Abstract
IgMFcR (FcμR) are expressed on B cell and B cell subsets. Mice deficient in secreted IgM and FcμR share properties of impaired specific antibody response and autoimmunity with patient with selective IgM deficiency (SIGMD). Intravenous immunoglobulin (IGIV) regulates immune response, including modulation of IgGFc receptors. However, there are no data on the expression of FcμR in patients with SIGMD, and the effects of IGIV on FcμR. In this study, we investigated FcμR expression in naïve marginal zone (MZ), IgM memory, and class-switched memory B cells in patients with selective IgM deficiency and healthy controls. Furthermore, we examined the direct effect of IGIV on FcμR expression and on the upregulation of FcμR by TLR2 agonist (Pam3). Finally, we examined the effect of IVIG on spontaneously produced IgM and natural IgM anti-phosphorylcholine (PC) antibodies by B cells and B1 cells. FcμR expression is significantly decreased in MZ B cells in patients with SIGMD as compared to control. IGIV, at immunomodulatory concentrations, inhibited FcμR upregulation by Pam3 in MZ B cells, and IgM-depleted IGIV inhibited spontaneous secretion of natural IgM anti-PC antibodies and not total IgM by B1 cells. These data suggest that decreased FcμR expression on MZ B cells may play a role in the pathogenesis of SIGMD, and an inhibition of TLR-2-induced upregulation of FcμR by IGIV may be one of the mechanisms of its anti-inflammatory action. IGIV-induced inhibition of natural IgM antibodies may be one of the mechanisms of IGIV-induced immunoregulation.
Keywords: FcμR, Marginal zone B cells, IGIV, natural antibodies, anti-phosphorylcholine IgM antibodies, TLR
1. INTRODUCTION
Although IgM Fc receptor (FcμR) was described on T cells and B cells more than 35 years ago [1–6], unlike other FcRs, it has defied genetic identification until recently when Kubagawa and colleague [7] identified a cDNA coding a bona fide FcμR that is defined as a 60 kd transmembrane sialoglycoprotein expressed predominantly on B and T cells, and weekly on NK cells.
Recent studies of mice deficient in FcμR have established a role of IgM in immune homeostasis and regulation of antibody response, therefore, in microbial defense, and suppression of autoantibody response via FcμR [8, 9]. These FcμR-deficient mice produce significantly less specific antibody response to protein antigens, and have impaired germinal center formations (B cells), and increased autoantibodies formation as the mice age. The phenotype of FcμR-deficient mice resembles that of mice lacking secreted IgM with regard to specific antibody responses and development of autoantibodies [10–14]. In humans, patients with selective IgM deficiency (SIGMD) are also more susceptible to infections, display impaired specific antibody response to pneumococcal polysaccharide, and develop autoimmune diseases; the latter is more common in adults than in children [15, 16].
Intravenous immunoglobulin (IVIG, current terminology IGIV, which will be used throughout this manuscript) has been used in a variety of autoimmune diseases and has been shown display immunomodulatory properties targeting a variety of cells of the immune system [17–19]. IGIV preparations contain a plethora of natural antibodies of IgG isotype against a variety of self antigens [20, 21]. The immunomodulatory effects of IgG are mediated via both Fab and Fc portion of IgG molecules, the latter via IgG FcR effects on a variety of cells types [17, 22, 23]. However, the effects of IGIV on FcμR, especially its expression in B cell subsets and the production of IgM natural antibodies, have not been reported. Furthermore, the expression of FcμR on B cells in patients with SIGMD has not been studied.
Our present study demonstrates that IGIV influences both the expression of FcμR and natural IgM antibody production by B1 cells. Furthermore, FcμR expression is decreased in marginal zone B cells from patients with SIGMD.
2. MATERIAL AND METHODS
2.1 Subjects
Peripheral blood mononuclear cells (PBMNCs) were isolated from blood of patient with primary SIGMD (serum IgM 4mg/dl to 32mg/dl) and age and gender matched healthy subjects by Ficoll-hypaque density gradient. Human Subject Committee of the Institution Review Board of the University of California, Irvine approved the protocol. Patients with selective IgM deficiency have been followed for at least last 5 years, and have reproducible selective IgM deficiency. All these patients presented with a history of recurrent upper respiratory tract infections. Their clinical and immunological characteristics have been published [15]
2.2 Antibodies and reagents
B cell subsets were identified with following anti-human antibodies: CD19 PE Cy5.5, anti-IgM APC, CD27 FITC, anti-IgD PerCP Cy7, CD21 PerCP Cy7, FCμR PE (clone HM14), mouse IgG1κPE (isotype), CD20 PE and CD43 APC, all from BD Pharmingen (San Jose, California). TLR2 (Pam3CSK4), CpG (ODN 2006) were purchased from InvivoGen (San Diego, California). In Initial experiments, HM14 mAb monoclonal antibody against FcμR [7] provided by Kubagawa was used. Thereafter, commercial antibodies (same clone) were used.
2.3 Depletion of contaminating IgM from IGIV
IGIV preparations are contaminated with IgM in quantities sufficient to interfere with natural IgM antibody assay, therefore, for natural IgM secretion experiments IGIV preparation used in this study (contained IgM12–15 ng/ml) was dialyzed, and contaminating IgM was removed from the IGIV preparation by absorption using magnetic particles coated with goat anti-human IgM antibody (Biomag anti human IgM beads, Bangs Laboratories, Fishers, IN). In brief, Biomag beads were washed to remove the storage buffer and incubated with IGIV solution for 3 hours at room temperature with end over end mixing. After incubation, the tube was placed in a magnetic stand; beads were allowed to migrate to the magnet and collected the IgM depleted solution. These preparations had undetectable levels of IgM and anti-PC antibodies.
2.4 Immunophenotyping
PBMNCs were incubated with various concentrations of IGIV ranging from replacement concentration (1.25 μg/ml) to immunomodulatory concentrations 10 μg/ml or more) in the presence or absence of TLR2 agonist Pam3 and TLR9 agonist CpG for 24 hours and stained for surface markers defining various subsets of B cells as below. Antibody panel for 5-color B cell Phenotype
|
| |||||
| Panel | FITC | PE | PE- Cy5.5 | APC | PerCP- Cy7 |
|
| |||||
| 1 | CD27 | FCμR | CD19 | anti-IgM | IgD |
|
| |||||
Following staining, cells were washed with phosphate buffered saline and analyzed. Flow cytometry was performed using BD LSR Flow (Becton-Dickinson, San Jose, CA) equipped with argon ion laser emitting at 488nm (for FITC, PE, and PerCP excitation) and a spatially separate diode laser emitting at 631 nm (for APC excitation). Forward and side scatters were used to gate and exclude cellular debris. Ten thousand cells were acquired and analyzed using Flowjo software (Treestar, Ashton, OR). Percent positive cells and mean fluorescence intensity (MFI) were determined. MFI data are expressed as Δ MFI after subtracting the isotype background control. B cell subsets were identified by following phenotype: naïve B cells-CD19+/CD27-/IgD+/IgM+, MZ B cells-CD19+/CD27+/IgD+/IgM+, IgM memory-CD19+/CD27+/IgD-/IgM+, Class switch memory-CD19+/CD27+/IgD-/IgM-, and B1 cells-CD20+/CD27+/CD43+/CD70-.
2.4 B Cell enrichment and B1 cell isolation
For experiments of natural IgM production, CD20+ B cells were isolated by negative selection with EasySep B cell enrichment cocktail and magnetic nanoparticles (Stem cell Technologies,Vancouver, BC, Canada). Briefly, unwanted cells were specifically labeled with bispecific tetrameric antibody complexes that recognize unwanted cells and dextran. Dextran-coated magnetic nanoparticles were added, and magnetically labeled cells were then separated from unlabeled target cells using a magnet. For B1 cells separation, cells were stained with CD20 PE and CD43 APC, and sorted for CD20+/CD27+/CD43+ (B1) and CD20+/CD27+/CD43- (B2) B cells by FACSAria II. Purity of B1 cells was >95%. B2 cells were contaminated with <5% B1 cells.
2.5 Detection of total IgM and IgM anti-phosphorylcholine (PC) antibodies
Sorted B1 and B2 cells (2.5 × 104/ml) were incubated with different doses of IgM-depleted IGIV ranging from replacement concentration (1.25 μg/ml) to immunomodulatory concentration (10 μg/ml) for 5 days to detect natural antibodies. Supernatants were collected and stored at −70°C for ELISA. PC-IgM ELISA (Alpha Diagnostic, San Antonio, TX) and IgM ELISA Kits (Genway, San Diego, CA) were used to detect spontaneously secreted anti-PC IgM antibodies and IgM as per manufacturer protocol.
Statistical analyses
Statistical analysis was performed using Graph pad prism (GraphPad Inc., San Diego, CA). Differences between patient and control subject delta MFI and percent positive were tested using paired t test. Differences between un-activated and activated condition were tested using paired t test. A p-value of <0.05 was considered statistically significant.
3. RESULTS
3.1 FcμR expression is decreased on MZ B cells in patients with SIGMD
PBMNCs from ten healthy controls and patients with SIGMD were stained with monoclonal antibodies defining various B cell subsets, and with anti-FcμR antibodies. Ten thousand cells were acquired, and analyzed for both proportions of FcμR positive B cell subsets and for MFI as an indicator of density of FcμR, using multicolor flow cytometry. The MFI of FcμR on MZ B cells from patients with SIGMD was significantly lower (P<0.05) as compared to that from normal control. Fig. 1A shows a representative flow cytometric profile and, Fig. 1B (right panel) shows data from 10 patients and 10 controls. However, no significant difference was observed in the proportions of FcμR+ B cell subsets (Fig. 1B left panel).
Figure 1. FcμR expression in subsets of B cells in selective IgM deficient patients and control subjects by Flow cytometry.
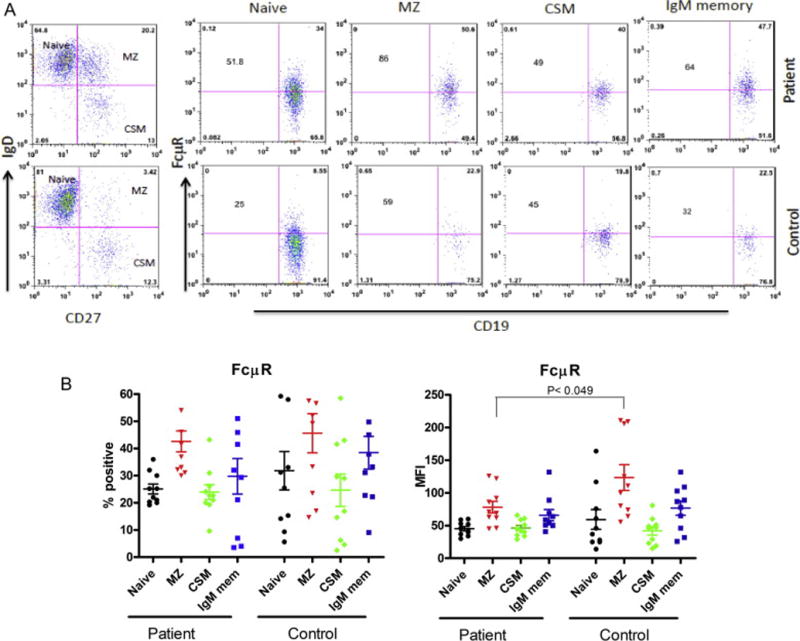
Fig. 1A shows a representative flow cytometric plot. Numbers in the corner of each quadrant indicate percentages and numbers in the center indicate the mean fluorescence intensity (MFI) of FcμR. Fig. 1B shows percentage of FcμR+ B cell subsets (left panel) and right panel shows the MFI of FcμR from 10 patients and 10 healthy matched controls. Data on MFI is expressed as Δ MFI after subtracting isotype background control (mean ± SEM).
3.2 TLR2 and TLR9 stimulation upregulates FcμR expression
We have demonstrated that B cells without co-stimulatory signals can be stimulated directly with TLRs [24]. Therefore, we examined the effect of TLR-stimulation on FcμR expression on B cell subsets. Two TLR agonists were selected, Pam3 for TLR2 and CpG for TLR9, since TLR2 and TLR9 among TLRs provide strong signals to B cells. PBMNCs were stimulated with Pam3 (5 μg/ml) and CpG (10 μg/ml) for 24 hours and FcμR expression was examined on various subsets of B cell subsets using monoclonal antibodies and corresponding isotype controls. Pam3 increased significantly the proportions of FcμR+ naïve B, MZ B, and memory B cells, whereas, CpG increased the proportions of naïve and IgM memory B cells (Fig. 2A). Pam3 upregulated the MFI of FcμR on naïve and MZ B cells but CpG had no effect on the FcμR density on MZ B cells (Fig. 2B). Therefore, Pam3 was used for subsequent experiments.
Figure 2. Effect of TLR2 and TLR9 agonists on the FcμR expression by B cell subsets.
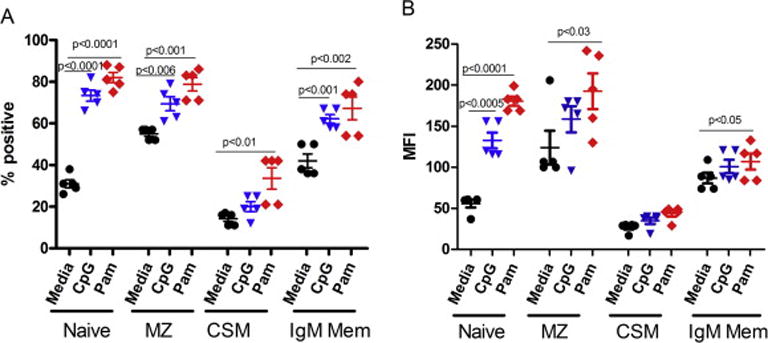
PBMCs from controls were cultured in the absence (media) or presence of CpG (10 μg/ml) and Pam3 (5 μg/ml) for 24 hours. Percentage of FcμR+ B cells (A) and MFI of FcμR (B) were determined in each B cell subset by multicolor flow cytometry (n = 5). Each symbol represents data from an individual. The horizontal wide and narrow bars indicate the arithmetic mean and 1 SE, respectively. Note the significant upregulation of FcμR expression, especially its density, on naive, MZ and IgM memory B cells by TLR2 agonist (Pam3) stimulation.
3.3 IGIV has no direct influence on FcμR in B cell subsets
PBMNCs were incubated with various concentrations of IGIV for 24 hrs. at 370C. The proportions of FcμR+ of B cell subsets and the density of FcμR (MFI) on various B cell subsets were measured with subsets-defining monoclonal antibodies and isotype controls, using multicolor flow cytometry. Pam3 was used as a positive. IGIV did not influence the proportions of FcμR+ B cell subsets (Fig. 3A) or the density (MFI) of FcμR on any of B cell subsets (Fig. 3B).
Figure 3. Direct effect of IGIV on FcμR expression on B cell subsets.
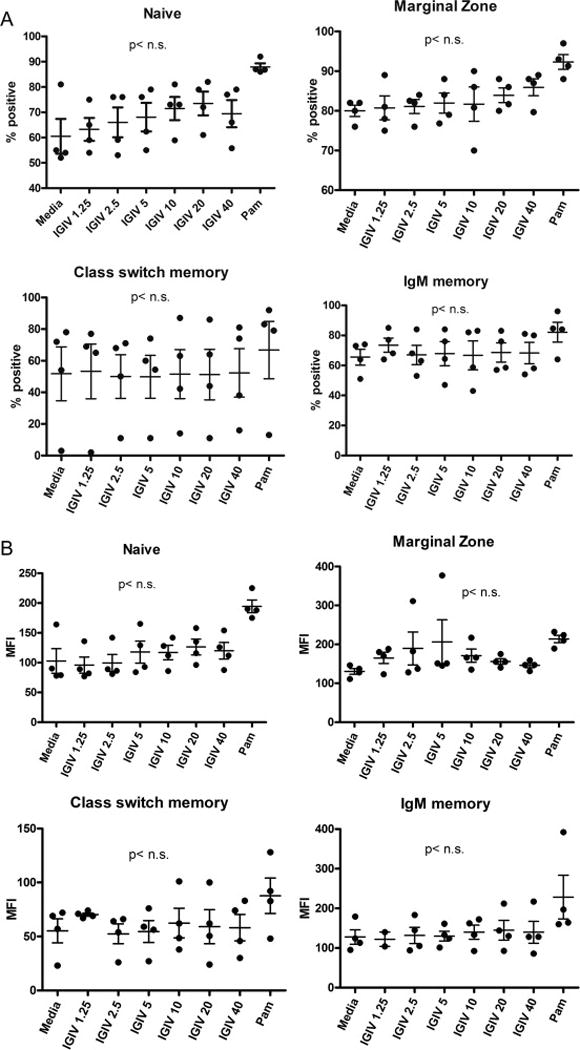
PBMCs from healthy individuals were incubated with different doses of IGIV (1.25 μg to 40μg/ml) and Pam3 for 24 hours. Frequency (%) of FcμR+ B cells (A) and MFI of FcμR (B) in each B cell subset were assessed by multicolor flow cytometry (n = 4). “n.s.” indicates not significant (p>0.05).
3.4 IGIV inhibits TLR2-induced upregulation of FcμR on marginal zone B cells
PBMNCs were pre-incubated with various concentrations of IGIV (1.25 μg-10 μg/ml) for 2 hours, and then Pam3 (5ug/ml) was added. Cells were cultured for 24 hours, washed, stained with monoclonal antibodies and corresponding isotype controls, and analyzed by multicolor flow cytometry. IGIV, in a concentration-dependent manner, inhibited upregulation of density of FcμR on MZ B cells [Fig. 4A]. A trend of inhibition was also observed on naïve B cells; however, differences were not significant. No effect was observed on the proportions of FcμR+ B cell subsets [Fig. 4B].
Figure 4. Effect of IGIV on TRL2-induced upregulation of FcμR expression by B cell subsets.
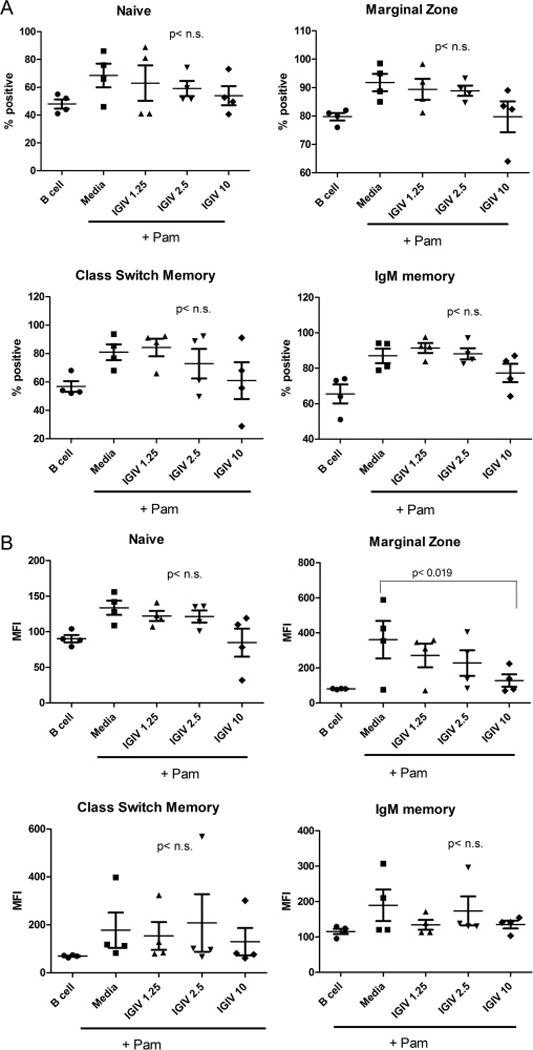
PBMCs from healthy individuals were incubated without (media) or with IGIV (1.25 μg to 10 μg/ml) for 2 hours, and then stimulated with Pam3 (5 μg/ml) for 24 hours. PBMNCs incubated without IGIV and Pam3 served another control. Frequency (%) of FcμR+ B cells (A) and MFI of FcμR (B) in each B cell subset were assessed by Flow cytometry (n = 4). Note that IGIV significantly inhibits the Pam3-induced upregulation of FcμR density by MZ B cells in a dose-dependent manner.
3.5 IGIV inhibits spontaneous secretion of total IgM and natural IgM anti-phosphorylcholine (PC) antibodies by B1 cells
IGIV has been shown to contain natural IgG autoantibodies against various self-antigens [20, 21, 25–27]. Natural antibodies or autoantibodies are spontaneously produced in absence of external stimuli, predominantly of IgM isotype, low affinity, and polyreactive with specificity against self-antigens including PC [28]. An effect of IGIV on natural IgM antibodies has not been evaluated. Therefore, we examined the effect of IgM-depleted IGIV preparation on spontaneously secreted IgM and IgM anti-PC antibodies. Since B1 cells are considered to produce natural antibodies, sorted B1 and B2 cells were incubated with various concentrations of IGIV for 5 days, and supernatants were analyzed for spontaneously secreted IgM and anti-PC IgM antibodies. IgM and anti-PC IgM antibodies were produced predominantly by B1 cells; a small amount of these antibodies produced B2 cell preparations may be due to contaminating B1 cells. Furthermore, IgM-depleted IGIV, in a concentration-dependent manner, inhibited significantly anti-PC IgM antibodies by B1 cells (Fig. 5A), but had no significant effect on total spontaneous IgM produced by B1 cells (Fig. 5B). IgM-depleted IGIV has no detectable anti-PC antibodies (data not shown).
Figure 5. Effect of IGIV on the secretion of spontaneously produced IgM and natural IgM anti-PC antibodies by B1 cells.
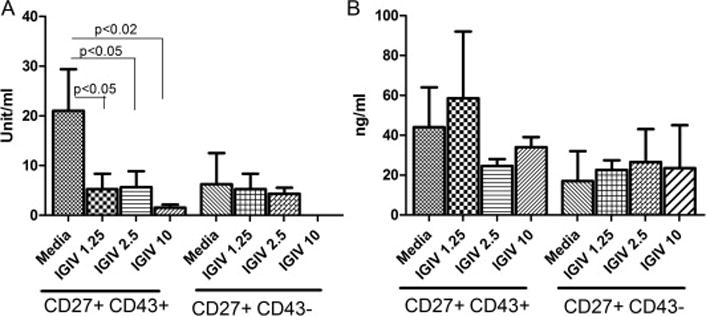
B1 (CD27+/43+) and B2 (CD27+/CD43-) cells sorted from normal PBMNCs (2 × 104/ml) were cultured with the indicated concentrations of IgM-depleted IGIV for 5 days. The resultant supernatants were assayed for IgM anti-PC antibodies (A) and secreted total IgM (B) by ELISA. Results are expressed by mean ± 1 S.E. from five different healthy individuals.
4. DISCUSSION
FcμR was recently defined as a 60 kd transmembrane sialoglycoprotein expressed predominantly on B and T cells, and weekly on NK cells [7]. The interaction of FcμR to its IgM ligand is distinct from that of pIgR and Fcα/μR with IgM and polymeric IgA. Finally FcμR recognizes a molecular configuration on IgM that conferred by Cμ3/Cμ4 domains; in contrast pIgFcR recognizes Cμ4 domain.
Recent studies of mice deficient in FcμR and secreted IgM have established that that IgM plays a role in immune homeostasis, microbial defense, and suppression of autoantibody response via FcμR [8, 9]. These FcμR-deficient mice produce significantly less specific antibody response to protein antigens, and have impaired germinal center formations, and increased autoantibodies formation as the mice age. In humans, patients with SIGMD are also more susceptible to infections, display impaired specific antibody response to pneumococcal polysaccharide, and develop autoimmune diseases in adults [15, 16], and decreased germinal center B cells (manuscript submitted). In this study, we have observed that FcμR are expressed on all subsets of B cells from healthy subjects, which is in agreement with data reported by Kubagawa et al [7]. Furthermore, we observed decreased expression on MZ B cells in patients with primary selective IgM deficiency. The pathogenesis of primary SIGMD remains unclear. A number of mechanisms have been proposed [16]. It is possible that decreased MZ B cells may also play a role in the pathogenesis of SIGMD.
IGIV has also been used as an immunomodulatory agent in a variety of autoimmune diseases [17, 18, 25, 26]. The mechanisms of IVIG-mediated immunoregulation have been extensively studied. Revetch’s group was first to report that immunomodulatory effect of IVIG is due to stimulation of inhibitory FcR for IgG (FcγRIIb) on B cells [20]. Kaveri and colleagues [19, 23] have demonstrated that IVIG induces the generation of Treg, diminishes IL-17, and inhibits the maturation and function of dendritic cells, thereby, regulating autoimmune response. Recently, B cells, such as B1 and MZ B cells, have been demonstrated to play a role in innate immunity. We have reported production of proinflammatory cytokines, chemokines, and growth factors by human B cells in response to direct stimulation of B cells by TLR agonists [24]. A role of IGIV in the regulation of expression of FcμR has not been studied. In this study, we have demonstrated that IGIV inhibits TLR-induced upregulation of FcμR, which may be one of the mechanisms for anti-inflammatory effects of IGIV.
Monomeric IgM is expressed as membrane bound antibody on all naïve B cells and is the first surface immunoglobulin to appear during ontogeny in human [28]. Secreted pentameric IgM generates 10 linked antigen binding sites resulting in higher valency than other immunoglobulins. Secreted IgM comes in two flavors, the natural IgM (innate) and antigen-induced IgM (adaptive or immune). The natural IgM is spontaneously produced by B1 cells in the absence of pathogens (antigen) and is present in germ-free mice and newborn humans, whereas immune IgM is produced by B2 cells following antigen encounter [21, 27, 29–33]. Natural IgM is found in both mice and humans, and constitutes the majority of total circulating IgM. Natural IgM is generated from germline configured transcripts in B cells, prior to class switched recombination (CSR) and somatic hypermutation (SHM), and is of low affinity; however, its high valency allows to bind to antigens with a wide range of avidities, therefore, are polyreactive [34]. Although discovered more than 100 years ago, natural antibodies were thought to recognize only self-antigens, and therefore, important in removal of self-antigens (products of apoptotic cells). However, studies of mutant mice deficient in IgM secretion generated by ablation of the μ heavy chain secretory exon, has established roles of both natural and adaptive IgMs in protection against microbes in infection and self-antigens in autoimmune diseases [12, 14, 35–37].
There has been much controversy regarding characterization of human B1 cells. CD5+ B cells are capable of producing autoantibodies and are expanded in autoimmune diseases (38–40), and therefore, CD5 was considered a marker of B1 cells; however, CD5 is also expressed on B2 cells following activation. Furthermore, both CD5+ and CD5- B cells can produce IgM autoantibodies (41, 42). Therefore, Griffin et al (43), using functional characteristics, identified the phenotype of human B1 cells as CD20+/CD27+/CD43+/CD70-. Therefore, we identified and sorted B1 cell using this phenotype.
Follicular B2 are generally involved in the response to T-dependent antigens, whereas B1 and MZ B cells, sometimes referred to as innate-like B cells based on their similarities, are involved in other functions, including production of natural IgM in the absence of an immune response (spontaneous), and response to T-independent antigens [44–46]. It is interesting to note that our study demonstrates that IGIV affects predominantly MZ B cells and IgM-depleted IGIV inhibits production of natural anti-PC IgM antibodies by B1 cells at high concentrations. IgM-depleted IGIV has no detectable anti-PC antibodies. Whether this effect of IGIV on natural autoantibodies play a role in immune homeostasis remains to be determined. In contrast, Berry et al (47) reported that replacement dose of IGIV (low dose) stimulates B cell proliferation and immunoglobulin, including IgM production in some patients with primary immunodeficiency. These patients have low levels of serum IgM. These investigators did not examine the effect on normal B cells. Furthermore, levels of serum IgM do not change in patients with primary immunodeficiency diseases that are treated with replacement dose of IGIV. Therefore, some of the effects observed by Berry et al (47) may have been contributed by contaminating IgM in IGIV or are in vitro artifact.
In summary, changes in FcμR in MZ B cells in SIGMD may play a role in its pathogenesis. A modulation of FcμR on B cell subsets, and regulation of natural IgM autoantibodies by IGIV may be one of the mechanisms for its anti-inflammatory and immunomodulatory effects.
Acknowledgments
This study was supported in part by an Investigator Initiated Grant BT13-21575 from Baxalta US, Inc.
Footnotes
CONFLICT OF INTEREST
Sudhir Gupta has participated in clinical trials and has served as an ad hoc advisor for Baxalta US, Inc. None of other authors have any conflict of interest.
References
- 1.Moretta L, Ferrarini M, Durante ML, Mingari MC. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975;5:565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Good RA. Subpopulations of human T lymphocytes. I. Studies in immunodeficient patients. Clin Exp Immunol. 1977;30:222–228. [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta S, Good RA. Subpopulations of human T lymphocytes. III. Distribution and quantitation in peripheral blood, cord blood, tonsils, bone marrow, thymus, lymph nodes and spleen. Cell Immunol. 1978;26:263–270. doi: 10.1016/0008-8749(78)90270-8. [DOI] [PubMed] [Google Scholar]
- 4.Parrott DM, Good RA, O’Neill GJ, Gupta S. Heterogeneity of locomotion in human T cell subsets. Proc Natl Acad Sci (USA) 1978;75:2392–2395. doi: 10.1073/pnas.75.5.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrarini M, Hoffman T, Fu SM, Winchester RJ, Kunkel HG. Receptors for IgM on certain human B lymphocytes. J Immunol. 1977;119:1525–1529. [PubMed] [Google Scholar]
- 6.Platsoucas CD, Kampin S, Karanas A, Clarkson BD, Good RA, Gupta S. Receptors for immunoglobulin isotype on T and B lymphocytes from patients with untreated chronic lymphocytic leukemia. Clin Exp Immunol. 1980;40:256–263. [PMC free article] [PubMed] [Google Scholar]
- 7.Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang D-W, et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J Exp Med. 2009;206:2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochida R, Mori H, Hase K, Takatsu H, Kurosaki T, Tokuhisa T, et al. Critical role of the IgMFc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci (USA) 2012;109:E2699–706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honjo K, Kubagawa Y, Jones DM, Dizon B, Dhu Z, Ohno H, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcR) Proc Natl Acad Sci (USA) 2012;109:15882–15887. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. 2010;184:3386–3393. doi: 10.4049/jimmunol.0902640. [DOI] [PubMed] [Google Scholar]
- 11.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 12.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune diseases in absence of secreted IgM. Proc Natl Acad Sci (USA) 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrenstein MR, O’keefe TL, Davies SL, Neuberger MS. Targeted gene disruption reveals a role of natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci (USA) 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenstein MR, Cook HT, Neuberger MS. Deficiency in serum immunoglobulin (Ig) M predisposes to development of IgG autoantibodies. J Exp Med. 2000;91:1253–1258. doi: 10.1084/jem.191.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yel L, Ramanuja S, Gupta S. Clinical and Immunological features in IgM deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]
- 16.Louis AG, Gupta S. Selective IgM deficiency: An ignored primary immunodeficiency. Clin Rev Allergy Immunol. 2014;46:104–111. doi: 10.1007/s12016-013-8375-x. [DOI] [PubMed] [Google Scholar]
- 17.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature Rev Immunol. 2013;13:176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 18.Aranson Y, Schoenfeld Y, Amital H. Intravenous immunoglobulin therapy for autoimmne Diseases. Autoimmunity. 2009;42:533–560. doi: 10.1080/08916930902785363. [DOI] [PubMed] [Google Scholar]
- 19.Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J. Moduation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–615. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Kaveri SV. Intravenous immunoglobulin: Exploring the potential of natural antibodies. Autoimmunity Rev. 2012;11:792–794. doi: 10.1016/j.autrev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 23.Bayry J, Lacroix-Desmazes S, Carbonneil G, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–765. doi: 10.1182/blood-2002-05-1447. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal S, Gupta S. TLR1/2, TLR7, and TLR9 signals directly activate human peripheral blood naive and memory B cell subsets to produce cytokines, chemokines, and hematopoietic growth factors. J Clin Immunol. 2011;31:89–98. doi: 10.1007/s10875-010-9456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gronwall C, Silverman G. Natural IgM: Beneficial autoantibodies for the control of inflammatory and autoimmune diseases? J Clin Immunol. 2014;34:S12–S27. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaveri SV, Lacroix-Desmazes S, Bayry J. The anti-inflammatory IgG. N Eng J Med. 2008;359:307–309. doi: 10.1056/NEJMcibr0803649. [DOI] [PubMed] [Google Scholar]
- 27.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umblical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Pahwa R, O’Reilly, Good RA, Siegal FP. Ontogency of lymphocyte subpopulations in human fetal liver. Proc Natl Acad Sci (USA) 1976;71:919–922. doi: 10.1073/pnas.73.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkon K, Casali P. Nature and function of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira P, Forni L, Larsson EL, Cooper M, Heusser C, Cutinho A. Autonomous activation of B and T cells in antigen-free mice. Eur J Immunol. 1986;16:685–688. doi: 10.1002/eji.1830160616. [DOI] [PubMed] [Google Scholar]
- 31.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 32.Baumgarth N. Double life of a B-1 cell: self reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 33.Boes M. Role of natural and immune IgM in immune responses. Mol Immunol. 2000;37:1141–1149. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 34.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol. 2004;25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nature Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 37.Mannoor K, Xu Y, Chen C. Natural autoantibodies and associated B Cells in immunity and autoimmunity. Autoimmunity. 2012;46:138–47. doi: 10.3109/08916934.2012.748753. [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi O, Miyajima H, Hirano T, Noguchi M, Ueda A, Hashimot H, Hirose S, Okumura K. The Leu-1 B cell pupolation in patients with rheumatoid arthritis. J Clin Immunol. 1987;7:441–448. doi: 10.1007/BF00915053. [DOI] [PubMed] [Google Scholar]
- 39.Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in sjogren’s syndrome. Arthritis Rheum. 1988;31:642–647. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- 40.Burastero SE, Casali P, Wilder RL, Notkins AL. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patient with rheumatoid arthritis. J Exp Med. 1988;168:1979–1992. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casali P, Notkins AL. Probing the human B cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Ann Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 42.Kasaian MT, Ikematsu H, Casali P. Identification and analysis of a novel human surface CD5= B lymphocyte subset producing nature antibodies. J Immunol. 1992;148:2690–2702. [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin DO, Rothstein TL. Human b1 cell frequency: isolation and frequency of human b1 cells. Front Immunol. 2012;3:122. doi: 10.3389/fimmu.2012.00122.eCollection2012. 2012 May 25;3:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerruti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nature Rev Immunol. 2013;13:118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X. Regulatory function of innate-like B cells. Cell Mol Immunol. 2013;10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry J, Fournier EM, Maddur MS, Vani J, Wootla B, Sibéril S, Dimitrov JD, Lacroix-Desmazes S, Berdah M, Crabol Y, Oksenhendler E, Lévy Y, Mouthon L, Sautès-Fridman C, Hermine O, Kaveri SV. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun. 2011;36:9–15. doi: 10.1016/j.jaut.2010.09.006. [DOI] [PubMed] [Google Scholar]


