Abstract
Mucin-type O-glycans are a class of glycans initiated with N-acetylgalactosamine (GalNAc) α-linked primarily to Ser/Thr residues within glycoproteins and often extended or branched by sugars or saccharides. Most secretory and membrane-bound proteins receive this modification, which is important in regulating many biological processes. Alterations in mucin-type O-glycans have been described across tumor types and include expression of relatively small-sized, truncated O-glycans and altered terminal structures, both of which are associated with patient prognosis. New discoveries in the identity and expression of tumor-associated O-glycans are providing new avenues for tumor detection and treatment. This chapter describes mucin-type O-glycan biosynthesis, altered mucin-type O-glycans in primary tumors, including mechanisms for structural changes and contributions to the tumor phenotype, and clinical approaches to detect and target altered O-glycans for cancer treatment and management.
1. INTRODUCTION
Altered glycosylation is a hallmark of cancer that has helped to shape the management and understanding of cancer. Currently, several glycan-based biomarkers are in use worldwide and glycans have been established as key participants in tumorigenesis and progression. In the 1950s, glycopep-tides isolated from transformed cells were found to be larger in size than those from their nontransformed counterparts (Buck, Glick, & Warren, 1971; Meezan, Wu, Black, & Robbins, 1969; Warren, Buck, & Tuszynski, 1978). Around the same time, some plant lectins were found to exhibit enhanced binding to tumor cells, and in the 1970s and 1980s, researchers discovered that many of the antitumor monoclonal antibodies (mAbs) generated against tumors recognized glycans (Aub, Tieslau, & Lankester, 1963; Feizi, 1985; Ozanne & Sambrook, 1971). These observations indicated that glycans are altered in cancer, setting the stage to investigate when, where, how, and what glycan structures are altered in cancer, which have led to new strategies that have fundamentally altered our view of cancer and approach to attack this deadly disease.
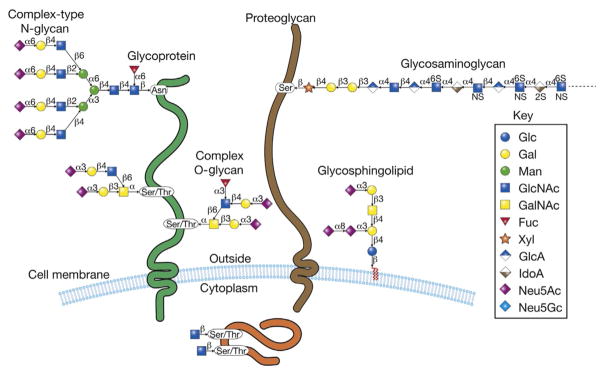
Glycans are present in all living organisms, required for life, and regulate a diversity of biological processes. In mammals, glycans are constructed from a combination of 10 monosaccharides (Gal, Glc, Man, Fuc, Xyl, N-acetylgalactosamine (GalNAc), GlcNAc, GlcA, IdoA, and N-acetylneuraminic or sialic acids (SAs)), which are attached via α or β gly-cosidic bond to form linear and/or branched structures (Fig. 1). Further structural diversity is obtained through modifications of the saccharides, e.g., phosphorylation, sulfation, and acetylation, as well as glycan linkage to various macromolecules. Such complex modifications occur in glycopro-teins, glycolipids, GPI-anchored proteins, and in free glycans as found in milk and secretions.
Figure 1.
Human cells are covered with a dense assortment of glycoproteins, proteogly-cans, and glycolipids, in addition to GPI-anchored glycoproteins (not shown here). The glycoproteins contain Asn-linked oligosaccharides (N-glycans) and Ser-/Thr-linked oligo-saccharides (O-glycans). The proteoglycans contain Ser-linked glycosaminoglycans, comprised of heparan sulfate, chondroitin sulfates, dermatan sulfate, and keratin sulfate. The glycolipids are largely glycosphingolipids, comprised of ceramide to which glucose is the linking sugar. In addition, O-linked GlcNAc is found in cytoplasmic, nuclear, and mitochondrial glycoproteins. The large repertoire of glycans in such glycoconjugates constitutes the glycome of the cell and each cell type expresses its own relatively unique glycome, which is also subject to development and disease-specific changes. The symbols used to represent the monosaccharides are indicated.
Glycoproteins can be broadly divided into two classes, N-glycans and O-glycans, although many types exist and 9 of the 20 amino acids can be modified with sugars. N-glycans are linked via an amide bond to asparagine in the Asn-X-Ser/Thr sequon where X is any amino acid except proline. O-glycans are linked most often to serine or threonine, and in some cases to tyrosine, and can be further subdivided into nuclear/cytoplasmic O-glycans, consisting of O-GlcNAc which functions in conjunction with phosphorylation to regulate signal transductions, and secreted or membrane-bound glycoproteins with O-glycans.
The most common O-glycan in both membrane and secretory proteins is the mucin-type or GalNAc-type O-glycan initiated by GalNAcα1-linked to Ser/Thr of both mucin and nonmucin glycoproteins (Ju, Aryal, Kudelka, Wang, & Cummings, 2014; Ju, Otto, & Cummings, 2011; Ju et al., 2013; Ohtsubo & Marth, 2006; Schjoldager & Clausen, 2012) (Fig. 1). Unlike N-glycans, no conserved glycosite sequon has been identified for O-GalNAc-linked glycans (Hansen et al., 1998; Julenius, Molgaard, Gupta, & Brunak, 2005; Steentoft et al., 2013). Other types of O-glycans include O-glucose, O-fucose, O-mannose, O-galactose, and O-xylose, the latter occurs in proteoglycans. In contrast to nuclear/cytoplasmic O-GlcNAc, which is dynamic, O-glycans in the secretory pathway are stable through the life of the glycoprotein, unless acted upon by glycosidases, such as sialidases (neuraminidases) derived from pathogens during infection. In addition to glycoproteins, glycolipids form a major component of cellular glycoconjugates and in mammals consist primarily of ceramide-linked gly-cans, forming what are called glycosphingolipids or GSLs (Fig. 1), divided into the lacto, globo, and ganglio series.
Mucin-type O-glycans were first observed on mucins but later shown to be ubiquitous. Eichenwald discovered that mucins contain carbohydrates in 1865, and Gottschalk and colleagues discovered that GalNAc links the carbohydrate to the mucin in the 1960s (Carubelli, Bhavanandan, & Gottschalk, 1965; Dahr, Uhlenbruck, & Bird, 1974; Gottschalk & Murphy, 1961; Schauer & Gottschalk, 1968; Tanaka, Bertolini, & Pigman, 1964). Recently, glycoproteomics and prediction algorithms identified mucin-type O-glycans on ~83% of proteins entering the ER–Golgi secretory apparatus, including many nonmucin proteins (Steentoft et al., 2013). O-glycoproteins contain hundreds of O-glycans, as on MUC2, a dozen or so O-glycans, as on the LDL receptor, or a single O-glycan, as on erythropoietin and the transferrin receptor (Cummings et al., 1983; Do & Cummings, 1992; Do, Enns, & Cummings, 1990; Hollingsworth & Swanson, 2004; Larsson, Karlsson, Sjovall, & Hansson, 2009; Sasaki, Bothner, Dell, & Fukuda, 1987).
O-glycans regulate various physiological processes. Blockage of extensions of O-glycans in mice is embryonically lethal, while tissue-specific deletion results in defects in platelets, endothelia, kidneys, GI tract, immune cells, and lipid metabolism, indicating that O-glycans regulate these processes (Alexander et al., 2006; An et al., 2007; Ellies et al., 1998; Fu et al., 2011; Priatel et al., 2000; Tenno et al., 2007; Wang et al., 2012, 2010; Xia et al., 2004; Yeh et al., 2001). Related defects have also been observed in humans, resulting in endocrine, immune, and developmental dysfunction, in addition to cancer. Nonmalignant diseases include familial tumoral calcinosis, dyslipidemia, Wiskott–Aldrich Syndrome, Tn syndrome, and congenital heart disease (Fakhro et al., 2011; Higgins, Siminovitch, Zhuang, Brockhausen, & Dennis, 1991; Ju & Cummings, 2005; Schjoldager et al., 2012; Teslovich et al., 2010; Topaz et al., 2004).
Like glycans in general, O-glycans on glycoproteins use a variety of mechanisms to regulate biological processes. These are broadly categorized into direct and indirect effects (Cummings & Pierce, 2014). Direct effects involve direct interaction of a glycan epitope with a glycan-binding protein (GBP). GBPs include soluble and cell surface proteins from self or microbes or parasites. Many classes of GBPs have been identified including lectins (C-type, P-type, I-type, L-type, R-type, galectins, etc.), GAG-binding proteins, antibodies, and others (Varki & Angata, 2006). Indirect effects of protein glycosylation include effects on protein conformation, stability, recycling, solubility, proteolysis, immune surveillance, etc. A classic example is the LDL receptor, which requires mucin-type O-glycans for protein stability and activity (Kingsley, Kozarsky, Hobbie, & Krieger, 1986; Kingsley & Krieger, 1984; Kozarsky, Kingsley, & Krieger, 1988).
Cancers express altered mucin-type O-glycans, in addition to altered N-glycans and glycolipids as described elsewhere (Bremer, Schlessinger, & Hakomori, 1986; Dall’Olio & Chiricolo, 2001; Dennis & Laferte, 1989; Dennis, Laferte, Waghorne, Breitman, & Kerbel, 1987; Dennis, Waller, Timpl, & Schirrmacher, 1982; Fernandes, Sagman, Auger, Demetrio, & Dennis, 1991; Fuster & Esko, 2005; Ganzinger & Deutsch, 1980; Granovsky et al., 2000; Guo, Lee, Kamar, Akiyama, & Pierce, 2002; Hakomori, 1996; Nagy et al., 2002; Partridge et al., 2004; Santer, Gilbert, & Glick, 1984; Tai, Paulson, Cahan, & Irie, 1983; van Beek, Smets, & Emmelot, 1973; Yamashita, Tachibana, Ohkura, & Kobata, 1985). These tumor O-glycans comprise (1) oncofetal antigens, which are rare in normal adult tissue but expressed embryonically; (2) neoantigens, which are novel structures not appreciably expressed either embryonically or in normal tissues; and (3) altered levels of normal antigens. Normal adult tissues do not express oncofetal or neoantigens, making these ideal for targeted diagnostics and therapeutics; however, all three types of alterations are important in tumor biology and can be useful in clinical management.
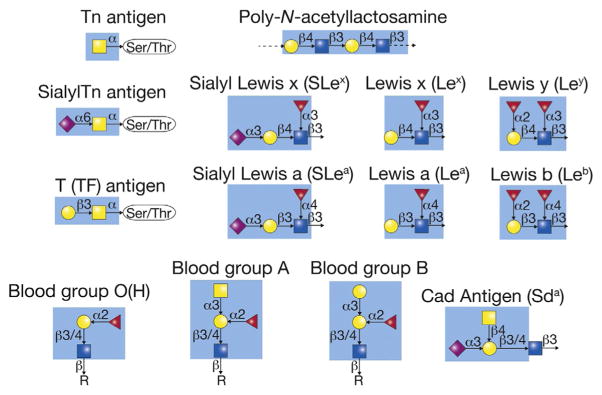
Tumor O-glycans consist of both relatively small and very extended structures, including the truncated glycans Tn, sialyl Tn, and T, as well as the extended glycans ABO(H) and sialylated Lewis antigens on poly-N-acetyllactosamine (Fig. 2). Tumors also express dysregulated post-glycosylational modifications, such as reduced sulfation and SA acetylation. In tumors, truncated O-glycans tend to be tumor-specific, or only found in tumors but not in normal cells, while altered terminal structures tend to be tumor-associated, with distinct changes noted in tumors but the structures themselves present in some normal tissues. Alterations in O-glycan terminal structures are also observed on N-glycans and glycolipids, in contrast to truncated O-glycans found only on O-glycans. Despite these differences, both small and extended tumor O-glycans are present across carcinomas and contribute to the tumor phenotype.
Figure 2.
The structures of many tumor-associated carbohydrate antigens are indicated. The colored (different gray shades) box in each structure represents the known antigenic determinant recognized by antibodies.
O-glycans are altered at the earliest stages of cellular transformation, and genetically engineered mouse models recapitulating some of these alterations suggest that these alterations are important in cancer initiation (An et al., 2007; Wargovich et al., 2004). Tumor O-glycans also correlate with cancer invasion and metastasis and can be engineered into cell lines, resulting in enhanced metastatic potential in xenotransplant studies. Altered O-glycans contribute to metastasis through various mechanisms, ranging from supporting tumor–endothelial interactions to survival in the blood via interaction with platelets and immune evasion (Biancone, Araki, Araki, Vassalli, & Stamenkovic, 1996; Fuster, Brown, Wang, & Esko, 2003; Kim, Borsig, Varki, & Varki, 1998; Takada et al., 1993).
Knowledge of altered O-glycan structures in cancer has led to the development of O-glycan-based biomarkers, including glycan- or glycoprotein-targeted antibodies, such as CA15-3, CA125, CA19-9, and B72.3, as well as autoantibody arrays and glycan-based imaging. Glycan-targeted therapeutics have also been developed or are in development including passive immuno-therapies, carbohydrate-based vaccines, and various strategies to block glycan–GBP interactions, such as sialyl Lewis x (SLex)–selectin interactions (Fuster et al., 2003).
This chapter introduces O-glycan biosynthesis, describes alterations observed in human tumors and possible mechanisms for these alterations, as well as how these alterations may contribute to tumor biology. Genetic and transcriptional alterations in genes contributing to O-glycosylation is also discussed as well as tissue and serum biomarkers, imaging, and glycan-targeted therapeutics. We conclude with our perspectives and where we believe the greatest opportunities are for translating what we know about altered O-glycans in cancer to improve patient care.
2. O-GLYCAN BIOSYNTHESIS
Overview
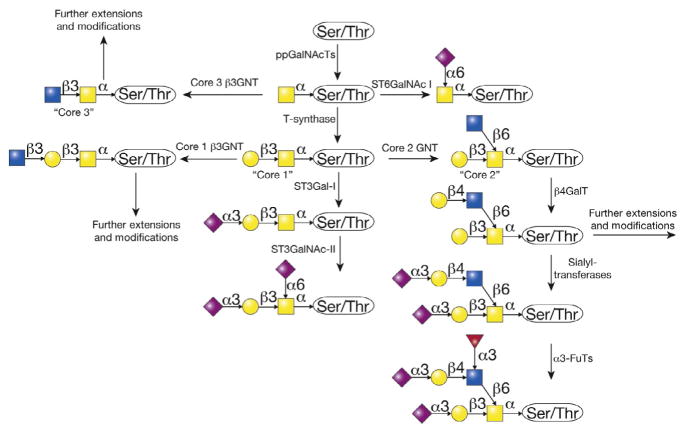
Mucin-type O-glycans consist of branched and linear arrangements of monosaccharides that are transferred by glycosyltransferases to glycoproteins on serine/threonine residues as they traverse the Golgi apparatus (Fig. 3). The synthesis of mucin-type O-glycans is complex and depends on many factors. (1) Expression of glycosyltransferase genes: Glycosyltransferases are first synthesized and undergo transcriptional regulation, which depends on tissue-specific, environmental, and pathologic factors. (2) Localization of glycosyltransferases: After transcription, glycosyltransferases must be translated in the rough endoplasmic reticulum and transported to the appropriate location in the secretory apparatus. The localization and levels of enzyme in the Golgi are regulated by retrograde and anterograde vesicular cycling, posttranslational modifications such as cytoplasmic tail phosphorylation, and also general Golgi regulation. (3) Golgi structure: The structure of Golgi stacks differs between cell types, under physiologic, environmental, and pharmacologic stress, and in different cellular states, such as proliferation or cytokinesis. These changes affect routes of protein export and glycosylation.
Figure 3.
The biosynthesis of O-GalNAc-type O-glycans is initiated and completed in the Golgi apparatus. The ppGalNAcT family of enzymes adds N-acetylgalactosamine from the nucleotide sugar donor UDP-GalNAc to proteins entering the Golgi to form the Tn antigen. The Tn antigen is normally a precursor to a wide variety of other structures, deriving from modifications of the GalNAc residue, to generate core 1, core 2, and core 3 O-glycans. The key reaction is the addition of galactose from UDP-Gal by the enzyme termed T-synthase, which generates the common core 1 O-glycan. The core 1 and/or core 2 O-glycans are found in all human cells. Such glycans are extended by various glycosyltransferases using specific nucleotide sugar donors, e.g., UDP-Gal, UDP-GlcNAc, UDP-GalNAc, GDP-Fuc, CMP-Sialic acid, etc.
Ultimately, glycosylation results in production of glycan structures from the cumulative enzymatic activity of many glycosyltransferases and perhaps host or foreign glycosidases. Glycosyltransferases exhibit varying activities on different glycoprotein or glycopeptide substrates and sometimes occupy distinct or overlapping compartments in the Golgi, enabling competition between glycosyltransferases in glycan synthesis. Availability and levels of sugar donors impact glycosylation, and congenital disorders of glycosylation have been observed due to defects in glycosyltransferases as well as defects in sugar transporters (Freeze & Ng, 2011).
In glycobiology, a nontemplate driven set of glycosyltransferase reactions results in glycosylation microheterogeneity: one glycosite on one type of protein contains various structurally distinct glycans. Microheterogeneity has been observed in various systems, for example, in the production of immunoglobulins for biopharmaceuticals, and is considered a principle of glycobiology. How this happens and what benefit microheterogeneity may confer to the cell is not completely clear. Nonetheless, protein conformation, structure, oligomerization, ratio of glycosyltransferase-to-substrate, and whether a protein is membrane-bound or secreted affect glycosylation and heterogeneity. Although glycosylation is complex and incompletely understood, much is known about how O-glycans are synthesized to produce a variety of structures, some of which are altered in cancer. Here, we outline key pathways, enzymes, and structures involved in mucin-type O-glycan biosynthesis as these are critical to informing our understanding of altered O-glycosylation in cancer.
2.1 Core structures 1–4
Mucin-type O-glycosylation initiates with transfer of GalNAc from UDP-GalNAc to Ser/Thr in a glycoprotein via an α-linkage to form GalNAcα1-Ser/Thr, which is also recognized as the Tn antigen (Ju et al., 2014, 2011, 2013). This reaction is catalyzed by a family of enzymes called polypeptide GalNAc-transferases (ppGalNAcTs), consisting of 20 members in humans (Fig. 3). In contrast, Drosophila has 14 and C. elegans has 9 members (Bennett et al., 2012). ppGalNAcTs are thought to initiate O-glycosylation in the cis-Golgi, although some reports indicate that these enzymes may be variably distributed in the medial and trans-Golgi, in addition to the cis-Golgi (Roth, Wang, Eckhardt, & Hill, 1994; Rottger et al., 1998). ppGalNAcTs are unique among glycosyltransferases in that many contain a lectin domain, facilitating interaction not just with peptide but also with glycans on the peptide. This has led to the idea that there are two classes of ppGalNAcTs: initiator glycosyltransferases and glycopeptide glycosyltransferases (Tabak, 2010). The first group transfers UDP-GalNAc to unglycosylated peptides, while the second group utilizes glycosylated peptides. Notably, some ppGalNAcTs have both activities, so these groups are not mutually exclusive.
Each mammalian cell does not express all ppGalNAcTs, but rather, different tissues have unique expression patterns of particular family members (Young, Holcomb, Ten Hagen, & Tabak, 2003). Similarly, different ppGalNAcTs are thought to modify different, though possibly overlapping, sets of glycoproteins and glycosites, although ppGalNAcTs can compensate to some degree for defects in other transferases (Gerken, Raman, Fritz, & Jamison, 2006; Wandall et al., 1997). These ideas are supported by evidence that deletion of individual ppGalNAcTs in mice result in viable mice with variable and sometimes subtle defects depending on the ppGalNAcT deleted (Orr et al., 2013). Similar findings are observed in humans in which defects in ppGalNAcT11 are associated with congenital heart disease and defects in ppGalNAcT3 are associated with calcium/phosphate dysregulation (Fakhro et al., 2011; Topaz et al., 2004). SNPs, mutations, and altered transcription of different ppGalNAcTs have been implicated in cancer as discussed later.
Synthesis of Tn antigen is normally followed by transfer of Gal, GlcNAc, or GalNAc to the Tn antigen to form core O-glycan structures 1–8 (Fig. 3). Cores 5–8 are rare structures, whereas cores 1–4 are common and are discussed here. Core 1 or the T antigen is Galβ1–3-GalNAcα-Ser/Thr. This structure is synthesized by the T-synthase (Core 1 β3-galactosyltransferase, C1GalT1), which transfers Gal from UDP-Gal to Tn in the cis- and medial-Golgi. T-synthase is ubiquitously expressed in all cells and in mammals requires its unique molecular chaperone Cosmc (core 1 β3-GalT-specific molecular chaperone or C1GalT1C1), which is also ubiquitously expressed (Aryal, Ju, & Cummings, 2010, 2012; Ju, Aryal, Stowell, & Cummings, 2008; Ju, Brewer, D’Souza, Cummings, & Canfield, 2002; Ju & Cummings, 2002). Cosmc is unique in the chaperone field in that it has a single specific client, and is unique in the glycobiology field in that it was the first and only chaperone identified for a glycosyltransferase (Fig. 4). Interestingly, Cosmc shares sequence similarity to the T-synthase, suggesting that it originally arose from a duplication and transposition of the T-synthase in an evolutionary ancestor. C. elegans and Drosophila T-synthase orthologs do not require Cosmc for proper folding, presumably due to the presence of N-glycans that were lost in mammalian T-synthase but may facilitate interaction with calnexin/calreticulin in the ER (Ju, Zheng, & Cummings, 2006). Mammalian Cosmc interacts with unfolded T-synthase, perhaps cotranslationally in the RER via a unique peptide region in T-synthase called the CBRT (Cosmc Binding Region of T-synthase; Aryal, Ju, & Cummings, 2014; Narimatsu et al., 2011). This results in proper folding of the T-synthase and production of an active enzyme, which is transported to the Golgi, preventing nonproductive aggregation, ubiquitination, retrotranslocation to the proteasome, and degradation (Ju, Aryal, et al., 2008). Loss of Cosmc or T-synthase activity results in expression of Tn in all cells described to date and various pathologies such as Tn syndrome (also known as permanent mixed-field polyagglutinability), possibly IgA nephropathy, and cancer as discussed below (Ju, Lanneau, et al., 2008; Ju et al., 2011, 2013; Wang et al., 2010; Xia et al., 2004). Also, deletion of Cosmc or the T-synthase in a mouse results in embryonic lethality and expression of the Tn antigen and also bleeding dysfunction when deleted in platelet and endothelial cells (Wang et al., 2012, 2010; Xia et al., 2004). T antigen is normally sialylated or modified by GlcNAc trans-ferases; however, it can be expressed in various pathologies, such as cancer, as well as on activated B cells during a germinal center reaction.
Figure 4.
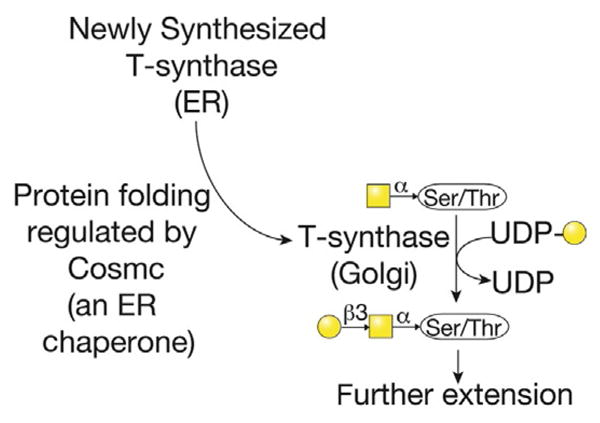
The T-synthase is the enzyme that generates core 1 O-glycan, also termed the T antigen. However, the formation of active T-synthase (the core 1 β3galactosyltransferase or C1GalT1), which is a Golgi enzyme, requires its correct folding in the endoplasmic reticulum by the specific molecular chaperone Cosmc (core 1 β3-GalT-specific molecular chaperone). Cosmc is encoded by a gene on the X-chromosome, and acquired alterations in expression of Cosmc, either by genetic mutation, epigenetic silencing, or by other mechanisms, can lead to expression of the Tn antigen.
Core 1 or the T antigen can be further converted to core 2 by one of three Core 2 GlcNAc Transferases (C2GnT1–3), which transfer GlcNAc from UDP-GlcNAc via a β1–6 linkage to form GlcNAcβ1–6(Galβ1–3) GalNAcα1-Ser/Thr (Bierhuizen & Fukuda, 1992; Schwientek et al., 1999, 2000; Stone et al., 2009; Yeh, Ong, & Fukuda, 1999)(Fig. 3). C2GnT1 and 3 only modify core 1 to form core 2 structures, whereas C2GnT2 can also modify core 3 to form core 4 structure, as described below. Hence, C2GnT2 is also called C2/4GnT. C2GnT1 is ubiquitously expressed, C2GnT2 or C2/4GnT is restricted to GI tract, pancreas, and kidney, and C2GnT3 is restricted to thymus and T cells (Tian & Ten Hagen, 2009). Presumably, distinct tissue distribution and activities, in the case of C2/4GnT, facilitate tissue-specific regulation and coregulation of different core structures, such as core 2 and 4. C2GnTs are related to other β6GnTs, including the I-GnTs involved in formation of the I blood group structure and GnTV involved in β1–6 branching of N-glycans.
Unlike T-synthase, which appears to be constitutively transcribed and expressed, core 2 appears to be more sensitive to cellular state and differentiation. Activation of mature T cells upregulates C2GnT1, resulting in increased core 2-based structures. In contrast, resting mature T cells contain primarily core 1-based structures (Fukuda, 2006). Transcriptional regulation of C2GnTs is complex with multiple transcripts and promoters per enzyme. C2GnT1, for example, uses alternative promoters to produce five different mRNAs (Falkenberg, Alvarez, Roman, & Fregien, 2003; Sekine, Nara, & Suzuki, 1997). In addition to transcriptional regulation, enzymatic competition regulates synthesis of core 2-based structures.
C2GnTs functionally colocalize with ST3Gal-I which transfers N-acetylneuraminic acid (SA) via α2–3 linkage to Gal in core 1 to form sialyl core 1 or sialyl T. Formation of sialyl T by ST3Gal-I inhibits transfer of GlcNAc by C2GnTs. Although only activated T cells normally express core 2, deletion of ST3Gal-I results in elevated expression of core 2 in naïve and activated T cells, suggesting that ST3Gal-I activity normally outcompetes C2GnT for substrate in naïve T cells (Priatel et al., 2000).
Core 2 forms a platform for polyLacNAc (−3Galβ1–4GlcNAcβ1-)n, which functions as a ligand for several galectins and as a substrate to form blood group antigens and various Lewis antigens. In addition to galectins, polyLacNAc-containing glycans interact with other lectins, such as selectins. Hence, regulation of core 2 is critical to the regulation of structures attached to core 2. Core 2 is elevated in immunopathologies, such as Wiskott-Aldrich syndrome and HIV, and expression of C2GnTs is elevated in many cancers and decreased in others, both correlating with progression of disease (Brockhausen, 2006; Higgins et al., 1991; Lefebvre et al., 1994).
Core 1- and core 2-based structures are ubiquitously expressed. In contrast, cores 3 and 4 are primarily expressed in the GI tract. Core 3 structure is synthesized by Core 3 N-acetylglucosaminyltransferase (C3GnT; β3GnT6) by transferring GlcNAc from UDP-GlcNAc to the Tn antigen in β1–3 linkage to form GlcNAcβ1–3GalNAcα1-Ser/Thr, which can then be further modified by the C2/4GnT branching enzyme which transfers an additional GlcNAc via β1–6 linkage to form GlcNAcβ1–3(GlcNAcβ1–6)GalNAcα1-Ser/Thr or core 4. Interestingly, although C3GnT is expressed in stomach>small intestine~colon in humans, core 3 is most often observed in the colon and appears less abundant in the stomach and not present in tissues outside of the GI tract (Iwai et al., 2002). This suggests that either transcript level does not completely correlate with the activity, that C3GnT may compete with other glycosyltransferases, such as T-synthase, for its substrate the Tn antigen, or that C3GnT may have unique acceptor specificities while T-synthase has broad substrates. In support of this idea, core 1 and 2 structures predominate in the stomach. Core 3- and 4-based structures are found on mucins in intestines and may be important in maintaining the mucus barrier and preventing pathological interactions between bacteria and luminal epithelial cells. Accordingly, deletion of C3GnT in mice increases susceptibility to DSS-induced colitis (An et al., 2007). Core 3 may play a role in suppressing tumor development as discussed below.
2.2 Extended O-glycans
Although O-glycan structures are typically smaller in size than N-glycans, core 1–4 structures are often extended to form various structures including polyLacNAc chains, Lewis antigens, and various blood group antigens including well-known ABO blood groups, as well as less well-known Cad (Sda) antigens (Fig. 3). Some of these terminal structures are also found on other glycoconjugates such as N-glycans and glycolipids. In some cases, terminal structures can confer biological activity whether on an O-glycan, N-glycan, or glycolipid, for example, in SLex-mediated sperm–egg interactions; however, in some cases, the class of glycan presenting a terminal structure is biologically important (Pang et al., 2011). For example, P-selectin requires a very specific glycopeptide epitope to engage its glycoprotein partner, PSGL1. This epitope includes SLex on a core 2 residue with nearby sulfated tyrosine (Leppanen et al., 1999; Somers, Tang, Shaw, & Camphausen, 2000). Deletion of O-glycans abrogates this binding (Ellies et al., 1998; Kumar, Camphausen, Sullivan, & Cumming, 1996). O-glycans likely share some glycosyltransferase machinery, such as β4GalT, with other classes of glycoconjugates to extend their O-glycans; however, O-glycan-specific extensions are also observed. In addition to glycosyltransferases, monosaccharide modifications, such as acetylation and sulfation, are critical to synthesize glycan-binding epitopes, whether for endogenous lectins or mAbs generated to recognize glycans or glycoconjugates.
2.3 Extended core 1
Core 1 is most often sialylated by ST3Gal-I and/or ST6GalNAc I–IV to form mono or disialyl core 1 and branched to form core 2. However, other modifications of core 1 are sometimes observed. Core 1 is classically defined as a type 3 chain (Galβ1–3GalNAc-R) and can serve as a platform for blood group antigens, such as H, A, and B antigens, as well as for O-glycan-specific modifications such as the Cad (Sda) antigen, which is also found on extended core 2, 3, and 4 structures (Fig. 3). Furthermore, core 1 can be elongated or extended by Core 1 β3-N-acetylglucoaminyltransferase (Core 1 GnT) by transferring GlcNAc from UDP-GlcNAc to form extended core 1, GlcNAcβ1–3Galβ1–3GalNAcα1-Ser/Thr (Yeh et al., 2001). This can be further modified by other glycosyltransferases to form sulfated SLex structures on extended core 1, which is expressed by activated endothelial for inflammatory leukocyte homing and recognized by mAB MECA-79 (Bruehl, Bertozzi, & Rosen, 2000; Hemmerich, Butcher, & Rosen, 1994; Yeh et al., 2001).
2.4 Extended core 2
Extended core 2 are quite common and mediated by alternating activity of β4GalTs and β3GnTs, which form polyLacNAc chains based on type 2, repeats (3Galβ1–4GlcNAcβ1-)n (Fig. 3). These structures can be expressed as linear chains, also called i antigen, branched by β1–6GnT-I to form branched structures, and/or modified by fucosyltransferases, sialyl transfer-ases, sulfotransferases, etc., to form various blood group antigens as well as Lewis, sialyl Lewis, and sulfo sialyl Lewis structures. PolyLacNAc are also substrates for a class of animal lectins called galectins, which are important in immunity, cell turnover, and growth factor activity (Yang, Rabinovich, & Liu, 2008). In addition to expression on O-glycans, poly-LacNAc are also found on N-glycans as the Galβ4Ts and β3GnTs responsible for synthesizing i antigen can function on O-glycans, N-glycans, and glycolipids (Clausen & Hakomori, 1989; Fukuda et al., 1985; Fukuda, Carlsson, Klock, & Dell, 1986; Inaba et al., 2003; Watanabe, Hakomori, Childs, & Feizi, 1979).
2.5 Extended core 3, 4
Cores 3, 4, and extended structures are less well detailed, in part because core 3 structures are restricted to the GI tract in humans, but by enzyme activity are reduced in GI cancers and generally not observed in cancer cell lines (Iwai et al., 2002; Yang et al., 1994). Further, although core 3-based structures are thought to be a major component of colonic glycans, based on studies of purified or partially purified mucins from the GI tract, core 3 is minimally expressed in the mouse GI tract (Thomsson et al., 2012). Evaluating enzyme activity as a supplement or correlate to structural data is difficult because C3GnT is an extremely unstable enzyme (Vavasseur, Yang, Dole, Paulsen, & Brockhausen, 1995). Nonetheless, a few studies have evaluated mucins from GI tract and observed core 3, core 4, and extended core 3 and 4 structures in human colonic mucins (Podolsky, 1985). Extended core 3 structures are most often observed with one of the most abundant structures being Siaα2–6 core 3 with SA on the GalNAc, extended by β1–3/4Gal and with variable extension of a few type 1 or type 2 chains and presence of fucosylation, sialylation, and sulfation. Additionally, branching off Galβ1–3 core 3 has been observed as well as core 4 structures, core 5 structures (GalNAcα1–3GalNAc), Cad/Sda antigen, blood group determinants, and Lewis structures (Capon, Maes, Michalski, Leffler, & Kim, 2001; Larsson et al., 2009; Podolsky, 1985).
2.6 ABO blood group antigens
Blood group antigens are observed on O-glycoproteins, N-glycoproteins, and glycolipids, both on red blood cells and various other cells of the body. Blood group antigens are synthesized on type 1, 2, 3, or 4 structures. Type 1 and 2 structures are Galβ1–3GlcNAc-R and Galβ1–4GlcNAc-R, respectively (Fig. 3). Both are present on O- and N-glycoproteins as well as on glycolipids. Type 3 and 4 structures are both Galβ1–3GalNAc-R, however the R group for types 3 and 4 differs. R for type 3 is Ser/Thr of an O-glycopeptide, and R for type 4 is a glycolipid moiety. Type 2 structures are ubiquitous, while type 1 structures are found in the GI tract. Types 1 and 2 can both be found in polymers of (Type 1)n and (Type 2)n, with the latter forming polyLacNAc chains, also called i blood group. In addition to forming a linear chain, i blood group can also be branched by various β1–6GnTs to form I blood group. I blood group predominates after embryonic development, increasing through adulthood (Marsh, 1961).
Synthesis of blood group antigens requires at least two steps. The first is synthesis of H antigen, the structure corresponding to O blood type. The second is synthesis of either A or B structure. The H antigen is generated by addition of fucose in α1,2 linkage to a terminal galactose on a type 1–4 chain. Two genetic loci encode the H transferase. The H loci is functional in red blood cells and the secretor loci is functional in GI epithelia, getting its name for the secreted blood group antigens produced from secreted glycoconjugates (Henry, Oriol, & Samuelsson, 1995). These transferases are also important in synthesizing some Lewis antigen as discussed below.
After synthesis of the H structure, the A and B transferases, which differ by four amino acids, utilize the H structure to synthesize A and B structures on type 1–4 chains. The A transferase transfers GalNAc from UDP-GalNAc via α3 linkage to the terminal Gal of the H structure, while the B transferase transfers Gal from UDP-Gal via α3 linkage also to the terminal Gal of the H structure. Individuals carrying mutated A/B transferases encode neither functional A or B transferase, making them O blood group; only one functional A or B transferase, making them AA/AO or BB/BO; or both functional transferases, making them AB+. More rarely, individuals can be H-, Se-, or H-/Se-, making them unable to synthesize AB/H or other blood group structures such as Lewis antigens.
Susceptibility or protection from various diseases, such as certain infections, has been associated with the presence of different blood group antigens. Pathogens contain GBPs that may recognize cells of an individual with one blood type but not another. Alternatively, individuals with a given blood type cannot mount an adaptive immune response to pathogens expressing the same blood group or blood group-like structures. Galectins appear to be able to fill this immunologic gap by recognizing and killing ABO-expressing bacteria (Stowell et al., 2010). In addition to infections, AB/H structures and changes in these structures are observed in cancers and contribute to the tumor phenotype, as discussed later.
2.7 Lewis antigens
Lewis antigens are synthesized primarily by endodermal epithelia, such as GI epithelia, but are found in endodermal epithelia and RBCs due to transfer of glycolipids to RBCs (Henry et al., 1995). Lewis structures are found on type 1 and 2 chains of O-glycans, N-glycans, and glycolipids. Type 1 chains contain Lewisa/b, while type 2 chains contain Lewisx/y (Fig. 3). The Lewis locus encodes the fucosyltransferases responsible for synthesizing the Lewis antigens (Kukowska-Latallo, Larsen, Nair, & Lowe, 1990). These transferases exhibit similar expression to the secretor loci.
The Lewis transferase is an α3/4FucT which transfers fucose from GDP-Fuc to GlcNAc in a type 1 or 2 chain. An α3 linkage is formed when transferred to a type 2 chain, and an α4 linkage is formed when attached to a type 1 chain due to prior occupancy of the Gal on the 4 or 3 position of the GlcNAc, respectively. Addition of the fucose forms the Lea (type 1) or Lex (type 2) structure. Transfer of fucose to the terminal galactose in α1–2 to form the H antigen prior to action of the α3/4FucT is responsible for forming the Leb (type 1) and Ley (type 2) antigens. Formation of the H antigen uses the same α1–2FucT responsible for synthesizing the H precursor to A and B blood groups (Stanley & Cummings, 2009). In summary, Lewis antigens are synthesized by addition of α3/4fucose to an unsubstituted type 1 or 2 chain to form Lea/x antigens or to an H type 1 or 2 chain to form Leb/y antigens.
Lewis antigens can also be sialylated and/or sulfated to form sialyl and sulfo Lewis antigens. Sialylation most often occurs at the 3 position of the terminal galactose of the type 1 or 2 chain to form SLea/x. Sulfation can also occur at the 3 position of the terminal galactose, denoted 3′ (′ indicates terminal galactose, whereas no ′ indicates modifications of the subterminal GlcNAc), the 6 position of the terminal galactose, denoted 6′, or the 6 position of the subterminal GlcNAc, denoted 6. Sialylation and sulfation on the terminal galactose are compatible, resulting in the possibility of structures such as 6,6′-bis-sulfo-Sialyl Lex (Stanley & Cummings, 2009). Sulfo, sialyl, and sulfo sialyl Lewis antigens are important in physiological processes such as inflammation, in particular, because of their role in leukocyte rolling and as selectin ligands. These antigens also play an important role in cancer, which additionally express dimeric Lewis antigens such as sialyl-dimeric Lewis x (Matsushita, Cleary, Ota, Hoff, & Irimura, 1990). Regulation of these structures is complex and involves coordinated synthesis and activity of multiple enzymes and careful regulation at both the genetic/transcriptional level as well as in the secretory apparatus.
2.8 Sialic acids
SAs are an important component of O-glycans as well as of N-glycans and glycolipids. Over 50 different SAs have been observed. Neu5Ac is the most common in humans, while Neu5Gc is common in lower mammals but normally absent in humans due to a mutation in the synthase. Interestingly, Neu5Gc is observed in pathologic conditions in humans, such as cancer, presumably due to dietary uptake (Hedlund, Padler-Karavani, Varki, & Varki, 2008; Tangvoranuntakul et al., 2003). SA can be acetylated, methylated, etc., and contributes to glycan-binding epitopes, such as sialyl Lewis antigens.
Approximately, 20 sialyltransferases mediate transfer of CMP-SA to glycoconjugates in mammals. These transfer SAs in α2–3 and α2–6 linkage to Gal, α2–6 linkage to GalNAc, and α2–8 to other SAs as observed in poly-sialic acid on N-glycans in N-CAM and on O-glycans in neuropilin-2. Four families of sialyltransferases catalyze these reactions including ST3Gal-I–VI, ST6Gal-I,II, ST6GalNAc-I–VI, and STSia-I–VI (Harduin-Lepers et al., 2001). Within a sialyltransferase family, enzymes are further divided based on properties of the acceptor, in particular the glycan structure, e.g., type I (Galβ1–3GlcNAc) or type II chains (Galβ1–4GlcNAc), and the class of glycoconjugate, e.g., O-glycans, N-glycans, and glycolipids.
Many sialyltransferases transfer SAs to O-glycans. These include ST3Gal-I,III–V; ST6Gal-II; and ST6GalNAc-I–IV. ST3Gal-I makes Siaα2–3 core 1, while ST3Gal-III–V forms Siaα2–3Galβ1–3/4GlcNAc-(i.e., on type 1 or type 2 chains), which is found on extended core 2 chains. ST6Gal-I–II makes Siaα2–6Galβ1–4GlcNAc- (i.e., on type 2 chains), which is also found on extended core 2 chains. ST6GalNAc-I–IV all modify GalNAc of core 1 to form sialyl or disialyl T but differ in substrate preference based on whether core 1 is unsubstituted or whether it is monosialylated by ST3Gal-I. ST6GalNAc-I modifies unsubstituted acceptors and is the only ST6GalNAc that synthesizes STn, ST6GalNAc-II modifies unsubstituted or monosialylated acceptors, and ST6GalNAc-III–IV modify mono-sialylated acceptors. ST6GalNAc-IV also modifies glycolipids.
2.9 Monosaccharide modifications
Monosaccharides in glycoconjugates can be sulfated, phosphorylated, acyl/deacylated, epimerized, and methylated by postglycosylational modifications (Muthana, Campbell, & Gildersleeve, 2012; Yu & Chen, 2007). All but methylation are observed in humans. These modifications increase the structural and functional diversity of glycans and are altered in disease. Sulfation, as discussed previously, generates sulfo Lewis and sulfo sialyl Lewis antigens, as well as GAGs. Acylation/deacylation utilizes common substituents such as acetyl or less common substituents, such as ferrulate and lactyl, through the action of human enzymes and sometimes microbial/parasitic enzymes. In colon cancer, a loss of SA O-acetylation is observed. Normally, ~50% of colonic mucin SAs are O-acetylated. Glycan phosphorylation is best characterized for the lysosomal sorting signal Man-6-P on N-glycans and O-Mannose glycans on α-dystroglycan (α-DG) as well as some microbial/parasitic organisms but probably modulate other glycan biology. Epimerization alters the stereochemistry of monosaccharides and converts glucuronic acid to iduronic acid in the synthesis of GAGs. Post-glycosylational modifications are not well studied and often difficult to assay, but observations to date indicate they are diverse and important in multiple classes of glycans in humans and other animals and organisms.
3. ALTERED O-GLYCAN STRUCTURES OBSERVED IN CANCER
Overview
Alterations in O-glycan structures were arguably first observed in the 1940s and 1950s with expression of immature blood group structures in gastric carcinoma (Oh-Uti, 1949). Later, purification and characterization of specificities of various lectins as well as generation of mAbs led to the identification of truncated and shortened O-glycans, such as Tn, STn, and T antigens, as well as identification and confirmation of altered terminal O-glycan structures, such as Lewis blood group and AB/H structures (Lee et al., 1991; Magnani et al., 1982; Miyake, Taki, Hitomi, & Hakomori, 1992; Nuti et al., 1982; Prokop & Uhlenbruck, 1969; Takahashi, Metoki, & Hakomori, 1988). Recent studies investigating glycan-binding specificities of many of these reagents through glycan microarrays have allowed improved interpretation of these early studies. Further evidence for altered O-glycans in cancer derived from immunologic studies evaluating autoantibody signatures and cellular immunity through glycopeptide arrays and delayed type hypersensitivity reactions (DTHR), while advances in physical methods, such as mass spectrometry, gas chromatography, and NMR, have revealed structural features of these altered O-glycans. The initial discovery of altered O-glycans in cancer led researchers to investigate the clinical applicability of these discoveries—including sensitivity and specificities, tissue localization, clinical stage of expression, and whether these structures correlate with survival/progression and/or contribute to the tumor phenotype. This section focuses on structural alterations observed in primary human tumors, including histology and mechanistic insights into structural alterations as well as potential contributions to the tumor phenotype.
3.1 Methods to identify altered O-glycosylation in cancer
There are three general approaches to identify alterations in glycosylation, including O-glycosylation, in human tumors. The first method uses affinity probes, the next method uses physical methods, such as mass spectrometry, and the third method involves indirect immunologic approaches, evaluating immunologic responses to altered glycosylation. There are advantages and limitations to all of these approaches.
Antibodies against carbohydrates have been generated through a variety of approaches. With the advent of mAbs, many researchers began immunizing mice against tumor and tumor cell extracts and screening against tumor cells and/or histologic specimens. Although the initial goal was to develop antitumor antibodies and not necessarily anticarbohydrate antibodies, many of the antibodies generated were against glycan or glycopeptide epitopes including O-glycoproteins or O-glycans, such as CA15-3 (MUC1), CA-125 (MUC16), B72.3/Tag-72 (STn), and CA19-9 (SLea)(Gendler et al., 1990; Magnani, Steplewski, Koprowski, & Ginsburg, 1983; Nuti et al., 1982; Yin & Lloyd, 2001). More recently, investigators have taken targeted approaches to generate anti-O-glycan tumor antibodies, such as immunizing mice with tumor cells, microorganisms, or glycoproteins containing defined tumor glycans, and screening antibodies against histo-logic specimens, tumor cell lines, defined glycoproteins, and/or glycopep-tide microarrays. Not all glycan structures are equally immunogenic, biasing the production of antibodies. Glycan determinates recognized by antibodies and other GBPs contain two to six monosaccharides, limiting the generation of mAbs against single monosaccharides, such as GalNAc (Cummings, 2009). However, antibodies against monosaccharide clusters or monosaccharide peptide epitopes have been developed, for example, to the Tn antigen (Heimburg-Molinaro et al., 2013).
Lectins have also been used as affinity reagents in conjunction with mAbs. Lectins form multimeric units, facilitating detection of low-affinity interactions through enhanced avidity. Altered binding of plant lectins to tumor cells, such as of wheat germ agglutinin, provided some of the earliest evidence that glycans are altered in cancer (Aub et al., 1963; Ozanne & Sambrook, 1971). Lectins differ from mAbs in that they tend to be more polyreactive, recognizing many related glycan structures with a gradient of affinities, in contrast to mAbs which tend to be more specific. Despite these limitations for lectins, careful use of GBP inhibitors and multiple GBPs can provide important structural information. In addition to classic use of lectins and mAbs, other affinity reagents may provide additional information, including VLR-Fcs generated from lampreys, which use unique binding domains to generate glycan reactivity (Han, Herrin, Cooper, & Wilson, 2008; Hong et al., 2013).
3.2 Truncated O-glycans
Overview
Tn, STn, and T antigens are biosynthetically related carbohydrate structures that are highly expressed in carcinomas but not present in normal tissues or cells (Tables 1 and 3) (Fig. 3). Various reagents have been used to assess these structures, including antibodies to all three structures and lectins that bind Tn and T antigens. Some of these reagents have been validated across platforms, including defined tissues for immunohistochemistry, hap-ten inhibition, column chromatography, and glycopeptide and glycan microarrays, whereas other reagents are less well-defined. For example, BaGs6 and TKH2 mAbs, recognizing Tn and STn, are highly specific, whereas lectins, such as HPA, and other mAbs, cross-react with normal structure, such as blood group A, or are poorly characterized (Hirohashi, Clausen, Yamada, Shimosato, & Hakomori, 1985; Ju et al., 2014).
Table 1.
Truncated O-glycans in cancer
| Antigen | Tissue (unless serum is noted) | % Tumor positive | % Normal positive | Stage of expression (pre-malig, primary, met) | Notes | Citation |
|---|---|---|---|---|---|---|
| Breast | ||||||
| Tn | Breast | 14/15 (93%) | 1/5 | In situ, grossly malig | By lysate absorption to antiserum, lectin | Springer, Desai, and Banatwala (1975) |
| Tn | Breast | 48/50 (96%) | Primary, metastatic | Adsorption | Springer, Murthy, Desai, and Scanlon (1980) | |
| STn | Breast | 13/21 (62%) | Metastatic | B72.3 | Nuti et al. (1982) | |
| STn | Breast | 19/41 (46%) | 2/13 (15%); 2 positives benign, 9 total benign, rest normal noncancer | Primary | B72.3 | Nuti et al. (1982) |
| STn | Breast | 37/44 (84%) | 6/20 (30%); benign lesions, weak staining in positives | Thor, Ohuchi, Szpak, Johnston, and Schlom (1986) | ||
| T | Breast | 15/15 (100%) | 2/5 | In situ, grossly malig | By lysate absorption to antiserum, lectin | Springer et al. (1975) |
| T | Breast | Present, unspecified | Present, unspecified | Differentiated, undifferentiated | PNA-binding tissue section | Klein et al. (1979) |
| T | Breast | 47/52 (90%) | 2/21 (10%); 2 positives pre-malig | Adsorption | Springer et al. (1980) | |
| Tn | Colorectal | 72% (n=29) cancer; 35% (n=25) Transitional mucosa |
0% (n=22) | ETn1.01; all tumor grades positive | Itzkowitz et al. (1989) | |
| Tn | Colorectal | 72% (n=29) cancer; 67% (n=25) Transitional mucosa |
14% (n=22) | VVA; all tumor grades positive | Itzkowitz et al. (1989) | |
| Tn | Colorectal | 81% (n=29) cancer; 61% (n=25) Transitional mucosa |
14% (n=22) | CU-1; all tumor grades positive | Itzkowitz et al. (1989) | |
| Tn | Colorectal polyps | 103/103 (100%) | 79 adenomatous; 24 hyperplastic | VVA | Itzkowitz, Bloom, Lau, and Kim (1992) | |
| Tn | Colorectal | 44/52 (85%) 5/20 (25%) 21/22 (95%) |
0/17 (0%) | Primary Transitional Liver met |
BaGS-6 Tec-02 |
Cao et al. (1995) |
| STn | Colorectal | 44/52 (85%) 11/20 (55%) 21/22 (95%) |
0/17 (0%) | Primary Transitional Liver met |
TKH-2 B72.3 |
Cao et al. (1995) |
| STn | Colorectal | 40/60 (67%) 29/46 (63%) |
7/46 (15%) | Carcinomas Transitional mucosa |
HBSTn-1 | Vazquez-Martin, Cuevas, Gil-Martin, and Fernandez-Briera (2004) |
| STn | Colorectal | 4/4 | B72.3 | Nuti et al. (1982) | ||
| STn | Colorectal | 51/54 (94%) | 5/27 (19%) | Of benign, highest is 20% of cells reactive in Crohn’s sample | Thor et al. (1986) | |
| STn | Colorectal | 96% (n=29) tumor; 38% (n=25) Transitional mucosa |
0% (n=22) | TKH2; all tumor grades positive | Itzkowitz et al. (1989) | |
| STn | Colorectal | 93% (n=29); 38% (n=25) Transitional Mucosa |
0% (n=22) | B72.3; all tumor grades positive | Itzkowitz et al. (1989) | |
| STn | Colorectal | 87.5% (112/128) | TKH2; STn(+) worse prognosis | Itzkowitz et al. (1990) | ||
| STn | Colon and Serum | 27.8% | RIA; 45 U/ml cutoff | Motoo et al. (1991) | ||
| STn | Colorectal polyps | 29% (7/24) | Hyperplastic | TKH2 | Itzkowitz et al. (1992) | |
| STn | Colorectal polyps | 56% | Adenomatous | TKH2 | Itzkowitz et al. (1992) | |
| T | Colorectal | 71% (n=29) Tumor; 47% Transitional mucosa (n=25) |
0% (n=22) | AH9-16; well/moderately differentiated positive; reduced in poorly differentiated | Itzkowitz et al. (1989) | |
| T | Colorectal | 8 ng/ug (n=11) | 3.3 ng/ug (n=5, UC) 1.5 ng/ug (n=9 normal) |
Units: ng TF/ug protein; Use O-glycanase to release and analyze by HPAEC |
Kakeji, Tsujitani, Mori, Maehara, and Sugimachi (1991) | |
| T | Colorectal | 31/52 (60%) 0/20 (0%) 20/22 (91%) |
0/17 (0%) | Primary Transitional Liver met |
TF-α/β A78-G/A7 PNA HB-T1 TF-α HH8 BM22 TF-β A-68-B/A11 |
Cao et al. (1995) |
| STn, T, Tn | 15/24 Tn/STn/T(+) 6/24 Tn/STn(+) 2/24 STn/T(+) 1/24 Tn/STn/T(−) |
Itzkowitz et al. (1989) | ||||
| Gastric | ||||||
| Tn | Gastric | 80/87 (91.9%) | 0/58 (0%); intracellular staining noted in all | David, Nesland, Clausen, Carneiro, and Sobrinho-Simoes (1992) | ||
| Tn | Gastric | 96/163 (59%) | HPA | Kakeji et al. (1991) | ||
| STn | Gastric | 3/4 | Thor et al. (1986) | |||
| STn | Gastric and Serum | 28.1% | RIA; 45U/ml cutoff | Motoo et al. (1991) | ||
| STn | Gastric | 69/87 (19.3%) | 0/58 (0%); 8 positive for intracellular staining | Discrepancy in manuscript, frequency not match % tumors positive | David et al. (1992) | |
| STn | Gastric | 53/85 (62.3%) | TKH2 | Ma et al. (1993) | ||
| STn | Gastric | 21/31 (68%) | TKH2; correlate with outcome | Werther, Rivera-MacMurray, Bruckner, Tatematsu, and Itzkowitz (1994) | ||
| STn | Gastric | 186/340 (54.7%) | TKH2; International study: Japan, Brazil, USA, Chile; cancer beyond stage I (advanced) express more frequently than stage I | Werther et al. (1996) | ||
| T | Gastric | 18/87 (20.7%) | 0/58 (0%) | David et al. (1992) | ||
| Pancreas | ||||||
| Tn | Pancreas | 36/36 (100%) IDC 5/5 IPT |
0/45 (0%) | CU-1, 91S8 (similar staining reported) 100% Tn/STn(+); Localization: 100% of cyto vs. 47% luminal surface, 31% luminal contents positive | Osako et al. (1993) | |
| Tn | Pancreas | 3/6 (50%); adenoma 2/7 (29%); hyperplastic duct | Benign | All Tn+STn- (adenoma) Tn/STn(+) (hyperplastic ducts) |
Osako et al. (1993) | |
| STn | Pancreas | 3/3 | Thor et al. (1986) | |||
| STn | Pancreas and Serum | 40.0% | RIA; 45U/ml cutoff | Motoo et al. (1991) | ||
| STn | Pancreas | 36/36 (100%); IDC 5/5; IPT |
0/45 (0%) | TKH2 | Osako et al. (1993) | |
| STn | Pancreas | 3/6 (50%); adenoma 2/7 (29%); hyperplastic duct | Benign | All Tn-/STn+ (adenoma) Tn/STn(+) (hyperplastic duct) |
Osako et al. (1993) | |
| STn | Pancreas | 77% (n=64) | 2% (n=58) | Infiltrating pancreatic ductal adenocarcinoma | TKH2, increase in advanced cancer, reduced in PanINs (PanIN 3=67% n =9 but <10% PanIN <3) | Kim et al. (2002) |
| T | Pancreas | 29/36 (81%) | 36% PDs 71% ACs 53% ICs PD panc duct AC Acinar cells IC Islet cells n total=45 |
PNA | Osako et al. (1993) | |
| Bladder | ||||||
| Tn | Bladder | 27/34 (77%) | 0/10 (0%) | Primary | BaGs2 | Langkilde, Wolf, Clausen, Kjeldsen, and Orntoft (1992) |
| STn | Bladder | 1/34 (3%) | 1/10 (10%) | Primary | TKH2 | Langkilde et al. (1992) |
| Respiratory | ||||||
| Tn | Lung | 84/93 (90%) | HPA | Laack et al. (2002) | ||
| STn | Lung | 26/27 (96%) | Thor et al. (1986) | |||
| T | Respiratory | 4/5 | Adsorption | Springer et al. (1980) | ||
| Ovarian | ||||||
| STn | Ovary | 40/40 (100%) | B72.3 | Thor et al. (1986) | ||
| STn | Ovary | 61/82 (74%) | Inoue, Ton, Ogawa, and Tanizawa (1991) | |||
| Endometrial | ||||||
| STn | Endometrial | 36/43 (84%) | 13/32; proliferative phase and menopausal negative; secretory phase, especially late were positive) | TKH2 | Inoue, Ogawa, et al., 1991 | |
| Cervical | ||||||
| Tn | Uterine cervix | 50/111 (45%) | VVA | Hirao, Sakamoto, Kamada, Hamada, and Aono (1993) | ||
| Tn | Uterine cervix | 24/29 (82.8%) 17/29 (58.5%) |
Met Primary |
VVA; more Tn in met than primary, but no difference in T antigen in primary v. met | Hamada, Furumoto, Kamada, Hirao, and Aono (1993) | |
| Salivary gland | ||||||
| Tn | Salivary gland | 12/13 (92%) 7/9 (78%) 7/18 (39%) 6/14 (43%) 1/6 (17%) |
Mucoepidermoid carcinoma Adenocarcinoma Carcinoma in pleomorphic adenoma Adenoid cystic carcinoma Acinic cell carcinoma |
Tn: 1E3, HB-Tn-1; similar results for both; Tn and STn: 1C12; similar results to Tn | Therkildsen, Mandel, Christensen, and Dabelsteen (1993) | |
| STn | Salivary gland | 12/13 (92%) 7/9 (78%) 7/18 (39%) 6/14 (43%) 1/6 (17%) |
Mucoepidermoid carcinoma Adenocarcinoma Carcinoma in pleomorphic adenoma Adenoid cystic carcinoma Acinic cell carcinoma |
STn: TKH2, HBSTn1; similar results for both; Tn and STn: 1C12; similar results to STn | Therkildsen et al. (1993) | |
| GI unspecified | ||||||
| STn | Digestive (unspecified) and serum | 4.1% | RIA; 45 U/ml cutoff | Motoo et al. (1991) | ||
| T | GI tract | 5/5 | Adsorption | Springer et al. (1980) | ||
| Esophagus | ||||||
| STn | Esophagus and serum | 0% | RIA; 45 U/ml cutoff | Motoo et al. (1991) | ||
| Liver | ||||||
| STn | Liver and Serum | 7.1% | RIA; 45 U/ml cutoff | Motoo et al. (1991) | ||
| Biliary tract | ||||||
| STn | Bile/pancreas (serum) | 8/15 (53%) 5/9 (56%) |
2/14 (14%) | Bile duct Pancreas |
<45 U/ml (normal); ≥45 U/ml (high) | Nanashima et al. (1999) |
| STn | Biliary tract and Serum | 25.0% | RIA; 45 U/ml cutoff | Motoo et al. (1991) | ||
| Melanoma | ||||||
| Tn | Melanoma | 0/2 | Adsorption | Springer et al. (1980) | ||
| STn | Melanoma | 0/2 | Thor et al. (1986) | |||
| T | Melanoma | 0/4 | Adsorption | Springer et al. (1980) | ||
| Nonepithelial solid tumor | ||||||
| STn | Osteogenic sarcoma | 0/1 | Thor et al. (1986) | |||
| STn | Glioblastoma multiforme | 0/1 | Thor et al. (1986) | |||
| Blood cells | ||||||
| Tn | Blood cells (bone marrow aspirates) | 5/725 (<1%) | 0/35 (0%) | FBT3 | Roxby, Pfeiffer, Morley, and Kirkland (1992) | |
| STn | Lymphoma | 0/4 | Thor et al. (1986) | |||
| STn | Leukemia | 0/1 | Thor et al. (1986) | |||
| STn | Thymoma | 0/1 | Thor et al. (1986) | |||
| Various | ||||||
| Tn | Various | 7/8 (88%) | Adsorption | Springer et al. (1980) | ||
| STn | Various normal, from noncancer | 0/25 (0%) | B72.3 | Nuti et al. (1982) | ||
| STn | 33 normal organ and tissue type | Minimal | Thor et al. (1986) | |||
| T | Various nonbreast benign | 0/7 (0%) | Adsorption | Springer et al. (1980) | ||
| T | Various nonbreast, healthy | 0/11 (0%) | Adsorption | Springer et al. (1980) | ||
Frequency of positive staining reported as number positive samples out of all samples unless indicated otherwise; % reported in parentheses for n>5; literature search performed for tumor antigen.
IDC, invasive ductal carcinoma; IPT, intraductal papillary tumor; malig, malignant; met, metastasis; RIA, radioimmunoassay, PNA, Peanut agglutinin; VVA, Vicia villosa agglutinin; HPAEC, high-performance anion exchange chromatography; HPA, Helix pomatia agglutinin; PanIN, pancreatic intraepithelial lesion; cyto, cytoplasm; (+), positive; (−), negative.
Table 3.
Frequency of altered Tn, STn, T, SLea, and SLex in cancer
| Tissue | Tn | STn | T | SLea | SLex |
|---|---|---|---|---|---|
| Breast | >90 (1/5) | 60–85 (<30) | 90–100 (10–40) | 5 | 25 |
| Colon | 70–100 (0–15) | 65–100 (0–20) | 60–90 (0) | 55–80 (0) | 40–90 |
| Gastric | 60–90 (0) | 55–75 (0) | 20 (0) | 80–90 (37) | >90 |
| Pancreas | 100 (0) | 80–100 (<2) | 80 | 55 (70) | >90 |
| Bladder | 80 (0) | 3 (10) | |||
| Respiratory | 90 | 95 | 4/5 | ||
| Lung | 40 | 60 | |||
| Esophagus | 0 | 50 | |||
| Cervical | 45–80 | ||||
| Ovarian | 75–100 | 50 | |||
| Endometrial | 84 | ||||
| Salivary | 20–90 | 20–90 | |||
| Liver | 10 | ||||
| Melanoma | 0/2 | 0/2 | 0/4 | ||
| Blood | <1 | 0/6 | |||
| Mesothelioma | 10 |
As discussed previously, Tn is synthesized by a family of ppGalNAcTs and normally extended to form core O-glycan structures 1–4. Alterations in biosynthetic machinery and other factors may contribute to expression of the truncated O-glycans, Tn, STn, and T. Further, these structures have been shown to contribute to the tumor phenotype in various model systems.
3.2.1 Tn antigen
3.2.1.1 Background
Tn antigen is expressed on a majority of carcinomas arising from every tissue evaluated to date and not expressed on normal adult tissues. The Tn antigen was first identified by Dausset in 1959 on red blood cells from a patient with a rare autoimmune hemolytic anemia and polyagglutinability, later shown to result from anti-Tn antibodies in serum (Dausset, Moullec, & Bernard, 1959). Tn, or T nouvelle, was named in reference to T antigen, which was discovered earlier through a similar RBC polyagglutinability. However, in contrast to Tn, T is present on all individuals after treatment with neur-aminidase. Dahr described GalNAc-Ser/Thr as the Tn antigen, and Springer and others identified the biosynthetic relationship between Tn and T (Dahr et al., 1974; Springer, 1984). Tn was first observed on tumor cells in 1969 but established as a pan-carcinoma antigen in the 1970s–1980s through the work of Springer (Prokop & Uhlenbruck, 1969). Many groups have since verified these early observations. Tn is highly expressed on carcinomas but less common on blood cancers (Ju et al., 2014). Interestingly, many of the tissues that express Tn also express other truncated O-glycans, such as STn, and T.
3.2.1.2 Histology
Tn antigen is a pan-carcinoma antigen. It is expressed in ≥90% of carcinomas of breast, pancreas, and lung, ≥60% of carcinomas of colon, stomach, and bladder, 45–80% of cervical carcinomas, 20–90% of salivary carcinomas, depending on the tumor type, and <1% of primary melanomas and blood cancers (Cao et al., 1995; David et al., 1992; Hamada et al., 1993; Hirao et al., 1993; Itzkowitz et al., 1992, 1989; Kakeji et al., 1991; Laack et al., 2002; Langkilde et al., 1992; Osako et al., 1993; Roxby et al., 1992; Springer et al., 1975, 1980; Therkildsen et al., 1993). Tn is expressed in <20% of normal tissues, with lower percentages observed with more specific reagents (Cao et al., 1995; David et al., 1992; Itzkowitz et al., 1989; Langkilde et al., 1992; Osako et al., 1993; Roxby et al., 1992; Springer et al., 1975). Furthermore, Tn is expressed highly on carcinoma cell surfaces and in luminal content, in addition to being upregulated in the cytoplasm of cancers. Expression of Tn on the cell surface and in luminal content is extremely specific for carcinomas as normal tissues do not express Tn in these locations (Osako et al., 1993). Tn is a normal biosynthetic precursor but not a normal terminal product. Therefore, Tn is occasionally observed in the secretory apparatus but not found on cell surface or secreted proteins, except in cancer.
Tn antigen is expressed early in cancer development and its expression correlates with clinical progression. In the colon, Tn is found on 25–70% of transitional tissues and also in premalignant lesions and hyperplastic polyps (Cao et al., 1995; Itzkowitz et al., 1992, 1989; Yuan, 1989). In carcinogen-induced rodent models of colorectal and breast cancers, Tn is observed in precursor lesions (Babino et al., 2000; Berriel et al., 2005). The ratio of Tn to T increases during cancer progression, largely due to increased Tn, and accordingly, Tn predicts cancer invasion more readily than T (Desai, 2000). In comparing Tn-positive and -negative tumors, Tn expression correlates with poor survival in carcinomas of the cervix, lung, colon, and stomach (Hirao et al., 1993; Kakeji et al., 1991; Konno, Hoshino, Terashima, Motoki, & Kawaguchi, 2002; Laack et al., 2002).
3.2.1.3 Mechanisms for expression
Tn is highly expressed in carcinomas, expressed early in tumorigenesis, and correlates with disease progression and survival. However, the mechanism for Tn expression is less clear. Approximately, 10 mechanisms have been proposed for expression of Tn antigen in cancer and some of these have been observed in primary tumors. The proposed mechanisms include genetic/transcriptional alterations in proteins required for extension of the Tn antigen, alterations—either genetic or cell biologic—of the enzymes that synthesize the Tn antigen, changes in expression of nucleotide sugars or transporters, and changes in vesicular transport, retention, and structure of the Golgi/ER as well as alterations in glycosyltransferase oligomerization (Ju et al., 2014).
Tn is normally extended by the T-synthase or C3GnT to form core 1 or core 3 structures. Loss of T-synthase and C3GnT activity has been observed in tumor cells and tissue (Ju, Lanneau, et al., 2008; Vavasseur et al., 1995; Yang et al., 1994). Defects in Cosmc, rather than T-synthase, appear to result in loss of T-synthase activity. Cosmc mutations, LOH, and epigenetic silencing have been observed in cancer cell lines, and mutations have been observed in cervical cancer specimens (Ju, Lanneau, et al., 2008; Mi et al., 2012). Recently, methylation of the Cosmc promoter was observed in ~40% of a series of pancreatic adenocarcinoma samples, correlating with Tn expression and loss of T-synthase protein (Radhakrishnan et al., 2014).
Various mechanisms that result in altered Golgi structure or altered glycosyltransferase localization within the Golgi have also been suggested to result in Tn expression. These include growth-factor-induced relocalization of ppGalNAcTs to the ER, relocalization of ppGalNAcTs to the trans-Golgi, alterations in proteins required for glycosyltransferase retention, and altered Golgi pH (Gill, Chia, Senewiratne, & Bard, 2010; Kellokumpu, Sormunen, & Kellokumpu, 2002; Petrosyan, Ali, & Cheng, 2012). However, unlike loss of T-synthase and C3GnT activities, the potential roles of altered Golgi structure and glycosyltransferase localization in Tn expression have not been evaluated in primary tumors so that their contributions to Tn expression are unknown.
3.2.2 Sialyl-Tn
3.2.2.1 Background
Sialyl-Tn (STn), or Neu5Acα2,6Tn, is a pan-carcinoma antigen that is expressed early in tumorigenesis and correlates with disease expression. STn is often coexpressed with Tn and, similar to Tn, not expressed on normal cells or tissues (Fig. 3). STn was first identified along with Tn in Tn syndrome but discovered in tumors with generation of an mAb, B72.3, generated from immunization with cell membrane fractions from metastatic breast cancer (Colcher, Hand, Nuti, & Schlom, 1981). B72.3 reacts with a sialidase-sensitive epitope on STn-containing ovine submaxillary mucin (OSM) and binding to OSM is blocked by STn-Ser (Johnson et al., 1986; Kjeldsen et al., 1988). Other STn antibodies, such as MLS102 and TKH2, were also generated and have been used to evaluate STn in tumors (Julien, Videira, & Delannoy, 2012).
3.2.2.2 Histology
Most carcinomas express STn, including >60% of carcinomas of the breast, colon, stomach, pancreas, lung, ovaries, and endometrium, 20–90% of salivary carcinomas, and, in contrast to Tn, few bladder carcinomas (Cao et al., 1995; David et al., 1992; Inoue, Ogawa, et al., 1991; Inoue, Ton, et al., 1991; Itzkowitz et al., 1990, 1992, 1989; Kim et al., 2002; Langkilde et al., 1992; Ma et al., 1993; Motoo et al., 1991; Nanashima et al., 1999; Nuti et al., 1982; Osako et al., 1993; Therkildsen et al., 1993; Thor et al., 1986; Vazquez-Martin et al., 2004; Werther et al., 1994, 1996). STn is also expressed early in tumorigenesis and correlates with poor survival. Aberrant crypt foci (the earliest sign of cellular atypia) and ulcerative colitis lesions that will progress to colorectal cancer express STn (Itzkowitz et al., 1995, 1996; Wargovich et al., 2004). Similar to Tn, STn is also expressed in precursor lesions in carcinogen-induced rodent models of breast and colorectal cancer, and STn has been shown to correlate with histologic and clinical cancer progression, including poor survival in colorectal, gastric, and ovarian cancers (Babino et al., 2000; Berriel et al., 2005; Itzkowitz et al., 1990, 1995, 1996; Kobayashi, Terao, & Kawashima, 1992; Ma et al., 1993; Werther et al., 1994, 1996). In contrast to Tn, STn is not a normal biosynthetic precursor. Therefore, both intracellular and cell surface expression are necessarily pathologic.
3.2.2.3 Mechanisms for expression
STn is often coexpressed with Tn and therefore mechanisms that result in Tn expression likely apply to STn, including alteration in Cosmc/T-synthase, defects in C3GnT, and alterations in Golgi structure and glycosyltransferase dynamics, as discussed previously. Tn-independent mechanisms for STn expression have been proposed, including ST6GalNAc-I upregulation and de-esterification of acetyl STn to form STn. Engineered overexpression of ST6GalNAc-I in cell lines results in STn expression (Julien et al., 2001; Marcos et al., 2004). While some tumors exhibit elevated ST6GalNAc-I transcript, protein, or activity correlating with STn expression, other tumors, such as colorectal carcinoma, have a reduction in ST6GalNAc-I activity despite robust STn expression, and tissues expressing STn almost always coexpress Tn, which could not be explained by ST6GalNAc-I (Marcos et al., 2011; Vazquez-Martin et al., 2004; Yang et al., 1994). Another Tn-independent mechanism for STn expression derives from studies using alkaline, de-esterifying conditions, which results in STn expression in normal colon (where it is usually absent) but not in other normal tissues such as pancreas (Jass, Allison, & Edgar, 1994; Julien et al., 2012). This suggests that in some tissues acetyl STn may normally conceal STn antigen, and therefore STn may arise in tumors from increased esterase activity. However, this assumes that all STn antibodies evaluated in normal tissue to date are blocked by STn acetylation, which is unlikely but untested.
3.2.3 T antigen
3.2.3.1 Background
T antigen is highly expressed on tumors, but reagents used to define T are more variable and less defined than those used for Tn and STn, resulting in more cross-reactivity with normal tissues and non-T structures. Parts of the CNS and germinal center B cells normally express T antigen (Butcher et al., 1982; Desai, 2000). Structurally, T consists of Galβ1–3GalNAcα1-Ser/Thr, which forms after transfer of Gal to Tn by the T-synthase (Fig. 3). Historically, T was sometimes considered Galβ1–3GalNAc in α or β linkage to a glycoprotein or -lipid, in contrast to the current definition of α linkage to a glycoprotein. Normally, T is extended by addition of Neu5Ac and/or GlcNAc to form mono or disialyl T and core 2-based structures. The T antigen was first discovered by Hübener, Thomsen, and Friedenreich in the 1920s and 1930s when studying blood agglutination (Reepmaker, 1952). They found that a microbial contaminant, later attributed to neuraminidase, results in cold agglutination of RBCs when mixed with serum due to normal anti-T antibodies. Georg Springer first identified T as a pan-carcinoma antigen, along with his studies of Tn in the 1970s (Springer, 1984; Springer et al., 1975, 1980; Springer, Desai, Tegtmeyer, Spencer, & Scanlon, 1993).
3.2.3.2 Histology
T antigen is a pan-carcinoma antigen that is expressed in greater than 60% of tumors of breast, colon, pancreas, and lung, and ~20% of gastric tumors. Normal tissues express 0–40% of T, likely due to cross-reactivity of anti-T reagents, some of which react with α- and β-linked Galβ1–3GalNAc on glycoprotein or lipids (Cao et al., 1995; David et al., 1992; Itzkowitz et al., 1989; Klein et al., 1979; Osako et al., 1993; Springer et al., 1975, 1980). Nonetheless, chemical and enzymatic studies have confirmed that T is a genuine carcinoma antigen (Campbell, Finnie, Hounsell, & Rhodes, 1995). In contrast to Tn and STn, T expression is less helpful to prognosticate tumors (Desai, 2000). Early studies from Springer using DTHRs suggested that cellular immune responses to T are extremely sensitive and specific for carcinoma (Desai, 2000; Springer, 1984).
3.2.3.3 Mechanisms for expression
The expression of T antigen could occur through a variety of mechanisms. Colon tumors upregulate UDP-Gal transporters, resulting in elevated T antigen, as well as SLea/x, in cancer cells (Kumamoto et al., 2001). Reduction of sulfotransferase activity in colon tumors has been proposed to result in conversion of endogenous sulfo-T in colon to T antigen (Yu, 2007). Alterations in Golgi pH increase T expression in cell lines, presumably due to changes in Golgi structure and glycosyltransferase localization (Kellokumpu et al., 2002). In breast cancer, decrease in C2GnT results in a shift from core 2- to core 1-based structures, which along with other mechanisms may enhance T expression (Brockhausen, Yang, Burchell, Whitehouse, & Taylor-Papadimitriou, 1995). However, the importance of any of these mechanisms in T expression in primary tumors is currently unclear.
3.2.4 Comparing Tn, STn, and T expression and function in tumor biology
3.2.4.1 Expression
The truncated O-glycans Tn, STn, and T are pan-carcinoma antigens and many tumors coexpress these structures. Over half of colorectal cancers express all three antigens, while most pancreatic cancers express both Tn and STn (Itzkowitz et al., 1989; Osako et al., 1993). Interestingly, benign lesions sometimes express one of these antigens but rarely coexpress multiple antigens (Osako et al., 1993). Some tumors, such as breast, exhibit a shift from core 2- to core 1-based structures including elevation of normal mono and disialylated T antigens (Brockhausen et al., 1995). Hence, Tn, STn, T, and normal core 1-based structures can all be expressed on the same tumor. This probably reflects heterogeneity across some tumors, in which different cells express different antigens, as well as coexpression of these antigens on individual cells and individual proteins within a tumor. Whether one of these situations predominates is unclear but may highlight which mechanism(s) drive expression of truncated O-glycans.
3.2.4.2 Function
Tn, STn, and T are highly expressed in tumors and Tn and STn expression correlates with disease progression, suggesting that these antigens may contribute to the tumor phenotype. Deletion of C3GnT in a mouse results in expression of Tn and STn in the GI tract and increased susceptibility to chemically induced colorectal cancer (An et al., 2007). Further, deletion of Cosmc in pancreatic cancer cells or an organotypic tissue model results in features of cellular transformation and tumorigenesis in vitro as well as increased tumor growth in xenotransplant studies (Radhakrishnan et al., 2014). Alterations in cell adhesion and oncogenic signaling were also observed in these engineered cells, consistent with the behavioral alterations. Expression of Tn or STn may be immunomodulatory. STn facilitates resistance to NK cell killing, and Tn interacts with MGL on dendritic cells and inhibits migration of immature APCs (Ogata, Maimonis, & Itzkowitz, 1992; van Vliet, Paessens, Broks-van den Berg, Geijtenbeek, & van Kooyk, 2008). T antigen interacts with galectin-3, facilitating tumor cell interaction with endothelia and platelets (Glinsky et al., 2001). Many additional mechanisms likely contribute to the participation of truncated O-glycans in tumor biology, given that ~83% of proteins entering the secretory pathway are O-glycoproteins (Steentoft et al., 2013). Identifying these mechanisms may provide new therapeutic strategies.
3.3 Altered terminal and extended structures
Overview
Cancers exhibit alterations in terminal glycans, including Lewis antigens, blood group structures, as well as recently identified terminal α-GlcNAc on core 2 (Tables 2 and 3). Many tumors overexpress sialyl Lewisa/x and delete, overexpress, or ectopically express blood group structures, while gastric carcinomas delete terminal α-GlcNAc on core 2. Many of these changes correlate with survival; however, unlike truncated O-glycans and terminal α-GlcNAc, alterations in Lewis and blood group antigens are observed on N-glycoproteins and glycolipids in addition to O-glycoproteins. Determining the glycan carrier is not always possible, but a few examples highlight the importance of O-glycans as platforms for these alterations. O-glycans are major carriers of sialylated Lewis antigens and ABH structures in some tumors and of sialylated Lewis antigens in serum, and expression of these structures on O-glycans correlates with poor prognosis (Gupta et al., 1985; Izumi et al., 1995; Shimodaira et al., 1997; St Hill et al., 2009). Further, expression of SLex on core 2 O-glycans facilitates interaction with E-selectin, which is important for tumor metastasis (St Hill, Baharo-Hassan, & Farooqui, 2011).
Table 2.
Altered terminal O-glycans in cancer
| Antigen | Tissue | Change | % Tumor positive | % Normal positive | Outcome | Notes | Citation |
|---|---|---|---|---|---|---|---|
| Colorectal | |||||||
| SLea | Colorectal | 12/21 (57%) | CA19-9, CA52a; RIA | Magnani et al. (1982) | |||
| SLea | Colorectal | 40/68 (59%) | 0/15 (0%) | CA19-9 | Atkinson et al. (1982) | ||
| SLea | Colorectal |
|
|
CA19-9; SLea(+) in primary predict met to regional LN | Nakayama, Watanabe, Katsumata, Teramoto, and Kitajima (1995) | ||
| SLea | Colorectal | 110/159 (69.2%) | Yes; 5-yr—DFS: +=73%, −=84.7% | CA19-9 | Nakamori et al. (1997) | ||
| Lewis | Colorectal |
|
|
|
SLex predictor of nonpolyploid growth type vs. polyploid growth type | Nakagoe et al. (2001) | |
| SLex | Colorectal | 76% (n=17) | Weak staining | CSLEX1; IHC | Fukushima et al. (1984) | ||
| SLex | Colorectal |
|
|
|
Itzkowitz et al. (1986) | ||
| Sialyl-dimeric Lex | Colorectal | FH6; increased in met | Matsushita et al. (1990) | ||||
| SLex | Colorectal | 50/132 (37.9%) | Rare in normal or transitional mucosa | Yes; 5-yr survival: SLex(+) 58.3%, SLex(−) 93.0% | FH6; 6/8 liver mets had greater % cells FH6(+), 20/25 mets to LNs (80%) stronger FH6 staining; SLex correlate with depth of invasion, LN met, LN invasion, tumor stage; SLex(+) greater recurrence and recurrence to distant sights than SLex(−) | Nakamori et al. (1993) | |
| SLex | Colorectal | 58/159 (36.5%) | Yes, 5 yr—DFS: SLex(+)=55.6%, SLex(−)=89% | FH6 | Nakamori et al. (1997) | ||
| Lex | Colorectal |
|
|
|
Itzkowitz et al. (1986) | ||
| Incompatible BG-A or B | Colorectal | >50% | Yuan et al. (1985) | ||||
| Deletion of BG structure | Colorectal | Yuan et al. (1985) | |||||
| Precursor BG-H accumulation | Colorectal | 80% (n=25) | Yuan et al. (1985) | ||||
| ABH | Colorectal | 46/82 (56.1%) expressors | Proximal: 18/23 Distal: 57/59 (78.3%) “deleted” (96.6%) “deleted” | Yes, 5-yr survival: ABH “expressors” 33.5% “deletors” 75.4%, ABH | Nakagoe et al. (2000) | ||
| ABH | Colorectal |
|
|
A expression predictor of nonpolyploid growth vs. polyploid growth- type | Nakagoe et al. (2001) | ||
| Gastric | |||||||
| SLea | Gastric | 4/5 | CA19-9, CA52a; RIA | Magnani et al. (1982) | |||
| SLea | Gastric | 16/18 (89%) | 7/19 (37%) | CA19-9 | Atkinson et al. (1982) | ||
| SLex | Gastric | 94% (n=17) | CSLEX1 | Fukushima et al. (1984) | |||
| Pancreas | |||||||
| SLea | Pancreas | 4/7 (57%) | CA19-9, CA52a; RIA | Magnani et al. (1982) | |||
| SLea | Pancreas | 19/22 (86%) | 7/10 (70%) | CA19-9 | Atkinson et al. (1982) | ||
| SLex | Pancreas | 100% (n=3) | Weak staining | CSLEX1 | Fukushima et al. (1984) | ||
| Esophagus | |||||||
| SLea | Esophagus | 0/5 | CA19-9, CA52a; RIA | Magnani et al. (1982) | |||
| SLex | Esophagus | 50% (n=4) | Strong staining | CSLEX1 | Fukushima et al. (1984) | ||
| Liver | |||||||
| SLea | Liver | 1/11 (9%) | 7/11 (64%) | CA19-9 | Atkinson et al. (1982) | ||
| Gall bladder | |||||||
| SLea | Gall bladder | 2/5 | 6/11 (54%) | CA19-9 | Atkinson et al. (1982) | ||
| Lung | |||||||
| SLea | Lung | 28/66 (42%) | CA19-9 | Atkinson et al. (1982) | |||
| SLex | Lung | 63% (n=16) | CSLEX1 | Fukushima et al. (1984) | |||
| BG-A | Lung (NSCLC) | Loss | 43/71 with A or AB BG retain expression A in tumor; 28/71 (39%) lose expression A in tumor | Yes | Median survival—lose A: 15 months, retain A in tumor: 71 months; survival of B, O = same as retain A and loss of B or H not change survival | Lee et al. (1991) | |
| BG-A | Lung | Loss | 35/62 lose A (56%) | No | Gwin et al. (1994) | ||
| BG-A | Lung | Loss | Yes, of A/AB BG patients, median survival: A loss (n=36) 38 months v. A(+)(n=54) 98 months | Graziano, Tatum, Gonchoroff, Newman, and Kohman (1997) | |||
| H/Ley/Leb | Lung | MIA15-5(+)(n=91): 20.9% 5-yr survival v. Ag-(n=58) 58.6% 5-yr survival | Yes | MIA15-5 Ab; when segregate by blood group A (i.e., A or AB) significant difference in survival but not for B or O | Miyake et al. (1992) | ||
| Breast | |||||||
| SLea | Breast | 1/18 (6%) | CA19-9 | Atkinson et al. (1982) | |||
| SLex | Breast | 25% (n=8) | CSLEX1 | Fukushima et al. (1984) | |||
| Mesothelioma | |||||||
| SLea | Mesothelioma | 1/12 (8%) | CA19-9 | Atkinson et al. (1982) | |||
| Ovary | |||||||
| SLex | Ovary | 50% (n=6) | CSLEX1 | Fukushima et al. (1984) | |||
| Kidney | |||||||
| SLex | Renal tubules | Strong staining | CSLEX1 | Fukushima et al. (1984) | |||
| Various epithelia | |||||||
| Serum Ca19-9 | Various (colon, gastric, breast, pancreas) | CEA more sensitive, except for pancreas | Gupta et al. (1985) | ||||
| Myeloid | |||||||
| ABH | Myeloid | Loss |
|
|
Reason for reduced A, B: 8/29 (29%) primary loss of A or B, 5/29 (17%) indirect due to loss of H, 3/29 (10%) loss of A or B and H | Bianco, Farmer, Sage, and Dobrovic (2001) | |
Frequency of positive staining reported as number positive samples out of all samples unless indicated otherwise; % reported in parentheses for n>5; literature search performed for tumor antigen.
BG, blood group; NSCLC, nonsmall-cell lung cancer; LN, lymph node; yr, year; met, metastasis; DFS, disease-free survival; RIA, radioimmunoassay.
3.3.1 Terminal α-GlcNAc on core 2
Gastric cancer is the fourth most common malignancy and the second leading cause of cancer deaths worldwide (Thun, DeLancey, Center, Jemal, & Ward, 2010). Helicobacter pylori is a major risk factor for gastric cancer and interacts with surface but not gland mucins of the stomach (An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group, 1993; Nakayama, 2014; Parsonnet et al., 1991). A unique glycan structure—α1–4GlcNAc terminating core 1 and core 2 branches of core 2 O-glycans—was identified on gland mucins, suggesting that this structure may prevent H. pylori colonization. Accordingly, terminal α1–4GlcNAc on a soluble protein inhibits H. pylori growth through disrupting the cell wall (Kawakubo et al., 2004). Interestingly, deletion of α1–4GlcNAcT in a mouse results in spontaneous gastric adenocarcinoma, independent of H. pylori, along with protumorigenic immune activation (Karasawa et al., 2012). Hence, α1–4GlcNAc on core 2 prevents gastric cancer through its antibiotic properties toward H. pylori and by preventing protumorigenic inflammation in the stomach. In line with these mouse studies, loss of α1–4GlcNAc has been observed in patients with gastric carcinoma and Barrett’s adenocarcinoma and found to correlate with worse survival in gastric cancer (Iwaya et al., 2014; Shiratsu, Higuchi, & Nakayama, 2014).
3.3.2 Lewis antigens
3.3.2.1 Background
Sialyl Lewis antigens consist of sialylated and fucosylated type 1, 2 chains (Fig. 2), as previously described. Sialyl Lea/x (SLea/x) are upregulated in cancer; however, tumors express long-chain and polyfucosylated forms, such as dimeric SLex, in contrast to short-chain and monofucosylated forms observed in inflammation and normal tissues and cells. Lewis antigens were first discovered in an RBC agglutination reaction, however, later observed as a synthetic product of internal epithelia and not RBCs, having been transferred to RBCs on glycolipids in serum (Marcus & Cass, 1969). Generation of mAbs against tumor cells in the 1970s and 1980s resulted in identification of tumor-associated SLea/x antigens.
3.3.2.2 SLea
SLea is a tumor-associated carbohydrate antigen as first identified by Magnani and Koprowski using an antibody, CA19-9, generated from immunization with SW1116 cells (Magnani et al., 1982, 1983) (Fig. 2). SLea is expressed by >50% of carcinomas of the colon, stomach, pancreas, ~40% of carcinomas of the lung, and ≤10% of carcinomas of esophagus, liver, breast and mesotheliomas. Normal colon does not express SLea in contrast to >30% of normal stomach, pancreas, and liver (Atkinson et al., 1982; Magnani et al., 1982; Nakagoe et al., 2001; Nakamori et al., 1997; Nakayama et al., 1995). CA19-9 is mainly used as a serum test for pancreatic cancer. Although normal pancreas expresses CA19-9, pancreatic carcinomas secrete elevated levels of this antigen and its expression correlates with poor survival (Berger et al., 2008). CA19-9 is not useful for all patients as 10–20% of the population is Lewis-negative and therefore cannot synthesize SLea.
3.3.2.3 SLex
SLex is a tumor marker and ligand for leukocyte adhesion. As described above, monofucosylated short-chain SLex is present in normal tissue and blood cells, whereas long-chain polyfucosylated SLex, such as sialyl-dimeric Lex, is tumor specific. In colon, for example, antibodies against short-chain SLex bind 60–80% of normal tissues, whereas antibodies against long-chain structures bind 5–10% of normal tissues (Itzkowitz et al., 1986). Expression of SLex in tumors largely resembles expression of SLea. SLex is expressed in >90% of tumors of stomach and pancreas, ≥40% of carcinomas of colon, esophagus, and ovaries, and ~25% of breast carcinomas (Fukushima et al., 1984; Itzkowitz et al., 1986; Matsushita et al., 1990; Nakagoe et al., 2001; Nakamori et al., 1993, 1997). In colon, SLex expression correlates with poor survival (Nakamori et al., 1993, 1997).
3.3.2.4 Functions of SLex and SLea in tumor biology
Sialyl Lewis antigens facilitate metastasis in model systems and patients. SLea/x are selectin ligands, mediating tumor cell attachment to endothelia, platelets, and leukocytes (Takada et al., 1991, 1993). Attachment to endothelia contributes to vessel invasion, whereas attachment to platelets, and possibly leukocytes, contributes to survival in the vasculature. Cancer cells that express SLea/x more readily metastasize in xenotransplants, and overexpression of E-selectin in the liver results in metastatic redirection of SLea/x(+) cells to the liver (Biancone et al., 1996; Fukuda et al., 2000; Fuster et al., 2003). In patients, expression of SLea/x correlates with worse survival and using agents that block selectin expression or interactions with SLea/x improves survival (Berger et al., 2008; Lee et al., 2005; Matsumoto et al., 2002; Nakamori et al., 1993, 1997; Nakayama et al., 1995).
3.3.2.5 Mechanisms for overexpression
Expression of SLea/x arises from glycan truncation or overexpression (Kannagi, 2004). Some tissues contain 6-sulfated and 2–6 sialylated SLea/x. However, tumorigenesis results in reduced sulfation and 2–6 sialylation of SLea/x and increased expression of SLea/x (Kannagi, 2004). SLea/x overexpression results from oncogene or environmental induction of biosynthetic enzymes, such as Cox-2 expression and hypoxia (Kannagi, 2004). Various glycosyltransferases can be affected although upregulation of sialyltransferases appears to be a major mechanism for SLea/x expression. Fut3, the major fucosyltransferase responsible for synthesizing SLea, is not usually upregulated in tumors (Kannagi, 2004). In addition to affecting glycosyltransferases, hypoxia induces UDP-Gal transporter overexpression, resulting in elevated levels of SLea/x and T antigen (Kumamoto et al., 2001).
3.3.3 ABH structures
3.3.3.1 Background
Alterations in AB/H structures are observed in tumors with loss of blood groups in tissues normally containing structures (Fig. 2), gain of blood groups in tissues normally deficient in structures, and occasionally mismatched expression of blood groups, e.g., expression of A in a BB/BO individuals. Some of these changes correlate with clinical progression. Landsteiner first described the ABO blood groups in the early 1900s, Watkins later characterized the structure and genetics of blood groups, and Masumune first observed alterations in ABO blood groups in tumors in the 1940s (Oh-Uti, 1949). AB/H structures are synthesized on type 1–4 chains, as previously discussed.
3.3.3.2 Histology
Colon and lung carcinomas exhibit different alterations in blood group structures due to different levels of normal expression. Colons (especially distal) are deficient in ABH, while lungs express these structures. Accordingly, colon cancers exhibit ectopic and mismatched expression of blood groups structures, while lung carcinomas exhibit deletion of normal structures and expression of truncated structures, such as H in A individuals. A/B incompatibility is observed in 0% to >50% of colorectal carcinomas, depending on the study, and accumulation of blood group ABH structures is observed in 5–40% of tumors, depending on the structure, in contrast to 0–5% ABH expression in normal tissues (Nakagoe et al., 2001, 2000; Yuan et al., 1985). Lung carcinomas exhibit a loss of ABH structures, for example, 40–55% of tumors exhibit a loss of A with a gain of H/Ley/Leb structures, due to precursor accumulation (Graziano et al., 1997; Gwin et al., 1994; Lee et al., 1991; Miyake et al., 1992) (Fig. 2). In addition to carcinomas, myeloid cancers exhibit ~55% loss of A or B in A, B, A/B blood types and 21% loss of H in O blood types (Bianco et al., 2001). Further, altered ABH structures correlate with disease progression. In particular, deletion of A in lung and ectopic expression of ABH in colon correlate with poor survival.
3.3.3.3 Mechanisms for altered expression
Loss of blood group structures appears to occur at the level of the A and B transferases. Loss of A/B transferase activities and epigenetic silencing at the A, B, H loci correlate with blood group deletion in carcinomas and myeloid cancers, respectively (Bianco-Miotto, Hussey, Day, O’Keefe, & Dobrovic, 2009; Orntoft, Wolf, & Watkins, 1988). Mechanisms for incompatible blood group expression are less clear. A and B glycosyltransferases differ by four amino acid residues, so mutation could result in conversion from A to B or vice versa. Alternatively, cross-reactivity of antiblood group reagents with other tumor-associated antigens such as Forssman or Tn—both of which express terminal GalNAc—may result in apparent incompatible ABH expression. However, many antiblood group reagents are specific so this is not likely.
3.3.3.4 Function of blood group structures in tumor biology
Correlation of survival with altered blood group structures suggests that these changes contribute to tumor biology. However, the mechanistic contributions of altered ABH to the tumor phenotype may differ, depending on the tissue, since some tumors, such as colon, ectopically express ABH while others, such as lung, delete AB structures. In line with deleted AB structures in lung, A−/H+ cells display greater motility and proliferation in vitro as compared to A+/H− cells as well as changes in integrin function (Ichikawa, Handa, & Hakomori, 1998).
3.4 Genetic associations with glycogenes and cancer
Genomic studies including, GWAS, linkage analysis, transcriptomics, etc., have provided invaluable insights in biomedicine, including in cancer glycobiology, where it has been applied to investigate ppGalNAcTs in disease. ppGalNAcTs constitute a 20 enzyme family in mammals that expanded in recent evolutionary history. These enzymes exhibit partially overlapping specificities and expression, resulting in coordinated activity, which is poorly understood. Deletion of individual enzymes in higher organisms often results in subtle defects that are lethal in simpler model systems, such as Drosophila, and some mammalian GalNAcTs are not able to compensate for their lower homologs bringing into question conservation of biological function across evolutionary history (Bennett et al., 2010; Orr et al., 2013; Ten Hagen & Tran, 2002; Tran et al., 2012). To address these limitations, researchers have performed genomic studies in humans to better understand roles of ppGalNAcTs and have observed a range of associations in cardiac development, obesity, dyslipidemia, calcium/phosphate imbalance, and cancer. SNPs in ppGalNAcT1 have been associated with ovarian cancer, mutations in ppGalNAcT12 have been observed in colorectal carcinoma, elevations in transcripts have been observed for ppGalNAcT6 and ppGalNAcT14 in breast and gastric cancers, and alterations in ppGalNAcT14 have been associated with apoptotic signaling in cancer cells, connecting enzyme level to cancer biology (Berois et al., 2006; Freire et al., 2006; Gomes et al., 2009; Patani, Jiang, & Mokbel, 2008; Phelan et al., 2010; Sellers et al., 2008; Wagner et al., 2007; Wu et al., 2010). However, it is currently unclear how or whether these observed alterations/associations in ppGalNAcTs contribute to altered glycan structure or tumor biology. More studies will be needed to confirm these initial observations and provide additional mechanistic insight.
3.5 Mucins
Mucins comprise a large gene family of ~20 molecules, which can broadly be divided into secreted and membrane-associated mucins. Some mucins exhibit tissue-restricted expression, while others are broadly expressed in a range of epithelia. Biochemically, mucins are large molecules characterized by tandem repeats rich in serine, threonine, and proline. These tandem repeats are highly O-glycosylated and mucins themselves exhibit >50% O-glycosylation by weight (Hollingsworth & Swanson, 2004; Kufe, 2009). O-glycosylation is important in regulating mucin biology as defects in O-glycosylation appear to result in decreased expression of mucins in vivo and enhanced bacterial degradation in vitro (An et al., 2007; Fu et al., 2011; van der Post et al., 2013). Alterations in mucin structure, expression, and glycosylation have been observed in a range of carcinomas, and these alterations have been hypothesized to be important in the tumor phenotype (Hollingsworth & Swanson, 2004; Kufe, 2009). Changes in glycosylation include both underglycosylation and misglycosylation, for example, expression of tumor glycans Tn, STn, and SLea. Mucins maintain barrier function, retain growth factors and signaling molecules, and engage in receptor-mediated signaling. Altered mucin structure is thought to result in alterations in all of these functions. Mucins play two opposing roles in cancer—as tumor suppressors and tumor promoters—probably depending on the tissue and mucin type. Deletion of MUC2 in mice results in spontaneous tumorigenesis in intestine, colon, and rectum, while overexpression of MUC1 in mouse mammary tissue results in spontaneous mammary carcinoma (Schroeder et al., 2004; Spicer, Rowse, Lidner, & Gendler, 1995; Velcich et al., 2002).
4. CLINICAL APPLICATIONS
Overview
Discovery of tumor-associated O-glycans has led to development of clinical tools to target these antigens for detection and therapy. Application of tumor O-glycans for detection has been focused in three major areas: (1) tissue and serum biomarkers, (2) assessment of antitumor O-glycan immune responses, and (3) tumor O-glycan imaging. The second major application of tumor O-glycans is in cancer therapies. Tumor-associated O-glycans provide a unique epitope for targeted therapeutics. Cytotoxic antibodies and antibodies conjugated to radionuclides or toxins as well as therapeutic vaccines have been developed to target these TACAs. Further, efforts to interfere with the biologic contributions of tumor O-glycans to the cancer phenotype have been investigated, including interruption of sialyl Lewis–selectin interaction important in metastasis. Although many of the efforts focused on tumor detection and therapeutics are investigative, some have proved to be efficacious in patient treatment and/or management, including a few examples in which these technologies have become standard in patient management.
4.1 Cancer detection
4.1.1 Serum biomarkers
Serum biomarkers are important for diagnostics, prognostics, and following tumor response and burden. The first tests to identify tumor-associated O-glycans used immunohistochemistry to stain tumor samples. These include antibodies and lectins against truncated O-glycans Tn, STn, and T as well as altered terminal structures, such as SLex, SLea, and altered ABH antigens, as discussed above (Fig. 2). Identification of tumor antigens in tissues led to the idea that these antigens may be present in serum, allowing noninvasive tumor detection.
Healthy epithelia are polarized with apical membranes often facing a luminal structure and basolateral membranes attached to basement membrane and extracellular matrix. This polarized architecture prevents secretion of large glycoproteins into serum. In contrast, transformation results in a loss of epithelial polarity and aberrant secretion of glycoproteins into serum. These include mucins and other aberrantly glycosylated glycoproteins which contain altered O-glycans.
Some of the earliest serum TACAs identified are CA15-3, CA125, CA19-9, and STn (CA72-4) (Table 4; Reis, Osorio, Silva, Gomes, & David, 2010). These are glycoproteins or glycans, and all but STn are widely used in clinic to follow tumor progression. CA15-3 and CA125 were cloned and discovered to recognize MUC1 and MUC16, which are highly O-glycosylated and aberrantly glycosylated in patient serum (Gendler et al., 1990; Kufe et al., 1984; Siddiqui et al., 1988; Swallow et al., 1987; Yin & Lloyd, 2001). CA19-9, in contrast, recognizes SLea (Magnani et al., 1982) (Fig. 2). CA15-3, CA125, and CA19-9 are most useful in the management of breast, ovarian, and pancreatic cancer, respectively. However, none of these antigens are sufficiently specific to be used for diagnostics. Further, expression of SLea requires a functional Lewis transferase. STn is thought to be an extremely specific marker; however, STn is expressed in most carcinomas (e.g., stomach, colon, pancreas, biliary tract, ovaries, and cervix), limiting its utility in identifying tissue of origin. Recent strategies have sought to increase specificity of mucin biomarkers as well as localization of STn by identifying glycoproteins in serum containing both of these antigens. Proof-of-principle experiments using sandwich ELISAs or arrays with STn and CA125 have established increased specificity of ovarian cancer detection as compared to CA125 alone (Akita et al., 2012; Chen et al., 2013).
Table 4.
Serum biomarkers
| Biomarker | Glycan/glycoprotein antigen | Tissue | Use |
|---|---|---|---|
| CA15-3 | MUC1 | Breast | Clinical, tumor burden |
| CA125 | MUC16 | Ovarian | Clinical, tumor burden |
| CA19-9 | SLea | Pancreas | Clinical, tumor burden |
| CA72-4 | STn on Tag-72 | Pan-carcinoma | Not in general use |
| STn/CA125 | STn and CA125 | Ovarian | In development |
4.1.2 Imaging
Tumor imaging facilitates diagnosis, management, and planning as well as intra/postoperative assessment. Technologies such as MRI and X-ray evaluate features of the tissue, whereas scintigraphy, SPECT, and PET utilize radiopharmaceuticals, such as immunoradionuclides and metabolites, to assess tumor properties. Radionuclides have been optimized for various purposes and in general are transferable across probes, i.e., antibodies or antibody fragments as well as metabolites such as FDG. Pharmacokinetic properties of antibodies have revealed that antibody fragments, such as scFvs, exhibit enhanced tissue penetration and reduced Fc-dependent clearance as compared to full-length antibodies. Nonetheless, radio and pharmacokinetic properties of an agent can be optimized once a selective probe is identified.
Antitumor O-glycan antibodies provide a unique opportunity for tumor imaging and as such have been developed for underglycosylated MUC1, Tn, STn, and STn/ST (Table 5). Reagents against all of these epitopes have been evaluated in rodents and humans, and a reagent targeting STn/ST has been shown to be useful in radioguided immunosurgery (RIGS; Agnese et al., 2004; Alisauskus, Wong, & Gold, 1995; Beresford, Pavlinkova, Booth, Batra, & Colcher, 1999; Cardillo et al., 2004; Chaturvedi et al., 2008; Chinn et al., 2006; Colcher et al., 1987, 1988; Danussi et al., 2009; Esteban et al., 1987; Forero et al., 2003; Goel et al., 2001, 2000; Gold, Alisauskas, & Sharkey, 1995; Gold, Cardillo, Goldenberg, & Sharkey, 2001; Gold, Cardillo, Vardi, & Blumenthal, 1997; Gold et al., 2008; Gulec et al., 2011; Karacay et al., 2009; Mariani et al., 1995; Milenic et al., 1991; Nakamoto et al., 1998; Pavlinkova, Beresford, Booth, Batra, & Colcher, 1999; Pavlinkova, Booth, Batra, & Colcher, 1999; Salouti, Rajabi, Babaei, & Rasaee, 2008; Slavin-Chiorini et al., 1993, 1997, 1995; Xiao et al., 2005; Yao et al., 1995; Yokota, Milenic, Whitlow, & Schlom, 1992; Yokota et al., 1993; Zhang et al., 1998; Zou et al., 2010). Probes and radionuclides have been optimized for many of the antitumor O-glycans tested to date. Interestingly, one of the most researched antibodies for imaging is CC49, which reacts with ST>STn on TAG-72(Colcher et al., 1988; Hanisch, Uhlenbruck, Egge, & Peter-Katalinic, 1989; O’Boyle et al., 1996).
Table 5.
Tumor imaging
| Antigen | Antibody/agent | Probe/source | Application | Stage of development | Tissue | Citations |
|---|---|---|---|---|---|---|
| MUC1 | ||||||
| MUC1 | hPam4 | 111In/125I/131I | SPECT, gamma planar imaging | Rodents, humans | PAC xenograft, in situ | Gulec et al. (2011), Alisauskus et al., 1995, Gold et al. (1995, 2001, 1997) and Mariani et al., 1995 |
| MUC1 | TF10 | 125I | SPECT, gamma planar imaging | Rodents | PAC xenograft | Gold et al. (2008) |
| MUC1 | IMP-288 (TF10—pretarget) | 90Y | SPECT, gamma planar imaging | Rodents | PAC xenograft | Karacay et al. (2009) |
| MUC1 | bsPAM4 | 125I | SPECT, gamma planar imaging | Rodents | PAC xenograft | Cardillo et al. (2004) |
| MUC1 | IMP-192 (bsPAM4-pretarget) | 99mTc | SPECT, gamma planar imaging | Rodents | PAC xenograft | Cardillo et al. (2004) |
| MUC1 | IMP-166 (bsPAM4—pretarget) | 111In | SPECT, gamma planar imaging | Rodents | PAC xenograft | Cardillo et al. (2004) |
| MUC1 | PR81 | 99mTc | SPECT, gamma planar imaging | Rodents | Breast xenograft | Salouti et al. (2008) |
| Tn | ||||||
| Tn | Anti-Tn 2154F12A4 mAb | Qdot 800 | Near-infrared fluorescence optical imaging | Rodents | Breast xenograft | Danussi et al. (2009) |
| Tn | MLS128 | 111In | SPECT, gamma planar imaging | Rodents | CRC xenograft | Yao et al. (1995) |
| Tn | MLS128 | 125I/131I | SPECT, gamma planar imaging | Rodents | CRC xenograft | Yao et al. (1995) |
| Tn | MLS128 (biotin-Ab, streptavidin-probe) | 125I | SPECT, gamma planar imaging | Rodents | CRC xenograft | Zhang et al. (1998) |
| Tn | MLS128 (biotin-Ab, streptavidin, biotin-probe) | 111In | SPECT, gamma planar imaging | Rodents | CRC xenograft | Nakamoto et al. (1998) |
| STn | ||||||
| B72.3 | 131I | Gamma planar imaging | Rodents, humans | CRC, CRC xenograft | Colcher et al. (1987, 1988) and Esteban et al. (1987) | |
| ST>STn | ||||||
| ST>STn | CC49 (scFv)2 | 125I/131I | SPECT, gamma planar imaging | Rodents | CRC xenograft | Beresford et al. (1999), Pavlinkova et al., 1999 and Pavlinkova et al., 1999 |
| ST>STn | CC49 Humanized ΔCH2 | 125I/131I | SPECT, gamma planar imaging | Rodents, humans | Xiao et al. (2005), Slavin-Chiorini et al. (1993, 1997, 1995), Forero et al. (2003) and Agnese et al. (2004) | |
| ST>STn | CC49 Fab′ | 125I | SPECT, gamma planar imaging | Rodents | Milenic et al. (1991) and Yokota et al. (1992, 1993) | |
| ST>STn | CC49 sc(Fv)2 | 99mTc | SPECT, gamma planar imaging | Rodents | CRC xenograft | Goel et al. (2001) |
| ST>STn | CC49 sc(Fv)2 | I125 | SPECT, gamma planar imaging | Rodents | CRC xenograft | Beresford et al. (1999) |
| ST>STn | CC49 Humanized ΔCH2 | 111In | SPECT, gamma planar imaging | Rodents | CRC xenograft | Chinn et al. (2006) |
| ST>STn | CC49 [sc(Fv)2]2 | 125I/131I | SPECT, gamma planar imaging | Rodents | CRC xenograft | Goel et al. (2000) |
| ST>STn | CC49 (Fab′)2 | 125I/131I | SPECT, gamma planar imaging | Rodents | CRC xenograft | Milenic et al. (1991) |
| ST>STn | CC49 | 124I | PET | Rodents | CRC xenograft | Zou et al. (2010) |
| T | ||||||
| T | JAA-F11 | 124I | PET | Rodents | Breast xenograft | Chaturvedi et al. (2008) |
PAC, pancreatic adenocarcinoma; SPECT, single-photon emission computed tomography; PET, positron emission tomography; CRC, colorectal carcinoma
CC49 has been validated in RIGS and was initially developed as a second-generation antibody against TAG-72 (tumor-associated glycoprotein-72)(Agnese et al., 2004). CC49 binds TAG-72 and is inhibited by ST but not STn (Hanisch et al., 1989). Further, it binds ST>STn in vitro, with some reactivity against both antigens (O’Boyle et al., 1996). Although ST is not generally considered to be a TACA, its presentation on TAG-72 appears to be tumor-associated. In contrast to CC49, the antibody B72.3 reacts with STn on TAG-72(O’Boyle et al., 1996). Based on the high expression of STn on tumors, this begs the question as to why there has been tremendous investment in CC49 as compared with B72.3 for imaging.
A major line of evidence that likely contributed to this shift in evaluation of CC49 highlights an important lesson in tumor glycobiology—that tumor cells are not always representative of tumor tissue glycosylation. Second-generation CC series anti-TAG-72 antibodies were developed and tested in a colorectal cancer cell xenotransplant model (Colcher et al., 1988). Many of the CC series, especially CC49, exhibited greater reactivity with the colorectal cancer cell in vivo. Importantly, the cell line evaluated was LS174T. LS174T comprise a mixed population of cells with a small fraction of cells exhibiting a loss of Cosmc and expression of Tn and STn antigens while the larger fraction exhibits intact O-glycosylation (Ju, Lanneau, et al., 2008). This differs from real tumor biology in which STn, along with Tn, is highly expressed as a TACA in contrast to ST which is generally considered a normal O-glycan structure. Whether B72.3 or other STn/Tn immunoradionuclides would outperform CC49 in RIGS or other applications requires further investigation.
4.1.3 Assessing anti-O-glycan immune responses
The discovery that tumors elicit autoantibodies has led to attempts to use these autoantibodies and other immunologic parameters for clinical evaluation and diagnosis (Sahin et al., 1995; Wang et al., 2005). Most of the early attempts to identify tumor autoantibodies focused on protein recognitions; however, early evidence from Springer suggested that tumors elicit glycan-specific immune responses, which result in changes in antiglycan antibodies and induction of antiglycan cellular immunity (Springer, 1984).
Recently, autoantibodies produced against tumor O-glycans have been evaluated in well-defined glycopeptide arrays and suggest that a portion of cancer patients with breast, ovarian, and prostate cancers generate IgG against MUC1-Tn, STn, T, and core 3 tumor glycopeptides (Wandall et al., 2010). Antibodies appeared to be glycopeptide-specific, not reacting with peptide or glycan alone. Autoantibodies against core 3 were surprising, as core 3 has not previously been shown to be expressed in many of the tissues evaluated and it is believed to be a normal O-glycan in the GI tract. In contrast to IgG, antiglycan and glycopeptide IgM antibodies were present in patients and controls, confirming Springer’s early work that normal gut bacteria induce antiglycan antibodies.
Autoantibody arrays have also been used to predict clinical course. In a subset of breast cancer patients, elevated autoantibodies against STn-MUC1 and core 3-MUC1 correlated with survival (Blixt et al., 2011). This supports the intriguing possibility that normal immune responses against glycopeptides may contribute to cancer clearance.
4.2 Cancer therapeutics
Generation of mAbs against tumor antigens led to the idea that tumors express unique antigens that may be targeted by antibodies or therapeutic vaccines. These approaches are currently being evaluated for a variety of tumor O-glycans and have resulted in a variety of important lessons in the glycoimmunology and cancer glycoimmunology fields.
4.2.1 Passive immunotherapies
Passive cancer immunotherapies include unconjugated antibodies with intrinsic cytolytic activity (antibody-dependent cell-mediated cytotoxicity, ADCC), antibodies conjugated to radionuclides (radioimmunotherapy), and antibodies-conjugated toxins (immunotoxins). Reagents targeting tumor O-glycans have been developed for unconjugated antibodies and immunotoxins; however, the emerging success of radiolabeled antibodies for imaging highlight the potential for radioimmnotherapies.
Antibodies against Tn, STn, and mucins have been generated which exhibit ADCC-dependent and independent cytolytic activities, some of which have been evaluated in vivo (Ando et al., 2008; Hubert et al., 2011; Ibrahim et al., 2011; Kubota, Matsushita, Niwa, Kumagai, & Nakamura, 2010; Morita, Yajima, Asanuma, Nakada, & Fujita-Yamaguchi, 2009; Pegram et al., 2009; Welinder, Baldetorp, Borrebaeck, Fredlund, & Jansson, 2011). A major limitation of this approach, however, is that full-length antibodies with Fc are often required for cytolytic activities but exhibit poor tissue penetration. Further, not all highly specific antibodies exhibit cytolytic activities, although these can sometimes be engineered, for example, by isotype swapping.
To address some of these challenges, researchers have developed immunotoxins in which antibody or antibody fragments have been conjugated to bacterial toxins. When these antibodies bind the cell surface, they are endocytosed and the toxins are activated within the cell, resulting in cell death. This is a powerful approach but requires the antibodies to recognize antigens that are selectively expressed on the tumor surface and not normal cells. Therefore, this approach would theoretically be promising for tumor-specific O-glycans like Tn and STn but are less likely to be successful for tumor-associated O-glycans, such as Lewis or blood group antigens. Nonetheless, an antibody specific for Ley, which is elevated in some carcinomas, was generated and tested in vivo. Initial results were promising but dose-limiting toxicities, including gastritis, were observed and most likely resulted from reaction with low levels of antigen present in normal tissues (Pai, Wittes, Setser, Willingham, & Pastan, 1996; Pastan, Hassan, Fitzgerald, & Kreitman, 2006; Pastan et al., 1991).
4.2.2 Therapeutic vaccines
Although long controversial, landmark studies with melanoma and prostrate cancer established therapeutic vaccination as a viable approach for cancer treatment (Kantoff et al., 2010; Schwartzentruber et al., 2011). Further, early work from Springer suggested that vaccination with T/Tn containing substance (in his case RBC) could elicit antiglycan immune responses and may improve patient survival (Springer, 1984). More recent work suggests that anticancer immune response may correlate with patient survival. Together, these lines of evidence suggest that therapeutic vaccination using tumor-associated O-glycans may be able to generate antiglycan and antitumor immunity.
In contrast to the traditional view that glycans elicit T-cell-independent immunity, producing IgM and not IgG, and that glycoconjugates rely on peptide–MHC and not glycopeptide–MHC interactions for presentation, work over the last 10–20 years has established that (1) certain classes of glycans, such as zwitterionic polysaccharides, elicit T-cell-dependent help through ROS-mediated endolysosomal processing and presentation by MHC-II to CD4 T cells, (2) glycopeptides are generated and processed in the endolysosome and presented as a glycopeptide in the MHC-II for presentation to glycopeptide-specific TCRs of T helper cells, so-called Tcarbs, (3) glycopeptides can be presented by MHC-I and recognized by glycopeptide-specific cytolytic T cells, and (4) antiglycan IgG is abundant in human sera (Avci, Li, Tsuji, & Kasper, 2011; Cobb, Wang, Tzianabos, & Kasper, 2004; Haurum et al., 1994; Springer et al., 1993; Stowell et al., 2014). Further, Tn and STn can elicit natural T-cell-dependent antiglycan or glycopeptide responses in cancer patients as well as in immunization while linked to carrier proteins or presented as glycopeptides (Gilewski et al., 2007; Ingale, Wolfert, Gaekwad, Buskas, & Boons, 2007; Julien et al., 2012; Napoletano et al., 2007; Pedersen et al., 2011; Sabbatini et al., 2007; Sorensen et al., 2006; von Mensdorff-Pouilly et al., 2000; Wandall et al., 2010). Although early attempts to generate antiglycan and anti-O-glycan immune responses capable of impacting clinical course were empirical and exhibited mixed success, lessons learned over the last 20 years as well proof-of-principle glycovaccine engineering with other diseases and tumor models provide a basis for systematic development and evaluation of therapeutic vaccines targeting tumor-associated O-glycans (Avci et al., 2011).
4.2.3 Selectin–Lewis interactions
SLea and SLex interact with selectins on endothelial cells, platelets, and leukocytes. Tumors overexpress sialyl Lewis antigens, facilitating metastasis via enhanced platelet interactions in blood and enhanced endothelial interaction during vascular extravasation. Platelets bind tumor in blood, forming a “cloak” that protects tumor cells from antitumor cellular immunity. Blocking SLea/x–selectin interactions in model systems through decoy disaccharides or peptide mimics has provided proof-of-concept that blocking SLe–selectin interactions can block metastasis (Fukuda et al., 2000; Fuster et al., 2003). Further, work in mice and clinical trials suggested that interactions of P-selectin and SLea/x could be blocked by heparin, resulting in improved course of disease in mice and patients (Borsig et al., 2001; Lee et al., 2005). These results appear independent of anticoagulant effects as benefits were observed to a lesser degree in patients treated with coumarin anticoagulant. Cimetidine, an H2 blocker, was also shown to increase survival among patients with colorectal cancer through downregulating E-selectin expressed on endothelia. This effect was most pronounced among patients with high level of SLea/x expression in tumors and least pronounced among patients with low SLea/x expression (Matsumoto et al., 2002). Results from mouse models using specific SLe–selectin inhibitors as well as human studies using heparin and cimetidine provide strong evidence that SLe–selectin interactions may be a viable target for cancer treatment; however, no conclusions can be made until specific inhibitors are tested in patients in randomized controlled prospective studies.
5. CONCLUSIONS
Since the discovery of altered glycosylation in cancer through lectin binding, mAbs, and glycopeptide analysis, altered glycosylation has been observed in most every cancer to date establishing it as a hallmark of cancer. Altered O-glycosylation was arguably first observed with alterations in blood group structures in gastric cancer, but validated for truncated pan-carcinoma O-glycans T/Tn with the work of Springer and colleagues. Alterations include expression of truncated, tumor-specific O-glycans such as Tn, STn, and T antigens, which are only found on O-glycans, loss of terminal α-GlcNAc on core 2 in gastric cancer, and altered terminal tumor-associated structures, such as ABH and SLea/x structures, which are found on various classes of glycoconjugates.
Recent advances have highlighted possible mechanisms for altered expression of tumor O-glycans, including deletion, epigenetic silencing, and LOH of Cosmc resulting in Tn/STn expression, hypoxia-induced transcriptional changes, resulting in increased expression of SLea/x and T antigens, as well as epigenetic silencing of A, B transferases. The extent of these and other mechanistic alterations is currently unclear and will require further investigation.
New insights into whether and how altered O-glycans contribute to the tumor phenotype have also developed. Expression of Tn and STn results in increased susceptibility to tumorigenesis in core 3 knockout mice, and loss of α-GlcNAc contributes to gastric tumorigenesis in H. pylori-dependent and -independent manners. T antigen expression has been implicated in tumorigenesis in xenotransplant studies as have deletion of ABH structures and overexpression of SLea/x. Tn, STn, α-GlcNAc, ABH, and SLea/x correlate with patient survival across studies, and blocking SLea/x through heparin or cimetidine increases patient survival in clinical studies.
Antibodies against glycoproteins and glycans, including CA15-3, CA19-9, and CA125, are in clinical use but used for prognostics and not diagnostics due to poor specificities. Development of new antibodies against tumor O-glycans and use of multiple antibodies, such as CA125 and anti-STn, will increase sensitivities and specificities, allowing broader application of these reagents. Imaging with radiolabeled antiglycan antibodies provides a unique opportunity to localize tumors and has proved useful in surgical planning. Although no agent targeting tumor O-glycans is in standard clinical use, various approaches including radioimmunotherapies, immunotoxins, therapeutic vaccines, and glycan-GBP inhibitors are promising in preclinical and some clinical studies. The discovery of tumor O-glycans and associated applications for tumor detection, evaluation, and therapeutics offer great promise in the management of this terrible disease.
Acknowledgments
The work of the authors was supported by NIH Grant U01CA168930 to T. J. and R. D. C.
References
- Agnese DM, Abdessalam SF, Burak WE, Jr, Arnold MW, Soble D, Hinkle GH, et al. Pilot study using a humanized CC49 monoclonal antibody (HuCC49DeltaCH2) to localize recurrent colorectal carcinoma. Annals of Surgical Oncology. 2004;11(2):197–202. doi: 10.1245/aso.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Akita K, Yoshida S, Ikehara Y, Shirakawa S, Toda M, Inoue M, et al. Different levels of sialyl-Tn antigen expressed on MUC16 in patients with endometriosis and ovarian cancer. International Journal of Gynecological Cancer. 2012;22(4):531–538. doi: 10.1097/IGC.0b013e3182473292. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Viney EM, Zhang JG, Metcalf D, Kauppi M, Hyland CD, et al. Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16442–16447. doi: 10.1073/pnas.0607872103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alisauskus R, Wong GY, Gold DV. Initial studies of monoclonal antibody PAM4 targeting to xenografted orthotopic pancreatic cancer. Cancer Research. 1995;55(23 Suppl):5743s–5748s. [PubMed] [Google Scholar]
- An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. The Journal of Experimental Medicine. 2007;204(6):1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, et al. Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biological & Pharmaceutical Bulletin. 2008;31(9):1739–1744. doi: 10.1248/bpb.31.1739. [DOI] [PubMed] [Google Scholar]
- Aryal RP, Ju T, Cummings RD. The endoplasmic reticulum chaperone Cosmc directly promotes in vitro folding of T-synthase. The Journal of Biological Chemistry. 2010;285(4):2456–2462. doi: 10.1074/jbc.M109.065169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal RP, Ju T, Cummings RD. Tight complex formation between Cosmc chaperone and its specific client non-native T-synthase leads to enzyme activity and client-driven dissociation. The Journal of Biological Chemistry. 2012;287(19):15317–15329. doi: 10.1074/jbc.M111.312587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal RP, Ju T, Cummings RD. Identification of a novel protein binding motif within the T-synthase for the molecular chaperone Cosmc. The Journal of Biological Chemistry. 2014;289(17):11630–11641. doi: 10.1074/jbc.M114.555870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson BF, Ernst CS, Herlyn M, Steplewski Z, Sears HF, Koprowski H. Gastrointestinal cancer-associated antigen in immunoperoxidase assay. Cancer Research. 1982;42(11):4820–4823. [PubMed] [Google Scholar]
- Aub JC, Tieslau C, Lankester A. Reactions of normal and tumor cell surfaces to enzymes. I. wheat-germ lipase and associated mucopolysaccharides. Proceedings of the National Academy of Sciences of the United States of America. 1963;50:613–619. doi: 10.1073/pnas.50.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nature Medicine. 2011;17(12):1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babino A, Oppezzo P, Bianco S, Barrios E, Berois N, Navarrete H, et al. Tn antigen is a pre-cancerous biomarker in breast tissue and serum in n-nitrosomethylurea-induced rat mammary carcinogenesis. International Journal of Cancer. 2000;86(6):753–759. doi: 10.1002/(sici)1097-0215(20000615)86:6<753::aid-ijc1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bennett EP, Chen YW, Schwientek T, Mandel U, Schjoldager K, Cohen SM, et al. Rescue of Drosophila melanogaster l(2)35Aa lethality is only mediated by polypeptide GalNAc-transferase pgant35A, but not by the evolutionary conserved human ortholog GalNAc-transferase-T11. Glycoconjugate Journal. 2010;27(4):435–444. doi: 10.1007/s10719-010-9290-5. [DOI] [PubMed] [Google Scholar]
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford GW, Pavlinkova G, Booth BJ, Batra SK, Colcher D. Binding characteristics and tumor targeting of a covalently linked divalent CC49 single-chain antibody. International Journal of Cancer. 1999;81(6):911–917. doi: 10.1002/(sici)1097-0215(19990611)81:6<911::aid-ijc12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Berger AC, Garcia M, Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2008;26(36):5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berois N, Mazal D, Ubillos L, Trajtenberg F, Nicolas A, Sastre-Garau X, et al. UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferas e-6 as a new immunohistochemical breast cancer marker. The Journal of Histochemistry and Cytochemistry. 2006;54(3):317–328. doi: 10.1369/jhc.5A6783.2005. [DOI] [PubMed] [Google Scholar]
- Berriel E, Hill M, Barcia JJ, Ubillos L, Gonzalez M, Detjen G, et al. Simple mucin-type cancer associated antigens are pre-cancerous biomarkers during 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Oncology Reports. 2005;14(1):219–227. [PubMed] [Google Scholar]
- Bianco T, Farmer BJ, Sage RE, Dobrovic A. Loss of red cell A, B, and H antigens is frequent in myeloid malignancies. Blood. 2001;97(11):3633–3639. doi: 10.1182/blood.v97.11.3633. [DOI] [PubMed] [Google Scholar]
- Bianco-Miotto T, Hussey DJ, Day TK, O’Keefe DS, Dobrovic A. DNA methylation of the ABO promoter underlies loss of ABO allelic expression in a significant proportion of leukemic patients. PLoS One. 2009;4(3):e4788. doi: 10.1371/journal.pone.0004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancone L, Araki M, Araki K, Vassalli P, Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. The Journal of Experimental Medicine. 1996;183(2):581–587. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(19):9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M, et al. Auto-antibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Research. 2011;13(2):R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. The Journal of Biological Chemistry. 1986;261(5):2434–2440. [PubMed] [Google Scholar]
- Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: Glycodynamics and functions. EMBO Reports. 2006;7(6):599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhausen I, Yang JM, Burchell J, Whitehouse C, Taylor-Papadimitriou J. Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. European Journal of Biochemistry. 1995;233(2):607–617. doi: 10.1111/j.1432-1033.1995.607_2.x. [DOI] [PubMed] [Google Scholar]
- Bruehl RE, Bertozzi CR, Rosen SD. Minimal sulfated carbohydrates for recognition by L-selectin and the MECA-79 antibody. The Journal of Biological Chemistry. 2000;275(42):32642–32648. doi: 10.1074/jbc.M001703200. [DOI] [PubMed] [Google Scholar]
- Buck CA, Glick MC, Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Butcher EC, Rouse RV, Coffman RL, Nottenburg CN, Hardy RR, Weissman IL. Surface phenotype of Peyer’s patch germinal center cells: Implications for the role of germinal centers in B cell differentiation. Journal of Immunology. 1982;129(6):2698–2707. [PubMed] [Google Scholar]
- Campbell BJ, Finnie IA, Hounsell EF, Rhodes JM. Direct demonstration of increased expression of Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and ulcerative colitis mucin and its concealment in normal mucin. The Journal of Clinical Investigation. 1995;95(2):571–576. doi: 10.1172/JCI117700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76(10):1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Capon C, Maes E, Michalski JC, Leffler H, Kim YS. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. The Biochemical Journal. 2001;358(Pt 3):657–664. doi: 10.1042/bj3580657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo TM, Karacay H, Goldenberg DM, Yeldell D, Chang CH, Modrak DE, et al. Improved targeting of pancreatic cancer: Experimental studies of a new bispecific antibody, pretargeting enhancement system for immunoscintigraphy. Clinical Cancer Research. 2004;10(10):3552–3561. doi: 10.1158/1078-0432.CCR-03-0340. [DOI] [PubMed] [Google Scholar]
- Carubelli R, Bhavanandan P, Gottschalk A. Studies on glycoproteins. XIi. The O-glycosidic linkage of n-acetylgalactosamine to seryl and threonyl residues in ovine submaxillary gland glycoprotein. Biochimica et Biophysica Acta. 1965;101:67–82. doi: 10.1016/0926-6534(65)90031-x. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Heimburg J, Yan J, Koury S, Sajjad M, Abdel-Nabi HH, et al. Tumor immunolocalization using 124 I-iodine-labeled JAA-F11 antibody to Thomsen-Friedenreich alpha-linked antigen. Applied Radiation and Isotopes. 2008;66(3):278–287. doi: 10.1016/j.apradiso.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Gentry-Maharaj A, Burnell M, Steentoft C, Marcos-Silva L, Mandel U, et al. Microarray Glycoprofiling of CA125 improves differential diagnosis of ovarian cancer. Journal of Proteome Research. 2013;12(3):1408–1418. doi: 10.1021/pr3010474. [DOI] [PubMed] [Google Scholar]
- Chinn PC, Morena RA, Santoro DA, Kazules T, Kashmiri SV, Schlom J, et al. Pharmacokinetics and tumor localization of (111)in-labeled HuCC49DeltaC(H) 2 in BALB/c mice and athymic murine colon carcinoma xenograft. Cancer Biotherapy & Radiopharmaceuticals. 2006;21(2):106–116. doi: 10.1089/cbr.2006.21.106. [DOI] [PubMed] [Google Scholar]
- Clausen H, Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sanguinis. 1989;56(1):1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117(5):677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D, Esteban JM, Carrasquillo JA, Sugarbaker P, Reynolds JC, Bryant G, et al. Quantitative analyses of selective radiolabeled monoclonal antibody localization in metastatic lesions of colorectal cancer patients. Cancer Research. 1987;47(4):1185–1189. [PubMed] [Google Scholar]
- Colcher D, Hand PH, Nuti M, Schlom J. A spectrum of monoclonal antibodies reactive with human mammary tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(5):3199–3203. doi: 10.1073/pnas.78.5.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D, Minelli MF, Roselli M, Muraro R, Simpson-Milenic D, Schlom J. Radioimmunolocalization of human carcinoma xenografts with B72.3 second generation monoclonal antibodies. Cancer Research. 1988;48(16):4597–4603. [PubMed] [Google Scholar]
- Cummings RD. The repertoire of glycan determinants in the human glycome. Molecular BioSystems. 2009;5(10):1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S, Schneider WJ, Hobgood KK, Tolleshaug H, Brown MS, et al. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. The Journal of Biological Chemistry. 1983;258(24):15261–15273. [PubMed] [Google Scholar]
- Cummings RD, Pierce JM. The challenge and promise of glycomics. Chemical Biology. 2014;21(1):1–15. doi: 10.1016/j.chembiol.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahr W, Uhlenbruck G, Bird GW. Cryptic A-like receptor sites in human erythrocyte glycoproteins: Proposed nature of Tn-antigen. Vox Sanguinis. 1974;27(1):29–42. doi: 10.1111/j.1423-0410.1974.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Dall’Olio F, Chiricolo M. Sialyltransferases in cancer. Glycoconjugate Journal. 2001;18(11–12):841–850. doi: 10.1023/a:1022288022969. [DOI] [PubMed] [Google Scholar]
- Danussi C, Coslovi A, Campa C, Mucignat MT, Spessotto P, Uggeri F, et al. A newly generated functional antibody identifies Tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology. 2009;19(10):1056–1067. doi: 10.1093/glycob/cwp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dausset J, Moullec J, Bernard J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn) Blood. 1959;14:1079–1093. [PubMed] [Google Scholar]
- David L, Nesland JM, Clausen H, Carneiro F, Sobrinho-Simoes M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS. Supplementum. 1992;27:162–172. [PubMed] [Google Scholar]
- Dennis JW, Laferte S. Oncodevelopmental expression of—GlcNAc beta 1–6Man alpha 1–6Man beta 1—Branched asparagine-linked oligosaccharides in murine tissues and human breast carcinomas. Cancer Research. 1989;49(4):945–950. [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Dennis J, Waller C, Timpl R, Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982;300(5889):274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- Desai PR. Immunoreactive T and Tn antigens in malignancy: Role in carcinoma diagnosis, prognosis, and immunotherapy. Transfusion Medicine Reviews. 2000;14(4):312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- Do SI, Cummings RD. Presence of O-linked oligosaccharide on a threonine residue in the human transferrin receptor. Glycobiology. 1992;2(4):345–353. doi: 10.1093/glycob/2.4.345. [DOI] [PubMed] [Google Scholar]
- Do SI, Enns C, Cummings RD. Human transferrin receptor contains O-linked oligosaccharides. The Journal of Biological Chemistry. 1990;265(1):114–125. [PubMed] [Google Scholar]
- Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9(6):881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- Esteban JM, Colcher D, Sugarbaker P, Carrasquillo JA, Bryant G, Thor A, et al. Quantitative and qualitative aspects of radiolocalization in colon cancer patients of intravenously administered MAb B72.3. International Journal of Cancer. 1987;39(1):50–59. doi: 10.1002/ijc.2910390110. [DOI] [PubMed] [Google Scholar]
- Fakhro KA, Choi M, Ware SM, Belmont JW, Towbin JA, Lifton RP, et al. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2915–2920. doi: 10.1073/pnas.1019645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg VR, Alvarez K, Roman C, Fregien N. Multiple transcription initiation and alternative splicing in the 5′ untranslated region of the core 2 beta1-6 N-acetylglucosaminyltransferase I gene. Glycobiology. 2003;13(6):411–418. doi: 10.1093/glycob/cwg039. [DOI] [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW. Beta 1–6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Research. 1991;51(2):718–723. [PubMed] [Google Scholar]
- Forero A, Meredith RF, Khazaeli MB, Carpenter DM, Shen S, Thornton J, et al. A novel monoclonal antibody design for radioimmunotherapy. Cancer Biotherapy & Radiopharmaceuticals. 2003;18(5):751–759. doi: 10.1089/108497803770418292. [DOI] [PubMed] [Google Scholar]
- Freeze HH, Ng BG. Golgi glycosylation and human inherited diseases. Cold Spring Harbor Perspectives in Biology. 2011;3(9):a005371. doi: 10.1101/cshperspect.a005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire T, Berois N, Sonora C, Varangot M, Barrios E, Osinaga E. UDP-N-acetyl-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. International Journal of Cancer. 2006;119(6):1383–1388. doi: 10.1002/ijc.21959. [DOI] [PubMed] [Google Scholar]
- Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. The Journal of Clinical Investigation. 2011;121(4):1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Roles of mucin-type O-glycans synthesized by core2beta1,6-N-acetylglucosaminyltransferase. Methods in Enzymology. 2006;416:332–346. doi: 10.1016/S0076-6879(06)16022-X. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Bothner B, Ramsamooj P, Dell A, Tiller PR, Varki A, et al. Structures of sialylated fucosyl polylactosaminoglycans isolated from chronic myelogenous leukemia cells. The Journal of Biological Chemistry. 1985;260(24):12957–12967. [PubMed] [Google Scholar]
- Fukuda M, Carlsson SR, Klock JC, Dell A. Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. The Journal of Biological Chemistry. 1986;261(27):12796–12806. [PubMed] [Google Scholar]
- Fukuda MN, Ohyama C, Lowitz K, Matsuo O, Pasqualini R, Ruoslahti E, et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Research. 2000;60(2):450–456. [PubMed] [Google Scholar]
- Fukushima K, Hirota M, Terasaki PI, Wakisaka A, Togashi H, Chia D, et al. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Research. 1984;44(11):5279–5285. [PubMed] [Google Scholar]
- Fuster MM, Brown JR, Wang L, Esko JD. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Research. 2003;63(11):2775–2781. [PubMed] [Google Scholar]
- Fuster MM, Esko JD. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nature Reviews. Cancer. 2005;5(7):526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- Ganzinger U, Deutsch E. Serum sialyltransferase levels as a parameter in the diagnosis and follow-up of gastrointestinal tumors. Cancer Research. 1980;40(4):1300–1304. [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. The Journal of Biological Chemistry. 1990;265(25):15286–15293. [PubMed] [Google Scholar]
- Gerken TA, Raman J, Fritz TA, Jamison O. Identification of common and unique peptide substrate preferences for the UDP-GalNAc:Polypeptide alpha-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. The Journal of Biological Chemistry. 2006;281(43):32403–32416. doi: 10.1074/jbc.M605149200. [DOI] [PubMed] [Google Scholar]
- Gilewski TA, Ragupathi G, Dickler M, Powell S, Bhuta S, Panageas K, et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clinical Cancer Research. 2007;13(10):2977–2985. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- Gill DJ, Chia J, Senewiratne J, Bard F. Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. The Journal of Cell Biology. 2010;189(5):843–858. doi: 10.1083/jcb.201003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, et al. The role of Thomsen-Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Research. 2001;61(12):4851–4857. [PubMed] [Google Scholar]
- Goel A, Baranowska-Kortylewicz J, Hinrichs SH, Wisecarver J, Pavlinkova G, Augustine S, et al. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv’s: Novel imaging agents for rapid in vivo localization of human colon carcinoma. Journal of Nuclear Medicine. 2001;42(10):1519–1527. [PubMed] [Google Scholar]
- Goel A, Colcher D, Baranowska-Kortylewicz J, Augustine S, Booth BJ, Pavlinkova G, et al. Genetically engineered tetravalent single-chain Fv of the pancarcinoma monoclonal antibody CC49: Improved biodistribution and potential for therapeutic application. Cancer Research. 2000;60(24):6964–6971. [PubMed] [Google Scholar]
- Gold DV, Alisauskas R, Sharkey RM. Targeting of xenografted pancreatic cancer with a new monoclonal antibody, PAM4. Cancer Research. 1995;55(5):1105–1110. [PubMed] [Google Scholar]
- Gold DV, Cardillo T, Goldenberg DM, Sharkey RM. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Critical Reviews in Oncology/Hematology. 2001;39(1–2):147–154. doi: 10.1016/s1040-8428(01)00114-7. [DOI] [PubMed] [Google Scholar]
- Gold DV, Cardillo T, Vardi Y, Blumenthal R. Radioimmunotherapy of experimental pancreatic cancer with 131I-labeled monoclonal antibody PAM4. International Journal of Cancer. 1997;71(4):660–667. doi: 10.1002/(sici)1097-0215(19970516)71:4<660::aid-ijc24>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gold DV, Goldenberg DM, Karacay H, Rossi EA, Chang CH, Cardillo TM, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Research. 2008;68(12):4819–4826. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, Pinto-de-Sousa J, et al. Expression of UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. The Journal of Histochemistry and Cytochemistry. 2009;57(1):79–86. doi: 10.1369/jhc.2008.952283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A, Murphy WH. Studies on mucoproteins. IV. The linkage of the prosthetic group to the protein core in ovine submaxillary gland mucoprotein. Biochimica et Biophysica Acta. 1961;46:81–90. doi: 10.1016/0006-3002(61)90648-5. [DOI] [PubMed] [Google Scholar]
- Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nature Medicine. 2000;6(3):306–312. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- Graziano SL, Tatum AH, Gonchoroff NJ, Newman NB, Kohman LJ. Blood group antigen A and flow cytometric analysis in resected early-stage non-small cell lung cancer. Clinical Cancer Research: An official journal of the American Association for Cancer Research. 1997;3(1):87–93. [PubMed] [Google Scholar]
- Gulec SA, Cohen SJ, Pennington KL, Zuckier LS, Hauke RJ, Horne H, et al. Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: A phase I single-dose escalation trial. Clinical Cancer Research. 2011;17(12):4091–4100. doi: 10.1158/1078-0432.CCR-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Research. 2002;62(23):6837–6845. [PubMed] [Google Scholar]
- Gupta MK, Arciaga R, Bocci L, Tubbs R, Bukowski R, Deodhar SD. Measurement of a monoclonal-antibody-defined antigen (CA19-9) in the sera of patients with malignant and nonmalignant diseases. Comparison with carcinoembryonic antigen comparative study. Cancer. 1985;56(2):277–283. doi: 10.1002/1097-0142(19850715)56:2<277::aid-cncr2820560213>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gwin JL, Klein-Szanto AJ, Zhang SY, Agarwal P, Rogatko A, Keller SM. Loss of blood group antigen A in non-small cell lung cancer. Annals of Surgical Oncology. 1994;1(5):423–427. doi: 10.1007/BF02303816. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Research. 1996;56(23):5309–5318. [PubMed] [Google Scholar]
- Hamada S, Furumoto H, Kamada M, Hirao T, Aono T. High expression rate of Tn antigen in metastatic lesions of uterine cervical cancers. Cancer Letters. 1993;74(3):167–173. doi: 10.1016/0304-3835(93)90239-6. [DOI] [PubMed] [Google Scholar]
- Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321(5897):1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch FG, Uhlenbruck G, Egge H, Peter-Katalinic J. A B72.3 second-generation-monoclonal antibody (CC49) defines the mucin-carried carbohydrate epitope Gal beta(1–3) [NeuAc alpha(2–6)]GalNAc. Biological Chemistry Hoppe-Seyler. 1989;370(1):21–26. doi: 10.1515/bchm3.1989.370.1.21. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S. NetOglyc: Prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconjugate Journal. 1998;15(2):115–130. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001;83(8):727–737. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- Haurum JS, Arsequell G, Lellouch AC, Wong SY, Dwek RA, McMichael AJ, et al. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. The Journal of Experimental Medicine. 1994;180(2):739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(48):18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Priest JW, Live D, Boons GJ, Song X, Cummings RD, et al. Microarray analysis of the human antibody response to synthetic Cryptosporidium glycopeptides. International Journal for Parasitology. 2013;43(11):901–907. doi: 10.1016/j.ijpara.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. The Journal of Experimental Medicine. 1994;180(6):2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S, Oriol R, Samuelsson B. Lewis histo-blood group system and associated secretory phenotypes. Vox Sanguinis. 1995;69(3):166–182. doi: 10.1111/j.1423-0410.1995.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Higgins EA, Siminovitch KA, Zhuang DL, Brockhausen I, Dennis JW. Aberrant O-linked oligosaccharide biosynthesis in lymphocytes and platelets from patients with the Wiskott-Aldrich syndrome. The Journal of Biological Chemistry. 1991;266(10):6280–6290. [PubMed] [Google Scholar]
- Hirao T, Sakamoto Y, Kamada M, Hamada S, Aono T. Tn antigen, a marker of potential for metastasis of uterine cervix cancer cells. Cancer. 1993;72(1):154–159. doi: 10.1002/1097-0142(19930701)72:1<154::aid-cncr2820720129>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and −81 expressed in cancer of blood group O or B individuals: Its identification as Tn antigen. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(20):7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: Protection and control of the cell surface. Nature Reviews. Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Hong X, Ma MZ, Gildersleeve JC, Chowdhury S, Barchi JJ, Jr, Mariuzza RA, et al. Sugar-binding proteins from fish: Selection of high affinity “lambodies” that recognize biomedically relevant glycans. ACS Chemical Biology. 2013;8(1):152–160. doi: 10.1021/cb300399s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, et al. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Research. 2011;71(15):5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- Ibrahim NK, Yariz KO, Bondarenko I, Manikhas A, Semiglazov V, Alyasova A, et al. Randomized phase II trial of letrozole plus anti-MUC1 antibody AS1402 in hormone receptor-positive locally advanced or metastatic breast cancer. Clinical Cancer Research. 2011;17(21):6822–6830. doi: 10.1158/1078-0432.CCR-11-1151. [DOI] [PubMed] [Google Scholar]
- Ichikawa D, Handa K, Hakomori S. Histo-blood group A/B antigen deletion/reduction vs. continuous expression in human tumor cells as correlated with their malignancy. International Journal of Cancer. Journal international du cancer. 1998;76(2):284–289. doi: 10.1002/(sici)1097-0215(19980413)76:2<284::aid-ijc17>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Inaba N, Hiruma T, Togayachi A, Iwasaki H, Wang XH, Furukawa Y, et al. A novel I-branching beta-1,6-N-acetylglucosaminyltransferase involved in human blood group I antigen expression. Blood. 2003;101(7):2870–2876. doi: 10.1182/blood-2002-09-2838. [DOI] [PubMed] [Google Scholar]
- Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Robust immune responses elicited by a fully synthetic three-component vaccine. Nature Chemical Biology. 2007;3(10):663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Ogawa H, Tanizawa O, Kobayashi Y, Tsujimoto M, Tsujimura T. Immunodetection of sialyl-Tn antigen in normal, hyperplastic and cancerous tissues of the uterine endometrium. Virchows Archiv. A, Pathological Anatomy and Histopathology. 1991;418(2):157–162. doi: 10.1007/BF01600291. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ton SM, Ogawa H, Tanizawa O. Expression of Tn and sialyl-Tn antigens in tumor tissues of the ovary. American Journal of Clinical Pathology. 1991;96(6):711–716. doi: 10.1093/ajcp/96.6.711. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Bloom EJ, Kokal WA, Modin G, Hakomori S, Kim YS. Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer. 1990;66(9):1960–1966. doi: 10.1002/1097-0142(19901101)66:9<1960::aid-cncr2820660919>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Bloom EJ, Lau TS, Kim YS. Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut. 1992;33(4):518–523. doi: 10.1136/gut.33.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzkowitz SH, Marshall A, Kornbluth A, Harpaz N, McHugh JB, Ahnen D, et al. Sialosyl-Tn antigen: Initial report of a new marker of malignant progression in long-standing ulcerative colitis. Gastroenterology. 1995;109(2):490–497. doi: 10.1016/0016-5085(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Young E, Dubois D, Harpaz N, Bodian C, Chen A, et al. Sialosyl-Tn antigen is prevalent and precedes dysplasia in ulcerative colitis: A retrospective case–control study. Gastroenterology. 1996;110(3):694–704. doi: 10.1053/gast.1996.v110.pm8608878. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yuan M, Fukushi Y, Palekar A, Phelps PC, Shamsuddin AM, et al. Lewisx- and sialylated Lewisx-related antigen expression in human malignant and nonmalignant colonic tissues. Cancer Research. 1986;46(5):2627–2632. [PubMed] [Google Scholar]
- Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, et al. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Research. 1989;49(1):197–204. [PubMed] [Google Scholar]
- Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. The Journal of Biological Chemistry. 2002;277(15):12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- Iwaya Y, Hasebe O, Koide N, Kitahara K, Suga T, Shinji A, et al. Reduced expression of alphaGlcNAc in Barrett’s oesophagus adjacent to Barrett’s adenocarcinoma—A possible biomarker to predict the malignant potential of Barrett’s oesophagus. Histopathology. 2014;64(4):536–546. doi: 10.1111/his.12296. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, et al. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: Liver colonization and adhesion to vascular endothelial cells. Experimental Cell Research. 1995;216(1):215–221. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- Jass JR, Allison LM, Edgar S. Monoclonal antibody TKH2 to the cancer-associated epitope sialosyl Tn shows cross-reactivity with variants of normal colorectal goblet cell mucin. Pathology. 1994;26(4):418–422. doi: 10.1080/00313029400169112. [DOI] [PubMed] [Google Scholar]
- Johnson VG, Schlom J, Paterson AJ, Bennett J, Magnani JL, Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Research. 1986;46(2):850–857. [PubMed] [Google Scholar]
- Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomarkers. 2014;14(1):63–81. doi: 10.3233/CBM-130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Aryal RP, Stowell CJ, Cummings RD. Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. The Journal of Cell Biology. 2008;182(3):531–542. doi: 10.1083/jcb.200711151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 beta1,3-galactosyltransferase. The Journal of Biological Chemistry. 2002;277(1):178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD. Protein glycosylation: Chaperone mutation in Tn syndrome. Nature. 2005;437(7063):1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- Ju T, Lanneau GS, Gautam T, Wang Y, Xia B, Stowell SR, et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Research. 2008;68(6):1636–1646. doi: 10.1158/0008-5472.CAN-07-2345. [DOI] [PubMed] [Google Scholar]
- Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angewandte Chemie (International Ed. in English) 2011;50(8):1770–1791. doi: 10.1002/anie.201002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X, Kudelka MR, et al. Tn and sialyl-Tn antigens, aberrant O-glycomics as human disease markers. Proteomics. Clinical Applications. 2013;7(9–10):618–631. doi: 10.1002/prca.201300024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Zheng Q, Cummings RD. Identification of core 1 O-glycan T-synthase from Caenorhabditis elegans. Glycobiology. 2006;16(10):947–958. doi: 10.1093/glycob/cwl008. [DOI] [PubMed] [Google Scholar]
- Julenius K, Molgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15(2):153–164. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- Julien S, Krzewinski-Recchi MA, Harduin-Lepers A, Gouyer V, Huet G, Le Bourhis X, et al. Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc alpha2,6-sialyltransferase (ST6GalNac I) cDNA. Glycoconjugate Journal. 2001;18(11–12):883–893. doi: 10.1023/a:1022200525695. [DOI] [PubMed] [Google Scholar]
- Julien S, Videira PA, Delannoy P. Sialyl-tn in cancer: (How) Did we miss the target? Biomolecules. 2012;2(4):435–466. doi: 10.3390/biom2040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeji Y, Tsujitani S, Mori M, Maehara Y, Sugimachi K. Helix pomatia agglutinin binding activity is a predictor of survival time for patients with gastric carcinoma. Cancer. 1991;68(11):2438–2442. doi: 10.1002/1097-0142(19911201)68:11<2438::aid-cncr2820681119>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Kannagi R. Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression—The Warburg effect revisited review. Glycoconjugate Journal. 2004;20(5):353–364. doi: 10.1023/B:GLYC.0000033631.35357.41. [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England Journal of Medicine. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. Journal of Nuclear Medicine. 2009;50(12):2008–2016. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, et al. Essential role of gastric gland mucin in preventing gastric cancer in mice. The Journal of Clinical Investigation. 2012;122(3):923–934. doi: 10.1172/JCI59087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305(5686):1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- Kellokumpu S, Sormunen R, Kellokumpu I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: Dependence on intra-Golgi pH. FEBS Letters. 2002;516(1–3):217–224. doi: 10.1016/s0014-5793(02)02535-8. [DOI] [PubMed] [Google Scholar]
- Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123(4):1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Kozarsky KF, Hobbie L, Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, Krieger M. Receptor-mediated endocytosis of low density lipoprotein: Somatic cell mutants define multiple genes required for expression of surface-receptor activity. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(17):5454–5458. doi: 10.1073/pnas.81.17.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen T, Clausen H, Hirohashi S, Ogawa T, Iijima H, Hakomori S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2–6 alpha-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Research. 1988;48(8):2214–2220. [PubMed] [Google Scholar]
- Klein PJ, Newman RA, Muller P, Uhlenbruck G, Citoler P, Schaefer HE, et al. The presence and significance of the Thomsen-Friedenreich antigen in mammary gland. II. Its topochemistry in normal, hyperplastic and carcinoma tissue of the breast comparative study. Journal of Cancer Research and Clinical Oncology. 1979;93(2):205–214. doi: 10.1007/BF00406579. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Terao T, Kawashima Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. Journal of Clinical Oncology. 1992;10(1):95–101. doi: 10.1200/JCO.1992.10.1.95. [DOI] [PubMed] [Google Scholar]
- Konno A, Hoshino Y, Terashima S, Motoki R, Kawaguchi T. Carbohydrate expression profile of colorectal cancer cells is relevant to metastatic pattern and prognosis. Clinical & Experimental Metastasis. 2002;19(1):61–70. doi: 10.1023/a:1013879702702. [DOI] [PubMed] [Google Scholar]
- Kozarsky K, Kingsley D, Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, Matsushita T, Niwa R, Kumagai I, Nakamura K. Novel anti-Tn single-chain Fv-Fc fusion proteins derived from immunized phage library and antibody Fc domain. Anticancer Research. 2010;30(9):3397–3405. [PubMed] [Google Scholar]
- Kufe DW. Mucins in cancer: Function, prognosis and therapy. Nature Reviews. Cancer. 2009;9(12):874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3(3):223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- Kukowska-Latallo JF, Larsen RD, Nair RP, Lowe JB. A cloned human cDNA determines expression of a mouse stage-specific embryonic antigen and the Lewis blood group alpha(1,3/1,4)fucosyltransferase. Genes & Development. 1990;4(8):1288–1303. doi: 10.1101/gad.4.8.1288. [DOI] [PubMed] [Google Scholar]
- Kumamoto K, Goto Y, Sekikawa K, Takenoshita S, Ishida N, Kawakita M, et al. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen-Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Research. 2001;61(11):4620–4627. [PubMed] [Google Scholar]
- Kumar R, Camphausen RT, Sullivan FX, Cumming DA. Core2 beta-1,6-N-acetylglucosaminyltransferase enzyme activity is critical for P-selectin glycoprotein ligand-1 binding to P-selectin. Blood. 1996;88(10):3872–3879. [PubMed] [Google Scholar]
- Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, et al. Lectin histochemistry of resected adenocarcinoma of the lung: Helix pomatia agglutinin binding is an independent prognostic factor. The American Journal of Pathology. 2002;160(3):1001–1008. doi: 10.1016/S0002-9440(10)64921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkilde NC, Wolf H, Clausen H, Kjeldsen T, Orntoft TF. Nuclear volume and expression of T-antigen, sialosyl-Tn-antigen, and Tn-antigen in carcinoma of the human bladder. Relation to tumor recurrence and progression. Cancer. 1992;69(1):219–227. doi: 10.1002/1097-0142(19920101)69:1<219::aid-cncr2820690136>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19(7):756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- Lee AY, Rickles FR, Julian JA, Gent M, Baker RI, Bowden C, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. Journal of Clinical Oncology. 2005;23(10):2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ro JY, Sahin AA, Hong WK, Brown BW, Mountain CF, et al. Expression of blood-group antigen A—a favorable prognostic factor in non-small-cell lung cancer. The New England Journal of Medicine. 1991;324(16):1084–1090. doi: 10.1056/NEJM199104183241603. [DOI] [PubMed] [Google Scholar]
- Lefebvre JC, Giordanengo V, Limouse M, Doglio A, Cucchiarini M, Monpoux F, et al. Altered glycosylation of leukosialin, CD43, in HIV-1-infected cells of the CEM line. The Journal of Experimental Medicine. 1994;180(5):1609–1617. doi: 10.1084/jem.180.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen A, Mehta P, Ouyang YB, Ju T, Helin J, Moore KL, et al. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. The Journal of Biological Chemistry. 1999;274(35):24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- Ma XC, Terata N, Kodama M, Jancic S, Hosokawa Y, Hattori T. Expression of sialyl-Tn antigen is correlated with survival time of patients with gastric carcinomas. European Journal of Cancer. 1993;29A(13):1820–1823. doi: 10.1016/0959-8049(93)90529-o. [DOI] [PubMed] [Google Scholar]
- Magnani JL, Nilsson B, Brockhaus M, Zopf D, Steplewski Z, Koprowski H, et al. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. The Journal of Biological Chemistry. 1982;257(23):14365–14369. [PubMed] [Google Scholar]
- Magnani JL, Steplewski Z, Koprowski H, Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19–9 in the sera of patients as a mucin. Cancer Research. 1983;43(11):5489–5492. [PubMed] [Google Scholar]
- Marcos NT, Bennett EP, Gomes J, Magalhaes A, Gomes C, David L, et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Frontiers in Bioscience. 2011;3:1443–1455. doi: 10.2741/e345. [DOI] [PubMed] [Google Scholar]
- Marcos NT, Pinho S, Grandela C, Cruz A, Samyn-Petit B, Harduin-Lepers A, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Research. 2004;64(19):7050–7057. doi: 10.1158/0008-5472.CAN-04-1921. [DOI] [PubMed] [Google Scholar]
- Marcus DM, Cass LE. Glycosphingolipids with Lewis blood group activity: Uptake by human erythrocytes. Science. 1969;164(3879):553–555. doi: 10.1126/science.164.3879.553. [DOI] [PubMed] [Google Scholar]
- Mariani G, Molea N, Bacciardi D, Boggi U, Fornaciari G, Campani D, et al. Initial tumor targeting, biodistribution, and pharmacokinetic evaluation of the monoclonal antibody PAM4 in patients with pancreatic cancer. Cancer Research. 1995;55(23 Suppl):5911s–5915s. [PubMed] [Google Scholar]
- Marsh WL. Anti-i: A cold antibody defining the Ii relationship in human red cells. British Journal of Haematology. 1961;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis-A epitope expression on tumour cells. British Journal of Cancer. 2002;86(2):161–167. doi: 10.1038/sj.bjc.6600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita Y, Cleary KR, Ota DM, Hoff SD, Irimura T. Sialyldimeric Lewis-X antigen expressed on mucin-like glycoproteins in colorectal cancer metastases. Laboratory Investigation; A Journal of Technical Methods and Pathology. 1990;63(6):780–791. [PubMed] [Google Scholar]
- Meezan E, Wu HC, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Mi R, Song L, Wang Y, Ding X, Zeng J, Lehoux S, et al. Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing tn antigen. The Journal of Biological Chemistry. 2012;287(49):41523–41533. doi: 10.1074/jbc.M112.371989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenic DE, Yokota T, Filpula DR, Finkelman MA, Dodd SW, Wood JF, et al. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Research. 1991;51(23 Pt 1):6363–6371. [PubMed] [Google Scholar]
- Miyake M, Taki T, Hitomi S, Hakomori S. Correlation of expression of H/Le(y)/Le(b) antigens with survival in patients with carcinoma of the lung. The New England Journal of Medicine. 1992;327(1):14–18. doi: 10.1056/NEJM199207023270103. [DOI] [PubMed] [Google Scholar]
- Morita N, Yajima Y, Asanuma H, Nakada H, Fujita-Yamaguchi Y. Inhibition of cancer cell growth by anti-Tn monoclonal antibody MLS128. Bioscience Trends. 2009;3(1):32–37. [PubMed] [Google Scholar]
- Motoo Y, Kawakami H, Watanabe H, Satomura Y, Ohta H, Okai T, et al. Serum sialyl-Tn antigen levels in patients with digestive cancers. Oncology. 1991;48(4):321–326. doi: 10.1159/000226951. [DOI] [PubMed] [Google Scholar]
- Muthana SM, Campbell CT, Gildersleeve JC. Modifications of glycans: Biological significance and therapeutic opportunities. ACS Chemical Biology. 2012;7(1):31–43. doi: 10.1021/cb2004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P, Vereb G, Sebestyen Z, Horvath G, Lockett SJ, Damjanovich S, et al. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. Journal of Cell Science. 2002;115(Pt. 22):4251–4262. doi: 10.1242/jcs.00118. [DOI] [PubMed] [Google Scholar]
- Nakagoe T, Fukushima K, Nanashima A, Sawai T, Tsuji T, Jibiki MA, et al. Comparison of the expression of ABH/Lewis-related antigens in polypoid and non-polypoid growth types of colorectal carcinoma. Journal of Gastroenterology and Hepatology. 2001;16(2):176–183. doi: 10.1046/j.1440-1746.2001.02425.x. [DOI] [PubMed] [Google Scholar]
- Nakagoe T, Nanashima A, Sawai T, Tuji T, Ohbatake M, Jibiki M, et al. Expression of blood group antigens A, B and H in carcinoma tissue correlates with a poor prognosis for colorectal cancer patients. Journal of Cancer Research and Clinical Oncology. 2000;126(7):375–382. doi: 10.1007/pl00008485. [DOI] [PubMed] [Google Scholar]
- Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: Clinicopathological and immunohistochemical study. Cancer Research. 1993;53(15):3632–3637. [PubMed] [Google Scholar]
- Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, et al. Involvement of carbohydrate antigen sialyl Lewis(x) in colorectal cancer metastasis. Diseases of the Colon and Rectum. 1997;40(4):420–431. doi: 10.1007/BF02258386. [DOI] [PubMed] [Google Scholar]
- Nakamoto Y, Saga T, Sakahara H, Yao Z, Zhang M, Sato N, et al. Three-step tumor imaging with biotinylated monoclonal antibody, streptavidin and 111In-DTPA-biotin. Nuclear Medicine and Biology. 1998;25(2):95–99. doi: 10.1016/s0969-8051(97)00155-8. [DOI] [PubMed] [Google Scholar]
- Nakayama J. Dual roles of gastric gland mucin-specific O-glycans in prevention of gastric cancer. Acta Histochemica et Cytochemica. 2014;47(1):1–9. doi: 10.1267/ahc.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Watanabe M, Katsumata T, Teramoto T, Kitajima M. Expression of sialyl Lewis(a) as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer. 1995;75(8):2051–2056. doi: 10.1002/1097-0142(19950415)75:8<2051::aid-cncr2820750804>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Nanashima A, Yamaguchi H, Nakagoe T, Matsuo S, Sumida Y, Tsuji T, et al. High serum concentrations of sialyl Tn antigen in carcinomas of the biliary tract and pancreas. Journal of Hepato-Biliary-Pancreatic Surgery. 1999;6(4):391–395. doi: 10.1007/s005340050137. [DOI] [PubMed] [Google Scholar]
- Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, Bennett EP, Picco G, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Research. 2007;67(17):8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- Narimatsu Y, Kubota T, Furukawa S, Shimojima M, Iwasaki H, Tozawa Y, et al. Co-translational function of Cosmc, core 1 synthase specific molecular chaperone, revealed by a cell-free translation system. FEBS Letters. 2011;585(9):1276–1280. doi: 10.1016/j.febslet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Nuti M, Teramoto YA, Mariani-Costantini R, Hand PH, Colcher D, Schlom J. A monoclonal antibody (B72.3) defines patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. International Journal of Cancer. Journal international du cancer. 1982;29(5):539–545. doi: 10.1002/ijc.2910290509. [DOI] [PubMed] [Google Scholar]
- O’Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, Lloyd KO, et al. Specificity analysis of murine monoclonal antibodies reactive with Tn, sialylated Tn, T, and monosialylated (2–>6) T antigens. Hybridoma. 1996;15(6):401–408. doi: 10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- Ogata S, Maimonis PJ, Itzkowitz SH. Mucins bearing the cancer-associated sialosyl-Tn antigen mediate inhibition of natural killer cell cytotoxicity. Cancer Research. 1992;52(17):4741–4746. [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Oh-Uti K. Polysaccharides and a glycidamin in the tissue of gastric cancer. The Tohoku Journal of Experimental Medicine. 1949;51(3–4):297–304. [Google Scholar]
- Orntoft TF, Wolf H, Watkins WM. Activity of the human blood group ABO, Se, H, Le, and X gene-encoded glycosyltransferases in normal and malignant bladder urothelium. Cancer Research. 1988;48(15):4427–4433. [PubMed] [Google Scholar]
- Orr SL, Le D, Long JM, Sobieszczuk P, Ma B, Tian H, et al. A phenotype survey of 36 mutant mouse strains with gene-targeted defects in glycosyltransferases or glycan-binding proteins. Glycobiology. 2013;23(3):363–380. doi: 10.1093/glycob/cws150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71(7):2191–2199. doi: 10.1002/1097-0142(19930401)71:7<2191::aid-cncr2820710705>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ozanne B, Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nature: New Biology. 1971;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Pai LH, Wittes R, Setser A, Willingham MC, Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: An antibody linked to Pseudomonas exotoxin. Nature Medicine. 1996;2(3):350–353. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- Pang PC, Chiu PC, Lee CL, Chang LY, Panico M, Morris HR, et al. Human sperm binding is mediated by the sialyl-Lewis(x) oligosaccharide on the zona pellucida. Science. 2011;333(6050):1761–1764. doi: 10.1126/science.1207438. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England Journal of Medicine. 1991;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306(5693):120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nature Reviews. Cancer. 2006;6(7):559–565. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- Pastan I, Lovelace ET, Gallo MG, Rutherford AV, Magnani JL, Willingham MC. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Research. 1991;51(14):3781–3787. [PubMed] [Google Scholar]
- Patani N, Jiang W, Mokbel K. Prognostic utility of glycosyltransferase expression in breast cancer. Cancer Genomics Proteomics. 2008;5(6):333–340. [PubMed] [Google Scholar]
- Pavlinkova G, Beresford GW, Booth BJ, Batra SK, Colcher D. Pharmacokinetics and biodistribution of engineered single-chain antibody constructs of MAb CC49 in colon carcinoma xenografts. Journal of Nuclear Medicine. 1999;40(9):1536–1546. [PubMed] [Google Scholar]
- Pavlinkova G, Booth BJ, Batra SK, Colcher D. Radioimmunotherapy of human colon cancer xenografts using a dimeric single-chain Fv antibody construct. Clinical Cancer Research. 1999;5(9):2613–2619. [PubMed] [Google Scholar]
- Pedersen JW, Blixt O, Bennett EP, Tarp MA, Dar I, Mandel U, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. International Journal of Cancer. 2011;128(8):1860–1871. doi: 10.1002/ijc.25778. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Borges VF, Ibrahim N, Fuloria J, Shapiro C, Perez S, et al. Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Research. 2009;11(5):R73. doi: 10.1186/bcr2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosyan A, Ali MF, Cheng PW. Glycosyltransferase-specific Golgi-targeting mechanisms. The Journal of Biological Chemistry. 2012;287(45):37621–37627. doi: 10.1074/jbc.C112.403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan CM, Tsai YY, Goode EL, Vierkant RA, Fridley BL, Beesley J, et al. Polymorphism in the GALNT1 gene and epithelial ovarian cancer in non-Hispanic white women: The Ovarian Cancer Association Consortium. Cancer Epidemiology, Biomarkers & Prevention. 2010;19(2):600–604. doi: 10.1158/1055-9965.EPI-09-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK. Oligosaccharide structures of isolated human colonic mucin species. The Journal of Biological Chemistry. 1985;260(29):15510–15515. [PubMed] [Google Scholar]
- Priatel JJ, Chui D, Hiraoka N, Simmons CJ, Richardson KB, Page DM, et al. The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity. 2000;12(3):273–283. doi: 10.1016/s1074-7613(00)80180-6. [DOI] [PubMed] [Google Scholar]
- Prokop O, Uhlenbruck G. N-acetyl-D-galactosamine in tumor cell membranes: Demonstration by means of Helix agglutinins. Die Medizinische Welt. 1969;46:2515–2519. [PubMed] [Google Scholar]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(39):E4066–E4075. doi: 10.1073/pnas.1406619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reepmaker J. The relation between polyagglutinability of erythrocytes in vivo and the Hubener-Thomsen-Friedenreich phenomenon. Journal of Clinical Pathology. 1952;5(3):266–270. doi: 10.1136/jcp.5.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis CA, Osorio H, Silva L, Gomes C, David L. Alterations in glycosylation as biomarkers for cancer detection. Journal of Clinical Pathology. 2010;63(4):322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- Roth J, Wang Y, Eckhardt AE, Hill RL. Subcellular localization of the UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-mediated O-glycosylation reaction in the submaxillary gland. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(19):8935–8939. doi: 10.1073/pnas.91.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, et al. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. Journal of Cell Science. 1998;111(Pt. 1):45–60. doi: 10.1242/jcs.111.1.45. [DOI] [PubMed] [Google Scholar]
- Roxby DJ, Pfeiffer MB, Morley AA, Kirkland MA. Expression of the Tn antigen in myelodysplasia, lymphoma, and leukemia case reports. Transfusion. 1992;32(9):834–838. doi: 10.1046/j.1537-2995.1992.32993110755.x. [DOI] [PubMed] [Google Scholar]
- Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clinical Cancer Research. 2007;13(14):4170–4177. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(25):11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salouti M, Rajabi H, Babaei MH, Rasaee MJ. Breast tumor targeting with (99m)Tc-HYNIC-PR81 complex as a new biologic radiopharmaceutical. Nuclear Medicine and Biology. 2008;35(7):763–768. doi: 10.1016/j.nucmedbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Santer UV, Gilbert F, Glick MC. Change in glycosylation of membrane glycoproteins after transfection of NIH 3T3 with human tumor DNA. Cancer Research. 1984;44(9):3730–3735. [PubMed] [Google Scholar]
- Sasaki H, Bothner B, Dell A, Fukuda M. Carbohydrate structure of erythro-poietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. The Journal of Biological Chemistry. 1987;262(25):12059–12076. [PubMed] [Google Scholar]
- Schauer H, Gottschalk A. Studies on glycoproteins. XVII. Purification of O-seryl-N-acetylgalactosaminide glycohydrolase. Its complete separation from N-acetyl-beta-D-hexosaminidase and proteases. Biochimica et Biophysica Acta. 1968;156(2):304–310. [PubMed] [Google Scholar]
- Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing—deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochimica et Biophysica Acta. 2012;1820(12):2079–2094. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Schjoldager KT, Vakhrushev SY, Kong Y, Steentoft C, Nudelman AS, Pedersen NB, et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):9893–9898. doi: 10.1073/pnas.1203563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23(34):5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 Peptide vaccine and interleukin-2 in patients with advanced melanoma. The New England Journal of Medicine. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwientek T, Nomoto M, Levery SB, Merkx G, van Kessel AG, Bennett EP, et al. Control of O-glycan branch formation. Molecular cloning of human cDNA encoding a novel beta1,6-N-acetylglucosaminyltransferase forming core 2 and core 4. The Journal of Biological Chemistry. 1999;274(8):4504–4512. doi: 10.1074/jbc.274.8.4504. [DOI] [PubMed] [Google Scholar]
- Schwientek T, Yeh JC, Levery SB, Keck B, Merkx G, van Kessel AG, et al. Control of O-glycan branch formation. Molecular cloning and characterization of a novel thymus-associated core 2 beta1, 6-n-acetylglucosaminyltransferase. The Journal of Biological Chemistry. 2000;275(15):11106–11113. doi: 10.1074/jbc.275.15.11106. [DOI] [PubMed] [Google Scholar]
- Sekine M, Nara K, Suzuki A. Tissue-specific regulation of mouse core 2 beta-1,6-N-acetylglucosaminyltransferase. The Journal of Biological Chemistry. 1997;272(43):27246–27252. doi: 10.1074/jbc.272.43.27246. [DOI] [PubMed] [Google Scholar]
- Sellers TA, Huang Y, Cunningham J, Goode EL, Sutphen R, Vierkant RA, et al. Association of single nucleotide polymorphisms in glycosylation genes with risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(2):397–404. doi: 10.1158/1055-9965.EPI-07-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Research. 1997;57(23):5201–5206. [PubMed] [Google Scholar]
- Shiratsu K, Higuchi K, Nakayama J. Loss of gastric gland mucin-specific O-glycan is associated with progression of differentiated-type adenocarcinoma of the stomach. Cancer Science. 2014;105(1):126–133. doi: 10.1111/cas.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(7):2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin-Chiorini DC, Horan Hand PH, Kashmiri SV, Calvo B, Zaremba S, Schlom J. Biologic properties of a CH2 domain-deleted recombinant immunoglobulin. International Journal of Cancer. 1993;53(1):97–103. doi: 10.1002/ijc.2910530119. [DOI] [PubMed] [Google Scholar]
- Slavin-Chiorini DC, Kashmiri SV, Lee HS, Milenic DE, Poole DJ, Bernon E, et al. A CDR-grafted (humanized) domain-deleted antitumor antibody. Cancer Biotherapy & Radiopharmaceuticals. 1997;12(5):305–316. doi: 10.1089/cbr.1997.12.305. [DOI] [PubMed] [Google Scholar]
- Slavin-Chiorini DC, Kashmiri SV, Schlom J, Calvo B, Shu LM, Schott ME, et al. Biological properties of chimeric domain-deleted anticarcinoma immunoglobulins. Cancer Research. 1995;55(23 Suppl):5957s–5967s. [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103(3):467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Sorensen AL, Reis CA, Tarp MA, Mandel U, Ramachandran K, Sankaranarayanan V, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16(2):96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. The Journal of Biological Chemistry. 1995;270(50):30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- Springer GF. T and Tn, general carcinoma autoantigens. Science. 1984;224(4654):1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Springer GF, Desai PR, Banatwala I. Blood group MN antigens and precursors in normal and malignant human breast glandular tissue. Journal of the National Cancer Institute. 1975;54(2):335–339. [PubMed] [Google Scholar]
- Springer GF, Desai PR, Tegtmeyer H, Spencer BD, Scanlon EF. Pancarcinoma T/Tn antigen detects human carcinoma long before biopsy does and its vaccine prevents breast carcinoma recurrence. Annals of the New York Academy of Sciences. 1993;690:355–357. doi: 10.1111/j.1749-6632.1993.tb44029.x. [DOI] [PubMed] [Google Scholar]
- Springer GF, Murthy MS, Desai PR, Scanlon EF. Breast cancer patient’s cell-mediated immune response to Thomsen-Friedenreich (T) antigen. Cancer. 1980;45(12):2949–2954. doi: 10.1002/1097-0142(19800615)45:12<2949::aid-cncr2820451210>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- St Hill CA, Baharo-Hassan D, Farooqui M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS One. 2011;6(1):e16281. doi: 10.1371/journal.pone.0016281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hill CA, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, et al. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Cummings RD. Structures common to different glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. 2. New York: Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. The EMBO Journal. 2013;32(10):1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, et al. Glycosyltransferase function in core 2-type protein O glycosylation. Molecular and Cellular Biology. 2009;29(13):3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, et al. Innate immune lectins kill bacteria expressing blood group antigen. Nature Medicine. 2010;16(3):295–301. doi: 10.1038/nm.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, McBride R, Berger O, Razi N, Heimburg-Molinaro J, et al. Microbial glycan microarrays define key features of host-microbial interactions. Nature Chemical Biology. 2014;10(6):470–476. doi: 10.1038/nchembio.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow DM, Gendler S, Griffiths B, Corney G, Taylor-Papadimitriou J, Bramwell ME. The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature. 1987;328(6125):82–84. doi: 10.1038/328082a0. [DOI] [PubMed] [Google Scholar]
- Tabak LA. The role of mucin-type O-glycans in eukaryotic development. Seminars in Cell & Developmental Biology. 2010;21(6):616–621. doi: 10.1016/j.semcdb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T, Paulson JC, Cahan LD, Irie RF. Ganglioside GM2 as a human tumor antigen (OFA-I-1) Proceedings of the National Academy of Sciences of the United States of America. 1983;80(17):5392–5396. doi: 10.1073/pnas.80.17.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Takahashi N, Tsuyuoka K, Yago A, Zenita K, et al. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl Lewis A. Biochemical and Biophysical Research Communications. 1991;179(2):713–719. doi: 10.1016/0006-291x(91)91875-d. [DOI] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, et al. Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Research. 1993;53(2):354–361. [PubMed] [Google Scholar]
- Takahashi HK, Metoki R, Hakomori S. Immunoglobulin G3 monoclonal antibody directed to Tn antigen (tumor-associated alpha-N-acetylgalactosaminyl epitope) that does not cross-react with blood group A antigen. Cancer Research. 1988;48(15):4361–4367. [PubMed] [Google Scholar]
- Tanaka K, Bertolini M, Pigman W. Serine and threonine glycosidic linkages in bovine submaxillary mucin. Biochemical and Biophysical Research Communications. 1964;16(5):404–409. doi: 10.1016/0006-291x(64)90366-3. [DOI] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hagen KG, Tran DT. A UDP-GalNAc:Polypeptide N-acetylgalactosaminyltransferase is essential for viability in Drosophila melanogaster. The Journal of Biological Chemistry. 2002;277(25):22616–22622. doi: 10.1074/jbc.M201807200. [DOI] [PubMed] [Google Scholar]
- Tenno M, Ohtsubo K, Hagen FK, Ditto D, Zarbock A, Schaerli P, et al. Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity. Molecular and Cellular Biology. 2007;27(24):8783–8796. doi: 10.1128/MCB.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EUROGAST Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341(8857):1359–1362. [PubMed] [Google Scholar]
- Therkildsen MH, Mandel U, Christensen M, Dabelsteen E. Simple mucin-type Tn and sialosyl-Tn carbohydrate antigens in salivary gland carcinomas. Cancer. 1993;72(4):1147–1154. doi: 10.1002/1097-0142(19930815)72:4<1147::aid-cncr2820720403>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Thomsson KA, Holmen-Larsson JM, Angstrom J, Johansson ME, Xia L, Hansson GC. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology. 2012;22(8):1128–1139. doi: 10.1093/glycob/cws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor A, Ohuchi N, Szpak CA, Johnston WW, Schlom J. Distribution of oncofetal antigen tumor-associated glycoprotein-72 defined by monoclonal antibody B72.3. Cancer Research. 1986;46(6):3118–3124. [PubMed] [Google Scholar]
- Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–110. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, Ten Hagen KG. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconjugate Journal. 2009;26(3):325–334. doi: 10.1007/s10719-008-9162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nature Genetics. 2004;36(6):579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- Tran DT, Zhang L, Zhang Y, Tian E, Earl LA, Ten Hagen KG. Multiple members of the UDP-GalNAc: Polypeptide N-acetylgalactosaminyltransferase family are essential for viability in Drosophila. The Journal of Biological Chemistry. 2012;287(8):5243–5252. doi: 10.1074/jbc.M111.306159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek WP, Smets LA, Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells–a general phenomenon? Cancer Research. 1973;33(11):2913–2922. [PubMed] [Google Scholar]
- van der Post S, Subramani DB, Backstrom M, Johansson ME, Vester-Christensen MB, Mandel U, et al. Site-specific O-glycosylation on the MUC2 mucin protein inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB) The Journal of Biological Chemistry. 2013;288(20):14636–14646. doi: 10.1074/jbc.M113.459479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet SJ, Paessens LC, Broks-van den Berg VC, Geijtenbeek TB, van Kooyk Y. The C-type lectin macrophage galactose-type lectin impedes migration of immature APCs. Journal of Immunology. 2008;181(5):3148–3155. doi: 10.4049/jimmunol.181.5.3148. [DOI] [PubMed] [Google Scholar]
- Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology. 2006;16(1):1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- Vavasseur F, Yang JM, Dole K, Paulsen H, Brockhausen I. Synthesis of O-glycan core 3: Characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetylglucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology. 1995;5(3):351–357. doi: 10.1093/glycob/5.3.351. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin C, Cuevas E, Gil-Martin E, Fernandez-Briera A. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology. 2004;67(2):159–165. doi: 10.1159/000081003. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- von Mensdorff-Pouilly S, Petrakou E, Kenemans P, van Uffelen K, Verstraeten AA, Snijdewint FG, et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and n-acetylgalactosamine (GalNAc) peptides. International Journal of Cancer. 2000;86(5):702–712. doi: 10.1002/(sici)1097-0215(20000601)86:5<702::aid-ijc16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nature Medicine. 2007;13(9):1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Wandall HH, Blixt O, Tarp MA, Pedersen JW, Bennett EP, Mandel U, et al. Cancer biomarkers defined by autoantibody signatures to aberrant O-glycopeptide epitopes. Cancer Research. 2010;70(4):1306–1313. doi: 10.1158/0008-5472.CAN-09-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, et al. Substrate specificities of three members of the human UDP-N-acetyl-alpha-D-galactosamine:Polypeptide N-acetylgalactosaminyltransferase family, GalNAc-T1, –T2, and -T3. The Journal of Biological Chemistry. 1997;272(38):23503–23514. doi: 10.1074/jbc.272.38.23503. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jobe SM, Ding X, Choo H, Archer DR, Mi R, et al. Platelet biogenesis and functions require correct protein O-glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16143–16148. doi: 10.1073/pnas.1208253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, et al. Cosmc is an essential chaperone for correct protein O-glycosylation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yu J, Sreekumar A, Varambally S, Shen R, Giacherio D, et al. Autoantibody signatures in prostate cancer. The New England Journal of Medicine. 2005;353(12):1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- Wargovich MJ, Chang P, Velasco M, Sinicrope F, Eisenbrodt E, Sellin J. Expression of cellular adhesion proteins and abnormal glycoproteins in human aberrant crypt foci. Applied Immunohistochemistry & Molecular Morphology. 2004;12(4):350–355. doi: 10.1097/00129039-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Warren L, Buck CA, Tuszynski GP. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochimica et Biophysica Acta. 1978;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hakomori SI, Childs RA, Feizi T. Characterization of a blood group I-active ganglioside. Structural requirements for I and i specificities. The Journal of Biological Chemistry. 1979;254(9):3221–3228. [PubMed] [Google Scholar]
- Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B. A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti-tumor activity. Glycobiology. 2011;21(8):1097–1107. doi: 10.1093/glycob/cwr048. [DOI] [PubMed] [Google Scholar]
- Werther JL, Rivera-MacMurray S, Bruckner H, Tatematsu M, Itzkowitz SH. Mucin-associated sialosyl-Tn antigen expression in gastric cancer correlates with an adverse outcome. British Journal of Cancer. 1994;69(3):613–616. doi: 10.1038/bjc.1994.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werther JL, Tatematsu M, Klein R, Kurihara M, Kumagai K, Llorens P, et al. Sialosyl-Tn antigen as a marker of gastric cancer progression: An international study. International Journal of Cancer. Journal international du cancer. 1996;69(3):193–199. doi: 10.1002/(SICI)1097-0215(19960621)69:3<193::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wu C, Guo X, Wang W, Wang Y, Shan Y, Zhang B, et al. N-Acetylgalactosaminyltransferase-14 as a potential biomarker for breast cancer by immunohistochemistry. BMC Cancer. 2010;10:123. doi: 10.1186/1471-2407-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, et al. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. The Journal of Cell Biology. 2004;164(3):451–459. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Horst S, Hinkle G, Cao X, Kocak E, Fang J, et al. Pharmacokinetics and clinical evaluation of 125I-radiolabeled humanized CC49 monoclonal antibody (HuCC49deltaC(H)2) in recurrent and metastatic colorectal cancer patients. Cancer Biotherapy & Radiopharmaceuticals. 2005;20(1):16–26. doi: 10.1089/cbr.2005.20.16. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Tachibana Y, Ohkura T, Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induced by polyoma transformation. The Journal of Biological Chemistry. 1985;260(7):3963–3969. [PubMed] [Google Scholar]
- Yang JM, Byrd JC, Siddiki BB, Chung YS, Okuno M, Sowa M, et al. Alterations of O-glycan biosynthesis in human colon cancer tissues. Glycobiology. 1994;4(6):873–884. doi: 10.1093/glycob/4.6.873. [DOI] [PubMed] [Google Scholar]
- Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Reviews in Molecular Medicine. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- Yao Z, Sakahara H, Zhang M, Kobayashi H, Nakada H, Yamashina I, et al. Radioimmunoimaging of colon cancer xenografts with anti-Tn monoclonal antibody. Nuclear Medicine and Biology. 1995;22(2):199–203. doi: 10.1016/0969-8051(94)00092-x. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105(7):957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Yeh JC, Ong E, Fukuda M. Molecular cloning and expression of a novel beta-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. The Journal of Biological Chemistry. 1999;274(5):3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. The Journal of Biological Chemistry. 2001;276(29):27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Research. 1992;52(12):3402–3408. [PubMed] [Google Scholar]
- Yokota T, Milenic DE, Whitlow M, Wood JF, Hubert SL, Schlom J. Microautoradiographic analysis of the normal organ distribution of radioiodinated single-chain Fv and other immunoglobulin forms. Cancer Research. 1993;53(16):3776–3783. [PubMed] [Google Scholar]
- Young WW, Jr, Holcomb DR, Ten Hagen KG, Tabak LA. Expression of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase isoforms in murine tissues determined by real-time PCR: A new view of a large family. Glycobiology. 2003;13(7):549–557. doi: 10.1093/glycob/cwg062. [DOI] [PubMed] [Google Scholar]
- Yu LG. The oncofetal Thomsen-Friedenreich carbohydrate antigen in cancer progression. Glycoconjugate Journal. 2007;24(8):411–420. doi: 10.1007/s10719-007-9034-3. [DOI] [PubMed] [Google Scholar]
- Yu H, Chen X. Carbohydrate post-glycosylational modifications. Organic & Biomolecular Chemistry. 2007;5(6):865–872. doi: 10.1039/b700034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M. The expression of Tn and S-Tn antigens in cancer and pre-malignant lesion of colorectal tissues by enzyme immunohistochemical method. Zhonghua Bing Li Xue Za Zhi. 1989;18(3):211–213. [PubMed] [Google Scholar]
- Yuan M, Itzkowitz SH, Palekar A, Shamsuddin AM, Phelps PC, Trump BF, et al. Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, fetal, and malignant colonic tissue. Cancer Research. 1985;45(9):4499–4511. [PubMed] [Google Scholar]
- Zhang M, Yao Z, Sakahara H, Saga T, Nakamoto Y, Sato N, et al. Effect of administration route and dose of streptavidin or biotin on the tumor uptake of radioactivity in intraperitoneal tumor with multistep targeting. Nuclear Medicine and Biology. 1998;25(2):101–105. doi: 10.1016/s0969-8051(97)00157-1. [DOI] [PubMed] [Google Scholar]
- Zou P, Povoski SP, Hall NC, Carlton MM, Hinkle GH, Xu RX, et al. 124I-HuCC49deltaCH2 for TAG-72 antigen-directed positron emission tomography (PET) imaging of LS174T colon adenocarcinoma tumor implants in xenograft mice: Preliminary results. World Journal of Surgical Oncology. 2010;8:65. doi: 10.1186/1477-7819-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]