Abstract
During the last decade, the use of medical Cannabis has expanded globally and legislation is getting more liberal in many countries, facilitating the research on cannabinoids. The unique interaction of cannabinoids with the human endocannabinoid system makes these compounds an interesting target to be studied as therapeutic agents for the treatment of several medical conditions. However, currently there are important limitations in the study, production and use of cannabinoids as pharmaceutical drugs. Besides the main constituent tetrahydrocannabinolic acid, the structurally related compound cannabidiol is of high interest as drug candidate. From the more than 100 known cannabinoids reported, most can only be extracted in very low amounts and their pharmacological profile has not been determined. Today, cannabinoids are isolated from the strictly regulated Cannabis plant, and the supply of compounds with sufficient quality is a major problem. Biotechnological production could be an attractive alternative mode of production. Herein, we explore the potential use of synthetic biology as an alternative strategy for synthesis of cannabinoids in heterologous hosts. We summarize the current knowledge surrounding cannabinoids biosynthesis and present a comprehensive description of the key steps of the genuine and artificial pathway, systems biotechnology needs and platform optimization.
Keywords: Cannabis sativa, cannabinoids, synthetic biology, biotechnology, Saccharomyces cerevisiae
In this review, the authors explore the use of synthetic biology as an alternative approach for the synthesis of pharmaceutical cannabinoids in a heterologous host organism.
INTRODUCTION
Cannabinoids enclose a group of more than 100 chemical compounds (Ahmed et al.2008; ElSohly and Slade 2005; Radwan et al.2008) mainly found in the plant Cannabis sativa L., native from Central Asia (de Barge 1860; de Candolle 1886). Cannabis belongs to the family Cannabaceae that comprise only 11 genera, including Cannabis, Humulus (hop) and Celtis (hackberries). C. sativa is an annual plant with a dioecious breeding system (i.e. male and female flowers are found on separate plants), although monoecious plant also exist as result of special breeding (Clarke 1981; Raman 1998). The tendency in literature is to use the designation C. sativa L. to all species or varieties of the genera Cannabis. Despite this fact the number of species is not consensual, with some authors proposing a monotypic genus while others argue the existence of four (sativa, indica, ruderalis and afghanica) or even seven (ruderalis, sativa ssp. sativa, sativa ssp. spontanea, indica ssp. kafiristanica, indica spp. indica, indica ssp. afghanica and indica ssp. chinensis) species of Cannabis. This differentiation mainly reflects geographical differences, distinct chemical compositions and/or phenotypic variation of the Cannabis plants (McPartland, Clarke and Watson 2000; Hillig 2005; Linnaeus 1753).
Cannabis has been used by humans for different purposes for more than 5000 years. The fiber-type of Cannabis (hemp), where the major cannabinoid is cannabidiol (CBD), has been used as a source of fiber for textile production and oil seed. On the other hand, the drug-type of Cannabis (marijuana) have a higher content of the psychoactive compound Δ9-tetrahydrocannabinol (Δ9-THC) and for that reason has been used as a recreational drug but also in the treatment of pain and other medical conditions (for review see Russo 2011). Δ9-THC is widely used in pharmaceutical formulations for the treatment of several medical conditions. Several synthetic Cannabis-based preparations such as dronabinol (Marinol®, Unimed Pharmaceuticals, Inc, Marietta, GA, USA), nabilone (Cesamet®, Valeant Pharmaceuticals North America, Aliso Viejo, CA, USA), Δ9-THC and CBD (Sativex®, GW Pharmaceuticals plc, Histon Cambridge, United Kingdom) have been used in the USA, Canada and other countries as an authorized treatment for nausea and vomiting in cancer chemotherapy, appetite loss in acquired immune deficiency syndrome and symptomatic relief of neuropathic pain in multiple sclerosis. The number of applications for Δ9-THC, CBD and other cannabinoids is still increasing with more pharmaceutical applications being investigated (Carlini 2004; Pertwee 2009).
Cannabinoids are terpenophenolic compounds, produced from fatty acids and isoprenoids precursors as part of the secondary metabolism of Cannabis. The main cannabinoids produced by Cannabis are Δ9-tetrahydrocannabidiol (THC), CBD and cannabinol (CBN), followed by cannabigerol (CBG), cannabichromene (CBC) and other minor constituents (Flores-Sanchez and Verpoorte 2008). The biosynthesis of cannabinoids takes place mainly in the secretory head cells of the glandular trichomes, especially in the capitate-stalked glandular hairs (Happyana et al.2013). In the plant, Δ9-THC and CBD are involved in the defense response against pathogens (McPartland 1984), CBG and CBD are mildly antifungal (Elsohly et al.2017) and Δ9-THC is also involved in UV light protection (Russo 2011).
Phytocannabinoids are plant-based cannabinoids. Besides the genus Cannabis, there are other examples of plants that produce cannabinoid-like compounds. Helichrysum umbraculigerum, a flowering plant from Southern Africa, produces cannabigerol and cannabigerolic acid (CBGA) in its aerial parts (Bohlmann and Hoffmann 1979) and the moss Radula marginata produce perrottetinic acid (Toyota et al.2002). If not especially mentioned as C. sativa derived cannabinoids, structurally related compounds with a terpenophilic skeleton naturally synthesized by plants sources are also called phytocannabinoids. For a simplified designation, the term ‘cannabinoid’ will be used to refer to phytocannabinoids in this review.
Endocannabinoids are endogenous metabolites found in the members of the phylum Chordates, which bind to specific receptors of the endocannabinoid system (ECS). Recently, endocannabinoids were also discovered in algae, bryophytes and monilophytes (Gachet et al.2017). The main endocannabinoids are molecules derived from arachidonic acid, anandamide and 2-arachidonoylglycerol (Devane et al.1992; Mechoulam et al.1995; Sugiura et al.1995). These substances have a local effect and short life before being degraded by two well-characterized enzymes, the fatty acid amide hydrolase and monoacylglycerol lipase (Cravatt et al.1996; Dinh et al.2002). It is worth to note that synthetic compounds with no direct structural relation to plant cannabinoids have been designed in the past. These synthetic cannabinoids are chemicals developed to interact with the receptors of the ECS and are mostly known as illicit substances.
The ECS is a ubiquitous lipid signaling system with important homeostatic and physiological functions that include modulation of pain and inflammation. The name is derived from cannabinoids, because its first studies with cannabinoids led to the discovery and elucidation of the ECS and its biological functions. The isolation of Δ9-THC from Cannabis (Gaoni and Mechoulam 1964; Mechoulam 1970; Mechoulam and Gaoni 1965) led to the discovery and characterization of the specific mechanism of action of cannabinoids, by the identification of specific binding sites in the brain (Devane et al.1988; Herkenham et al.1991). This allowed the molecular cloning (Matsuda et al.1990) of cannabinoid CB1 receptor (CB1) as main cellular target. Later, a second peripheral receptor CB2 was identified (Munro, Thomas and Abu-Shaar 1993). Endocannabinoids are natural metabolites that stimulate this receptor type. Δ9-THC is a potent activator of the CB1 receptor, while the non-psychoactive CBD is a very low-affinity CB1 ligand. Despite this fact, CBD modulate the effect of Δ9-THC via direct blockade of CB1 receptor (McPartland et al.2015). This modulation attenuates some of the side effects of Δ9-THC such as anxiety, dysphoria, panic reactions and paranoia, and is also known to improve the Δ9-THC therapeutic activity (Izzo et al.2009; Russo 2011).
The global legislation for the use of medical Cannabis changes rapidly, and currently Cannabis is legal as therapeutic agent in 23 states of the United States as well as in the Netherlands, Germany, Czech Republic, Canada and Israel. There are still several drawbacks in the production of medical THC and other cannabinoids, especially related to the legal regulations for the cultivation of Cannabis in most countries. Furthermore, chemical synthesis of cannabinoids has failed to be a cost-effective alternative mainly because of complex synthesis leading to high production cost and low yields. A new alternative is the use of a biotechnology-based synthetic biology approach for a cost-effective, environmentally friendly, high-quality and reliable source of cannabinoids.
Agricultural production of cannabinoids faces several challenges such as plant susceptibility to climate and diseases, no GAP standardization, low content of less-abundant cannabinoids, need for extraction of cannabinoids by chemical processing and legal and social factors related to the potential for illicit use of the plant. Currently, Δ9-THC and CBD used as therapeutic agents are either extracted from the plant or chemically synthesized. Biosynthesis of cannabinoids by engineered microbial strains could be an alternative strategy for the production of cannabinoids.
The identification of the enzymes involved in the cannabinoids biosynthetic pathway enables the reconstruction of the pathway using a suitable heterologous host system. A synthetic biology approach can be especially interesting for the production of less-abundant cannabinoids, but also work as a platform for the discovery and testing of unknown enzymes responsible for the biosynthesis of various rare cannabinoids and derivatives thereof. Microbial production can provide a competitive and efficient way for easy and high yield biosynthesis of rare cannabinoids. Some examples of such compounds are tetrahydrocannabivarin, cannabigerol and cannabichromene that are cannabinoids with therapeutic interest, which are difficult to obtain (Appendino et al.2008; Bolognini et al.2010; Davis and Hatoum 1983; Elsohly et al.2017; Hill et al.2010; Ligresti 2006; Wilkinson et al.2007).
Recently, important developments in the microbial biosynthesis of cannabinoids were achieved. The expression of THCA synthase in the yeast Komagataella phaffii allowed the bioconversion of CBGA to Δ9-tetrahydrocannabinolic acid (Δ9-THCA) (Zirpel, Stehle and Kayser 2015). In addition, a patent application was filed, referring the microbial biosynthesis of cannabinoids in genetically engineered microorganisms (Poulos and Farnia 2016). In this review, we will explore synthetic biology as an alternative approach for the biosynthesis of cannabinoids in heterologous systems. We present microbial biosynthesis as a novel biotechnological solution for the production of pharmaceutical cannabinoids. The review will start with a brief overview of Cannabis phytochemistry focusing on the biosynthesis of cannabinoids. This will be followed by a comprehensive description of the key steps of the pathway, precursor needs and the desired features of the chassis organism.
BIOSYNTHESIS IN CANNABIS SATIVA
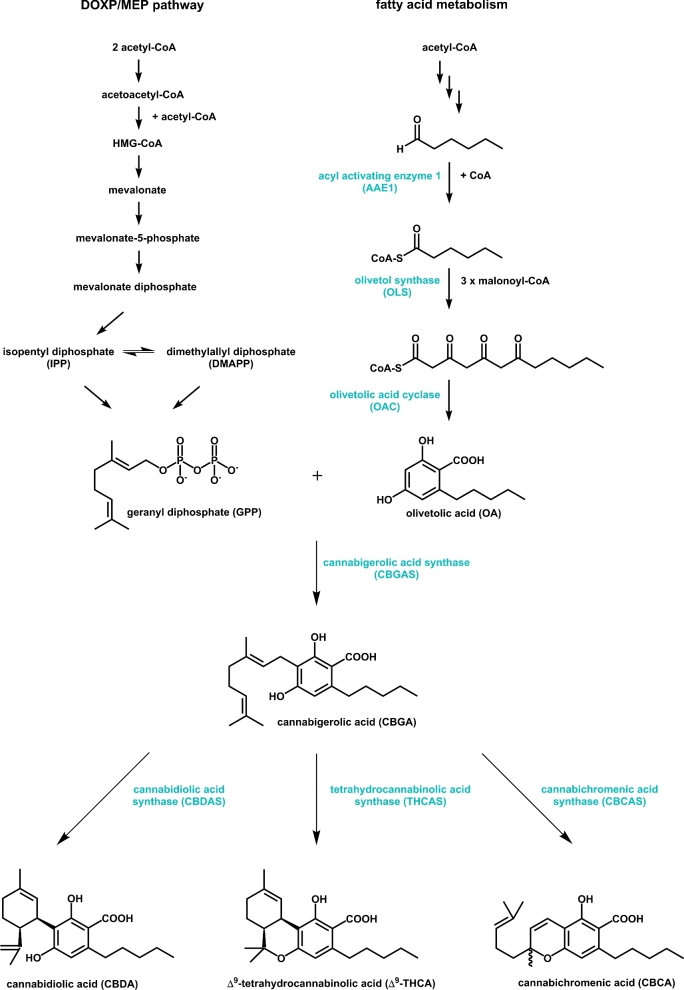
The biosynthesis of cannabinoids starts with the short-chain fatty acid, hexanoic acid. Initially, the fatty acid is converted to its coenzyme A (CoA) form by the activity of an acyl activating enzyme (Stout et al.2012). Subsequently, olivetolic acid (OA) is biosynthesized by the action of a type III polyketide synthase (PKS) and a polyketide cyclase (olivetolic acid cyclase [OAC]). The PKS olivetol synthase (OLS) converts one molecule of hexanoyl-CoA and three molecules of malonyl-CoA to olivetol followed by the C2-C7 aldol cyclization to OA by the OAC (Gagne et al.2012; Raharjo et al.2004). A geranyl diphosphate:olivetolate geranyltransferase, named cannabigerolic acid synthase (CBGAS), is responsible for the C-alkylation by geranyl diphosphate (GPP) to CBGA (Fellermeier and Zenk 1998). Finally, three different oxidocyclase enzymes catalyze the oxidative cyclization of the monoterpene moiety of CBGA for the biosynthesis of Δ9-THCA, cannabidiolic acid (CBDA) and cannabichromenic acid (CBCA) (Morimoto et al.1998; Sirikantaramas et al.2004; Taura et al.2007b). The enzymes involved in cannabinoids biosynthesis in C. sativa L. are summarized in Table 1 and the biosynthetic pathway is described in Fig. 2. Neutral form of these cannabinoids is the result of a non-enzymatic decarboxylation that usually happens during the plant material storage, by heat (smoking or baking) or sunlight exposure (de Meijer et al.2003).
Table 1.
List of the enzymes involved for the biosynthesis of cannabinoids in C. sativa L.
| Enzyme | Abbreviations | Accession no.a | EC no. | References |
|---|---|---|---|---|
| Acyl activating enzyme 1 | AAE1 | AFD33345.1 | 6.2.1.1 | (Stout et al.2012) |
| Olivetol synthase | OLS | AB164375 | 2.3.1.206 | (Taura et al.2009) |
| Olivetolic acid cyclase | OAC | AFN42527.1 | 4.4.1.26 | (Gagne et al.2012) |
| Cannabigerolic acid synthase | CBGAS | US8884100B2b | 2.5.1.102 | (Fellermeier and Zenk 1998) |
| (Page and Boubakir 2012) | ||||
| Tetrahydrocannabinolic acid synthase | THCAS | AB057805 | 1.21.3.7 | (Sirikantaramas et al.2004) |
| Cannabidiolic acid synthase | CBDAS | AB292682 | 1.21.3.8 | (Taura et al. 2007b) |
| Cannabichromenic acid synthase | CBCAS | WO 2015/196275 A1c | 1.3.3- | (Morimoto et al.1998) |
| (Page and Stout 2015) |
Genbank
Patent number
application number
Figure 2.
Biosynthetic pathway of cannabinoids in C. sativa. Highlighted enzymes have to be transferred into a heterologous host as S. cerevisiae exhibiting a mevalonate pathway.
Polyketide pathway for the biosynthesis of olivetolic acid and biosynthesis of cannabinoids terpene precursor geranyl diphosphate
The synthesis of OA starts with hexanoic acid. In the Cannabis plant, the origin of this fatty acid in the trichomes has not yet been elucidated. The formation of hexanoic acid by a de novo biosynthesis route is suggested by data showing high expression of an acyl carrier protein (ACP) and a 3-keto-ACP reductase enzyme in the glandular trichomes in comparison with plant leaves. According to this hypothesis, hexanoic acid would be synthesized by an early termination of the fatty acid biosynthesis and the action of a specific acyl-ACP thioesterase (Marks et al.2009). Notwithstanding, it is also hypothesized that hexanoic acid might be derived from the lipoxygenase pathway through the degradation of C18 unsaturated fatty acids (Marks et al.2009; Stout et al.2012).
The hexanoyl-CoA synthetase 1 isolated from the transcriptome of glandular trichomes is most likely the enzyme responsible for the formation of hexanoyl-CoA for the cannabinoid pathway. Several findings support this hypothesis. This enzyme is specifically expressed in trichomes and is localized in the cytosol, as also suggested for OLS and OAC (Gagne et al.2012; Stout et al.2012; Taura et al.2009).
Olivetol and OA are classified as resorcinolic lipids (alkylresorcinol, resorcinolic acid) and are both biosynthesized via a polyketide pathway. The polyketide synthase OLS catalyzes the aldol condensation of hexanoyl-CoA with three malonyl-CoA units towards olivetol and the α-pyrones pentyl diacetic lactone and hexanoyl triacetic acid lactone. Recent reports show that OLS only produces OA in combination with the cyclase enzyme OAC (Fellermeier et al.2001; Gagne et al.2012; Raharjo et al.2004; Taguchi et al.2008; Taura et al.2009; Yang et al.2016). The OAC catalyzes the C2-C7 intramolecular aldol cyclization to OA by preserving the carboxylate moiety (Gagne et al.2012; Raharjo et al.2004).
The terpenoid part of the cannabinoid can be derived from two different precursor pathways, namely the mevalonate pathway (MVA) localized in the cytosol and the plastid localized non-MVA, also termed as 2-C-methyl-d-erythritol 4-phosphate or 1-deoxy-d-xylulose 5-phosphate pathway (MEP/DOXP pathway). In higher plants, the MVA pathway is mainly involved with the plant primary metabolism, whereas MEP pathway is the main contributor for secondary metabolism, including terpene production (in detail elsewhere, e.g. Eisenreich et al.2004; Hunter 2007). The MVA and MEP pathways are both responsible for the production of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP), the two early precursors of terpenes. The condensation of IPP and DMAPP to produce GPP is catalyzed by the enzyme geranyl diphosphate synthase (Burke, Wildung and Croteau 1999). According to the information currently available, the most likely source of GPP for the production of the monoterpene moiety of cannabinoids in the plant is the MEP/DOXP pathway.
Cannabinoids biosynthesis
The central precursor for cannabinoid biosynthesis, CBGA, is synthesized by the aromatic prenyltransferase CBGAS (Fellermeier and Zenk 1998; Page and Boubakir 2012) by the condensation of GPP and OA. CBGAS is mainly expressed in glandular trichomes of female flowers and young leaves of Cannabis, and the coding sequence could be functionally expressed in Saccharomyces cerevisiae cells, verifying the CBGAS activity in the microsomal fractions (Page and Boubakir 2012). Subsequently, the monoterpene moiety of CBGA is stereoselectively cyclized by the three different enzymes cannabichromenic acid synthase (Page and Stout 2015), cannabidiolic acid synthase (CBDAS) and tetrahydrocannabinolic acid synthase (THCAS). The CBDAS and THCAS belong to the berberine bridge enzyme family, oxidoreductases with a covalently bound flavin adenine dinucleotide (FAD) (Kutchan and Dittrich 1995). Both enzymes use molecular oxygen for the regeneration of the FAD cofactor, releasing equimolar amounts of hydrogen peroxide and a particular product (Sirikantaramas et al.2004; Taura et al.2007a). The THCAS is well characterized, and a crystal structure is available (Shoyama et al.2012). The enzyme possesses a signal peptide for the secretory pathway, one disulfide bond and eight possible Asn glycosylation sites. Additionally, the enzyme was already functionally expressed in Spodoptera frugiperda (Sf9) insect cells (Sirikantaramas et al.2004), K. phaffii (Taura et al.2007b; Zirpel, Stehle and Kayser 2015) and S. cerevisiae (Zirpel, Stehle and Kayser 2015).
HETEROLOGOUS SYSTEMS FOR THE BIOSYNTHESIS OF CANNABINOIDS
Special requirements of the host organism
Choosing a suitable host organism for production of a heterologous metabolite needs some general considerations, regarding the tools available for the expression of heterologous proteins (strains, vectors, promoters and signal peptides), genetic information and availability of classical genetic approaches as well as the accessibility of modern molecular biological tools. Other specific requirements would relate more to the specific pathway that needs to be introduced. Such factors could be the suitability of the host organism for production of particular precursors or cofactors as well as suitability of the host to express the necessary types of pathway enzymes. In Table 2, we compare a selection of potential microbial production hosts and evaluate their suitability within different categories. In the case of the cannabinoid biosynthesis, both precursors GPP and hexanoic acid should be provided in sufficient amounts by the chassis organism. The isoprenoid production has already been extensively studied and optimized for the production of artemisinin in Escherichia coli and S. cerevisiae, favoring the yeast as better platform organism (Paddon and Keasling 2014). Nevertheless, early stage optimizations of isoprenoid production are also documented for the oleaginous yeast Yarrowia lipolytica (Sharpe, Ye and Zhu 2014) and the methylotrophic yeast K. phaffii (Liu et al.2014).
Table 2.
Comparison of different microbial expression hosts regarding their capacity of heterologous cannabinoid biosynthesis.
| Genetic tools available | Strains, promoters, vectors | Plant protein expression capacity | Posttranslational modifications | GPP engineering | Hexanoic acid engineering | Acetyl-CoA pool engineering | |
|---|---|---|---|---|---|---|---|
| Escherichia coli | +++ | +++ | + | – | ++ | + | + |
| Saccharomyces cerevisiae | +++ | +++ | ++ | ++ | +++ | ++ | +++ |
| Komagataella phaffii (Pichia pastoris) | + | ++ | +++ | ++ | + | ||
| Kluyveromyces marxianus | ++ | + | ++ | ++ | ++ | ||
| Yarrowia lipolytica | + | + | ++ | ++ | + | ++ |
+++, many publications available, well established; ++, publications available, optimization potential; +, first publications available, not yet established/not working; –, not possible; ‘empty’, not yet described.
A clear challenge in heterologous production of cannabinoids is the production of hexanoic acid. The most efficient microbial hexanoic acid formation reported to date is by Cheon et al. (2014), who described the production of up to 142 mgL−1 of hexanoic acid in Kluyveromyces marxianus using a pathway that may be transferrable to other yeasts.
Looking at the characteristics of the enzymes of the late cannabinoid pathway, a prokaryotic host seems not to be feasible. CBGAS is an integral membrane protein, making high titer of functional expressed protein in E. coli rather unlikely. In addition, the FAD-dependent oxygenases THCAS and CBDAS possess a disulfide bond and several N-glycosylation sites excluding thereby the use of prokaryotic hosts (Shoyama et al.2012; Zirpel, Stehle and Kayser 2015).
All precursors needed for the cannabinoid biosynthesis, GPP, malonyl-CoA and hexanoyl-CoA, are derived from acetyl-CoA. Therefore, a strategy to boost the acetyl-CoA pool is essential to reach high yields. Recently, S. cerevisiae was used to rewire the central carbon metabolism (Meadows et al.2016). By the addition of four genes only, the engineered yeast is able to produce high amounts of cytosolic acetyl-CoA accompanied with an improved pathway redox balance, a reduced ATP requirement and CO2 loss. Since S. cerevisiae also is one of the most developed host organisms in respect to knowledge and molecular biology tools, this organism therefore is a promising chassis organism for the heterologous biosynthesis of cannabinoids. In the following, we will therefore focus on the establishment of S. cerevisiae as a platform organism for the heterologous biosynthesis of cannabinoids. A serious alternative, however, would be K. phaffii, which is well known for secreted heterologous synthesized enzymes. Zirpel, Stehle and Kayser (2015) showed that this yeast is also able to produce high levels of intracellular accumulated THCAS.
Metabolic engineering of Saccharomyces cerevisiae to produce cannabinoids
In order to rewire the Saccharomyces cerevisiae metabolism for the biosynthesis of cannabinoids, a combined approach comprising the adaptation of native metabolic pathways, the assembly of heterologous metabolic pathways and protein engineering is needed to obtain a cell factory that produces cost-effective cannabinoids. Looking at the biosynthetic pathway of THCA in C. sativa, the pathway can be divided into three parts: (i) GPP supply, (ii) synthesis of OA and (iii) the actual cannabinoid formation.
GPP supply
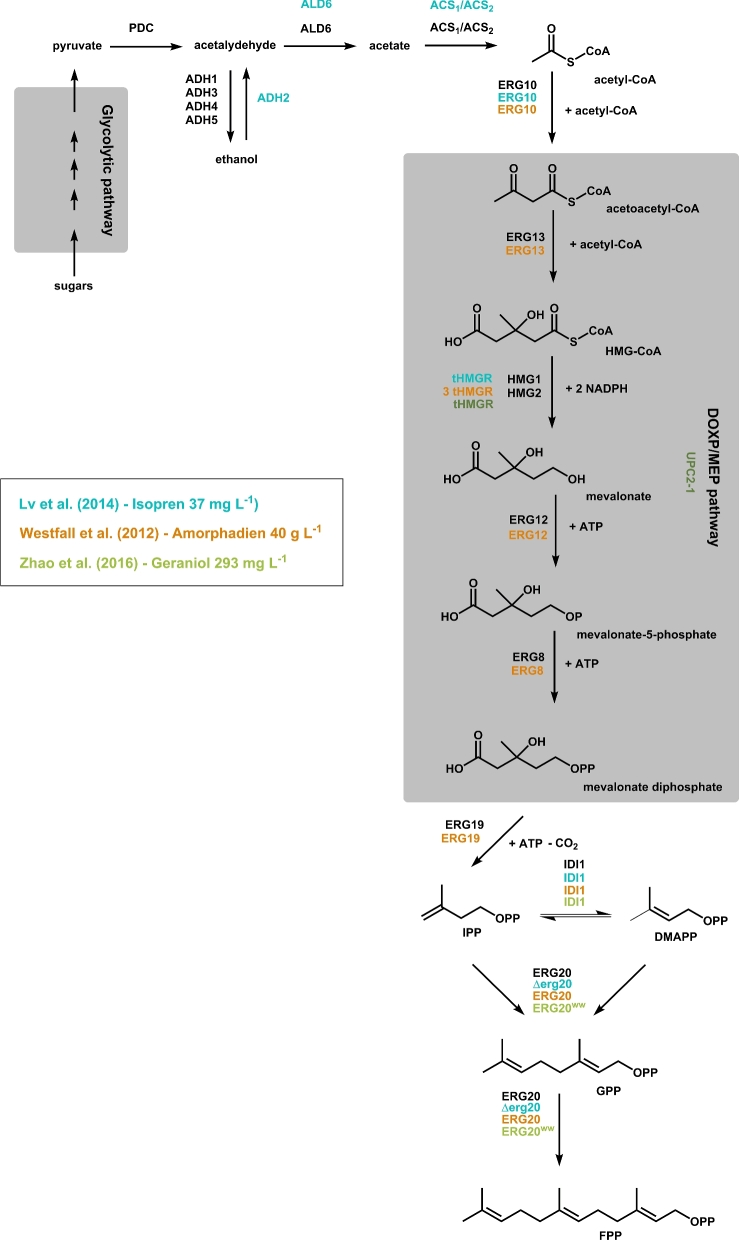
Saccharomyces cerevisiae serves as a model and excellent organism for heterologous isoprenoid production (Nevoigt 2008), and several promising metabolic engineering strategies were identified by in silico profiling (Gruchattka et al.2013). Up to date, many strategies were tested to improve the isoprenoid production in yeast, whereas most are focusing on MVA pathway engineering (Fig. 1). In most cases, a truncated version of 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) is overexpressed since HMGR was identified as the rate-limiting enzyme of the MVA pathway (Ohto et al.2009). Recently, the co-overexpression of all MVA pathway genes resulted in the production of 40 gL−1 amorphadiene, a precursor of the antimalarial agent artemisinin (Westfall et al.2012). Lv et al. (2014) optimized additionally the native acetyl-CoA pathway. The co-overexpression of pyruvate decarboxylase, alcohol dehydrogenase 3, aldehyde dehydrogenase and the both acetyl-coA synthetases resulted in an enhanced isoprene biosynthesis and might be an option to improve isoprenoid biosynthesis further.
Figure 1.
Isoprenoid formation in S. cerevisiae. The isoprenoid biosynthesis starts with acetyl-CoA, which is derived from the glycolytic pathway. At the end of the MVA, the both isoprenoids IPP and DMAPP are formed. Subsequently, GPP and FPP are formed by the ERG20 protein. The different colors represent the different strategies applied for improved isoprenoid production. HMG-CoA—3-hydroxy-3-methylglutaryl coenzyme A, IPP—isopentenyl diphosphate, DMAPP—dimethylallyl diphosphate, GPP—geranyl diphosphate, FPP—farnesyl diphosphate, PDC—pyruvate decarboxylase, ADH1-5—alcohol dehydrogenase, ALD6—aldehyde dehydrogenase, ACS1/ACS2—acetyl-coA synthetase, ERG10—acetyl-CoA C-acetyltransferase, ERG13—3-hydroxy-3-methylglutaryl-CoA synthase, tHMGR—truncated 3-hydroxy-3-methylglutaryl-CoA reductase, ERG12—mevalonate kinase, ERG8—phosphomevalonate kinase, ERG19—mevalonate diphosphate decarboxylase, IDI1—isopentenyl diphosphate:dimethylallyl diphosphate isomerase, ERG20—farnesyl diphosphate synthetase, ERG20WW—ERG20-F96W-N127W, Δerg20—ERG20 knock out, UPC2-1—sterol regulatory element binding protein.
In contrast to plants, yeasts usually do not carry a specific GPP synthase (GPPS). In yeast, the farnesyldiphosphate synthase (FPPS; ERG20) possesses a GPPS activity, while GPP occurs exclusively as an intermediate of farnesyl diphosphate synthesis. The highest yields so far were obtained with the production of sesquiterpenes or even larger terpenes like miltiradiene (Zhou et al.2012) or artemisinin (Paddon et al.2013). Less effort was made on the optimization in monoterpene production and the overall yields reported are lower. However, for cannabinoid production in yeast a high production rate of GPP is necessary. Saturated mutagenesis of Lys197 residue of the ERG20 protein resulted in six strains (K197G, C, S, T, D, E) with an improved monoterpenol production but also with some growth impairment (Fischer et al.2011). Even better results were obtained by engineering the ERG20 protein into a geranyl diphosphate synthase (ERG20-F96W-N127W = ERG20WW) (Ignea et al.2014). The introduction of a larger side chain in position 96 (F96W) blocks the part of the active site, thereby hindering the FPP synthase activity without affecting the synthesis of GPP. ERG20 is a homodimeric protein whereas the N127 residue of one subunit is part of the active site of the other subunit (Fernandez, Kellogg and Poulter 2000; Ignea et al.2014). The replacement of the N127 residue by a tryptophan results in an abolished FPP synthesis, but the GPP activity remains. The overexpression of the N127W variant in a wild-type yeast strain leads to the formation of heterodimeric ERG20 proteins (endogenous ERG20/ERG20WW) reducing thereby the overall FPP synthase activity and eliminates the need for mutation of the endogenous ERG20 gene to reduce the FPP pool (Ignea et al.2014). Finally, the co-overexpression of IDI1, tHMG1 and UPC2-1 together with the ERG20WW protein resulted in a significantly improved geraniol production (Zhao et al.2016). A similar approach could be adapted to improve the GPP supply for heterologous cannabinoid biosynthesis in S. cerevisiae.
Olivetolic acid synthesis
Saccharomyces cerevisiae does not typically metabolize fatty acids as substrate, therefore its production relies on endogenous biosynthesis. The fatty acid composition in S. cerevisiae is rather simple, consisting mostly of C16 and C18 fatty acids (Klug and Daum 2014; Oh and Martin 2006). Trace amounts of short-chain fatty acids (SCFA) or medium-chain fatty acids (MCFA) are naturally produced by S. cerevisiae and certain strains used in wine and sake fermentation are known to produce higher levels (Aritomi et al.2004; Patel and Shibamoto 2002). The biosynthesis of fatty acids requires several substrates and cofactors such as acetyl-CoA, ATP and NAD(P)H. The first committed step of fatty acid biosynthesis is the conversion of acetyl-CoA to malonyl-CoA. This reaction is performed by the enzyme acetyl-CoA carboxylase and its activity is highly regulated (Hablacher et al.1993; Woods et al.1994). De novo biosynthesis of fatty acid in yeast is carried out by the fatty acid synthase (FAS), a multifunction protein complex in which all steps of fatty acid synthesis are integrated. The yeast FAS is classified as a eukaryote type I and this enzyme complex is divided in 2 subunits (Fas1p and Fas2p), each exhibiting more than one enzymatic activity. Fas1p harbors acetyl transferase, enoyl ACP reductase, hydroxyl acyl ACP dehydratase and malonyl transferase activities; and Fas2p contains the ACP, ketoacyl reductase, ketoacyl synthase and phosphopantheteine transferase activities (Jenni et al.2007; Klug and Daum 2014; Tehlivets, Scheuringer and Kohlwein 2007).
Up to date, several strategies were tested to produce SCFA in yeast species, usually involving the introduction of heterologous enzymes. Several examples of the production of SCFA were demonstrated by the introduction of heterologous FAS. The introduction of Homo sapiens type I fatty acid synthase and specific short-chain thioesterase in S. cerevisiae increased in vivo octanoic acid and total SCFA production (Leber and Da Silva 2014). A similar strategy could be adapted for the production of hexanoic acid by testing different heterologous short-chain thioesterases. Many fungal secondary metabolites are fatty-acid-derived molecules biosynthesized by the interaction of dedicated FAS with a PKS. One of the most notorious examples of this type of secondary metabolites is the biosynthesis of aflatoxin (AF) and sterigmatocystin (ST) in some species of the filamentous fungus Aspergillus. Previous studies have shown that the synthesis of AF and ST begins with the assembly of a C6 fatty acid from acetyl-CoA and 2 units of malonyl-CoA catalyzed by a specialized FAS, hexanoate synthase (Hitchman 2001).
Recently, different studies demonstrated the production of extracellular SCFA and MCFA by the modification of the native FAS or construction of a synthetic FASs in S. cerevisiae. Rational engineering of the cytosolic FAS allowed the reprogramming of the chain-length control with successful biosynthesis of SCFA and MCFA (Gajewski et al.2017). In another study, the creation of a synthetic FASs by the integration of heterologous enzymes showed the capability to biosynthesize SCFA, MCFA and methyl ketones (Zhu et al.2017).
As mentioned before, the biosynthesis of OA starts with the conversion of the free fatty acid hexanoic acid to hexanoyl-CoA. In Cannabis, this step is done by an acyl-CoA synthetase with high specificity for hexanoic acid. Saccharomyces cerevisiae contains four fatty acyl-CoA synthetases (FAA) named FAA1, FAA2, FAA3 and FAA4. FAA1, FAA3 and FAA4 have preference for long-chain fatty acids (C12:0 to C18:0), in contrast with FAA2 that accepts a wide range of fatty acid chain lengths with a preference for medium chains (C9:0-C13:0) (Johnson et al.1994). Since S. cerevisiae has no specific acyl-CoA synthetase for SCFA, it may be beneficial to introduce a Cannabis hexanoyl-CoA synthetase or a homolog from other organism to achieve an efficient conversion of hexanoic acid to hexanoyl-CoA in yeast.
The biosynthesis of OA from hexanoyl-CoA is performed by the combination of two cytosolic-located enzymes, OLS and OAC (Fig. 2). These two enzymes have been demonstrated to be active in vivo in S. cerevisiae with resulting formation of both olivetol and OA (Gagne et al.2012).
Implementation of cannabinoid forming enzymes
With GPP and OA available in the production host, only one enzymatic step is necessary to obtain CBGA, which is the central intermediate of the cannabinoid pathway (Fig. 2). The native enzyme responsible for the prenylation of OA is an integral membrane enzyme of which functional overexpression has turned out to be challenging. Nevertheless, the native enzyme has been expressed in Saccharomyces cerevisiae cells, but the overall activity is low and a high side-product formation (5-geranyl-olivetolate) was observed (Page and Boubakir 2015). Besides the integral membrane prenyltransferases in plants, soluble prenyltransferases are known from fungi and bacteria. Kuzuyama, Noel and Richard (2005) published a crystal structure of a soluble prenyltransferase NphB from Streptomyces sp. strain CL190 that is specific for GPP as prenyl donor and exhibits a broad substrate specificity towards aromatic substrates. They were able to show that the enzyme accepts olivetol and OA as prenyl acceptor. Furthermore, it was shown that NphB prenylates olivetol in the C2 and C4 position (Kumano et al.2008). Thus, NphB represents a potential alternative to replace the native CBGAS in a biotechnological production of cannabinoids. Indeed, the co-expression of the nphB and thca coding sequences in K. phaffii resulted in the successful synthesis of THCA in enzyme extracts containing OA and GPP (unpublished results).
Once the expression of an OA prenylating enzyme is established in yeast, the addition of one respective enzyme leads to the formation of different cannabinoids like THCA, CBDA and CBCA. For the THCAS, a whole cell bioconversion is already established in S. cerevisiae and K. phaffi (Zirpel, Stehle and Kayser 2015). Yeast cells expressing thcas were able to produce up to 360 mgL−1 THCA after CBGA feeding, demonstrating the capacity of yeast in the biosynthesis of cannabinoids. As the sequence and expression for CBDAS is already described (Taura et al.2007a), the THCAS can be easily exchanged with a CBDAS. However, expression levels are lower than reported for THCAS (unpublished data).
Diversified cannabinoids
The microbial production facilitates the possibility to design new cannabinoids with novel activities or improved pharmacokinetics through the implementation of tailoring enzymes. Cannabinoids are extremely hydrophobic compounds, for medical formulations cannabinoids are usually dissolved in oil or solvents, which may not be well tolerated by users (Scully 2007). The introduction of hydroxyl, carbonyl, carboxyl or glycosyl groups enhances the solubility of molecules in general. In 1980s, fungal and bacterial strains were already used to transform THC into polar derivatives (Binder and Meisenberg 1978; Binder and Popp 1980; Fukuda, Archer and Abbott 1977), as well as plant cell suspension cultures were used for the biotransformation of cannabinoids (Akhtar, Mustafa and Verpoorte 2015; Braemer and Paris 1987; Hartsel, Loh and Robertson 1983). Nevertheless, the overall yield of the metabolites was too low for pharmacological evaluation of the activity.
In humans, THC is metabolized in the liver mainly by microsomal hydroxylation and oxidation, but also allylic oxidation, epoxidation, aliphatic oxidation, decarboxylation and conjugation reactions were reported (Grotenhermen 2003). This leads to nearly 100 different identified metabolites for THC (Harvey and Brown 1991). THC is mainly hydroxylated on position C-11 by cytochrome P450 enzymes resulting in 11-hydroxy-tetrahydrocannabinol (11-OH-THC) and further oxidation leads to 11-nor-9-carboxy-tetrahydrocannabinol, the most important non-psychotropic metabolite (Fig. 3). Since 11-OH-THC seems to be three to seven times more potent than THC in animal tests (Karler and Turkanis 1987), and 11-OH-THC possesses still anti-inflammatory and analgesic properties (Burstein 1999), the elongation of the heterologous pathway by P450 enzymes might be a promising approach to produce THC derivatives. Glycosylation may also be an interesting strategy to alter the physicochemical properties. Plant glycosyltransferases (UGTs) are known for their relaxed substrate specificity (Bowles et al.2006; Hansen et al.2009). Recently, the UGT76G1 from Stevia rebaudiana was used to produce primary, secondary and tertiary glycosylations cannabinoid glycosides (Hardman, Brooke and Zipp 2017). The use of a second enzyme from Oryza sativa resulted in the transfer of a second glucose residue onto cannabinoid monoglycosides with a greatly improved water solubility.
Figure 3.
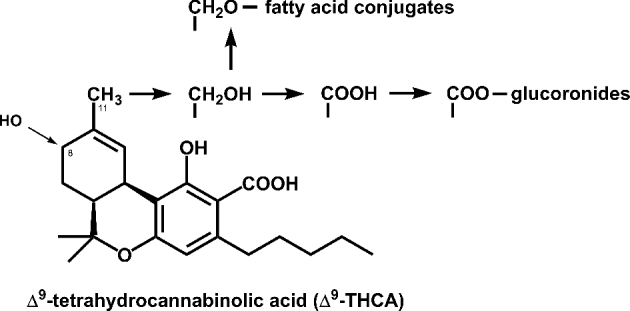
THCA metabolization products in humans. The C11 position is the major attacked site, but C8 position can be also hydroxylated (Grotenhermen 2003).
The implementation of the cannabinoid biosynthesis in a heterologous host provides a platform where some of these tailoring enzymes can be functionally expressed. This enables the production of significant amounts of these derivatives and may be the key to unlock their pharmacological potential.
OUTLOOK
The discovery and characterization of all key enzymes involved in the biosynthesis of the main cannabinoids Δ9-THC and CBD, allows for the production of these compounds by heterologous host organisms. The use of synthetic biology for the microbial biosynthesis of cannabinoids can revolutionize the production of medical cannabinoid drugs. Besides the main cannabinoids Δ9-THC and CBD, more than 100 cannabinoids compounds are described with little knowledge available regarding their producing pathways and potential applications. Synthetic biology could be used to create a chassis organism for the study and characterization of the enzymes involved in the biosynthesis of these less-abundant cannabinoids or derivatives thereof and allows the production of these compounds by a scalable fermentation process. This can have an enormous impact on the availability of rare cannabinoids to be tested in clinical trials to evaluate their efficacy as medical drugs. Furthermore, microbial production can support the design of novel cannabinoids with enhanced properties by the incorporation of tailoring enzymes. Together, these strategies will help to support the potential value of cannabinoids as pharmaceutical drugs.
Conflict of interest. None declared.
REFERENCES
- Ahmed SA, Ross SA, Slade D et al. . Structure determination and absolute configuration of cannabichromanone derivatives from high potency Cannabis sativa. Tetrahedron Lett 2008;49:6050–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar MT, Mustafa NR, Verpoorte R. Hydroxylation and glycosylation of Δ9-tetrahydrocannabinol by Catharanthus roseus cell suspension culture. Biocatal Biotransfor 2015;33:279–86. [Google Scholar]
- Appendino G, Gibbons S, Giana A et al. . Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod 2008;71:1427–30. [DOI] [PubMed] [Google Scholar]
- Aritomi K, Hirosawa I, Hoshida H et al. . Self-cloning yeast strains containing novel FAS2 mutations produce a higher amount of ethyl caproate in Japanese sake. Biosci Biotech Bioch 2004;68:206–14. [DOI] [PubMed] [Google Scholar]
- Binder M, Meisenberg G. Microbial transformation of cannabinoids - part 2; a screening of different microorganisms. Eur J Appl Microbiol 1978;5:37–50. [Google Scholar]
- Binder M, Popp A. Microbial transformation of cannabinoids. Part 3: major metabolites of (3R, 4R)-Δ1-tetrahydrocannabinol. Helv Chim Acta 1980;63:2515–8. [Google Scholar]
- Bohlmann F, Hoffmann E. Cannabigerol-ähnliche verbindungen aus Helichrysum umbraculigerum. Phytochemistry 1979;18:1371–4. [Google Scholar]
- Bolognini D, Costa B, Maione S et al. . The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol 2010;160:677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D, Lim E-K, Poppenberger B et al. . Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 2006;57:567–97. [DOI] [PubMed] [Google Scholar]
- Braemer R, Paris M. Biotransformation of cannabinoids by a cell suspension culture of Cannabis sativa L. Plant Cell Rep 1987;6:150–2. [DOI] [PubMed] [Google Scholar]
- Burke CC, Wildung MR, Croteau R. Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer. P Natl Acad Sci USA 1999;96:13062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SH. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol Therapeut 1999;82:87–96. [DOI] [PubMed] [Google Scholar]
- Carlini E. The good and the bad effects of (−) trans-delta-9-tetrahydrocannabinol (Δ9-THC) on humans. Toxicon 2004;44:461–7. [DOI] [PubMed] [Google Scholar]
- Cheon Y, Kim J-S, Park J-B et al. . A biosynthetic pathway for hexanoic acid production in Kluyveromyces marxianus. J Biotechnol 2014;182–183:30–6. [DOI] [PubMed] [Google Scholar]
- Clarke. Marijuana Botany: An Advanced Study, the Propagation and Breeding of Distinctive Cannabis. Oakland, CA: Ronin Publishing, 1981. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP et al. . Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996;384:83–7. [DOI] [PubMed] [Google Scholar]
- Davis WM, Hatoum NS. Neurobehavioral actions of cannabichromene and interactions with Δ9-tetrahydrocannabinol. Gen Pharmacol 1983;14:247–52. [DOI] [PubMed] [Google Scholar]
- de Barge A. Lettre de M. Alex. de Bunge À M. Decaisne. Botanique de France, 1860. [Google Scholar]
- de Candolle A. Origin of Cultivated Plants by Alphonse de Candolle, 2nd ed London: Paul, Trench, 1886. [Google Scholar]
- de Meijer EPM, Bagatta M, Carboni A et al. . The inheritance of chemical phenotype in cannabis sativa L. Genetics 2003;163335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR et al. . Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 1988;34:605–13. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A et al. . Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–9. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM et al. . Brain monoglyceride lipase participating in endocannabinoid inactivation. P Natl Acad Sci USA 2002;99:10819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Bacher A, Arigoni D et al. . Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci 2004;61:1401–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly HN, Turner CE, Clark AM et al. . Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds. J Pharm Sci 2017;71:1319–23. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48. [DOI] [PubMed] [Google Scholar]
- Fellermeier M, Eisenreich W, Bacher A et al. . Biosynthesis of cannabinoids. Eur J Biochem 2001;268:1596–604. [DOI] [PubMed] [Google Scholar]
- Fellermeier M, Zenk MH. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett 1998;427:283–5. [DOI] [PubMed] [Google Scholar]
- Fernandez SMS, Kellogg BA, Poulter CD. Farnesyl diphosphate synthase. Altering the catalytic site to select for geranyl diphosphate activity. Biochemistry 2000;39:15316–21. [DOI] [PubMed] [Google Scholar]
- Fischer MJC, Meyer S, Claudel P et al. . Metabolic engineering of monoterpene synthesis in yeast. Biotechnol Bioeng 2011;108:1883–92. [DOI] [PubMed] [Google Scholar]
- Flores-Sanchez IJ, Verpoorte R. Secondary metabolism in cannabis. Phytochem Rev 2008;7:615–39. [Google Scholar]
- Fukuda D, Archer RA, Abbott BJ. Microbiological transformations of δ6a,10a-tetrahydrocannabinol. Appl Environ Microb 1977;33:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet MS, Schubert A, Calarco S et al. . Targeted metabolomics shows plasticity in the evolution of signaling lipids and uncovers old and new endocannabinoids in the plant kingdom. Sci Rep 2017;7:41177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne SJ, Stout JM, Liu E et al. . Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. P Natl Acad Sci USA 2012;109:12811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski J, Buelens F, Serdjukow S et al. . Engineering fatty acid synthases for directed polyketide production. Nat Chem Biol 2017;13:363–5. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 1964;86:1646–7. [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42:327–60. [DOI] [PubMed] [Google Scholar]
- Gruchattka E, Hädicke O, Klamt S et al. . In silico profiling of Escherichia coli and Saccharomyces cerevisiae as terpenoid factories. Microb Cell Fact 2013;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hablacher M, Ivessa AS, Paltauf F et al. . Acetyl-coA carboxylase from yeast is an essential enzyme and is regulated by factors that control phospholipid metabolism. J Biol Chem 1993;268:10946–52. [PubMed] [Google Scholar]
- Hansen EH, Osmani SA, Kristensen C et al. . Substrate specificities of family 1 UGTs gained by domain swapping. Phytochemistry 2009;70:473–82. [DOI] [PubMed] [Google Scholar]
- Happyana N, Agnolet S, Muntendam R et al. . Analysis of cannabinoids in laser-microdissected trichomes of medicinal Cannabis sativa using LCMS and cryogenic NMR. Phytochemistry 2013;87:51–9. [DOI] [PubMed] [Google Scholar]
- Hardman JM, Brooke RT, Zipp BJ. Cannabinoid glycosides: In vitro production of a new class of cannabinoids with improved physicochemical properties. bioRxiv 2017, DOI: https://doi.org/10.1101/104349. [Google Scholar]
- Hartsel SC, Loh WH, Robertson LW. Biotransformation of cannabidiol to cannabielsoin by suspension cultures of cannabis sativa and Saccharum officinarum. Planta Med 1983;48:17–9. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Brown NK. Comparative in vitro metabolism of the cannabinoids. Pharmacol Biochem Be 1991;40:533–40. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR et al. . Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 1991;11:563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Weston SE, Jones NA et al. . Δ9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 2010;51:1522–32. [DOI] [PubMed] [Google Scholar]
- Hillig KW. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet Resour Crop Ev 2005;52:161–80. [Google Scholar]
- Hitchman T. Hexanoate synthase, a specialized type i fatty acid synthase in aflatoxin b1 biosynthesis. Bioorg Chem 2001;29:293–307. [DOI] [PubMed] [Google Scholar]
- Hunter WN. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J Biol Chem 2007;282:21573–7. [DOI] [PubMed] [Google Scholar]
- Ignea C, Pontini M, Maffei ME et al. . Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase. ACS Synth Biol 2014;3:298–306. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R et al. . Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009;30:515–27. [DOI] [PubMed] [Google Scholar]
- Jenni S, Leibundgut M, Boehringer D et al. . Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science 2007;316:254–61. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Knoll LJ, Levin DE et al. . Saccharomyces cerevisiae contains four fatty acid activation (FAA) genes: an assessment of their role in regulating protein N-myristoylation and cellular lipid metabolism. J Cell Biol 1994;127:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karler R, Turkanis SA. Different cannabinoids exhibit different pharmacological and toxicological properties. NIDA Res Mg 1987;79:96–107. [PubMed] [Google Scholar]
- Klug L, Daum G. Yeast lipid metabolism at a glance. FEMS Yeast Res 2014;14:369–88. [DOI] [PubMed] [Google Scholar]
- Kumano T, Richard SB, Noel JP et al. . Chemoenzymatic syntheses of prenylated aromatic small molecules using Streptomyces prenyltransferases with relaxed substrate specificities. Bioorgan Med Chem 2008;16:8117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan TM, Dittrich H. Characterization and mechanism of the berberine bridge enzyme, a covalently flavinylated oxidase of benzophenanthridine alkaloid biosynthesis in plants. J Biol Chem 1995;270:24475–81. [DOI] [PubMed] [Google Scholar]
- Kuzuyama T, Noel JP, Richard SB. Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. Nature 2005;435:983–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber C, Da Silva N. Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol Bioeng 2014;111:347–58. [DOI] [PubMed] [Google Scholar]
- Ligresti A. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 2006;318:1375–87. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. Species plantarum. 1753;1. [Google Scholar]
- Liu Y, Zhu X, Li W et al. . Enhancing production of ergosterol in Pichia pastoris GS115 by over-expression of 3-hydroxy-3-methylglutaryl CoA reductase from Glycyrrhiza uralensis. Acta Pharm Sinic B 2014;4:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X, Xie W, Lu W et al. . Enhanced isoprene biosynthesis in Saccharomyces cerevisiae by engineering of the native acetyl-CoA and mevalonic acid pathways with a push-pull-restrain strategy. J Biotechnol 2014;186:128–36. [DOI] [PubMed] [Google Scholar]
- Marks MD, Tian L, Wenger JP et al. . Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa. J Exp Bot 2009;60:3715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ et al. . Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990;346:561–4. [DOI] [PubMed] [Google Scholar]
- McPartland J. Pathogenicity of Phomopsis ganjae on Cannabis sativa and the fungistatic effect of cannabinoids produced by the host. Mycopathologia 1984;87:149–53. [Google Scholar]
- McPartland JM, Clarke RC, Watson DP. Hemp Diseases and Pests: Management and Biological Control - an Advanced Treatise. Wallingford, UK: CABI Publishing, 2000. [Google Scholar]
- McPartland JM, Duncan M, Di Marzo V et al. . Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015;172:737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows AL, Hawkins KM, Tsegaye Y et al. . Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016;537:694–7. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana Chemistry. Science 1970;168:1159–66. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L et al. . Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995;50:83–90. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. Hashish—IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron 1965;21:1223–9. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Komatsu K, Taura F et al. . Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998;49:1525–9. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–5. [DOI] [PubMed] [Google Scholar]
- Nevoigt E. Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol R 2008;72:379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CS, Martin CE. Candida albicans Spt23p controls the expression of the Ole1p Δ9-fatty acid desaturase and regulates unsaturated fatty acid biosynthesis. J Biol Chem 2006;281:7030–9. [DOI] [PubMed] [Google Scholar]
- Ohto C, Muramatsu M, Obata S et al. . Overexpression of the gene encoding HMG-CoA reductase in Saccharomyces cerevisiae for production of prenyl alcohols. Appl Microbiol Biot 2009;82:837–45. [DOI] [PubMed] [Google Scholar]
- Paddon CJ, Keasling JD. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Micro 2014;12:355–67. [DOI] [PubMed] [Google Scholar]
- Paddon CJ, Westfall PJ, Pitera DJ et al. . High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013;496:528–32. [DOI] [PubMed] [Google Scholar]
- Page JE, Boubakir Z. Aromatic prenyltransferase from Cannabis. 2012. US 20120144523. https://www.google.com/patents/US20120144523 (31 March 2017, date last accessed).
- Page JE, Boubakir Z. Aromatic prenyltransferase from Cannabis. 2015. US 20150128301 A1. www.google.com/patents/US20150128301 (31 March 2017, date last accessed).
- Page JE, Stout JM. Cannabichromenic acid synthase from Cannabis sativa. 2015. WO 2015196275 A1. www.google.com/patents/WO2015196275A1?cl=en (31 March 2017, date last accessed).
- Patel S, Shibamoto T. Effect of different strains of Saccharomyces cerevisiae on production of volatiles in napa gamay wine and petite sirah wine. J Agric Food Chem 2002;50:5649–53. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 2009;147:S163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos JL, Farnia AN. Production of cannabinoids in yeast. 2016. WO 2016010827 A1. www.google.com/patents/WO2016010827A1?cl (31 March 2017, date last accessed).
- Radwan MM, Ross SA, Slade D et al. . Isolation and characterization of new Cannabis constituents from a high potency variety. Planta Med 2008;74:267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raharjo TJ, Chang W Te, Choi YH et al. . Olivetol as product of a polyketide synthase in Cannabis sativa L. Plant Sci 2004;166:381–5. [Google Scholar]
- Raman A. The cannabis plant: botany, cultivation and processing for use. In: Brown DT. (ed). Cannabis: The Genus Cannabis. Amesterdam: Harwood Academic Publishers, 1998, 29–54. [Google Scholar]
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011;163:1344–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C. Cannabis: adverse effects from an oromucosal spray. Brit Dent J 2007;203:E12–12. [DOI] [PubMed] [Google Scholar]
- Sharpe PL, Ye RW, Zhu QQ. Carotenoid production in a recombinant oleaginous yeast. 2014. US 8846374 B2. www.google.com/patents/US8846374 (31 March 2017, date last accessed).
- Shoyama Y, Tamada T, Kurihara K et al. . Structure and function of Δ1-tetrahydrocannabinolic acid (thca) synthase, the enzyme controlling the psychoactivity of Cannabis sativa. J Mol Biol 2012;423:96–105. [DOI] [PubMed] [Google Scholar]
- Sirikantaramas S, Morimoto S, Shoyama Y et al. . The gene controlling marijuana psychoactivity. Molecular cloning and heterologous expression ofΔ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J Biol Chem 2004;279:39767–74. [DOI] [PubMed] [Google Scholar]
- Stout JM, Boubakir Z, Ambrose SJ et al. . The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J 2012;71:353–65. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A et al. . 2-arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Bioph Res Co 1995;215:89–97. [DOI] [PubMed] [Google Scholar]
- Taguchi C, Taura F, Tamada T et al. . Structural biology and crystallization communications crystallization and preliminary x-ray diffraction studies of polyketide synthase-1 (PKS-1) from Cannabis sativa. Acta Crystallogr F 2008;64:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura F, Sirikantaramas S, Shoyama Y et al. . Phytocannabinoids in Cannabis sativa: recent studies on biosynthetic enzymes. Chem Biodivers 2007a;4:1649–63. [DOI] [PubMed] [Google Scholar]
- Taura F, Sirikantaramas S, Shoyama Y et al. . Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett 2007b;581:2929–34. [DOI] [PubMed] [Google Scholar]
- Taura F, Tanaka S, Taguchi C et al. . Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett 2009;583:2061–6. [DOI] [PubMed] [Google Scholar]
- Tehlivets O, Scheuringer K, Kohlwein SD. Fatty acid synthesis and elongation in yeast. BBA Mol Cell Biol L 2007;1771:255–70. [DOI] [PubMed] [Google Scholar]
- Toyota M, Shimamura T, Ishii H et al. . New bibenzyl cannabinoid from the New Zealand liverwort Radula marginata. Chem Pharm Bull 2002;50:1390–2. [DOI] [PubMed] [Google Scholar]
- Westfall PJ, Pitera DJ, Lenihan JR et al. . Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. P Natl Acad Sci USA 2012;109:E111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JD, Williamson EM, Fisher GJ et al. . Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J Dermatol Sci 2007;45:87–92. [DOI] [PubMed] [Google Scholar]
- Woods A, Munday MR, Scott J et al. . Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem 1994; 269:19509–15. [PubMed] [Google Scholar]
- Yang X, Matsui T, Kodama T et al. . Structural basis for olivetolic acid formation by a polyketide cyclase from Cannabis sativa. FEBS J 2016;283:1088–106. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bao X, Li C et al. . Improving monoterpene geraniol production through geranyl diphosphate synthesis regulation in Saccharomyces cerevisiae. Appl Microbiol Biot 2016;100:4561–71. [DOI] [PubMed] [Google Scholar]
- Zhou YJ, Gao W, Rong Q et al. . Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc 2012;134:3234–41. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Zhou YJ, Krivoruchko A et al. . Expanding the product portfolio of fungal type I fatty acid synthases. Nat Chem Biol 2017; 13:360–2. [DOI] [PubMed] [Google Scholar]
- Zirpel B, Stehle F, Kayser O. Production ofΔ9-tetrahydrocannabinolic acid from cannabigerolic acid by whole cells of Pichia (Komagataella) pastoris expressingΔ9-tetrahydrocannabinolic acid synthase from Cannabis sativa L. Biotechnol Lett 2015;37:1869–75. [DOI] [PubMed] [Google Scholar]