Abstract
In this review, we highlight promising new discoveries that may generate useful and clinically relevant insights into the mechanisms that link exercise with growth during critical periods of development. Growth in childhood and adolescence is unique among mammals, and is a dynamic process regulated by an evolution of hormonal and inflammatory mediators, age-dependent progression of gene expression, and environmentally modulated epigenetic mechanisms. Many of these same processes likely affect molecular transducers of physical activity. How the molecular signaling associated with growth is synchronized with signaling associated with exercise is poorly understood. Recent advances in “omics,” namely, genomics and epigenetics, metabolomics, and proteomics, now provide exciting approaches and tools that can be used for the first time to address this gap. A biologic definition of “healthy” exercise that links the metabolic transducers of physical activity with parallel processes that regulate growth will transform health policy and guidelines that promote optimal use of physical activity.
Keywords: physical activity, genomics, epigenetics, proteomics, metabolomics
I. INTRODUCTION
Children, like most mammals during growth and development, are naturally physically active. Developmental biologists, pediatricians, and other child health care specialists have long suspected that exercise during infancy, childhood, and adolescence is not merely play, but is essential for healthy growth and development. The pattern and intensity of physical activity during key stages of growth and development can provide the organism with mechanical and other thermodynamic inputs, essential information that could optimally adapt the growing child to his or her specific environment. Precisely how the molecular transducers of physical activity interact with the molecular transducers of normal growth and development remains poorly understood. But recent advances in “omics,” namely, genomics and epigenetics, metabolomics, and proteomics, now provide exciting approaches and tools that can be used for the first time to address this gap. In this review, we highlight promising new discoveries that may generate useful and clinically relevant insights into the mechanisms that link exercise with growth during critical periods of development.
II. GROWTH AND EXERCISE: FINDING BIOMARKER CLUES IN CIRCULATING BLOOD FOR “BRIDGING THE GAPS”
A conceptual framework is emerging in which we can begin to test specific hypotheses, as well as probe particular mechanisms and pathways that, ultimately, can lead to maps that link the omics of naturally-occurring growth with the omics of exercise (acute bout), training (chronic exercise) and habitual physical activity. Growth in humans is unique among mammals. The duration of childhood and adolescence, i.e., the period of development before reproduction is possible, is long. Moreover, it is the general consensus among auxologists and comparative developmental biologists that we are the only mammals that exhibit so profound a pubertal growth spurt (bone and muscle) and sexual dimorphism (29). Human growth is a dynamic process regulated by an evolution of hormonal and inflammatory mediators, age-dependent progression of gene expression, and environmentally modulated epigenetic mechanisms. What is particularly intriguing is that many of the hormones and mediators involved in natural growth in children also appear to play substantial roles in the adaptive responses to exercise and physical fitness (Table 1). Our challenge is to elucidate the biologic processes that link growth with exercise using new omics tools now available.
Table 1.
Examples of biologic mediators, cytokines, and hormones common to the Exercise Adaptation and the Natural Process of Growth–Clues for “Bridging the Gaps.” These proteins, found predominantly in the circulating blood, provide clues for a deeper exploration of the genomic and epigenetic mechanisms that link acute exercise and physical fitness with growth during critical periods of development in children and adolescents.
| Molecular Transducers | Role in Exercise Adaptation | Role in Natural Process of Growth |
|---|---|---|
| Growth Factors and Hormones | ||
| Growth Hormone (GH) | Brief exercise stimulates GH; GH activates IGF-1 (and its binding proteins) leading to myriad anabolic and metabolic effects on muscle and other tissues (18) | GH pulse frequency and amplitude dramatically increase at the onset of puberty |
| Insulin Growth Factor (IGF) family of mediators | Exercise directly stimulates gene expression and protein production of IGF-1 in muscle tissue (18) | IGF-1 levels in the blood increase with muscle mass and growth throughout puberty in males and females |
| Glucocorticoid | Heavy exercise stimulates endogenous glucocorticoid secretion | Glucocorticoids are powerful regulators of growth in bone and muscle |
| Inflammatory and Stress Mediators and Cytokines | ||
| Interleukin-6 (IL-6) and inflammatory cytokines | Exercise stimulates IL-6 production from muscle; IL-6 may influence glucoregulatory mechanisms systemically | IL-6 (and other inflammatory mediators) directly inhibits the actions of IGF-1 (and other growth mediators); in infants, as IL-6 levels decrease, IGF-1 and GH binding protein levels increase (2) |
| Bone Specific Remodeling Factors | ||
| Fibroblast Growth Factors (FGF) | Circulating FGF virtually disappears with brief exercise (9) | FGF family involved in growth plate regulation of long bones |
| Brain/Neuronal Growth Factors | ||
| Brain Derived Neurotrophic Factor (BDNF) | BDNF levels are associated with fitness in adolescents (20) | Associated with cognitive development during growth (13) |
III. MAPPING THE GENOMIC LINKS BETWEEN GROWTH AND PHYSICAL ACTIVITY–BABY STEPS
The sequencing of the human genome (completed in 2003 by the Human Genome Project) launched a new era of discovery in human biology. Technologies such as microarrays and sequencing permitted investigators to interrogate the expression of the whole human genome and to identify specific genes, or interacting pathways of genes, that could play a role in health and disease. More recently, these techniques have been used to explore an intriguing aspect of transcription mechanisms, namely, the timing of gene expression across the lifespan (Figure 1).
Figure 1.
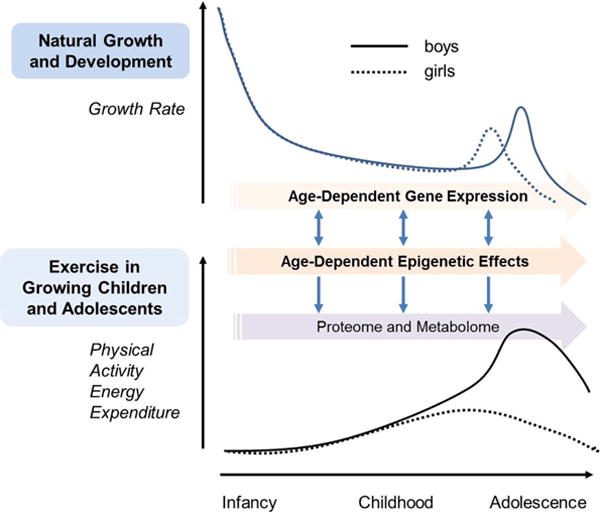
Interacting mechanisms of growth and physical activity in children and adolescents. Top Panel: Human growth is unique in the duration of childhood and in the magnitude of the pubertal growth spurt. Bottom Panel: Levels of physical activity (highly variable -shown here are estimates culled from the literature) are also dynamic during growth. Sexual dimorphism is also prominent in both human growth and levels of physical activity. Pioneering work is revealing how specific genes and epigenetic mechanisms influence growth at different stages. Exercise also influences genes and epigenetic processes. Ultimately, genetic and epigenetic activity drives the proteome and metabolome leading to dynamic phenotypes during childhood and across the lifespan. The challenge for the future is to map the link between these two processes and use this information to shape policies and guidelines around the optimal use of physical activity in healthy children and in children with chronic disease and disability.
Our studies aimed at uncovering the gene expression of circulating immune cells in response to brief exercise in children, adolescents, and adults have also begun to reveal age-dependent changes in gene expression related to exercise. We showed that a relatively brief bout of exercise, designed to mimic natural patterns of physical activity in children, induced a remarkable change in peripheral blood mononuclear cells (PBMCs) gene expression in healthy early and late pubertal boys (24) and girls (23) with the least affected number of genes in the early pubertal boys group. We found that many genes were altered by brief exercise in both groups, and while many of the same genes were affected by exercise in both the early- and late-pubertal subjects, our data also showed distinct, presumably age-dependent, differences (Figure 2) (23).
Figure 2.
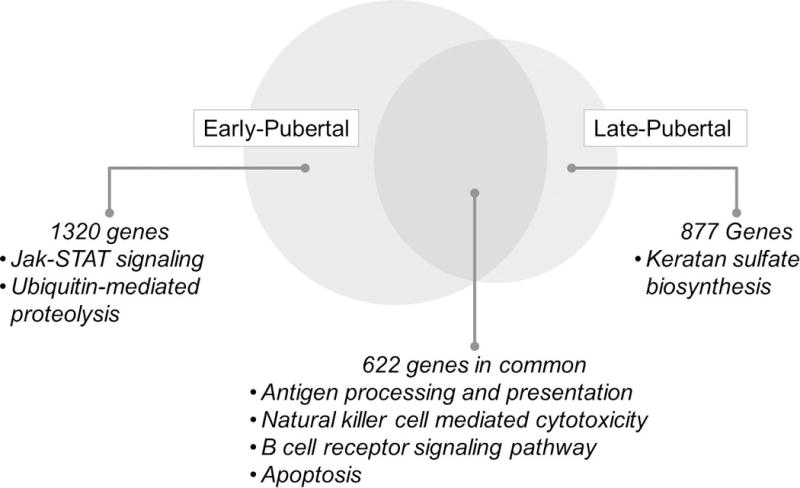
Differences and overlaps of gene expression in circulating PBMCs in response to brief exercise in early and late pubertal girls. We show selected statistically significant exercise changes in PBMC gene KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways. Note the relevance of these pathways to growth functions and inflammation. Data from (23).
In an elegant study, Stevens et al. (29) recently showed that growth in children is associated with distinct patterns of gene expression in evolutionarily conserved networks. His group looked at changes in gene expression that accompany development in children and correlated them with biological pathways at different ages and stages of development. They found that the expression of clusters of genes varies in a development-dependent manner in multiple human tissues (lymphoid, PBMCs, skeletal muscle, bone marrow, conjunctival endothelium, and temporal lobe brain). In an exercise of hypothesis development, we compared the Stevens data with very different but related acute exercise experiments from our laboratory. Of the 1450 PBMC genes from Stevens study identified as having age-dependent alterations in gene expression, 427 were also shown to be altered by exercise in our studies. Future research should focus on these genes and their associated pathways to determine more precisely how exercise in children can interact with, and possibly modify, the very process of growth and development or alternatively, how growth and development affect the response to exercise.
IV. BEYOND GENETICS–THE EPIGENETIC REVOLUTION
It quickly became apparent that the prodigious task of sequencing the human genome would not in and of itself resolve key questions of how the genomic map translated into the key proteins and metabolic processes that govern all biologic processes essential for life. Intriguingly, it was the exploration of gene silencing and time-dependence of gene expression during growth and development that led to another major breakthrough, namely epigenetics, the identification of a family of mechanisms (DNA methylation, histone modification, chromatin remodeling and non-coding RNAs) that could turn genes “on” or “off” without changing the DNA sequence and control the specificity, rate, and timing of gene transcription and translation (see Figure 3). Broadly defined epigenetics is “…the field of study surrounding stable alterations to the DNA and histone proteins that alter gene expression. Epigenetic modifications are responsible for tightly regulated tissue and cell-type specific gene expression patterns. Aberrations in these expression patterns can give rise to certain diseases, most notably some types of cancer. Interestingly, certain environmental factors can influence the expression of genes within a cell without mutations to the genome, but instead through modifying epigenetic marks. These environmental changes to the epigenome are so robust that even monozygotic twins can be identified by analyzing their unique epigenetic patterns.” (30)
Figure 3.
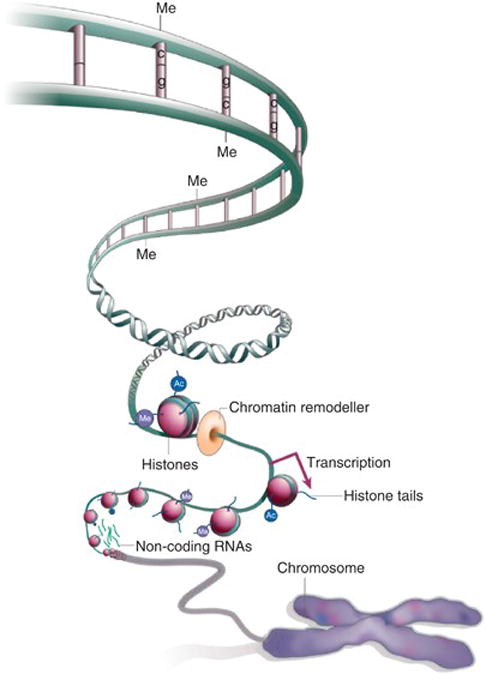
Schematic representation (reproduced from AACR Cancer Research Human Epigenome Task Force, Nature 2008; 454: 711–755) (1) of the principal epigenetic mechanisms: DNA methylation, histone modifications, chromatin remodeling and non-coding RNA (mainly, microRNAs), each of which alters how genes are expressed without altering the underlying DNA sequence. DNA methylation refers to the addition of a methyl (CH3) group to the DNA strand itself, often to the fifth carbon atom of a cytosine ring which causes gene silencing. Histones are core protein components of chromatin complexes which provide the structural backbone around which DNA wraps at regular intervals. Post-translational modifications of the basic side-chains in histone tails include methylation, acetylation, phosphorylation and ubiquitinylation which are critical for regulating chromatin function. The ncRNAs-associated silencing is a type of post-transcriptional gene modification during which the expression of one or more genes is downregulated or suppressed by microRNAs: small non-coding stretches of RNA.
In recent years, there is mounting evidence that a major mechanism through which environmental factors (e.g., diet, stress, exercise) can alter the proteome and metabolome is through epigenetic regulation of gene expression. These epigenetic changes can influence the organism across its lifespan. Until recently there has been limited knowledge about whether exercise induces epigenetic modifications in humans. Most studies conducted so far in exercise and epigenetics have been focused on DNA methylation. Voisin et al. summarized in a recent review 25 human studies of both acute exercise and training that significantly impact DNA methylation in a highly tissue- and gene-specific manner (31). Only three of these studies (all in adults) examined the effect of exercise training on genome-wide methylation status. Two studies showed that six months of aerobic exercise for one hour, twice a week, had distinct genome-wide changes in DNA methylation with an overlapping changes in mRNA expression in skeletal muscle (19) and adipose tissue (27) of middle-aged healthy sedentary men. The third study showed small (<8%) yet significant change in DNA methylation level in blood from breast cancer patients following 6-month exercise training. Fewer investigations have studied exercise in relation to histone modifications. McGee et al. showed that a single bout of cycling for 60 min at 76±2% of peak VO2 promotes chromatin remodeling and histone acetylation in young adult skeletal muscle (15). A set of studies from our laboratory showed the impact of acute exercise on gene and microRNA expression profile of young adult circulating PBMCs (22), neutrophils (25), natural killer cells (21), and monocyte (26). A pattern of genomic change is emerging from these studies that suggests a very specific exercise-induced priming of leukocytes. This priming may better prepare the organism to deal with injury or infections, more likely to occur when our ancestors were chasing prey or evading predators. To our knowledge, there are no experimental studies that have examined epigenetic mechanisms in response to exercise in the pediatric age range in humans.
V. THE BRAVE NEW WORLD OF PROTEOMICS AND METABOLOMICS
PUncovering key protein biomarkers that link the processes of growth and and exercise is essential for transforming the field of exercise as medicine in children and adolescents. This exploration has been constrained by technological limitations in the ability to identify protein products of genomic and epigenetic processes. More recently, proteomics and metabolomics have aided the study of protein structure, function, and abundance, and the examination of the metabolites, the end products of cellular processes, respectively. Proteomics is defined as the large-scale analysis of proteins enabled by technological advances such as tandem mass spectroscopy. The metabolome is defined as the complete set of low molecular weight (typically<1500 Da) metabolites produced by an organism, which are the end products of gene expression. Metabolomic discovery is enabled by technological advances such as nuclear magnetic resonance and advanced mass spectrometry.
Proteomic and metabolomic applications to the discovery of the molecular transducers of physical activity and exercise are still in their infancy, but progress is being made rapidly. Morris et al. demonstrated a relationship between fitness level and the amino acid profile in adult population (17), while Goto-Inoue et al. showed phospholipid compositional changes in rat muscle in response to chronic exercise and high-fat diet (11). Daskalaki et al. showed an acute impact of heavy exercise on purine metabolites as well as on key sex hormones such as the androgens (8). Just as our initial studies of leukocyte genomic responses to exercise have begun to show differences between early and late pubertal children, we believe that by using modern proteomic and metabolomic approaches to exercise in children, transformative discoveries in how physical activity influences growth and development will be made.
VI. THE CHALLENGES AHEAD–GAPS IN OUR UNDERSTANDING
As noted, the number and range of mechanisms that likely affect both 1) molecular transducers of physical activity and 2) normal growth and development (e.g., GH and IGF-1 family of mediators) are striking. Consequently, a key conceptual challenge for future research is to identify common mediators associated with both growth and physical activity, and map the molecular links between them. Successful mapping ultimately depends on accurate and reproducible definition of phenotypes in the growing child. This mapping will be most valuable if considered in three contexts: 1) habitual physical activity, 2) short term responses to brief exercise challenges, and 3) longer term adjustments and adaptation to exercise training.
VI.1. The Dynamic Phenotype of the Fit Child or Adolescent
The natural process of growth and development render children and adolescents “moving targets” for phenotyping. Defining and quantifying the “fit” phenotype in the growing child is particularly challenging. It is axiomatic that “children are not just miniature adults”, and as such there is a need to study exercise and physical activity in the pediatric population mindful of these key developmental differences. A recent review by Armstrong et al. (4) summarizes some of the developmentally related physiological responses (i.e., phenotype) to exercise in children compared to adults, among them: enhanced ability to oxidize lipids and spare glycogen in the muscle, relying more on oxidative metabolism during high-intensity exercise, may be disadvantaged in activities predominantly supported by anaerobic metabolism, at the onset of exercise have a higher potential for oxidative metabolism, and recover from short-term maximal-intensity and high-intensity exercise faster than adults.
It will be necessary to expand analysis of the data available from traditional cardiopulmonary exercise testing (CPET) beyond the “gold standard” of peak or maximal oxygen uptake. Current technologies used to assess exercise and physical activity biomarkers are based on biological concepts that are a century old and that were designed to optimally quantify physiological responses at the extreme limits of the exercise capacity of healthy adult human beings. Novel technologies and data management capabilities have opened new doors that can generate exercise and physical activity biomarkers from protocols that mimic more naturally occurring exercise in children and adolescents, and can provide insights into pathophysiological constraints imposed by disease (e.g., sickle cell disease) or factors related to therapy (e.g., survivors of childhood malignancies) in previously unimaginable ways. In-laboratory exercise testing is one of the few instances in biomarker discovery where the physiological perturbation (the “input”) can be measured in thermodynamically incontrovertible units (i.e., watts) and can be scaled to the individual child’s capabilities. Consequently, exercise testing can now be used in children not only to measure metabolism, neuromuscular function, and cardiac and respiratory function, but also to assess genomic and epigenetic immune responses in leukocytes or complex neurobehavioral responses such as executive function. It may prove useful, for example, to consider the value of dynamic interactions (e.g., slopes) (7) or response and recovery times, as we better define the exercise phenotype in the growing child. A better definition of the exercise phenotype will enhance our ability to create molecular maps.
VI.2. Human Growth in Childhood and Adolescence–Unique Among Mammals: Implications for Physical Activity and Exercise Impact on Genomics, Proteomics, and Metabolomics in Boys and Girls
No other mammal has so long a duration from birth through reproductive maturity as do human beings. Further, no other mammal exhibits so robust a pubertal growth spurt as do humans (see Figure 1). These long intervals have likely contributed to the unique construct of human society in which learning and acculturation during childhood and adolescence play incomparably critical roles in the individual organism’s ultimate survival and ability to reproduce. From an omics perspective, these long periods of “critical growth and development” provide a mechanism whereby environmental factors can play a role in subsequent programming of growth in a manner unprecedented in any other living species. While there is increasing recognition that physical activity in children is not merely play but rather an essential component of healthy growth and development, we are just in the beginning phases of applying genomic, epigenetic, proteomic, and metabolomic tools. However, the exact molecular mechanisms by which physical activity promotes healthy growth and development in infant, toddler, child, or teen remain poorly understood.
Closely tied to the unique patterns of growth and development seen in homo sapiens is the wide range of sexual dimorphism seen. From patterns of physical activity in childhood and adolescence, to autoimmune disease in young adulthood, to cardiovascular disease later in life, we are just beginning to understand the profound impact of the sexually determined silenced X-chromosome and the contrasting biological effects of testosterone and estradiol family of mediators (28). Many of these effects are epigenetic in nature. For example, in examining DNA-methylation mechanisms active in immune cells, Mamrut et al. found that sex-specific differentially methylated regions (DMRs) were overrepresented in CpG islands [areas of the genome containing an atypically high frequency of CpG (cytosine-phophate-guanine] sites and often associated with the control of transcription], suggesting that the epigenetic regulatory mechanisms of sex and immune cell specificity may differ. Further, sex-specific DMRs were particularly enriched in networks of early development and linked to estrogen receptor and immune-related molecules (14).
VI.3. Exercise, Physical Activity, and Critical Periods of Growth and Development: Can Epigenetic Changes that are Induced Early in Life Persist?
Epidemiological studies suggest that the early-life environment can affect epigenetic regulation of specific genes and make an important contribution to disease risk factors later in life. For example, exposure to famine in utero is associated with altered methylation status in blood in genes related to the cardio-metabolic disease (CMD) phenotype in elderly individuals (12). Furthermore, methylation of the retinoid-x-receptor-a in umbilical cord predicted >25% of the variation in age- and sex-adjusted fat mass in children at 6 and 9 years old (10). Such findings imply that epigenetic changes that are induced by environmental exposures early in life can persist beyond the period of challenge. Alisch et al. investigated pediatric age-associated DNA methylation changes in peripheral blood mononuclear cells (PBMCs) of 398 healthy males (3-17 y/o) and reported 2078 loci that exhibit age-associated DNA methylation differences (3). This shows that even within the relatively narrow age-range of childhood and adolescence, the “epigenome” is changing. Which specific childhood associated changes in epigenetics persist across the lifespan remain unknown.
Clarke-Harris et al. conducted a study to test whether methylation of the gene PGC-1α in blood could predict adiposity. Methylation of several sites in the promoter of PGC-1α in 40 children between 5 and 14 years of age were annually measured. Four sites were found in the promoter of PGC-1α that could predict adiposity. However, no interaction between physical activity and PGC-1α promoter methylation was found. The idea to study gene methylation status during a period of growth and development is compelling. And while pioneering, the Clarke-Harris study examined only a few target genes and might have missed important information that could be generated if the global whole-genome approach would have been used (6).
In a pioneering study conducted by Menni et al. (16), metabolomic markers revealed novel pathways of ageing and early years development in human populations in 6055 people. The authors examined untargeted epigenetic and metabolomic markers. They concluded, “Observational and experimental evidence increasingly supports a relation between growth and development during fetal and infant life and health in later years, termed the developmental origins of health and disease. The data from the present study provide specific molecular insights for this hypothesis. The results illustrate how metabolomic profiling joined by epigenetic studies may help to identify novel molecular mechanisms implicated in subtle early life influences which produce long-term physiological changes that influence human health.”
VI.4. The Challenge of Constantly Changing “Omics” Technologies
Despite enormous potential, omics technologies are works-in-progress, and the logistical and methodological limitations of these approaches must be recognized and carefully considered. Microarrays which have been a powerful tool in examining gene expression are now being replaced by more precise techniques like ultra-high throughput sequencing technology, involving direct gene sequencing (e.g., RNAseq). The technologies used for proteomics and metabolomics are less evolved. Indeed, application of proteomics and metabolomics technology to exercise physiology research has been confounded by a number of challenges, including a lack of standard protocols and molecular standards, the number of tissues involved, and the transient nature of the response to brief exercise. All such targets require validation testing. In addition, bioinformatics methods to generate the most appropriate biological interpretation from a database are still under development.
VII. BRIDGING THE GAPS–POTENTIAL STRATEGIES
Studies that utilize cutting edge genomic, epigenetic, proteomic and metabolomic research require substantial resources, often beyond the scope of even the best pediatric exercise scientists. But on June 11, 2015, National Institutes of Health (NIH) Director Francis Collins announced a transformative new NIH Common Fund (https://commonfund.nih.gov/index) initiative to support research that would “lay the foundation for our understanding of how physical activity affects the human body, and ultimately, advance our understanding of how activity improves and preserves health.” A particularly exciting component of the exercise Common Fund initiative, Molecular Transducers of Physical Activity, was the specific mandate to use “genomics, epigenetics, proteomics and metabolomics for the discovery of the entire range of molecular transducers that underlie the effects of physical activity in humans.” Further, the initiative noted, “To ensure that the results from this program will apply to people broadly, participants in the study will range from children to older adults; include a variety of fitness levels, racial, and ethnic groups; and be equally distributed between males and females.” This bold research activity has the potential to create the knowledge foundation needed as we continue to develop a better understanding of the effects of exercise on healthy growth and development.
VIII. INTEGRATIVE OMICS SYSTEMS APPROACH—IMPLICATIONS FOR POLICY THAT AFFECTS HEALTH IN GROWING CHILDREN
Exercise, training and habitual physical activity for healthy growth and development must be better understood in a system context. From a biological perspective, Professor Claude Bouchard recently called for a paradigm shift in this field. From “ focusing on candidate genes typically defined by authors’ preference or from biases in the published scientific literature, and the reliance on small, statistically underpowered, observational studies.” to “unbiased exploration of the genome using all the power of genomics, epigenomics and transcriptomics in combination with large observational but preferably experimental study designs, including Mendelian randomization” (5).
Human beings no longer live in the ecological niche into which they evolved. Survival for our hunting-gathering human ancestors depended on a remarkably efficient integration of neuromotor, cardiovascular, and respiratory systems. Being “fit” meant the capability to quickly escape predation and the ability to expend just enough energy to access food while remaining in net positive energy balance. Modern, more sedentary humans, are facing the ominous fact that absent repeated bouts of exercise (each a sizeable physiological challenge to cellular homeostasis), fitness dissipates and the risk of serious disease is increased. Exercise activates a complex set of mechanisms that can affect panoply of tissues ranging from mitochondria to bone. The molecular transducers of exercise in large measure prepare the organism to better deal with the thermodynamic, oxidative, and inflammatory stress of physical work.
The immediate implications and long term consequences of insufficient physical activity are particularly concerning for child health. Childhood is a critical period of growth and development in which environmentally modifiable mechanisms (likely involving epigenetic reprogramming), can shape health across the lifespan. In the context of child health, the information gained from omics mapping of acute exercise and training responses can transform health policy that promotes optimal exercise in communities, schools, and in individual families, and begin to mitigate the current epidemic of childhood obesity. This approach will also impact an equally challenging and looming health problem—namely, the role of exercise in children with chronic disease and disability. As medicine has advanced in recent years, there is an increasingly large population of child survivors of previously often fatal diseases and conditions (e.g., prematurity, cystic fibrosis, sickle cell anemia, childhood malignancies, and congenital heart disease). These conditions inevitably limit the child’s ability to engage in exercise, and a better biologic definition of “healthy” exercise will pave the way for more precise and effective exercise prescriptions for the child or adolescent with chronic disease and disability.
Acknowledgments
Funding sources
This work was supported in part by National Institutes of Health grants P01HD-048721, the UCI Institute for Clinical and Translational Science (CTSA grant) UL1 TR001414, and the UC Irvine Pediatric Systems Biology Research Fund.
Reference List
- 1.Moving AHEAD with an international human epigenome project. Nature. 2008;454:711–715. doi: 10.1038/454711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad I, Zaldivar F, Iwanaga K, Koeppel R, Grochow D, Nemet D, Waffarn F, Eliakim A, Leu SY, Cooper DM. Inflammatory and growth mediators in growing preterm infants. J Pediatr Endocrinol Metab. 2007;20:387–396. doi: 10.1515/jpem.2007.20.3.387. [DOI] [PubMed] [Google Scholar]
- 3.Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST. Age-associated DNA methylation in pediatric populations. Genome Res. 2012;22:623–632. doi: 10.1101/gr.125187.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong N, Barker AR, McManus AM. Muscle metabolism changes with age and maturation: How do they relate to youth sport performance? British Journal of Sports Medicine. 2015;49:860–864. doi: 10.1136/bjsports-2014-094491. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C. Exercise genomics‐‐ paradigm shift is needed: a commentary. British Journal of Sports Medicine. 2015;49:1492–1496. doi: 10.1136/bjsports-2015-095294. [DOI] [PubMed] [Google Scholar]
- 6.Clarke-Harris R, Wilkin TJ, Hosking J, Pinkney J, Jeffery AN, Metcalf BS, Godfrey KM, Voss LD, Lillycrop KA, Burdge GC. PGC1α Promoter Methylation in Blood at 5–7 Years Predicts Adiposity From 9 to 14 Years (EarlyBird 50) Diabetes. 2014;63:2528–2537. doi: 10.2337/db13-0671. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DM, Leu SY, Galassetti P, Radom-Aizik S. Dynamic Interactions of Gas Exchange, Body Mass, and Progressive Exercise in Children. Med Sci Sports Exerc. 2014;46:877–886. doi: 10.1249/MSS.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daskalaki E, Blackburn G, Kalna G, Zhang T, Anthony N, Watson DG. A Study of the Effects of Exercise on the Urinary Metabolome Using Normalisation to Individual Metabolic Output. Metabolites. 2015;5:119–139. doi: 10.3390/metabo5010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliakim A, Oh Y, Cooper DM. Effect of single wrist exercise on fibroblast growth factor-2, insulin-like growth factor, and growth hormone. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;279:R548–R553. doi: 10.1152/ajpregu.2000.279.2.R548. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, Emerald BS, Gale CR, Inskip HM, Cooper C, Hanson MA. Epigenetic Gene Promoter Methylation at Birth Is Associated With Child’s Later Adiposity. Diabetes. 2011;60:1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto-Inoue N, Yamada K, Inagaki A, Furuichi Y, Ogino S, Manabe Y, Setou M, Fujii NL. Lipidomics analysis revealed the phospholipid compositional changes in muscle by chronic exercise and high-fat diet. Sci Rep. 2013;3:3267. doi: 10.1038/srep03267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iughetti L, Casarosa E, Predieri B, Patianna V, Luisi S. Plasma brain-derived neurotrophic factor concentrations in children and adolescents. Neuropeptides. 2011;45:205–211. doi: 10.1016/j.npep.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Mamrut S, Avidan N, Staun-Ram E, Ginzburg E, Truffault F, Berrih-Aknin S, Miller A. Integrative analysis of methylome and transcriptome in human blood identifies extensive sex- and immune cell-specific differentially methylated regions. Epigenetics. 2015;10:943–957. doi: 10.1080/15592294.2015.1084462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol. 2009;587:5951–5958. doi: 10.1113/jphysiol.2009.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menni C, Kastenmuller G, Petersen AK, Bell JT, Psatha M, Tsai PC, Gieger C, Schulz H, Erte I, John S, Brosnan MJ, Wilson SG, Tsaprouni L, Lim EM, Stuckey B, Deloukas P, Mohney R, Suhre K, Spector TD, Valdes AM. Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol. 2013;42:1111–1119. doi: 10.1093/ije/dyt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris C, Grada CO, Ryan M, Roche HM, De Vito G, Gibney MJ, Gibney ER, Brennan L. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol Nutr Food Res. 2013;57:1246–1254. doi: 10.1002/mnfr.201200629. [DOI] [PubMed] [Google Scholar]
- 18.Nindl BC. Insulin-Like Growth Factor-I, Physical Activity, and Control of Cellular Anabolism. Medicine & Science in Sports & Exercise. 2010;42 doi: 10.1249/MSS.0b013e3181b07c39. [DOI] [PubMed] [Google Scholar]
- 19.Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson E, Yang BT, Lang S, Parikh H, Wessman Y, Weishaupt H, Attema J, Abels M, Wierup N, Almgren P, Jansson PA, Ronn T, Hansson O, Eriksson KF, Groop L, Ling C. Impact of an Exercise Intervention on DNA Methylation in Skeletal Muscle From First-Degree Relatives of Patients With Type 2 Diabetes. Diabetes. 2012;61:3322–3332. doi: 10.2337/db11-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pareja-Galeano H, Brioche T, Sanchis-Gomar F, Montal A, Jovani C, Martinez-Costa C, Gomez-Cabrera MC, Vina J. Impact of exercise training on neuroplasticity-related growth factors in adolescents. J Musculoskelet Neuronal Interact. 2013;13:368–371. [PubMed] [Google Scholar]
- 21.Radom-Aizik S, Zaldivar F, Haddad F, Cooper DM. Impact of brief exercise on peripheral blood NK cell gene and microRNA expression in young adults. Journal of Applied Physiology. 2013;114:628–636. doi: 10.1152/japplphysiol.01341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radom-Aizik S, Zaldivar F, Leu SY, Adams GR, Oliver S, Cooper DM. Effects of Exercise on microRNA Expression in Young Males Peripheral Blood Mononuclear Cells. Clin Transl Sci. 2012;5:32–38. doi: 10.1111/j.1752-8062.2011.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radom-Aizik S, Zaldivar F, Leu SY, Cooper DM. A brief bout of exercise alters gene expression and distinct gene pathways in peripheral blood mononuclear cells of early- and late-pubertal females. Journal of Applied Physiology. 2009;107:168–175. doi: 10.1152/japplphysiol.00121.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radom-Aizik S, Zaldivar F, Leu SY, Cooper DM. Brief Bout of Exercise Alters Gene Expression in Peripheral Blood Mononuclear Cells of Early- and Late-Pubertal Males. Pediatr Res. 2009;65:447–452. doi: 10.1203/PDR.0b013e3181993473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radom-Aizik S, Zaldivar F, Oliver S, Galassetti P, Cooper DM. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. Journal of Applied Physiology. 2010;109:252–261. doi: 10.1152/japplphysiol.01291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radom-Aizik S, Zaldivar J, Haddad F, Cooper DM. Impact of brief exercise on circulating monocyte gene and microRNA expression: Implications for atherosclerotic vascular disease. Brain, Behavior, and Immunity. 2014;39:121–129. doi: 10.1016/j.bbi.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronn T, Volkov P, Davegardh C, Dayeh T, Hall E, Olsson AH, Nilsson E, Tornberg, Dekker Nitert M, Eriksson KF, Jones HA, Groop L, Ling C. A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue. PLoS Genet. 2013;9:e1003572. doi: 10.1371/journal.pgen.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seifarth JE, McGowan CL, Milne KJ. Sex and Life Expectancy. Gender Medicine. 2012;9:390–401. doi: 10.1016/j.genm.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Stevens A, Hanson D, Whatmore A, Destenaves B, Chatelain P, Clayton P. Human growth is associated with distinct patterns of gene expression in evolutionarily conserved networks. BMC Genomics. 2013;14:547. doi: 10.1186/1471-2164-14-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tammen SA, Friso S, Choi SW. Epigenetics: The link between nature and nurture. Molecular Aspects of Medicine. 2013;34:753–764. doi: 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiol. 2015;213:39–59. doi: 10.1111/apha.12414. [DOI] [PubMed] [Google Scholar]


