis but also other production pathways, like the synthesis of terpenoids or polyketides, would benefit from elimination of glycogen storage. Importantly, the deletion of GSY1 causes not only a redirection of the carbon flux that is normally bound for glycogen synthesis towards FA production. Rather, it induces an additional increase in TAG synthesis beyond what would be expected from the mere rearrangement of carbon storage pools, resulting in higher rates and yields. Moreover, the results of this study suggest that the loss of glycogen storage has no significant impact on the growth rate and viability of yeast strains. Indeed, for both the respiratory and oleaginous Y. lipolytica as well as for the fermentative S. cerevisiae, the storage of lipids seems to play a more important role than of glycogen.
Supplementary Material
Supplementary data are available at FEMSYR online.
Acknowledgments
The authors thank Gerold Barth for the Yarrowia lipolytica wild-type strain H222, Peter Koetter for the Saccharomyces cerevisiae strain CEN.PK 113–7D and Gabi Gogg and Florian Hofer for technical assistance.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSYR online.
FUNDING
This work was supported by the Austrian Science Fund, FWF, project TRP 240-B21 (Translational Research Programme). R. Ledesma-Amaro received financial support from the European Union in the framework of the Marie-Curie FP7 COFUND People Program (FP7-267196) in the form of an AgreenSkills' Fellowship.
Conflict of interest. None declared.
REFERENCES
- Abghari A, Chen S. Yarrowia lipolytica as an oleaginous cell factory platform for production of fatty acid-based biofuel and bioproducts. Front Energy Res 2014;2:1–21. [Google Scholar]
- Barth G, Gaillardin C. Yarrowia lipolytica. In: Nonconv. Yeasts Biotechnol. SE - 10. Berlin Heidelberg: Springer, 1996, 313–88. [Google Scholar]
- Beopoulos A, Haddouche R, Kabran P et al. . Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA:diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl Microbiol Biot 2012;93:1523–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazeck J, Hill A, Liu L et al. . Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 2014;5:3131. [DOI] [PubMed] [Google Scholar]
- Bozaquel-Morais BL, Madeira JB, Maya-Monteiro CM et al. . A new fluorescence-based method identifies protein phosphatases regulating lipid droplet metabolism. PLoS One 2010;5:e13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ et al. . Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998;14:115–32. [DOI] [PubMed] [Google Scholar]
- Conrad M, Schothorst J, Kankipati HN et al. . Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 2014;38:254–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B, Sherman D, Fischer G et al. . Genome evolution in yeasts. Nature 2004;430:35–44. [DOI] [PubMed] [Google Scholar]
- Dulermo T, Lazar Z, Dulermo R et al. . Analysis of ATP-citrate lyase and malic enzyme mutants of Yarrowia lipolytica points out the importance of mannitol metabolism in fatty acid synthesis. Biochim Biophys Acta 2015;1851:1107–17. [DOI] [PubMed] [Google Scholar]
- Dulermo T, Nicaud J-M. Involvement of the G3P shuttle and β-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab Eng 2011;13:482–91. [DOI] [PubMed] [Google Scholar]
- Entian K-D, Koetter P. Yeast genetic strain and plasmid collections, In: Stansfield I, Stark MJR, (eds). YEAST GENE Anal. Second Ed. 525 B STREET, SUITE 1900, SAN DIEGO, CA 92101-4495 USA: ELSEVIER ACADEMIC PRESS INC. Methods in Microbiology, 2007, Vol. 36, pp. 629–66. [Google Scholar]
- Evans CT, Ratledge C. Phosphofructokinase and the regulation of the flux of carbon from glucose to lipid in the oleaginous yeast Rhodosporidium toruloides. Microbiology 1984;130:3251–64. [Google Scholar]
- Fickers P, Le Dall MT, Gaillardin C et al. . New disruption cassettes for rapid gene disruption and marker rescue in the yeast Yarrowia lipolytica. J Microbiol Methods 2003;55:727–37. [DOI] [PubMed] [Google Scholar]
- François J, Parrou JL. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 2001;25:125–45. [DOI] [PubMed] [Google Scholar]
- Friedlander J, Tsakraklides V, Kamineni A et al. . Engineering of a high lipid producing Yarrowia lipolytica strain. Biotechnol Biofuels 2016;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y et al. . Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 2009;6:343–5. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 2002;350:87–96. [DOI] [PubMed] [Google Scholar]
- Güldener U, Heck S, Fielder T et al. . A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res 1996;24:2519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanscho M, Ruckerbauer DE, Chauhan N et al. . Nutritional requirements of the BY series of Saccharomyces cerevisiae strains for optimum growth. FEMS Yeast Res 2012;12:796–808. [DOI] [PubMed] [Google Scholar]
- Hardy TA, Huang D, Roach PJ. Interactions between cAMP-dependent and SNF1 protein kinases in the control of glycogen accumulation in Saccharomyces cerevisiae. J Biol Chem 1994;269:27907–13. [PubMed] [Google Scholar]
- Hong K-K, Nielsen J. Adaptively evolved yeast mutants on galactose show trade-offs in carbon utilization on glucose. Metab Eng 2013;16:78–86. [DOI] [PubMed] [Google Scholar]
- Kavšček M, Bhutada G, Madl T et al. . Optimization of lipid production with a genome-scale model of Yarrowia lipolytica. BMC Syst Biol 2015;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers D, van Biezen N, Martens D et al. . Selection of oleaginous yeasts for fatty acid production. BMC Biotechnol. 2016;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar Z, Dulermo T, Neuvéglise C et al. . Hexokinase-a limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab Eng 2014;26:89–99. [DOI] [PubMed] [Google Scholar]
- Le Dall MT, Nicaud JM, Gaillardin C. Multiple-copy integration in the yeast Yarrowia lipolytica. Curr Genet 1994;26:38–44. [DOI] [PubMed] [Google Scholar]
- Ledesma-Amaro R, Nicaud J-M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res 2016;61:40–50. [DOI] [PubMed] [Google Scholar]
- Mauersberger S, Wang H-J, Gaillardin C et al. . Insertional Mutagenesis in the n-Alkane-Assimilating Yeast Yarrowia lipolytica: Generation of Tagged Mutations in Genes Involved in Hydrophobic Substrate Utilization. J Bacteriol 2001;183:5102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicaud J-M. Yarrowia lipolytica . Yeast 2012;29:409–18. [DOI] [PubMed] [Google Scholar]
- Osório H, Silles E, Maia R et al. . Influence of chronological aging on the survival and nucleotide content of Saccharomyces cerevisiae cells grown in different conditions: occurrence of a high concentration of UDP-N-acetylglucosamine in stationary cells grown in 2% glucose. FEMS Yeast Res 2005;5:387–98. [DOI] [PubMed] [Google Scholar]
- Park WS, Murphy PA, Glatz BA. Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can J Microbiol 1990;36:318–26. [DOI] [PubMed] [Google Scholar]
- Parrou JL, Enjalbert B, Plourde L et al. . Dynamic responses of reserve carbohydrate metabolism under carbon and nitrogen limitations in Saccharomyces cerevisiae. Yeast 1999;15:191–203. [DOI] [PubMed] [Google Scholar]
- Petschnigg J, Wolinski H, Kolb D et al. . Good Fat, Essential Cellular Requirements for Triacylglycerol Synthesis to Maintain Membrane Homeostasis in Yeast. J Biol Chem 2009;284:30981–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prein B, Natter K, Kohlwein SD. A novel strategy for constructing N-terminal chromosomal fusions to green fluorescent protein in the yeast Saccharomyces cerevisiae. FEBS Lett 2000;485:29–34. [DOI] [PubMed] [Google Scholar]
- Preiss J, Romeo T. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog Nucleic Acid Re 1994;47:299–329. [DOI] [PubMed] [Google Scholar]
- Rakicka M, Lazar Z, Dulermo T et al. . Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. 2015;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz-Claret C, Jolivet P, Chardot, T. et al. Time-co-ordinated control of glycogen synthase, protein phosphatase 2A and protein kinase CK2 during culture growth in Yarrowia lipolytica in relation to glycogen metabolism. C R Acad Sci III 2000;323:257–66. [DOI] [PubMed] [Google Scholar]
- Ratledge C. The role of malic enzyme as the provider of NADPH in oleaginous microorganisms: a reappraisal and unsolved problems. Biotechnol Lett 2014;36:1557–68. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, 3rd edn Cold Spring Harbour, NY, USA: Cold Spring Harbor Laboratory Press, 2001, 1–3. [Google Scholar]
- Sandager L, Gustavsson MH, Ståhl U et al. . Storage lipid synthesis is non-essential in yeast. J Biol Chem 2002;277:6478–82. [DOI] [PubMed] [Google Scholar]
- Schellenberger J, Que R, Fleming RMT et al. . Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2.0. Nat Protoc 2011;6:1290–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CM, Hussain MS, Blenner M et al. . Synthetic RNA polymerase III promoters facilitate high-efficiency CRISPR-Cas9-mediated genome editing in Yarrowia lipolytica. ACS Synth Biol 2016;5:356–9. [DOI] [PubMed] [Google Scholar]
- Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol 1981;147:195–7. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Alberti‐Segui C et al. . Heterologous HIS3 marker and GFP reporter modules for PCR‐targeting in Saccharomyces cerevisiae. Yeast 1997;13:1065–75. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wilson WA, Fujino MA et al. . Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol 2001;21:5742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WA, Wang Z, Roach PJ. Systematic identification of the genes affecting glycogen storage in the yeast Saccharomyces cerevisiae: implication of the vacuole as a determinant of glycogen level. Mol Cell Proteomics 2002;1:232–42. [DOI] [PubMed] [Google Scholar]
- Woods A, Munday M, Scott J et al. . Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem 1994;269:19509–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSYR online.


