Abstract
BACKGROUND
Results from the second CONCORD study (CONCORD-2) indicated that 5-year net survival for lung cancer was low (range, 10%–20%) between 1995 and 2009 in most countries, including the United States, which was at the higher end of this range.
METHODS
Data from CONCORD-2 were used to analyze net survival among patients with lung cancer (aged 15–99 years) who were diagnosed in 37 states covering 80% of the US population. Survival was corrected for background mortality using state-specific and race-specific life tables and age-standardized using International Cancer Survival Standard weights. Net survival was estimated for patients diagnosed between 2001 and 2003 and between 2004 and 2009 at 1, 3, and 5 years after diagnosis by race (all races, black, and white); Surveillance, Epidemiology, and End Results Summary Stage 2000; and US state.
RESULTS
Five-year net survival increased from 16.4% (95% confidence interval, 16.3%–16.5%) for patients diagnosed 2001–2003 to 19.0% (18.8%–19.1%) for those diagnosed 2004–2009, with increases in most states and among both blacks and whites. Between 2004 and 2009, 5-year survival was lower among blacks (14.9%) than among whites (19.4%) and ranged by state from 14.5% to 25.2%.
CONCLUSIONS
Lung cancer survival improved slightly between the periods 2001–2003 and 2004–2009 but was still low, with variation between states, and persistently lower survival among blacks than whites. Efforts to control well established risk factors would be expected to have the greatest impact on reducing the burden of lung cancer, and efforts to ensure that all patients receive timely and appropriate treatment should reduce the differences in survival by race and state.
Keywords: cancer registries, disparities, lung neoplasms, population-based survival, race, stage, survival, trends
INTRODUCTION
In the United States, lung cancer accounts for approximately 14% of all invasive cancers diagnosed each year and for 27% of all cancer-related deaths.1 Lung cancer incidence and mortality rates vary by sex and race.1,2 Among males, lung cancer incidence peaked in 1982, and mortality peaked in 1991.2 Among females, mortality peaked in 2003, and incidence peaked in 2006.2 Lung cancer incidence and mortality rates are currently decreasing slowly, but the rates are higher among black males than among white males and are lower among black females than among white females.1,2 By 2020, the numbers of lung cancer cases and deaths in the United States are projected to increase because of the aging white population and population growth in the black population.3,4
Population-based cancer survival provides an indicator of the overall effectiveness of the health care system to deliver screening, early diagnosis, and evidenced-based treatment services to all individuals in the population being served.5 Survival differences between populations may be attributable to disparities in access to early diagnosis and optimal treatment.6
The second CONCORD study (CONCORD-2) reported survival for patients diagnosed with cancer between 1995 and 2009 in 67 countries, enabling comparison of survival of patients in the United States with other countries.6,7 The CONCORD-2 study is the largest study to date on lung cancer survival, both in the United States and worldwide. Between 1995 and 2009, 5-year net survival for patients diagnosed with lung cancer was low in most countries (range, 10%–20%).6 Survival in the United States was at the higher end of this range.6
In the current study, we conduct a more detailed analysis of US data from the CONCORD-2 study. We describe and discuss trends in net survival among patients diagnosed with lung cancer by race, stage, and state. We also discuss how population-based lung cancer survival might be used to help inform comprehensive cancer control.8
MATERIALS AND METHODS
Data Source
We analyzed the US lung cancer data from the CONCORD-2 study, which included cases reported by 37 state-wide cancer registries funded by CDC’s National Program of Cancer Registies (NPCR) and/or the National Cancer Institute’s Surveillance Epidemiology and End Results Program (SEER), and that covered approximately 80% of the US population and consented to the inclusion of their data in the more detailed analyses reported here.7,8 We analyzed individual tumor records for adults (men and women, aged 15–99 years) who were diagnosed with a primary, invasive cancer of the lung or bronchus (International Classification of Diseases for Oncology, third edition,9 topography codes: C34.0–C34.3 and C34.8–C34.9) between 2001 and 2009 and were followed until December 31, 2009, regardless of whether the patient had had a previous cancer. If a patient was diagnosed with 2 or more cancers of the lung between 2001 and 2009, then we only considered the first cancer in the survival analyses.
We grouped patients by year of diagnosis into 2 calendar periods (2001–2003 and 2004–2009) to reflect changes in the methods used by US registries to collect Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000 (SS2000) at diagnosis. Between 2001 and 2003, most registries coded stage to SS2000 directly from the medical records.10 Between 2004 and 2009, all registries derived SS2000 using the Collaborative Staging System.11
Survival Analyses
We analyzed net survival by race (all races, black, white), by stage (localized, regional, distant, unknown), state, and calendar period of diagnosis. We estimated net survival at 1, 3, and 5 years after diagnosis with 95% confidence intervals (CIs), using the Pohar Perme estimator.7,12 Net survival can be interpreted as the probability of surviving up to a given time since diagnosis, after controlling for other causes of death (background mortality).7 To control for wide differences in background mortality between participating registries and over time, we constructed life tables of all-cause mortality in the general population of each state from the number of deaths and the population, by single year of age, sex, calendar year, and, where possible, by race (black or white), using a flexible Poisson model.13 Methods for constructing life tables have been published.14
We estimated net survival using 2 different methods, since follow time was different in the 2 calendar periods. For patients diagnosed between 2001 and 2003, we used the cohort approach, because all patients had been followed for at least 5 years by December 31, 2009. We used the complete approach to estimate net survival for patients who were diagnosed between 2004 and 2009, because 5 years of follow-up data were not available for all patients. We estimated net survival for 5 age groups (ages 15–44, 45–54, 55–64, 65–74, and 75–99 years). We obtained age-standardized survival estimates using the International Cancer Survival Standard weights.15 If 2 or more of the 5 age-specific estimates could not be obtained, then we present only the pooled, unstandardized survival estimate for all ages combined. We identify unstandardized survival estimates using italics in tables.
We present trends, geographic variations, and differences in age-standardized survival by race in bar charts and funnel plots.16 Funnel plots of net survival for the 2 periods (2001–2003 and 2004–2009) provide insight into the variability of lung cancer survival by race and state. They indicate how much a particular survival estimate deviates from the pooled estimate for all registries combined, given the precision of each estimate.7,16 The pooled estimate for all US registries combined is shown as the “target” (horizontal line) in the funnel plot. More details on data and methods are provided in the accompanying article by Allemani et al.7
RESULTS
We present the overall results for lung cancer in Tables 1–3. State-specific results are reported in Supporting Tables 1–3.
TABLE 1.
Lung Cancer: Number of Patients (Men and Women Aged 15–99 Years) Diagnosed Between 2001 and 2009 and Distribution (%) by Surveillance, Epidemiology, and End Results Summary Stage 2000 at Diagnosis, by Race and Calendar Period of Diagnosis
| 2001–2003 | 2004–2009 | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SS2000a | All Races | White | Black | All Races | White | Black |
| No. of patients | 449,540 | 393,257 | 44,455 | 955,184 | 827,550 | 98,404 |
| Localized, % | 17.5 | 17.8 | 14.8 | 17.7 | 18.1 | 14.9 |
| Regional, % | 24.2 | 24.2 | 25.0 | 23.4 | 23.5 | 23.1 |
| Distant, % | 46.8 | 46.3 | 49.6 | 50.9 | 50.3 | 54.5 |
| Unknown, % | 11.5 | 11.7 | 10.6 | 8.0 | 8.1 | 7.5 |
Information on stage was not available for 2 states (Maryland and Wisconsin) or for Rhode Island for patients diagnosed between 2004 and 2009.
TABLE 3.
Lung Cancer: 5-Year, Age-Standardized Net Survival (NS, %) for Adult Patients (Men and Women Aged 15–99 Years) Diagnosed Between 2001 and 2009, by Surveillance, Epidemiology, and End Results Summary Stage 2000 at Diagnosis, Race, and Calendar Period of Diagnosis
| 2001–2003 | 2004–2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
| ||||||||||||
| SS2000 | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| All stages | 16.4 | 16.3–16.5 | 16.8 | 16.6–16.9 | 13.1 | 12.8–13.5 | 19.0 | 18.8–19.1 | 19.4 | 19.2–19.5 | 14.9 | 14.5–15.2 |
| Localized | 50.2 | 49.8–50.6 | 51.0 | 50.5–51.4 | 41.5 | 40.1–42.9 | 55.1 | 54.7–55.5 | 55.8 | 55.3–56.2 | 45.9 | 44.4–47.4 |
| Regional | 20.2 | 20.0–20.5 | 20.5 | 20.2–20.8 | 17.1 | 16.3–17.9 | 26.4 | 26.0–26.7 | 26.7 | 26.4–27.0 | 22.0 | 21.0–23.0 |
| Distant | 3.6 | 3.5–3.7 | 3.5 | 3.4–3.6 | 3.4 | 3.2–3.7 | 4.8 | 4.7–4.9 | 4.7 | 4.6–4.8 | 4.4 | 4.1–4.7 |
| Unknown | 13.0 | 12.6–13.4 | 13.1 | 12.6–13.5 | 11.2 | 10.2–12.3 | 13.8 | 13.4–14.3 | 14.0 | 13.5–14.5 | 11.0 | 10.0–12.1 |
Abbreviations: CI, confidence interval.
Distribution of Cases by Stage, Race, and Calendar Period
In total, 1,404,724 patients were diagnosed with lung cancer during 2001–2009; of these, 86.9% were white, and 10.2% were black (Table 1). The proportion of patients diagnosed with disease at distant stage increased from 46.8% between 2001 and 2003 to 50.9% between 2004 and 2009. In contrast, during the same periods, the proportions of localized stage (17.5% and 17.7%, respectively) and regional stage (24.2% and 23.4%, respectively) remained relatively stable over time (Table 1). In both calendar periods, the proportion of blacks diagnosed with localized stage disease was lower than that for whites, whereas a higher proportion of blacks was diagnosed at distant stage. Between 2004 and 2009, the proportion of patients diagnosed at each stage ranged between states as follows: localized (range, 13.1%–21.9%), regional (20.3%–26.3%), and distant (45.4%–59.4%) (Supporting Table 1).
One-Year, 3-Year, and 5-Year Net Survival by Race and Calendar Period
Between 2001 and 2003, the pooled estimate of net survival for all patients combined was 42.5% (95% CI, 42.4%–42.7%) at 1 year, 21.6% (21.4%–21.7%) at 3 years, and 16.4% (16.3–16.5) at 5 years. For patients who were diagnosed between 2004 and 2009, net survival had risen to 45.6% (45.5%–45.7%) at 1 year, 24.5% (24.4%–24.6%) at 3 years, and 19.0% (18.8%–19.1%) at 5 years (Table 2). Net survival for whites was similar to the overall US net survival at 1, 3, and 5 years, whereas net survival among blacks was approximately 4% to 5% lower than that among whites at 1, 3, and 5 years. Because the general direction and magnitude of racial disparities were similar at 1, 3, and 5 years, we present only 5-year net survival estimates in the remainder of the results.
TABLE 2.
Lung Cancer: Age-Standardized Net Survival (NS, %) at 1, 3, and 5 Years for Patients (Men and Women Aged 15–99 Years) Diagnosed Between 2001 and 2009 by Race and Calendar Period of Diagnosis
| 2001–2003 | 2004–2009 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| All Races | White | Black | All Races | White | Black | |||||||
|
| ||||||||||||
| Years | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI | NS (%) | 95% CI |
| 1 | 42.5 | 42.4–42.7 | 42.9 | 42.7–43.1 | 39.0 | 38.5–39.5 | 45.6 | 45.5–45.7 | 45.9 | 45.8–46.0 | 42.1 | 41.7–42.4 |
| 3 | 21.6 | 21.4–21.7 | 21.9 | 21.8–22.1 | 17.9 | 17.5–18.2 | 24.5 | 24.4–24.6 | 24.9 | 24.8–25.0 | 20.3 | 19.9–20.6 |
| 5 | 16.4 | 16.3–16.5 | 16.8 | 16.6–16.9 | 13.1 | 12.8–13.5 | 19.0 | 18.8–19.1 | 19.4 | 19.2–19.5 | 14.9 | 14.5–15.2 |
Abbreviations: CI, confidence interval.
Among whites in 37 states between 2004 and 2009, 5-year net survival ranged by state from 15.1% to 25.7% (Supporting Table 2). Among blacks in 36 states between 2004 and 2009, 5-year net survival ranged from 7.0% to 22.7% (data for 5-year net survival among blacks were not available for Montana).
The pooled estimates of 5-year net survival for the US increased from 50.2% between 2001 and 2003 to 55.1% between 2004 and 2009 for localized stage; from 20.2% to 26.4%, for regional stage; and from 3.6% to 4.8%, for distant stage (Table 3). In both calendar periods, the US estimate of 5-year net survival was 9% to 10% lower among blacks than among whites for localized stage and 3% to 5% lower for regional stage. Among 34 states between 2004 and 2009, the range in state-specific, 5-year net survival was 39.4% to 66.4% for patients diagnosed at localized stage, 19.1% to 34.0% for those diagnosed at regional stage, and 2.8% to 10.1% for those diagnosed at distant stage (Supporting Table 3) (5-year survival estimates by stage were not available for Maryland, Wisconsin, or Rhode Island for 2004–2009).
Absolute Change in 5-Year Net Survival Between 2001 to 2003 and 2004 to 2009
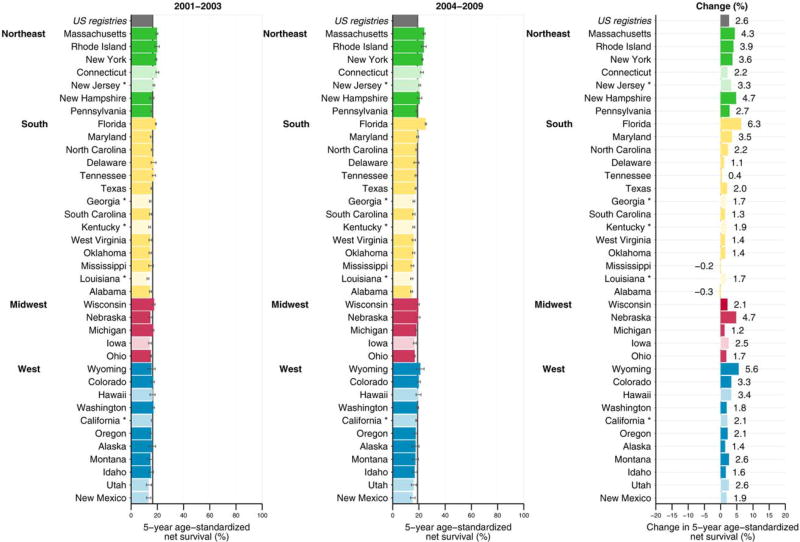
Five-year net survival in most Northeastern states was higher than the US pooled estimate between both 2001–2003 and 2004–2009 (Fig. 1). In contrast, 5-year net survival in many states in the South, Midwest, and West was lower than the US estimate during both time periods. Between the periods 2001–2003 and 2004–2009, the absolute change in 5-year net survival increased 0.4% to 6.3% in 35 states, with a small decrease (range, 0.2%–0.3%) in 2 states. The absolute increase was greater than 2.6% (the increase in the pooled US estimate) in most states in the Northeast. In contrast, the absolute increase was less than 2.6% in many states in the South, Midwest, and West.
Figure 1.
5-year, age-standardized net survival (%) is illustrated for adult patients (men and women aged 15–99 years) diagnosed with lung cancer between 2001 and 2003 and between 2004 and 2009 with the absolute change (%). States are grouped by US Census Region and are ranked within Census Region by the survival estimate for 2004 to 2009. Dark colors indicate states affiliated with the National Program of Cancer Registries; pale colors, states affiliated with the Surveillance, Epidemiology, and End Results program. An asterisk indicates registries affiliated with both federal surveillance programs. Change (%) is not plotted if the survival estimate for 1 or both periods was not age standardized.
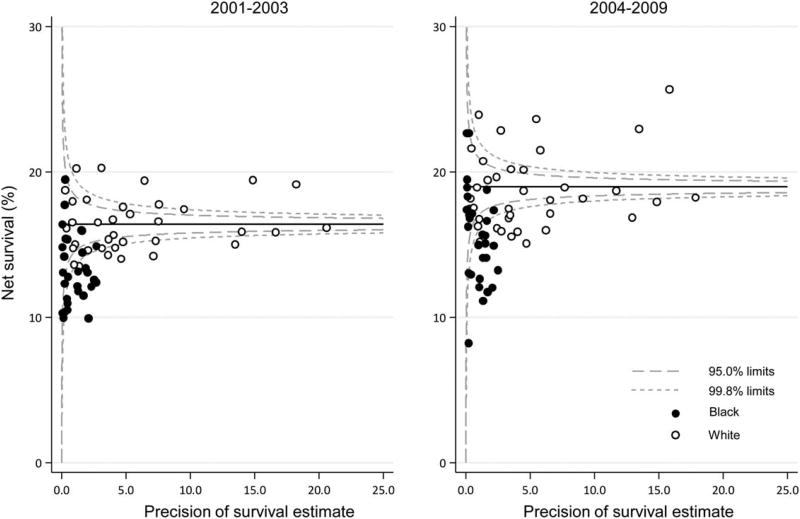
Funnel Plots of 5-Year Net Survival by State
Figure 2 shows geographic and racial variation in 5-year net survival by state. Although net survival for lung cancer was generally low in all states, in both calendar periods, survival for black patients was lower than that for white patients; and, in most states, it was lower than the pooled estimate of US registries (see Fig. 2, horizontal line in the funnel plot).
Figure 2.
Lung cancer 5-year, age-standardized net survival (%) is illustrated for adult patients (men and women aged 15–99 years) by state, race, and calendar period of diagnosis. The pooled US survival for each calendar period is indicated by the horizontal (solid) line.
DISCUSSION
This study provides lung cancer survival estimates by race and stage for 37 states, including 80% of the US population. Between 2004 and 2009, the US lung cancer 5-year net survival was at the high end of the range for many countries in the CONCORD-2 study6 and was consistent with the 5-year relative survival estimates previously reported in the National Program of Cancer Registries and SEER registries.2
Even for a lethal cancer like lung cancer, survival for blacks was lower than for whites (Table 2), and this was true especially for those with localized cancer (Table 3), for which surgery is the main treatment of curative intent. We observed that age-standardized 5-year net survival for lung cancer (19.0%) was 4.2% higher than that for liver cancer (14.8%)18 but lower than for the other cancers addressed in this Supplement, including stomach cancer (29.0%),19 ovarian cancer (41.0%),20 cervical cancer (62.8%),21 rectal cancer (64.0%),22 colon cancer (64.6%),23 acute lymphoblastic leukemia in children (88.1%),24 breast cancer (88.6%),25 and prostate cancer (96.9%).26
Between 2004 and 2009, we observed that 5-year net survival for all stages was 4.5% lower among blacks than among whites. The racial differences were even more marked for lung cancer diagnosed at local stage (9.9% lower among blacks), 4.7% lower among blacks diagnosed at regional stage, but essentially the same for patients diagnosed at distant stage (only 0.3% lower among blacks). Our results are consistent with other reports of racial disparities in lung cancer.2,17,27–29 Reviews suggest that the reasons for the wide racial disparities in lung cancer survival are complex and multifactorial, with contributions from treatment-related factors, such as physician-patient encounters and decision-making, and barriers to access to high-quality care, such as lower patient income or insurance coverage limits.30–32 Unfortunately, although our study highlights 2 key determinants (race and stage) of survival differences between US states, it does not provide definite conclusions about all of the factors that may contribute to differences, because information on these factors is not available for all patients with cancer at a population-based level. It would be interesting to analyze the availability or receipt of optimal treatment by US state and race. This may be possible during the next cycle of CONCORD (CONCORD-3).
In most states, we observed small but consistent increases in 5-year lung cancer net survival between 2001 to 2003 and 2004 to 2009, although the study only covered a single decade. Overall, the increase in 5-year net survival was 2.6% among whites and 1.8% among blacks. We also observed considerable variation in lung cancer survival between US states. Overall, 5-year net survival ranged widely by state from 14.5% to 25.2% between 2004 and 2009, and ranges in survival were more extensive by stage: 39.4%–66.4% for patients diagnosed at localized stage, 19.1%–34.0% for those diagnosed at regional stage, and 2.8%–10.1% for those diagnosed at distant stage. We also observed that the change in survival between 2001–2003 and 2004–2009 ranged from decreased survival in 2 states to an increase of up to 6.3% in the other 35 states. Like the differences by race, our study does not enable definite conclusions about the explanation for the differences we observed over time and by geography.
Clinical Perspective
Between 2001 and 2009, several improvements occurred in clinical care for lung cancer; for example, increased use of video-assisted thoracic surgery,33 intensity-modulated radiation therapy,34 and targeted therapy, also referred to as precision or personalized treatment.35,36 We could not directly assess whether differential access to treatment by race contributed to the racial disparities in survival, because we did not have data on the treatment received by each patient; however, if “equal treatment [for lung cancer] yields equal outcome regardless of race,”37 then that is a plausible conclusion.
Survival can be improved if treatment can be provided when lung cancer is diagnosed at localized stage.35,38 For the cancers discussed in this Supplement, only patients diagnosed at localized stage with liver cancer had lower 5-year survival (25.7%)18 between 2004 and 2009 than patients diagnosed at localized stage with lung cancer (55.1%). In contrast, cancers commonly identified through screening tests (colon, breast, and prostate cancers) had the highest 5-year survival for localized stage between 2004 and 2009 (89.7%,23 98.3%,25 and 99.9%,26 respectively). For the other cancers in this Supplement, only ovarian cancer had a higher proportion of cases (56.8%)20 diagnosed at distant stage between 2004 and 2009 than lung cancer (50.9%). Cancers commonly identified through screening tests (colon, breast, and prostate cancers) between 2004 and 2009 had lower proportions of cases diagnosed at distant stage, at 19.3%,23 5.2%,25 and 3.7%,26 respectively.
Screening with low-dose computed tomography (CT) is now recommended by the US Preventive Services Task Force (USPSTF)39 for individuals at high risk for lung cancer, and is covered by Medicare.40 However, early detection of lung cancer by screening is unlikely to have contributed to the increase in 5-year net survival that we observed for lung cancer, because low-dose CT scans were not broadly available or recommended between 2001 and 2009,41 and the USPSTF recommendations and Medicare regulations were only issued later.
In our study, the overall proportion of cases with unknown stage decreased from 11.5% between 2001 and 2003 to 8.0% between 2004 and 2009. This is an encouraging finding, because accurate lung cancer staging35 is needed to guide therapy selection. Although this decrease is consistent with an increase in accuracy over time in lung cancer staging, the observed changes could be an artifact related to changes in staging methods between the 2 calendar periods. In the alternative, cases with unknown stage could be missing at random when some centers did not provide the data; the overall survival for these cases likely would be similar to the average survival for all cases. Cases with unknown stage also could reflect data from patients who were not completely staged because they were not good candidates for clinical workup and treatment; the overall survival for these cases would be similar to that of patients with more advanced stage disease. Between 2004 and 2009, survival for patients with unknown stage in our study was lower than that for patients with local or regional stage disease but higher than that for those with distant stage disease; this pattern suggests that many of the unknown cases were likely similar to cases with a more advanced stage.
CDC Cancer Prevention and Control Programs
The National Comprehensive Cancer Control Program (NCCCP) supports state, tribal, and territory programs to develop cancer plans that design and implement activities in cancer prevention and control.8,42 State-specific data are critical to inform these cancer-control plans and activities.
Although research is conducted to improve clinical care and to reduce racial disparities, the greatest impact to reduce lung cancer incidence will come from cancer-control efforts directed at primary prevention of established risk factors, such as cigarette smoking,43 the inhalation of secondhand smoke by nonsmokers,43 indoor radon (a leading cause of lung cancer among nonsmokers),44 occupational exposures to carcinogens,45 and air pollution.45
To address lung cancer prevention, incidence and mortality, NCCCP programs develop detailed plans to prevent and control cancer for their communities, and most include objectives for reducing tobacco use and indoor radon exposure. For example, two-thirds of NCCCP programs include funding for tobacco control, such as supporting cessation services and smoke-free policies.46 NCCCP programs work with a national network of partner organizations to reach populations that tend to be heavy smokers.47 Continued and expanded access to tobacco-cessation services could increase abstinence rates and decrease lung cancer incidence further.
Several NCCCP programs support activities related to lung cancer screening, including awareness through health-care provider education, media campaigns, and surveys to better understand the status of lung cancer screening in their communities.48 Continued and expanded incorporation of objectives related to USPSTF lung cancer screening recommendations into NCCCP cancer plans could increase early detection of lung cancer, thereby improving lung cancer survival.39 NCCCP programs could use their experiences from other cancer screening programs to impact lung cancer screening rates at a population level. Furthermore, as lung cancer screening begins to be fully implemented in the United States, NCCCP programs might explore the use of patient navigators to coordinate and improve compliance with follow-up visits and annual repeated lung cancer screening.49
In the future, NCCCP programs also might consider the feasibility of monitoring and evaluating the quality of diagnostic, treatment, and survivorship services for patients with lung cancer. By improving understanding of lung cancer care and whether advances in care are differentially accessed, NCCCP programs may identify effective ways to improve lung cancer survival and reduce disparities in various communities.
NCCCP programs can use lung cancer net survival estimates for their states as an additional data resource to support cancer prevention and control.5,38,50 Combined with data on cancer incidence and death rates, cancer survival measures can provide a more comprehensive picture of the burden of cancer in a population and can support public health efforts to reduce cancer health disparities.5,38,50
Strengths and Limitations
The overview article5 by Weir et al in this Supplement of Cancer describes the strengths and limitations that apply to all of the articles in the Supplement, including this analysis of lung cancer survival.
Conclusions
We observed that lung cancer survival improved slightly from 16.4% between 2001 and 2003 to 19.0% between 2004 and 2009 in the United States overall and in most states. It was low even for individuals diagnosed at localized stage (55.1%) between 2004 and 2009, and it was even lower among blacks (14.9%) than among whites (19.4%). We also observed considerable variation (range, 14.5%–25.2%) in state-specific lung cancer survival between 2004 and 2009. Between 2001 and 2009, lung cancer incidence and mortality in the United States slowly decreased.1,2 Lung cancer mortality trends mirror lung cancer incidence trends because of the high fatality rate and low survival for patients with lung cancer.51 Given the low survival observed in all states, cancer-control efforts directed at primary prevention through control of well established risk factors would be expected to have the greatest impact on reducing the burden of lung cancer in the long term. Efforts directed at improving equality of access to treatment would be expected to reduce the racial differences in survival in the short to medium term.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Funding support for Claudia Allemani: US Centers for Disease Control and Prevention (CDC: 12FED03123, AC012036).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the CDC.
This Supplement edition of Cancer has been sponsored by the U.S. Centers for Disease Control and Prevention (CDC), an Agency of the Department of Health and Human Services.
The CONCORD-2 study was approved by the Ethics and Confidentiality Committee of the UK’s statutory National Information Governance Board (now the Health Research Authority) (ref ECC 3-04(i)/2011) and by the National Health Service Research Ethics Service (Southeast; 11/LO/0331).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Thomas B. Richards: Writing–original draft. S. Jane Henley: Writing–review and editing. Mary C. Puckett: Writing–review and editing. Hannah K. Weir: Writing–review and editing, overall project administration. Bin Huang: Writing–review and editing. Thomas C. Tucker: Writing–review and editing. Claudia Allemani: Conceptualization, data validation, analysis, visualization, writing, and funding acquisition.
References
- 1.US Cancer Statistics (USCS) Working Group. US Cancer Statistics: 1999–2013 Incidence and Mortality Web-Based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2016. [Accessed May 9, 2017]. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2014, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Bethesda, MD: National Cancer Institute; 2017. [Accessed May 9, 2017]. Available at: https://seer.cancer.gov/csr/1975_2014/ [Google Scholar]
- 3.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer. 2015;121:1827–1837. doi: 10.1002/cncr.29258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir HK, Thompson TD, Soman A, Moller B, Leadbetter S, White MC. Meeting the Healthy People 2020 objectives to reduce cancer mortality [serial online] Prev Chronic Dis. 2015;12:E104. doi: 10.5888/pcd12.140482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir HK, Stewart S, Allemani C, et al. Population-based cancer survival (2001–2009) in the United States: findings from the CONCORD-2 study. Cancer. 2017;123:4963–4968. doi: 10.1002/cncr.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CON-CORD-2) [and Supplemental Web Table 4] Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allemani C, Harewood R, Johnson CJ, et al. Population-based cancer survival in the United States: data, quality control, and statistical methods. Cancer. 2017;123:4982–4993. doi: 10.1002/cncr.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White MC, Babcock F, Hayes NS, et al. The history and use of cancer registry data and public health cancer control programs in the United States. Cancer. 2017;123:4969–4976. doi: 10.1002/cncr.30905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz AG, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology (ICD-O) 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 10.Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA. SEER Summary Staging Manual-2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 11.National Cancer Institute; Surveillance, Epidemiology, and End Results Program. Collaborative Stage. Bethesda, MD: National Cancer Institute; 2004. [Accessed May 9, 2017]. Available at: http://seer.cancer.gov/tools/collabstaging/ [Google Scholar]
- 12.Pohar Perme M, Stare J, Esteve J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 13.Rachet B, Maringe C, Woods LM, Ellis L, Spika D, Allemani C. Multivariable flexible modelling for estimating complete, smoothed life tables for sub-national populations [serial online] BMC Public Health. 2015;15:1240. doi: 10.1186/s12889-015-2534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spika D, Bannon F, Bonaventure A, et al. Life tables for global surveillance of cancer survival (the CONCORD programme): data sources and methods. BMC Cancer. 2017;17:159. doi: 10.1186/s12885-017-3117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corazziari I, Quinn MJ, Capocaccia R. Standard cancer patient population for age standardizing survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Quaresma M, Coleman MP, Rachet B. Funnel plots for population-based cancer survival: principles, methods and applications. Stat Med. 2014;33:1070–1080. doi: 10.1002/sim.5953. [DOI] [PubMed] [Google Scholar]
- 17.Henley SJ, Singh SD, King J, Wilson RJ, O’Neil ME, Ryerson AB. Invasive cancer incidence and survival—United States, 2012. MMWR Morb Mortal Wkly Rep. 2015;64:1353–1358. doi: 10.15585/mmwr.mm6449a1. [DOI] [PubMed] [Google Scholar]
- 18.Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5059–5078. doi: 10.1002/cncr.30820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:4994–5013. doi: 10.1002/cncr.30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart SL, Harewood R, Matz M, et al. Disparities in ovarian cancer survival in the United States (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5138–5159. doi: 10.1002/cncr.31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benard V, Watson M, Saraiya M, et al. Cervical cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5119–5137. doi: 10.1002/cncr.30906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph DA, Johnson CJ, White A, Wu M, Coleman MP. Rectal cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5037–5058. doi: 10.1002/cncr.30882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White A, Joseph DA, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5014–5036. doi: 10.1002/cncr.31076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai E, Ward K, Bonaventure A, Siegel D, Coleman MP. Survival among children diagnosed with acute lymphoblastic leukemia in the United States by race and age, 2001 to 2009: findings from the CONCORD-2 study. Cancer. 2017;123:5178–5189. doi: 10.1002/cncr.30899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JW, Lee Smith J, Ryerson AB, et al. Disparities in breast cancer survival in the United States (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5100–5118. doi: 10.1002/cncr.30988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steele CB, Li J, Huang B, Weir HK. Prostate cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123:5160–5177. doi: 10.1002/cncr.31026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 28.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–418. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 29.Nadpara P, Madhavan SS, Tworek C. Guideline-concordant timely lung cancer care and prognosis among elderly patients in the United States: a population-based study. Cancer Epidemiol. 2015;39:1136–1144. doi: 10.1016/j.canep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lathan CS. Lung cancer care: the impact of facilities and area measures. Transl Lung Cancer Res. 2015;4:385–391. doi: 10.3978/j.issn.2218-6751.2015.07.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haider AH, Dankwa-Mullan I, Maragh-Bass AC, et al. Setting a national agenda for surgical disparities research: recommendations from the National Institutes of Health and American College of Surgeons Summit. JAMA Surg. 2016;151:554–563. doi: 10.1001/jamasurg.2016.0014. [DOI] [PubMed] [Google Scholar]
- 32.Lathan CS, Waldman LT, Browning E, Gagne J, Emmons K. Perspectives of African Americans on lung cancer: a qualitative analysis. Oncologist. 2015;20:393–399. doi: 10.1634/theoncologist.2014-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vest MT, Herrin J, Soulos PR, et al. Use of new treatment modalities for non-small cell lung cancer care in the Medicare population. Chest. 2013;143:429–435. doi: 10.1378/chest.12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen AB, Li L, Cronin A, Schrag D. Comparative effectiveness of intensity-modulated versus 3D conformal radiation therapy among Medicare patients with stage III lung cancer. J Thorac Oncol. 2014;9:1788–1795. doi: 10.1097/JTO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network (NCCN) Non-Small Cell Lung Cancer. Version 3. Fort Washington, PA: NCCN; 2017. [Accessed May 9, 2017]. NCCN Clinical Practice Guidelines in Oncology. Available at: http:www.nccn.org. [Google Scholar]
- 36.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. International Agency for Research on Cancer. WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart. 4. Geneva, Switzerland: World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 37.Brawley OW. Lung cancer and race: equal treatment yields equal outcome among equal patients, but there is no equal treatment. J Clin Oncol. 2006;24:332–333. doi: 10.1200/JCO.2005.03.7077. [DOI] [PubMed] [Google Scholar]
- 38.Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. 2014;2014:187–197. doi: 10.1093/jncimonographs/lgu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyer VA US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Medicare and Medicaid Services (CMS) Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N) Baltimore, MD: CMS; 2015. [Accessed May 9, 2017]. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. [Google Scholar]
- 41.The National Academies of Sciences, Engineering, and Medicine. Implementation of Lung Cancer Screening: Proceedings of a Workshop. Washington, DC: The National Academies Press; 2016. [Accessed May 9, 2017]. Available at: http://www.nap.edu/23680. [PubMed] [Google Scholar]
- 42.Major A, Stewart SL. Celebrating 10 years of the National Comprehensive Cancer Control Program, 1998 to 2008 [serial online] Prev Chronic Dis. 2009;6:A133. [PMC free article] [PubMed] [Google Scholar]
- 43.US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 44.Neri A, Stewart SL, Angell W. Radon control activities for lung cancer prevention in national comprehensive cancer control program plans, 2005–2011 [serial online] Prev Chronic Dis. 2013;10:E132. doi: 10.5888/pcd10.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards TB, White MC, Caraballo RS. Lung cancer screening with low-dose computed tomography for primary care providers. Prim Care. 2014;41:307–330. doi: 10.1016/j.pop.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunne K, Henderson S, Stewart SL, et al. An update on tobacco control initiatives in comprehensive cancer control plans [serial online] Prev Chronic Dis. 2013;10:E107. doi: 10.5888/pcd10.120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC) Consortium of National Networks to Impact Populations Experiencing Tobacco-Related and Cancer Health Disparities. Atlanta, GA: Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC; 2016. [Accessed May 9, 2017]. Available at: https://www.cdc.gov/tobacco/about/coop-agreements/national-networks/ [Google Scholar]
- 48.Centers for Disease Control and Prevention (CDC) Lung Cancer Screening Programs. Atlanta, GA: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, CDCl; 2015. [Accessed May 9, 2017]. Policies and Practices for Cancer Prevention. Available at: http://www.cdc.gov/cancer/ncccp/pdf/lungcancerscreeningprograms.pdf. [Google Scholar]
- 49.Hunnibell LS, Slatore CG, Ballard EA. Foundations for lung nodule management for nurse navigators. Clin J Oncol Nurs. 2013;17:525–531. doi: 10.1188/13.CJON.525-531. [DOI] [PubMed] [Google Scholar]
- 50.Ellis L, Woods LM, Estève J, Eloranta S, Coleman MP, Rachet B. Cancer incidence, survival and mortality: explaining the concepts. Int J Cancer. 2014;135:1774–1782. doi: 10.1002/ijc.28990. [DOI] [PubMed] [Google Scholar]
- 51.Ferlay J, Soerjomataram I, Ervik M, et al. International Agency for Research on Cancer, World Health Organization. [Accessed May 9, 2017];GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Default.aspx.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.