ABSTRACT
Saccadic intrusions are small involuntary saccadic movements that disrupt visual fixation. Among saccadic intrusions without intersaccadic intervals, ocular flutter and opsoclonus are prominent. The saccade amplitude can occasionally be very small, which is referred to as ocular microflutter. The authors present a patient with acute-onset oscillopsia following a non-specific viral condition. An ocular microflutter was subsequently detected using video-oculography. After extensive investigation, a diagnosis of isolated idiopathic or post-viral ocular microflutter was made. The evolution of the condition was favourable, and the progressive improvement of oscillopsia occurred during the following months; however, complete resolution was not achieved. Ocular microflutter is a saccadic intrusion that is rarely described in the literature and is likely go clinically unnoticed because of its small amplitude and the rare use of video-oculography in daily practice. In patients in whom this condition is suspected, the use of video-oculography is essential for a correct diagnosis.
KEYWORDS: Ocular flutter, ocular microflutter, opsoclonus, saccadic intrusions, video-oculography
Introduction
There are a number of eye movements that disrupt visual fixation. Specifically, during fixation, our eyes are never completely stable and may exhibit slow drifts, conjugate microsaccades, and eye tremors. These movements are considered physiological.1
On the other hand, there are several types of eye movements that interrupt visual fixation and that are considered, in general, to be pathological. The best example is saccadic intrusions that begin with a rapid movement (saccade) away from the fixation point, usually followed by another corrective saccade.2,3
Saccadic intrusions are basically divided into those with intersaccadic intervals and those where there is no such interval.3 Among the former type, square-wave saccadic intrusions, square-wave pulses (or square-wave macrosaccadic intrusions), and macrosaccadic oscillations are worth noting.3 Some of these intrusions, especially square-wave intrusions, are observed in healthy people, e.g., the elderly.3 Among saccadic intrusions without intersaccadic interval, ocular flutter and opsoclonus are prominent.3
Occasionally, the amplitude of the ocular flutter is very small, which is known as ocular microflutter.4 In these cases, the ocular oscillation is so small that it is difficult to detect with the human eye, and its correct characterisation requires the use of complementary techniques, such as video-oculography.4
We present a case of intermittent ocular microflutter in a patient with acute-onset oscillopsia after a non-specific influenza syndrome.
Clinical case
We present the case of a 47-year-old female patient who experienced acute-onset continuous oscillopsia and lightheadedness in September 2012. In the previous weeks, she experienced remittent fever with chills, which was compatible with influenza, accompanied by hearing loss in the right ear that resolved without sequelae with antipyretic treatment. The patient did not mention any personal or family history of interest.
At the initial examination, both eyes had normal visual acuity. The pupils were isocoric and normoreactive to light and accommodation. Eye pursuit movements, ductions, and versions did not show any alterations. However, in the lateral horizontal gaze, an intermittent ocular oscillation was observed, which became more evident with the use of a direct ophthalmoscope. The eye fundi and the rest of the neurological examination were normal. No dysmetria, myoclonus, or gait ataxia were detected.
We used a non-invasive, quantitative video-oculography device to measure and record the eye movements of the patient. The device uses an infrared illuminated eye image captured by a camera module with a sampling rate of 60 Hz and a dynamic range of approximately ±20 degrees.
The patient was asked to follow the changing position of one black dot displayed at 6 m on a uniform light-grey background. Later, the patient was required to make spontaneous eye movements in lighted conditions without a target. Finally, the patient was required to make spontaneous eye movements in complete darkness. No stimuli were presented during these latter two paradigms. The patient took approximately 30 minutes to complete the test.
Video-oculography showed small-amplitude saccades, mostly double saccadic pulses (DSPs), with rightward or leftward initial saccade and variable persistence.
Single saccadic pulses (SSPs) are brief saccadic intrusions that take the eye from the fixation point that is followed by a slow drift that returns the eye to the previous position. A DSP is a pair of back-to-back saccades.3
Saccadic intrusions increased with gaze fixation, especially in the central and left lateral positions, where they were frequently continuous, forming small-amplitude oscillations with a rightward or leftward initial saccade. These findings were compatible with ocular sawtooth microflutter bursts (which have amplitudes between 0.2 and 1 degrees and an approximate frequency of 15 Hz) (Figure 1).
Figure 1.
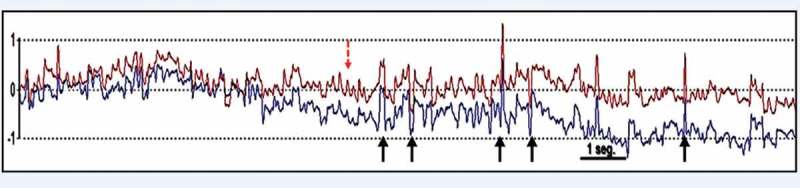
Video-oculography showing small-amplitude saccades, mostly DSPs (continuous arrows), during fixation in the left lateral position that consisted of small-amplitude oscillations leftward and rightward during eye movement recording, which were compatible with sawtooth ocular microflutter bursts (with an amplitude of 0.2–1 degrees and an approximate frequency of 15 Hz) (dashed arrow). Note: By convention, rightward and leftward ocular movements are represented by upward and downward tracings in eye movement recordings.
Given the diagnosis of ocular microflutter, an extensive study was performed, including (i) a complete blood count, coagulation test, biochemical analyses, thyroid profile, and vitamin B12 and folic acid levels; (ii) antinuclear antibodies and serology tests against Epstein-Barr, cytomegalovirus, herpes virus, varicella-zoster, Streptococcus, human immunodeficiency virus (HIV), Mycoplasma, Treponema pallidum, and Borrelia; (iii) a search for occult tumours (β2-microglobulin, α-fetoprotein, carcinogenic antigens (CEAs; CA 125, CA 15.3, and CA 19.9); (iv) brain and angio-magnetic resonance imaging as well as brain and body positron emission tomography; and (v) lumbar puncture (with biochemistry, cytology, microbiology, and oligoclonal band analysis). All results were compatible with normal values.
On the abdominopelvic computed tomography (CT) scan, a right adnexal cyst was observed. After assessment with transvaginal ultrasound, this finding was consistent with a persistent functional non-malignant cyst.
Ultimately, the diagnosis of idiopathic or post-viral isolated ocular microflutter was made. It was decided to observe the patient, whose oscillopsia progressively improved in the following months, without resolving completely.
Discussion
We report the case of a patient with acute-onset oscillopsia secondary to ocular microflutter after a non-specific influenza syndrome. Unlike other previously described cases, the oculomotor disorder was intermittent. The spontaneous evolution of the condition followed a favourable course, but it was not completely resolved. Some authors have defined ocular microflutter as a very small-amplitude ocular flutter.4 However, other authors believe that they are two completely different entities and that the amplitude is not the only differentiating factor.5,6
Cases of microflutter have been rarely described in the scientific literature.4–6 A study by Ashe et al., which described five cases of ocular microflutter, is notable. In these patients, the saccadic intrusions had a frequency of 15–30 Hz, an amplitude of 0.1–0.5 degrees, and a sawtooth morphology on video-oculography. Only in one of these patients was the microflutter was associated with an underlying disease (multiple sclerosis). In the rest of the cases, the cause was undetermined.4
In another publication, Foroozan and Brodsky6 described a female patient with oscillopsia and saccadic movements that were almost imperceptible to the human eye. Using video-oculography, the authors detected sawtooth saccadic movements with a frequency of 20 Hz and an amplitude of 0.2–1 degrees of multidirectional character (horizontal, vertical, and torsional). These saccadic intrusions were termed microsaccadic opsoclonus. In the patient’s overall study, colon cancer was detected and treated with surgery, chemotherapy, and radiation therapy. However, no onconeural antibodies were detected.
In our patient, video-oculographic recordings presented very similar amplitude and frequency characteristics to those described by Ashe et al.4 and Foroozan and Brodsky.6 The main difference is that our patient presented ocular microflutter bursts that alternated with other periods in which other saccadic intrusions predominated, mostly DSPs. In the recordings of the patients presented in the above-mentioned publications,4,6 the presence of microflutter appeared to be constant.
However, the presence of saccadic pulses intermixed with microflutter episodes in our patient shows that both types of saccadic intrusions are part of the same spectrum. Patients with isolated saccadic pulses would be at one end of the spectrum, whereas patients with continuous microflutter would be at the other end of the spectrum. Patients similar to ours, with combined saccadic pulses and microflutter bursts, would be between the two extremes.
Unlike microflutter, ocular flutter usually has a larger amplitude and a lower frequency (between 1 and 5 degrees and 10 and 25 Hz). This type of saccadic intrusion consists of rapid saccadic movements that divert the eye from its fixation point, followed immediately by another corrective saccadic movement.7,8 This pattern occurs continuously and in the horizontal direction. However, when saccadic intrusions occur in different directions, we associate opsoclonus or saccadomania.3
Fifty percent of children with opsoclonus have an underlying neuroblastoma9; in adults, a tumour will be found as a triggering cause in up to 20% of patients with opsoclonus (usually small cell lung carcinoma and ovarian, uterus, or breast malignancies).10 There are also other possible non-neoplastic causes, including post-traumatic effects, brainstem encephalitis, multiple sclerosis, phenytoin intoxication, etc.3 However, a cause cannot be established in up to 50% of patients with opsoclonus. However, because microflutter is such a rare entity, we can conclude from the cases reported in the literature that at least two patients had an underlying malignancy. This value would indicate that approximately 20% of microflutter patients have an underlying disease.
Paradoxically, although neoplastic causes generate a significant proportion of cases of opsoclonus, these cases are not often associated with onconeural antibodies. However, there have been isolated reports in the literature that are associated with anti-Ri,11 amphiphysin,12 Hu,12–14 Yo,15 and Ma216 antibodies. All of these tests were negative in our patient.
As for the anatomical mechanism of these saccadic intrusions, it is known that when an order is given to produce a voluntary saccade, the excitatory cells that project to the oculomotor nuclei are stimulated. At the same time, omnipause neurons, which control the activity of excitatory neurons between saccades, are inhibited. Within this circuit, the excitatory neurons have their own feedback system that informs them of the progression of the saccade through the eye position, which in turn reduces excitation. Ashe et al.4 proposed that ocular flutter could be the result of a joint failure of omnipause neurons and the feedback system of excitatory cells. However, an alteration of the omnipause cells would be sufficient to cause ocular microflutter.4,17–19
In summary, ocular microflutter is a saccadic intrusion that has rarely been described in the literature and is likely to go clinically unnoticed because of its small amplitude and because of the rare use of video-oculography in daily practice. In patients in whom this entity is suspected, the use of video-oculography is essential for a correct diagnosis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- [1].Abadi RV, Gowen E.. Characteristics of saccadic intrusions. Vision Res 2004;44:2675–2690. [DOI] [PubMed] [Google Scholar]
- [2].Leigh RJ, Das VE, Seidman SH.. A neurobiological approach to acquired nystagmus. Ann N Y Acad Sci 2002;956:380–390. [DOI] [PubMed] [Google Scholar]
- [3].Lemos J, Eggenberger E.. Saccadic intrusions: review and update. Curr Opin Neurol 2013;26:59–66. [DOI] [PubMed] [Google Scholar]
- [4].Ashe J, Hain TC, Zee DS, Schatz NJ.. Microsaccadic flutter. Brain 1991;114(Pt 1B):461–472. [DOI] [PubMed] [Google Scholar]
- [5].Carlow TJ. Medical treatment of nystagmus and ocular motor disorders. Int Ophthalmol Clin 1986;26:251–264. [DOI] [PubMed] [Google Scholar]
- [6].Foroozan R, Brodsky MC.. Microsaccadic opsoclonus: an idiopathic cause of oscillopsia and episodic blurred vision. Am J Ophthalmol 2004;138:1053–1054. [DOI] [PubMed] [Google Scholar]
- [7].Herishanu YO, Sharpe JA.. Saccadic intrusions in internuclear ophthalmoplegia. Ann Neurol 1983;14:67–72. [DOI] [PubMed] [Google Scholar]
- [8].Smith JL, Walsh FB.. Opsoclonus-ataxic conjugate movements of the eyes. Arch Ophthalmol 1960;64:244–250. [DOI] [PubMed] [Google Scholar]
- [9].Pranzatelli MR. The neurobiology of the opsoclonus-myoclonus syndrome. Clin Neuropharmacol 1992;15:186–228. [DOI] [PubMed] [Google Scholar]
- [10].Digre KB. Opsoclonus in adults: report of three cases and review of the literature. Arch Neurol 1986;43:1165–1167. [DOI] [PubMed] [Google Scholar]
- [11].Dropcho EJ, Kline LB, Antineuronal Riser J.. (anti-Ri) antibodies in a patient with steroid-responsive opsoclonus-myoclonus. Neurology 1993;43:207–211. [DOI] [PubMed] [Google Scholar]
- [12].Bataller L, Graus F, Saiz A, Vilchez JJ ; Spanish Opsoclonus-Myoclonus Study Group. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain 2001;124(Pt 2):437–443. [DOI] [PubMed] [Google Scholar]
- [13].Hersh B, Dalmau J, Dangond F, Gultekin S, Geller E, Wen PY.. Paraneoplastic opsoclonus-myoclonus associated with anti-Hu antibody. Neurology 1994;44:1754–1755. [DOI] [PubMed] [Google Scholar]
- [14].Senties-Madrid H, Vega-Boada F.. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J 2001;3:94–103. [PubMed] [Google Scholar]
- [15].Peterson K, Rosenblum MK, Kotanides H, Posner JB.. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology 1992;42:1931–1937. [DOI] [PubMed] [Google Scholar]
- [16].Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B, Saiz A, Meneses P, Rosenfeld MR.. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004;127(Pt 8):1831–1844. [DOI] [PubMed] [Google Scholar]
- [17].Horn AK, Büttner-Ennever JA, Suzuki Y, Henn V.. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol 1995;359:350–363. [DOI] [PubMed] [Google Scholar]
- [18].Horn AK, Büttner-Ennever JA, Wahle P, Reichenberger I.. Neurotransmitter profile of saccadic omnipause neurons in nucleus raphe interpositus. J Neurosci 1994;14:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ko MW, Dalmau J, Galetta SL.. Neuro-ophthalmologic manifestations of paraneoplastic syndromes. J Neuroophthalmol 2008;28:58–68. [DOI] [PubMed] [Google Scholar]


