Abstract
Purpose
Endoxifen concentrations have been associated with breast cancer recurrence in tamoxifen-treated patients. However, tamoxifen itself and other metabolites also show antiestrogenic anti-tumor activity. Therefore, the aim of this study was to develop a comprehensive Antiestrogenic Activity Score (AAS), which accounts for concentration and antiestrogenic activity of tamoxifen and three metabolites. An association between the AAS and recurrence-free survival was investigated and compared to a previously published threshold for endoxifen concentrations of 5.97 ng/mL.
Patients and methods
The antiestrogenic activities of tamoxifen, (Z)-endoxifen, (Z)-4-hydroxytamoxifen, and N-desmethyltamoxifen were determined in a cell proliferation assay. The AAS was determined by calculating the sum of each metabolite concentration multiplied by an IC50 ratio, relative to tamoxifen. The AAS was calculated for 1370 patients with estrogen receptor alpha (ERα)-positive breast cancer. An association between AAS and recurrence was investigated using Cox regression and compared with the 5.97 ng/mL endoxifen threshold using concordance indices.
Results
An AAS threshold of 1798 was associated with recurrence-free survival, hazard ratio (HR) 0.67 (95% confidence interval (CI) 0.47–0.96), bias corrected after bootstrap HR 0.69 (95% CI 0.48–0.99). The concordance indices for AAS and endoxifen did not significantly differ; however, using the AAS threshold instead of endoxifen led to different dose recommendations for 5.2% of the patients.
Conclusions
Endoxifen concentrations can serve as a proxy for the antiestrogenic effect of tamoxifen and metabolites. However, for the aggregate effect of tamoxifen and three metabolites, defined by an integrative algorithm, a trend towards improving treatment is seen and moreover, is significantly associated with breast cancer recurrence.
Keywords: Tamoxifen, Metabolites, Algorithm, Recurrence-free survival
Introduction
Five years of adjuvant treatment with the antiestrogenic drug tamoxifen lowers estrogen receptor alpha (ERα)-positive breast cancer recurrence and mortality rates [1]. Results from the Adjuvant Tamoxifen, Longer Against Shorter (ATLAS) trial, and the Adjuvant Tamoxifen Treatment offers more (aTTom) trial indicate that further decrease of recurrence and mortality rates can be achieved for a subset of patients by prolongation of tamoxifen treatment to up to 10 years [2, 3]. In the postmenopausal setting, aromatase inhibitors lower recurrence if given for 5 years or in sequence for 2–3 years, before or after tamoxifen [4]. In premenopausal woman, aromatase inhibitors alone do not work [5]. The combination of aromatase inhibition and ovarian suppression (either ablation or pharmacological suppression) has shown to improve disease-free survival compared to tamoxifen treatment [6, 7]. However, ovarian suppression can cause substantial side effects and the combination of an aromatase inhibitor and ovarian ablation did not show a difference in overall survival [6]. Therefore, tamoxifen remains an important treatment option. Despite tamoxifen’s effectiveness in reducing recurrence and mortality rates, resistance to tamoxifen often occurs and remains a major clinical challenge [8]. Multiple studies have investigated the variability in response to tamoxifen by focusing on patient-related factors to tailor treatment, such as cytochrome P450 (CYP) genotypes and serum concentrations of metabolites [9–14]. Tamoxifen is bioactivated by CYP enzymes, such as CYP2D6 and CYP3A4/5 (Fig. 1) [15]. CYP2D6 has most extensively been investigated, since it is responsible for bioactivation of tamoxifen’s most important metabolite, endoxifen [15]. Both the CYP2D6 genotype and endoxifen concentrations have been proposed as patient-related factors correlated with breast cancer outcome [13, 14, 16]. However, publications that correlate CYP2D6 genotype with breast cancer outcome have reported conflicting results [16]. Even though a clear association between CYP2D6 genotype and endoxifen concentration is reported, variability in plasma concentration of endoxifen could only partially be attributed to CYP2D6 polymorphisms [13, 14, 17–19]. Therefore, Therapeutic Drug Monitoring (TDM) of endoxifen seems the best way forward to tailor tamoxifen treatment, ensuring the true phenotype of patients [16, 20]. A threshold of 5.97 ng/mL endoxifen has been identified previously and could be applied to tailor tamoxifen treatment, recommending an increase in tamoxifen dose if endoxifen concentrations are below 5.97 ng/mL [13]. The results of that study indicated that patients with an endoxifen concentration above 5.97 ng/mL had 26% lower risk of developing an invasive breast cancer recurrence or new primary breast cancer compared to patients with a lower endoxifen concentration. However, TDM of endoxifen assumes that the antiestrogenic effect of tamoxifen is attributed solely to endoxifen, ignoring the possible contribution of other metabolites and of tamoxifen itself. Tamoxifen and metabolites have varying antiestrogenic activities towards the ERα and occur in different concentrations in patients, each potentially contributing to a different extent to the total antiestrogenic effect. The in vitro inhibitory potential of tamoxifen and many of its metabolites was previously evaluated, in ERα binding competition assays, as well as gene transcription and breast cancer cell growth assays [17, 21, 22]. Endoxifen and 4-hydroxytamoxifen are reported to be the most potent metabolites, with both exhibiting IC50 values in the low nanomolar range, while tamoxifen and N-desmethyltamoxifen are equally less potent with IC50 values in the micromolar range [17, 21, 22]. Previous studies reporting tamoxifen and metabolite concentrations indicate that endoxifen concentrations exceed 4-hydroxytamoxifen concentrations in human serum by approximately 6-fold [23–27]. Tamoxifen and N-desmethyltamoxifen are less potent than endoxifen, but have around 10- and 14-fold higher concentrations, respectively [23–27]. Therefore, it is plausible that the total antiestrogenic effect of tamoxifen depends on a cumulative, intrinsic effect of tamoxifen and active metabolites and their relative concentrations in blood.
Fig. 1.
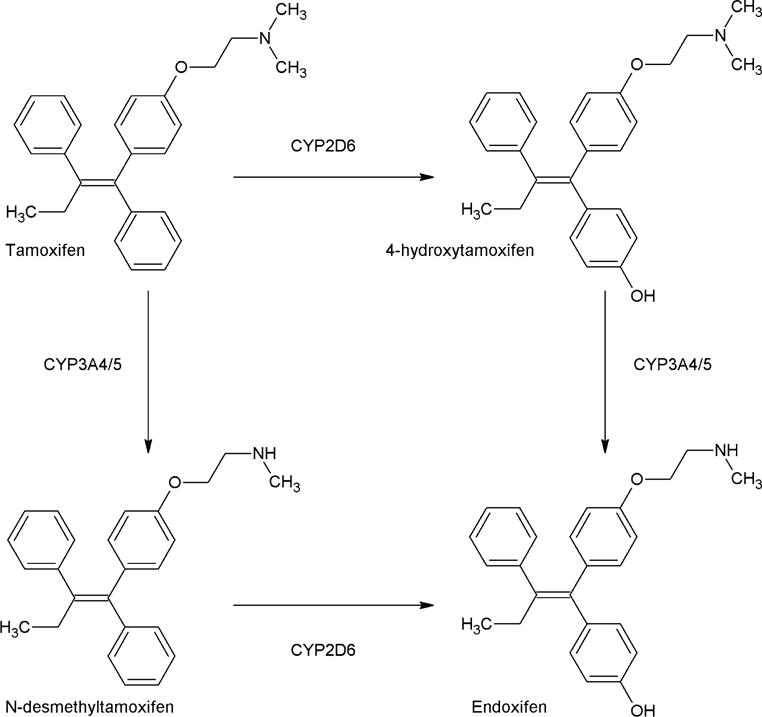
Part of the biotransformation of tamoxifen [15]
To our knowledge, an aggregate effect of tamoxifen together with its active metabolites on breast cancer outcome has never been investigated to date. The aim of this study was, therefore, to investigate if an aggregate Antiestrogenic Activity Score (AAS), which takes into account both concentration and antiestrogenic activity of tamoxifen and multiple active tamoxifen metabolites, is associated with breast cancer outcome. To have a more accurate comparison, the relative activities of tamoxifen, N-desmethyltamoxifen, (Z)-4-hydroxytamoxifen, and (Z)-endoxifen were assessed in vitro, using the same experimental setup. The calculated relative activities were then used to determine the AAS and tested for correlation with outcome.
Methods
Determination of in vitro relative antiestrogenic activity of tamoxifen and three metabolites
The antiestrogenic activities of tamoxifen, N-desmethyltamoxifen, (Z)-4-hydroxytamoxifen, and (Z)-endoxifen were determined using cell proliferation experiments. MCF-7 breast adenocarcinoma cells were regularly maintained in phenol-red free DMEM supplemented with L-glutamine and 10% fetal bovine serum. At 24 h prior to the experiment, cells were plated in clear bottom 384 well plates at a density of 600 cells per well. The cells were allowed to adhere for 24 h before an equal volume of two times the final concentration of the appropriate tamoxifen metabolite was added. Following compound addition, cell proliferation in the individual wells was monitored for 14 days using cell imaging for confluency assessment (IncuCyte®, Essen Bioscience). For each biological replicate, a metabolite serial dilution was carried out in DMSO, leading to a final range of tamoxifen (and metabolite) concentrations between 10−6 M to 10−11 M (10−6 M, 10−7 M, 10−8 M, 10−9 M, 10−10 M, and 10−11 M). For the control wells, an equivalent dilution of DMSO was applied (1:1000). The percentage growth inhibition versus metabolite concentration was plotted, sigmoidal dose–response curves were fitted, and the IC50 values were calculated using SigmaPlot (Systat Software, San Jose, CA). Calculation of the IC50 ratio was done for three independent biological replicates, and the average value was used for the AAS calculation. The AAS calculation was based on the antiestrogenic activity ratios relative to tamoxifen.
Patients
The analysis conducted in this study was based upon the data from 1370 patients with ERα-positive breast cancer who were selected from the Women’s Healthy Eating and Living (WHEL) study [28]. This dataset was previously analyzed by Madlensky et al. [13]. At study entry, the participants had been diagnosed with breast cancer <4 years earlier and had completed primary therapy without recurrence or development of a second primary breast cancer at onset of the study. A blood sample was taken from each patient at study entry. Data included quantifications of tamoxifen, N-desmethyltamoxifen, 4-hydroxytamoxifen and endoxifen serum concentrations, and recurrence-free survival time. To ensure steady-state blood concentrations, the patients included in the analysis had been taking tamoxifen for at least 4 months before the baseline survey. Recurrence-free survival was defined as the time from diagnosis of the original breast cancer to recurrence (including local and distant recurrences, metastatic disease, or new invasive primary breast cancer). The data are more extensively described elsewhere [13].
Calculation of AAS
We incorporated concentrations of tamoxifen and metabolites, corrected for antiestrogenic activity, into an algorithm. IC50 ratios for each metabolite (ICRmetabolite) were calculated by dividing the IC50 value of tamoxifen by the IC50 values of each metabolite using the following equation:
| (1) |
For example, the ICRendoxifen is calculated by dividing the IC50 of tamoxifen by the IC50 of endoxifen. For each patient, the Antiestrogenic Activity Score (AAS) was subsequently calculated as follows:
| (2) |
where [tam], [NDMtam], [4OHtam], and [endox] represent tamoxifen, N-desmethyltamoxifen, (Z)-4-hydroxytamoxifen, and endoxifen concentrations, respectively. Concentrations were reported in ng/mL, however converted to nmol/L (nM) for calculating the AAS, since an addition component is used in the algorithm. ICRNDMtam, ICR4OH-tam, and ICRendox, represent the calculated IC50 ratios, respectively. Tamoxifen and metabolite concentrations were measured in serum for each patient. The AAS was defined as the amount of tamoxifen antiestrogenic activity equivalents in nM, but was further treated as a dimensionless score. Development of this algorithm was based on a previously described comparable algorithm [29].
Statistical analysis
Patients in this analysis were selected based on the criterion that they had been taking tamoxifen for at least 4 months before the baseline survey. Time zero for an individual patient was defined as the date of the first tamoxifen administration. Patients who died or were lost to follow up before completing 4 months of tamoxifen were not included in this analysis. The data were therefore left truncated and were handled as such in Cox regression analysis, to assess the association between the AAS and recurrence-free survival. The AAS was first entered as a continuous variable and then as dichotomous, where (since a martingale residual plot did not show any particular pattern) the threshold was determined by dichotomizing potential optimal cutoff points and chosen such that the partial likelihood was maximal. Additionally, a bootstrap with replacement was performed (n = 1000) to validate the hazard ratio (HR) using the threshold obtained from the original dataset. The concordance index was calculated for both AAS and endoxifen. Data handling and statistical analyses were conducted using R (v.3.0.1) [30].
Results
Antiestrogenic activity of tamoxifen and metabolites
The results from the in vitro experiments are depicted in Table 1 and Fig. 2. To determine the effects of tamoxifen and three of its metabolites ((Z)-4-hydroxytamoxifen, (Z)-endoxifen, and N-desmethyltamoxifen) on general ERα activity without limiting analysis to single reporter genes, proliferation of the ERα-driven breast cancer cell line MCF-7 was used as a readout. As expected, (Z)-4-hydroxytamoxifen and (Z)-endoxifen were most potent at inhibiting MCF-7 cell proliferation. Tamoxifen and N-desmethyltamoxifen were far less potent. The IC50 ratios for each of the tamoxifen metabolites were calculated for each experiment and averaged, resulting in 0.38, 21.8, and 74.4 for N-desmethyltamoxifen, (Z)-4-hydroxytamoxifen, and (Z)-endoxifen, respectively. The IC50 ratio for tamoxifen was 1 by definition. The IC50 ratios were entered into Eq. (2) resulting in the following algorithm for AAS:
| (3) |
Table 1.
Results of in vitro growth experiments, IC50 values, calculated IC50 ratios and average
| Experiment 1
|
Experiment 2
|
Experiment 3
|
Average IC50 ratios | ||||
|---|---|---|---|---|---|---|---|
| IC50 (nM) | IC50 ratio | IC50 (nM) | IC50 ratio | IC50 (nM) | IC50 ratio | ||
| Tamoxifen | 106 | 1 | 88 | 1 | 188 | 1 | 1 |
| N-desmethyltamoxifen | 189 | 0.56 | 573 | 0.15 | 430 | 0.44 | 0.38 |
| (Z)-4-hydroxytamoxifen | 18 | 5.89 | 7 | 12.6 | 4 | 47 | 21.8 |
| (Z)-Endoxifen | 8 | 13.3 | 4 | 22 | 1 | 188 | 74.4 |
IC50 = half maximal inhibitory concentration
Fig. 2.
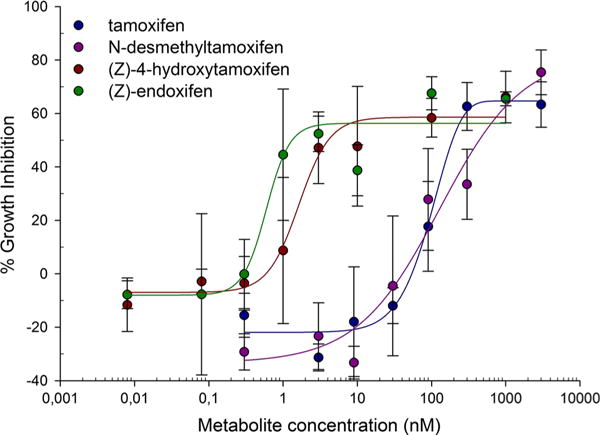
Dose-response curves for tamoxifen, N-desmethyltamoxifen, (Z)-4-hydroxytamoxifen, and (Z)-endoxifen
Implementation of the AAS score and association with outcome
The 1370 patients from the Madlensky analysis were included in the current study [13]. Boxplots for tamoxifen and metabolite concentrations are included in the Supplementary files (S1). Tamoxifen concentrations were around 10-fold higher than endoxifen with median values of 129 ng/mL [interquartile range (IQR): 74.8] and 12.9 ng/mL [IQR: 11.9], respectively. Endoxifen concentrations were 6.7-fold higher than 4-hydroxytamoxifen with median 1.9 ng/mL [IQR: 1.2] for 4-hydroxytamoxifen. N-desmethyltamoxifen concentrations exceeded tamoxifen concentrations by 1.9-fold and endoxifen concentrations by approximately 18-fold, with median 240 ng/mL [IQR: 121] for N-desmethyltamoxifen. These findings were in line with the concentrations reported by previous publications [23–27]. As expected from the metabolic pathway of tamoxifen (Fig. 1), correlations between tamoxifen concentrations and concentrations of its primary metabolites 4-hydroxytamoxifen and N-desmethyltamoxifen were seen, with correlation coefficients of 0.63 and 0.83 respectively. However, a weaker correlation between tamoxifen and endoxifen, a secondary metabolite of tamoxifen, was found (correlation coefficient 0.44). In addition, a stronger correlation between 4-hydroxytamoxifen and endoxifen concentrations was found, with a correlation coefficient of 0.86. A figure showing the correlations between tamoxifen and the three different metabolites is included in the Supplementary files (S1). Figure 3 shows the relative contribution of each compound to the AAS. The endoxifen concentration contributed to the largest extent to the AAS, followed by tamoxifen, N-desmethyltamoxifen, and lastly 4-hydroxytamoxifen.
Fig. 3.
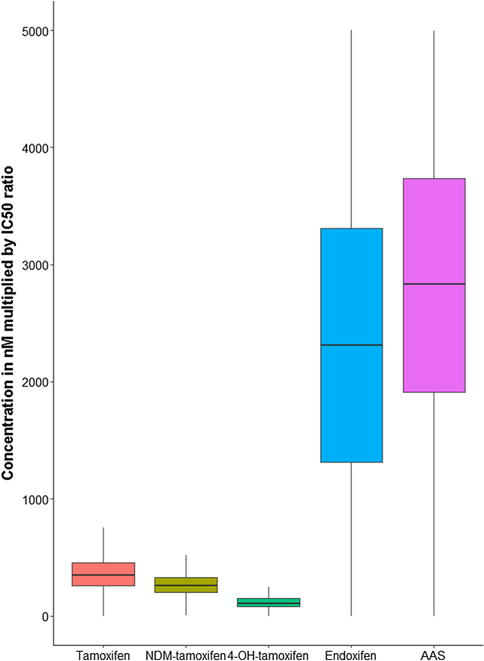
Boxplots for the concentration of each metabolite multiplied by its IC50 ratio representing the relative contribution to the AAS. Values greater than 1.5 times the upper value of the interquartile range are considered as outliers and removed from the plot
Menopausal status and breast cancer stage and grade were significantly associated with recurrence-free survival and were included in the Cox model as covariates. Out of 1370 patients, 178 patients experienced a recurrence, the median follow-up time was 7.3 years. No association between the AAS as a continuous variable and recurrence-free survival was found, HR 1.00 (95% confidence interval (CI) 0.99–1.00). The partial likelihood method identified a relevant threshold of 1798 for the AAS. In the Cox model, patients with an AAS ≥ 1798 had 33% lower risk at developing a secondary breast cancer event, HR of 0.67 (95% CI 0.47–0.96). The Kaplan–Meier curves for patients with AAS ≥ 1798 and AAS < 1798 are depicted in Fig. 4. After bootstrap resampling with replacement this result remained significant, HR 0.69 (95% CI 0.48–0.99). A table containing the type of recurrences per AAS group is added to the Supplementary files (S2).
Fig. 4.
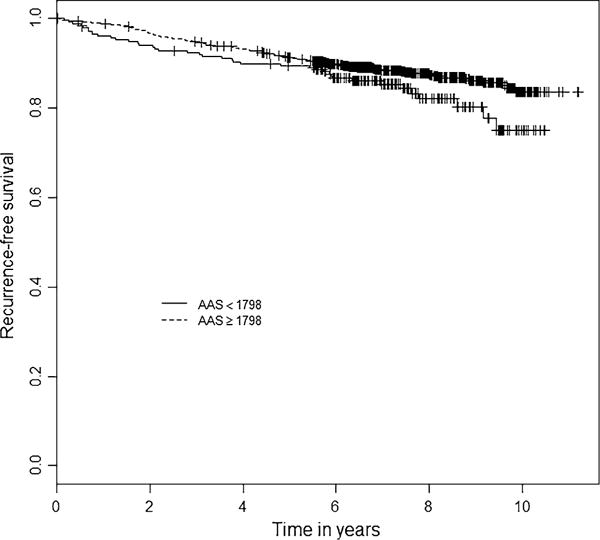
Kaplan–Meier curves for AAS. Dotted line: patients with AAS ≥ 1798, solid line: patients with AAS < 1798; p value = 0.031; AAS Antiestrogenic Activity Score; + = censored
The in vitro cell proliferation experiments showed some variability. Therefore, a sensitivity analysis was conducted. For 4-hydroxytamoxifen, N-desmethyltamoxifen, and endoxifen the IC50 ratios were multiplied by 1, 1.5, and 0.5, while for tamoxifen the IC50 ratio remained 1. Combining the multiplied IC50 ratios gave 26 different integrative algorithms to calculate the AAS, in addition to the algorithm defined in Eq. 3. For all these 26 algorithms the AAS calculation, including threshold finding and HR calculation, was performed. HRs were compared to the above reported HR based on the calculation of AAS with Eq. 3. All 26 HRs were significant, ranging between 0.60 and 0.69 indicating that the findings of this study were robust.
AAS score versus endoxifen
The AAS was compared to the endoxifen threshold in an additional analysis (Table 2). Of the 1370 patients included in the analysis, 1298 patients would be classified as above and below the threshold, either using the AAS or the endoxifen concentration threshold. Of the remaining 72 patients, 48 were identified with an AAS value above 1798, but would have been identified with an endoxifen concentration below 5.97 ng/mL (14.5% experienced recurrence). In addition, the remaining 24 patients were identified with an AAS below 1798, but would have been identified with an endoxifen concentration above 5.97 ng/mL (16.7% experienced recurrence). The concordance indices for AAS and endoxifen concentrations were similar, both with a value rounded to 0.71.
Table 2.
Threshold discriminatory value: comparison between endoxifen threshold and AAS threshold
| Amount of patients (% of 1370)
|
||
|---|---|---|
| Endoxifen ≥ 5.97 ng/mL | Endoxifen <5.97 ng/mL | |
| AAS ≥ 1798 | 1083 (79.1) | 48 (3.5) |
| AAS < 1798 | 24 (1.7) | 215 (15.7) |
AAS Antiestrogenic Activity Score; 5.97 ng/mL
Discussion
In this study, a novel measure for antiestrogenic efficacy for tamoxifen treatment was developed, showing that an integrative algorithm taking into consideration tamoxifen together with three active metabolites is associated with breast cancer outcome. The corrected HR of 0.69 (95% CI 0.49–0.99) implies that patients with an AAS ≥ 1798 are at 31% lower risk of developing a secondary breast cancer event, as compared to patients with an AAS < 1798. The data used for this analysis have been reported previously by Madlensky et al. [13], who identified a threshold for endoxifen concentrations of 5.97 ng/mL, HR = 0.70, (95% CI 0.52–0.94), bias corrected HR = 0.74 (95% CI 0.55–1.00). The corrected HR of 0.74 implies that patients with endoxifen concentrations above 5.97 ng/mL have 26% lower risk at developing a secondary breast cancer event. After bootstrap correction, the HR for the AAS threshold remained significant (this report), whereas the endoxifen threshold did not [13]. However, this difference might be the result of different bootstrap methods. The AAS threshold resulted in a lower HR, but the concordance indices for AAS and endoxifen were both 0.71. This suggests that AAS and endoxifen concentrations alone have similar discriminating ability. However, the cumulative effect of metabolites can theoretically be explained by comparing risk groups, identified by either the AAS or the endoxifen concentration threshold. In the 48 patients with an AAS above the threshold and an endoxifen concentration below the threshold, the low endoxifen concentration is compensated by the antiestrogenic effect of N-desmethyltamoxifen, 4-hydroxytamoxifen, and tamoxifen. In the 24 patients with an AAS below the threshold and an endoxifen concentration above the threshold, the antiestrogenic activity according to the AAS score is insufficient, regardless of an endoxifen concentration above 5.97 ng/mL. This suggests that endoxifen antiestrogenic activity can, to some extent, be mutually compensated by tamoxifen and different metabolites.
An additional finding was the low contribution of 4-hydroxytamoxifen to the AAS. The IC50 ratio for 4-hydroxytamoxifen was almost 22 and 58 times higher than the IC50 ratios for tamoxifen and N-desmethyltamoxifen, respectively. However, the AAS demonstrates that this high antiestrogenic activity is compensated by the low concentrations of 4-hydroxytamoxifen. Therefore, it can be concluded that 4-hydroxytamoxifen is far less important than previously expected.
Interpretation of our results should take into account several limitations. The antiestrogenic activities of tamoxifen and metabolites can be different when investigated in different cell lines, or in the presence of estrogen concentrations [31, 32]. However, the in vitro experiments were conducted to obtain the relative antiestrogenic activities of tamoxifen and three metabolites. Therefore, the ratios implemented in the AAS are not expected to be different in other cell lines or in the presence of estrogen. Second, estrogen concentrations can be associated with breast cancer outcome [7]. Estrogen concentrations were not included in the analysis, since these measurements were not available for a substantial part of the cohort. However, menopausal status was significantly associated with recurrence-free survival and included in the Cox regression. Thirdly, the analysis described is a post hoc analysis of the Madlensky study, based on a subset of patients included in the WHEL study. Thus, this study was not primarily designed to investigate the effect of tamoxifen and metabolite concentrations on breast cancer outcome. However, the data consisted of 1370 patients with ERα-positive breast cancer of whom breast cancer endpoints and metabolite concentrations were available; therefore, the data are suitable for the current analysis. Additionally, the study is limited because patients in the WHEL study were enrolled up to 4 years after diagnosis, therefore, patients who experienced recurrence soon after diagnosis are not taken into account in our analyses. The AAS does not take into account other metabolites that could potentially contribute to the total antiestrogenic effect of tamoxifen. However, the major metabolites of tamoxifen are included, with endoxifen and 4-hydroxytamoxifen as the most potent metabolites and N-desmethyltamoxifen as the most abundant metabolite. Additionally, the in vitro experiments showed variability in IC50 values. This variability was addressed by conducting a sensitivity analysis, which showed robust results. In addition, a selective bioanalytical method is pivotal to quantify tamoxifen and metabolite concentrations and to avoid overestimation of concentrations. Therefore, the absolute value of the AAS could deviate when using bioanalytical assays that lack high selectivity [33]. Lastly, the threshold was chosen such that the partial likelihood of the Cox model was maximal. A different threshold may be found by weighing a desired increase in recurrence-free survival time against the side effects of increasing the dose of tamoxifen.
In summary, this is the first analysis to demonstrate an aggregate effect of tamoxifen and three active metabolites on breast cancer outcome. Clinical decisions regarding dose adjustments based on either the AAS threshold or the endoxifen concentration threshold would be the same for 94.8% of patients. This implies, once again, that endoxifen is the most important metabolite. The results of this analysis demonstrate that endoxifen can serve as a proxy for the antiestrogenic effect of tamoxifen and three metabolites and that the AAS does not provide additional information, since the contribution of endoxifen is major and concordance indices are comparable for endoxifen and the AAS. However, for the AAS a trend towards improving treatment by measuring tamoxifen and three metabolites in comparison to measuring endoxifen is seen. Moreover, a threshold for the tamoxifen metabolite profile is identified at an AAS of 1798 with a corresponding HR of 0.67 (95% CI 0.47–0.96). In future prospective cohort studies, it would be evident to measure tamoxifen and metabolites in addition to endoxifen, in order to further elucidate this effect.
Supplementary Material
Acknowledgments
This work was supported by data from the WHEL study, which was initiated with the support of the Walton Family Foundation and was continued with funding from National Cancer Institute (CA69375). SC Linn received research funding from A Sister’s Hope. L Natarajan was partially supported by funding from the National Cancer Institute R01 (CA166293). BA Parker received (institutional) research funding from Genentech, Novartis, and GlaxoSmithKline.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s10549-016-4083-6) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival : an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray R, Rea D, Handley K. ATTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol. 2013;31(suppl) abstr 5. [Google Scholar]
- 4.Regan MM, Neven P, Giobbie-Hurder A, et al. Assessment of letrozole and tamoxifen alone and in sequence for post-menopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 2011;12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 6.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetz MP, Suman VJ, Hoskin TL, et al. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group Trial (ABCSG) 8. Clin Cancer Res. 2013;19:500–507. doi: 10.1158/1078-0432.CCR-12-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rae JM, Drury S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104:452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan MM, Leyland-Jones B, Bouzyk M, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saladores P, Mürdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15:84–94. doi: 10.1038/tpj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro : prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 16.de Vries Schultink AHM, Zwart W, Linn SC, et al. Effects of pharmacogenetics on the pharmacokinetics and pharmacodynamics of tamoxifen. Clin Pharmacokinet. 2015;54:797–810. doi: 10.1007/s40262-015-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mürdter TE, Schroth W, Bacchus-Gerybadze L, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 18.Teft WA, Gong IY, Dingle B, et al. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res Treat. 2013;139:95–105. doi: 10.1007/s10549-013-2511-4. [DOI] [PubMed] [Google Scholar]
- 19.Wu AHB, Lorizio W, Tchu S, et al. Estimation of tamoxifen metabolite concentrations in the blood of breast cancer patients through CYP2D6 genotype activity score. Breast Cancer Res Treat. 2012;133:677–683. doi: 10.1007/s10549-012-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jager NGL, Linn SC, Schellens JHM, Beijnen JH. Tailored tamoxifen treatment for breast cancer patients: a perspective. Clin Breast Cancer. 2015;15:1–4. doi: 10.1016/j.clbc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 22.Coezy E, Borgna J, Rochefort H. Tamoxifen and metabolites in MCF 7 cells : correlation between binding to estrogen receptor and inhibition of cell growth tamoxifen and metabolites in MCF7 cells : correlation between binding. Cancer Res. 1982;41:317–323. [PubMed] [Google Scholar]
- 23.Johnson MD, Zuo H, Lee K, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 25.Lim H-S, Ju Lee H, Seok Lee K, et al. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007;25:3837–3845. doi: 10.1200/JCO.2007.11.4850. [DOI] [PubMed] [Google Scholar]
- 26.Stearns V, Johnson M, Rae J, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 27.Teunissen SF, Jager NGL, Rosing H, et al. Development and validation of a quantitative assay for the determination of tamoxifen and its five main phase I metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2011;879:1677–1685. doi: 10.1016/j.jchromb.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barginear MF, Jaremko M, Peter I, et al. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: effect on active metabolite isomers and the antiestrogenic activity score. Clin Pharmacol Ther. 2011;90:605–611. doi: 10.1038/clpt.2011.153. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. https://www.R-project.org/ [Google Scholar]
- 31.Maximov PY, McDaniel RE, Fernandes DJ, et al. Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br J Pharmacol. 2014;171:5624–5635. doi: 10.1111/bph.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maximov PY, McDaniel RE, Fernandes DJ, et al. Pharmacological relevance of endoxifen in a laboratory simulation of breast cancer in postmenopausal patients. J Natl Cancer Inst. 2014;106:1–10. doi: 10.1093/jnci/dju283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager NGL, Rosing H, Linn SC, et al. Importance of highly selective LC-MS/MS analysis for the accurate quantification of tamoxifen and its metabolites: focus on endoxifen and 4-hydroxytamoxifen. Breast Cancer Res Treat. 2012;133:793–798. doi: 10.1007/s10549-012-2000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


