Abstract
The comparison of words and pseudowords has been extensively used in adult neuroimaging studies to inform neurocognitive models of reading but has rarely been used to inform models of reading acquisition. Using a rhyming judgment task, the current study examined age-related differences in the spelling to sound mapping mechanisms involved in word and pseudoword reading. We hypothesized a developmental increase in specialization of the brain mechanisms engaged for word and pseudoword processing. Consistent with adult studies, children in the current study demonstrated a greater activation for words as compared to pseudowords in the anterior left ventral occipito-temporal cortex (vOT). Inconsistent with adult studies, children also showed greater activation for words as compared to pseudowords in the mid-posterior left vOT, indicating a robust semantic influence on orthographic processing in young readers. Furthermore, our results did not indicate a lexicality by age interaction for 8- to 13-year-old children, suggesting that the adult-like specialization in the left vOT only appears later in development.
Keywords: Lexicality, pseudowords, reading, fMRI, children, brain development
1. Introduction
Cognitive models of visual word recognition aim to describe the ability to read both familiar words and pseudowords (pronounceable nonwords). Both word and pseudoword processing encompass integration of phonological and orthographic information. However, words differ from pseudowords both because they are orthographically familiar and because they possess semantic value (Cattinelli, Borghese, Gallucci, & Paulesu, 2013). The comparison of words and pseudowords has been extensively used in the adult neuroimaging literature to inform neurocognitive models of reading, but this contrast has rarely been used to inform models of reading acquisition. In the current study, we examined age-related differences in the spelling to sound mechanisms involved in word and pseudoword reading. We hypothesized a developmental increase in specialization of the brain mechanisms engaged for word and pseudoword processing.
1.1. Lexicality effect on brain activation of adult readers
Neuroimaging meta-analysis studies that examine the lexicality effect (the difference between words and pseudowords) on adults' brain activation suggest that the familiarity and the lexical status of written stimuli affect the degree to which different reading-related brain regions are involved (Cattinelli et al., 2013; Jobard, Crivello, & Tzourio-Mazoyer, 2003; McNorgan, Chabal, O'Young, Lukic, & Booth, 2015; Mechelli et al., 2005; Protopapas et al., 2016; Pugh et al., 2001, 2010; Taylor et al., 2013). Pseudowords as compared to words show greater activation in regions that are associated with spelling to sound mapping, such as the supramarginal gyrus (SMG) and inferior parietal lobule (IPL) (Graves, Binder, Desai, Conant, & Seidenberg, 2010; Jobard et al., 2003), and phonological processing, such as the superior temporal gyrus (STG) and dorsal inferior frontal gyrus (IFG) (Fiez & Petersen, 1998; Richlan, Kronbichler, & Wimmer, 2011; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003). Familiar words, on the other hand, show greater activation in regions that are associated with semantic processing or the integration of phonological/ orthographic information with semantic information, such as the angular gyrus (AG), inferior and middle temporal gyri (ITG and MTG), and ventral IFG (Fiebach, Friederici, Müller, & Cramon, 2002; Jobard et al., 2003). In sum, neuroimaging studies with adults indicate a dissociation between word and pseudoword brain activation. While pseudoword reading is more likely to engage spelling to sound mapping mechanisms, real word reading is more likely to engage ortho-lexical and semantic processing mechanisms. However, the engagement of one mechanism or the other seems to depend on the specific task. While lexical decision tasks tend to increase engagement of semantic and ortho-lexical processing mechanisms, naming tasks tend to increase engagement of spelling to sound mapping mechanisms (Carreiras, Mechelli, Estévez, & Price, 2007; McNorgan et al., 2015). The current study incorporated a phonological rhyming judgment task that is likely to enhance engagement of spelling to sound mapping mechanisms for both words and pseudowords (Pattamadilok et al., 2017). Using a single task that focuses on the conversion of orthography to phonology enables us to examine the lexicality effect on these mechanisms with no explicit requirement to access meaning-based representations (Booth & Burman, 2005). Neuroimaging studies with adults provide extensive evidence that the left ventral occipito-temporal (vOT) cortex is crucial to written word recognition in different orthographies (Dehaene, Cohen, Morais, & Kolinsky, 2015; Richlan et al., 2011). Studies with adults further show a dissociation for words and pseudowords in different parts of the left vOT, including the left fusiform gyrus (FG) and ITG. Pseudowords show a greater activation as compared to words in the mid-posterior part (MNI: -50 < Y < -70) (Bruno, Zumberge, Manis, Lu, & Goldman, 2008; Cattinelli et al., 2013; Jobard et al., 2003; McNorgan et al., 2015; Mechelli, Gorno-Tempini, & Price, 2003; Price & Mechelli, 2005; Taylor et al., 2013). This region includes the putative visual word form area (VWFA) and is associated with sub-lexical bigrams and single letter coding (Dehaene, Cohen, Sigman, & Vinckier, 2005; Kronbichler et al., 2004). In their interactive account of the left vOT, Price and Devlin (2011) suggest that the mid-posterior left vOT is involved in the integration of low-level orthographic stimuli with high-level semantic and phonological processing. The greater activation for pseudowords as compared to words in the mid-posterior part of the left vOT in adult readers can be explained by a greater prediction error from the top-down processes for the phonologically and semantically unfamiliar pseudowords. Real words, on the other hand, show greater activation as compared to pseudowords in the anterior part of the left vOT (MNI: -30 < Y < -50) (Cattinelli et al., 2013; Jobard et al., 2003; McNorgan et al., 2015; Price & Mechelli, 2005; Taylor et al., 2013). This region is associated with coding of larger orthographic units (morphemes, small words, and up) (Dehaene et al., 2005). The greater activation for words as compared to pseudowords in the anterior part of the left vOT is attributed to the role of this region in modality independent semantic processing (Price, 2000, 2012). Alternatively, it may reflect the role of the left anterior vOT in processing a higher level visual information and function as an orthographic lexicon, which contains whole word orthographic representations (Boukadi et al., 2016; Ludersdorfer et al., 2016). Both alternatives can explain the greater engagement of the anterior left vOT for word as compared to pseudoword reading (Taylor et al., 2013).
1.2. Developmental changes in the ventral occipito-temporal cortex
Neuroimaging studies with young children suggest that similar to adults, they are able to engage the left vOT in word recognition (Gaillard, Balsamo, Ibrahim, Sachs, & Xu, 2003; Houdé, Rossi, Lubin, & Joliot, 2010; Martin et al., 2015), even before formal reading instruction (Bach et al., 2010; Maurer et al., 2007; Raschle, Zuk, & Gaab, 2012). Furthermore, a left vOT dysfunction was found for both dyslexic children and adults (Richlan, Kronbichler, & Wimmer, 2011), and in children at-risk for dyslexia prior to reading onset (Vandermosten, Hoeft, & Norton, 2016), indicating an early involvement of the region in reading. Similar to adults, typically reading, but not dyslexic, children showed the posterior-to-anterior gradient of increasing selectivity for larger orthographic units in the left occipito-temporal cortex (Brem et al., 2009; Olulade, Flowers, Napoliello, & Eden, 2013, 2015). However, neuroimaging studies found age-related changes in sensitivity to word reading in the left vOT, with adult-like patterns appearing only by the age of 15 years (Ben-Shachar, Dougherty, Deutsch, & Wandell, 2011; Olulade et al., 2013). A direct comparison between children and adult activation revealed that word selectivity in children occurs more posteriorly than in adults. For children (7- to-13-year-old), word activation was greater than false font activation in regions near the middle fusiform gyrus (MNI: x,y,z = -36,-42,-23 and x,y,z = -42,-50,-14), while for adults greater word activation was found in anterior fusiform gyrus (MNI: x,y,z = -38,-26,-20 and x,y,z = -40,-34,-22), associated with semantic processing (Olulade et al., 2013). These findings suggest a greater semantic influence on the mid-vOT activation for children as compared to adults. This interpretation is consistent with models of reading development suggesting that greater reading experience facilitates spelling to sound mapping mechanisms, improves word recognition efficiency and in turn, reduces the reliance on semantic information (Booth & Burman, 2005; Harm & Seidenberg, 2004; Plaut & Booth, 2000; Stanovich, 1980). Consistent this hypothesis, Price and Devlin (2011) described three reading acquisition phases in the top-down effects on the left vOT. In pre-readers, activation in the left vOT is low because the stimuli are not yet familiar and cannot elicit the top-down activations. In the beginning stage of learning to read, activation in this region is the highest due to increased prediction error from the top-down processes that are yet to be efficient. In experienced readers, activation in this region decreases due to more efficient top-down processes and decreased prediction error. This model predicts that the top-down influence of semantics in beginning readers should be larger than the top-down influence in experienced readers.
1.3. Lexicality effect on brain activation of children readers
One indication of a specialization in the left vOT is the dissociation of greater activation for word reading in the anterior part and a greater activation for pseudowords in the mid-posterior part, as found in adults (Cattinelli et al., 2013; Jobard et al., 2003; McNorgan et al., 2015; Price & Mechelli, 2005; Taylor et al., 2013). Examining the lexicality effect on activation along the left vOT cortex in young readers has the potential to shed light on the developmental specialization in this region. Greater activation for words suggests a reliance on semantic information, while a greater activation for pseudowords suggests a reliance on phonological and sub-lexical processing. Alternatively, this pattern for pseudowords could be explained by increased prediction error from phonological and semantic top-down processes.
To date, only one functional Magnetic Resonance Imaging (fMRI) study has directly examined the main effect of lexicality on brain activation in children using a single task design, and this was in German speaking children (van der Mark et al., 2009). A few other studies used different tasks to examine phonological and semantic activation differences in children, using pseudowords and real words (Backes et al., 2002; Liebig et al., 2017). However, to the best of our knowledge, no fMRI study has directly examined the lexicality effect on brain activation in English speaking children, or examined age-related differences in the brain mechanisms involved in familiar word versus unfamiliar pseudoword processing using a single task. Consistent with the lexicality effect found in studies with adults, German speaking children (age 9.7-12.5 years) showed greater activation for word reading in left AG, and greater activation for pseudoword reading in left IFG and IPL, when performing a phonological lexical decision task (van der Mark et al., 2009). These results indicate that children at this age already show a specialization within these regions similar to adults. However, in contrast to findings from the meta-analyses with adults (Cattinelli et al., 2013; McNorgan et al., 2015; Taylor et al., 2013), this study showed a greater activation for pseudowords as compared to words in the left anterior FG (MNI: x,y,z = -42,-42,-21). Furthermore, their ROI analysis revealed the same pattern (pseudoword > word activation) along the entire left vOT. Studies with German speaking adolescents and adults, which incorporated the same phonological lexical decision task, also found greater activation for pseudohomophones as compared to words in the left anterior FG (MNI: x,y,z = -45,-48,-15/-18) (Kronbichler et al., 2007; Schurz et al., 2014). This effect was interpreted as indicating an increased demand on lexical search in this region for pseudohomophones. In contrast with the finding from German, adult English readers showed a lexicality effect (greater activation for nonwords and pseudohomophones as compared to words) only at the posterior part of the left vOT cortex (MNI: x,y,z = -46,-64,,-12) (Woollams, Silani, Okada, Patterson, & Price, 2011). In summary, it is likely that the discrepancies between the findings from adult studies and young German speakers are due to the nature of the language and task studied, rather than reflecting developmental changes. It might be that the transparent German orthography leads to a greater reliance on a top-down phonological as compared to a semantic effect on the left vOT. Alternatively, it might be that the phonological lexical decision task increased the demands on phonological and lexical search in the left vOT for pseudowords as compared to words. Hence, pseudowords increased the predictor error and therefore activation in the left vOT (Price & Devlin, 2011; Taylor et al., 2013).
1.4. The current study
In the current study, our investigation focused on the specialization of the vOT for word and pseudoword processing in children. Based on the results from English speaking adults (Cattinelli et al., 2013; Jobard et al., 2003; McNorgan et al., 2015; Price & Mechelli, 2005; Taylor et al., 2013), we predicted an age-related increased specialization of the left vOT as would be evident in a dissociation between word and pseudoword activation in the anterior and mid-posterior parts, respectively. Furthermore, we examined age-related specialization for word and pseudoword reading in other regions within the reading network.
2. Materials and Methods
2.1. Participants
Seventy-one (38 females) children included in the current paper analysis. Participants' age ranged between 8.5 and 13.5 years (M=10.9, SD=1.2). All participants are healthy, right-handed native English speakers. Participants were recruited from the Chicago metropolitan area. Informed consent was obtained from participants and their parents, and all procedures were approved by the Institutional Review Board at Northwestern University. Inclusion criteria for participants were: full scale intelligence of at least 80 standard score (M=113.1, SD=1.2) as measured by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999); reading ability within the normal range (between 85 and 130) as measured by the average age-corrected standard score of four reading skill sub-tests (M=104.2, SD=11.2): Sight Word Efficiency (M=103.9, SD=12.1) and Phonemic Decoding Efficiency (M=102.2, SD=15.1) subtests from the Test of Word Reading Efficiency (TOWRE; Torgesen, Wagner, & Rashotte, 1999), and Word Identification (M=105.2, SD=12.5) and Word Attack (M=105.4, SD=10.2) subtests from the Woodcock-Johnson-III Tests of Achievement (WJ-III; Woodcock, McGrew, Mather, & Schrank, 2001); average accuracy of at least 67% across all items of the behavioral in-scanner tasks, and at least 64% across lexical conditions for the specific conditions included in the analysis (O+P+ and O-P-). The original sample included 163 participants between the 8.5 and 13.5 years of age who had completed all the in-scanner tasks. Seventy-one participants were excluded due to movement in the scanner, for at least one of the runs. Another twenty-one participants were excluded due to either low intelligence, low reading ability, or low performance on the in-scanner tasks.
2.2. Experimental task and procedure
All participants underwent extensive training in a mock scanner prior to scanning and practiced the task outside the scanner immediately prior to the fMRI acquisition session. Thus, participants were familiar with the task and the scanning environment before the fMRI session. Stimuli were presented in an event-related design. Participants were presented with two runs of paired written words and two runs of paired written pseudowords and completed a phonological rhyme judgment task. Participants were asked to respond as quickly and as accurately as possible, using the right index finger for a yes (rhyme) response and right middle finger for a no (non-rhyme) response. The total duration of the word and pseudoword runs was ∼28 min (∼7 min per run). Each run included four different lexical conditions (12 trials per condition per run, 48 total lexical trials per run, 96 lexical trials across runs): O+P+ (orthographically similar and phonologically similar; e.g., CAGE-RAGE); O-P+ (orthographically non-similar and phonologically similar; e.g., GRADE-LAID); O+P- (orthographically similar and phonologically non-similar; e.g., STAMP-SWAMP); O-P- (orthographically and phonologically non-similar; e.g., THIEF-PLEAD). In addition, each run included two types of non-lexical control trials, 24 trials of a null baseline (fixation cross turned from red to blue) and 12 trials of perceptual baseline (non-alphabetic glyphs). In each lexical trial, the paired words/paired pseudowords were presented sequentially, and the order of which was counterbalanced across participants. Each trial began with the first word/pseudoword that was presented for 800ms, followed by a 200ms inter-stimulus interval, and then by the second word/pseudoword that was presented for 800ms, followed by a red cross that was presented for 2200ms. Response time and accuracy were recorded during the presentation of the second word and the red cross. Hence, the children had up to 3000ms to make their decision. The interval duration starting from the beginning of the red cross presentation of one trial until the beginning of the first stimulus in the next trial was jittered between 2200 and 2800ms. Responses made after the red cross disappeared from the screen were not recorded and counted as errors.
2.3. MRI data acquisition
Participants were positioned in the MRI scanner with their head orientation secured using foam pads. An optical response box was placed in the participant's right hand to log responses. Visual stimuli were projected onto a screen, which participants viewed via a mirror attached to the inside of the head coil. Images were acquired using a 3.0-Tesla Siemens Trio scanner. The blood oxygen- level-dependent (BOLD) signal was measured using a susceptibility weighted single-shot echo planar imaging (EPI) method. Functional images were interleaved from bottom to top in a whole-brain acquisition. The following parameters were used: TE = 20ms, flip angle = 80 degrees, matrix size = 128 × 120, field of view = 220 × 206.25 mm, slice thickness = 3 mm (0.48 mm gap), number of slices = 32, TR = 2000ms, number of volumes = 202. Before functional image acquisition, a high resolution T1-weighted 3D structural image was acquired for each subject (TR = 1570ms, TE = 3.36ms, matrix size = 256 × 256, field of view = 240 mm, slice thickness = 1 mm, number of slices = 160). All participants underwent extensive training in a mock scanner before scanning and practiced the task outside the scanner immediately before each fMRI acquisition session. Thus, participants were familiar with the task and the scanning environment before each fMRI session.
2.4. Behavioral data analysis
A linear mixed model was conducted to examine the lexicality effect on response time for correct responses. The mixed model enabled us to include each trial (rather than average across trials for each participant) and include both random and fixed effects. Participant number was defined as the random effect and lexicality (words vs. pseudowords) was defined as the fixed effect. The square root of the response time was defined as the independent variable. A logistic mixed model was conducted to examine the lexicality effect on accuracy. In this model, participant number was defined as the random effect and lexicality (words vs. pseudowords) was defined as the fixed effect. Accuracy was defined as the independent binomial variable. Further, for both response time and accuracy, a separate model included age as a covariate. The main effects and the interaction of lexicality and age were included as fixed variables in this model.
2.5. FMRI data analysis
FMRI data were analyzed using Statistical Parametric Mapping (SPM8 v5236, http://www.fil.ion.ac.uk/spm).
Pre-processing
ArtRepair software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html) was used to correct for participant movement. Images were realigned in ArtRepair, which identified and used interpolated values from the two adjacent non-outlier scans to replace outlier volumes, defined as those with head movement exceeding 4 mm in any direction or deviations of more than 1.5% from the mean global signal. No more than 10% of the volumes from each run and no more than four consecutive volumes for any individual were interpolated in this way. Slice timing was applied to minimize timing- errors between slices. Functional images were coregistered with the anatomical image and normalized to the Montreal Neurological Institute (MNI) ICBM152 T1 template. However, we did not implement the segmented anatomical images in the indirect normalization. The MNI adult template is based on an average of 152 normal adult MRI scans and is well defined with respect to a number of brain atlas tools. Moreover, using this template is justified as neuroanatomical differences between children within the age range included in our study and adults are unlikely to affect fMRI results (Burgund et al., 2002; Muzik, Chugani, Juhász, Shen, & Chugani, 2000; Poldrack, Mumford, & Nichols, 2011). Thus, it was deemed preferable to use the standard adult SPM template rather than create an average-based template, in order to compare to the previous findings with adults. Images were smoothed using a 2 × 2 × 4 nonisotropic Gaussian kernel.
First level (within-subject) analysis
The first six volumes of each run, during which a fixation cross was presented, were “dummy” scans and dropped from the analyses. Since we were interested in the lexicality effect, we modeled each lexical condition (words and pseudowords) collapsing across rhyme and non-rhyme conditions. We only included O+P+ and O-P- as conditions of interest to avoid using the inconsistent conditions (O+P- and O-P+) with pseudowords. Including only these conditions resulted in a total number of 48 lexical items (O+P+ and O-P-) and 48 fixation cross items per lexicality (words and pseudowords). The inconsistent conditions were avoided because those items for the pseudowords could have multiple pronunciations. The first level contrasts compared lexical to both explicit and implicit baselines activations within each lexical condition (words and pseudowords). In addition, the lexicality effect was determined by the direct words vs. pseudowords contrast. Hence, the explicit fixation baseline was included as a condition of interest in the general linear model (GLM) for each subject only for the comparison between the lexical and explicit baseline. All other lexical conditions and the perceptual conditions were included as conditions of non-interest.
Second level (between-subjects) analysis
A voxel-wise analysis was used to examine the general activation for each lexical condition and the lexicality effect, using three separate one-sample t-tests (words > explicit baseline; pseudowords > explicit baseline; words vs. pseudowords). Furthermore, second-level multiple regression analyses assessed group-level lexicality by age interactions, as measured by modeling the activation for words as compared to pseudowords (and vice-versa), and the age of each participant as a covariate. All these voxel-wise analyses were calculated within a functional mask of the words + pseudowords > explicit baseline contrast. Hence, the functional mask reflects the combination of activation for the words > fixation control and the pseudowords > fixation control, as presented in Figure 2a. This functional mask was, in turn, created within an anatomical mask of the left hemisphere language network, which included the left IFG, STG, MTG, ITG, IPL, SMG, AG, and FG. The significant cluster size within this mask was determined by using 3dClustSim which applies 10000 iterations in a Monte Carlo simulation (available as part of the AFNI fMRI analysis package, at http://afni.nimh.nih.gov/afni/download). We used 3dFWHMx to calculate Auto-Correlation Function (ACF) values (data smoothness estimates) for the group level (see Eklund, Nichols, & Knutsson, 2016). According to 3dClustSim the minimum cluster size at an uncorrected voxel threshold of p<0.001, was 15 voxels (k≥ 15) family wise error (FWE) corrected for cluster threshold of p<.05, and 33 voxels (k≥ 33) FWE corrected for cluster threshold of p<.01.
Figure 2.
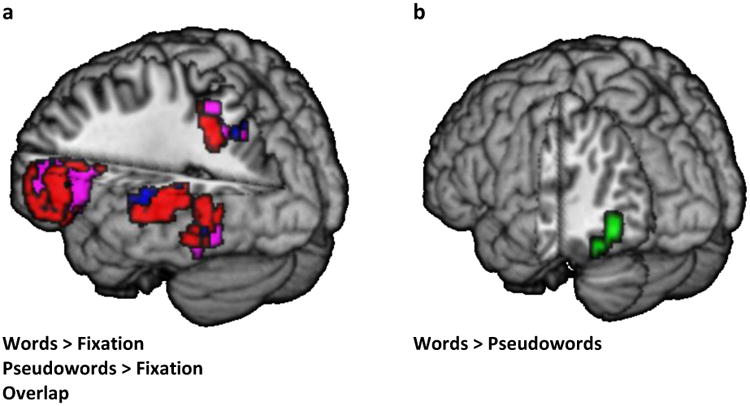
Voxel-wise significant activation for the contrasts of: a) words > fixation (red), pseudowords > fixation (blue) and overlap (violet); b) words > pseudowords (green). All results are presented in a voxel-level threshold of p<.001 uncorrected and a cluster level threshold of p<.05 (k>15) FWE corrected.
3. Results
3.1. Behavioral analysis results
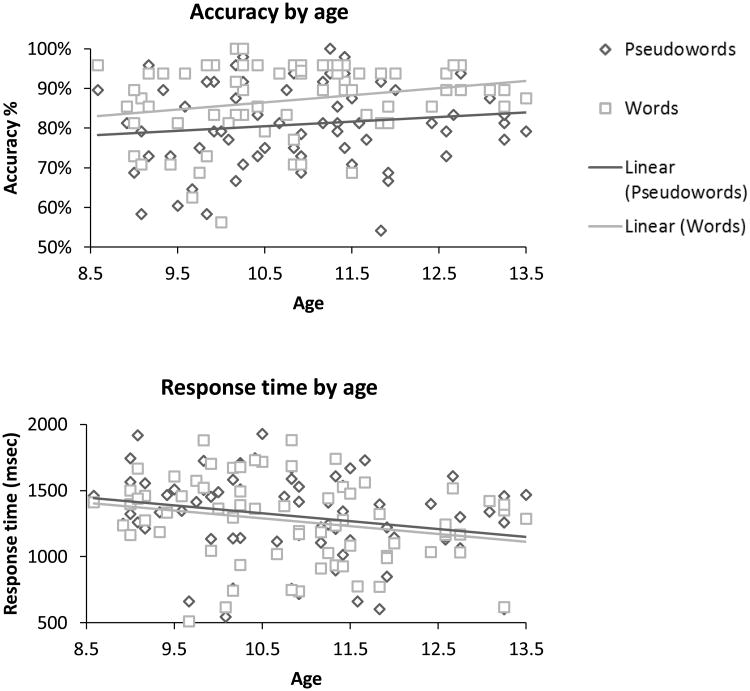
Means and standard deviations of words and pseudowords' accuracy and response time (for correct responses) are presented in Table 1. The linear mixed model revealed that participants were faster (F(1,5742.42) = 22.73, p<.001) when reading real words as compared to pseudowords. However, the main lexicality effect on response time was no longer significant when including age as a covariate in the model. In addition, age significantly affected response time (F(1,68.931) = 4.525, p<.05) across lexical conditions. The logistic mixed model revealed that participants were more accurate (F(1,6831) = 52.13, p<.001) when reading real words as compared to pseudowords. However, the main lexicality effect on accuracy was no longer significant when including age as a covariate in the model. Age had no significant effect on accuracy across lexical conditions.
Table 1. In-scanner task's behavioral results.
| Pseudowords | Words | |
|---|---|---|
| Accuracy (%) | 80.9 (10.5) | 87.2 (9.4) |
| Response time (ms) | 1311 (329) | 1272 (322) |
Note. The numbers in brackets represent standard deviation.
3.2. FMRI analysis results
All voxel-wise analysis significant results within the inclusive mask are presented in Table 2. Voxel-wise contrasts between lexical and fixation baseline (the null explicit baseline) conditions are presented in Figure 2a and show significant clusters in the left frontal, temporo-parietal, and occipito-temporal regions for both words and pseudowords (voxel threshold p<.001 uncorrected, cluster threshold p<.01, k≥33 FWE corrected). For pseudowords, there is an additional cluster in the left STG that extends to the left SMG. The left ventral occipito-temporal cluster extends to the left MTG for words but not for pseudowords. Finally, both word and pseudoword activation cover most of the left IFG. However, the peak activation for words is in the pars triangularis while the peak activation for pseudowords is in the pars opercularis. The direct contrast between word and pseudoword activation (each compared to the implicit baseline) is shown in Figure 2b and reveals significantly greater activation for words in two clusters in the left FG/ITG as compared to pseudowords (voxel threshold p<.001 uncorrected, cluster threshold p<.05, k≥15 FWE corrected), whereas there were not any significant clusters for the opposite contrast (pseudowords > words). There were no significant clusters for the lexicality by age interaction, as measured by modeling the activation for words as compared to pseudowords (and vice-versa) with the age of each participant as a covariate in a multiple regression analysis. Similar results were found when we repeated the voxel-wise analysis while controlling for in-scanner task accuracy.
Table 2. Voxel-wise analysis significant results within the inclusive mask.
| Brain region | BA | Z score | voxels | X | Y | Z | voxel level p value | cluster level p value | |
|---|---|---|---|---|---|---|---|---|---|
| Words > Fixation | L.FG/ITG/MTG | 37/19/22 | 7.51 | 840 | -44 | -52 | -18 | 0.001 | 0.01 |
| L.IFG | 44/45/47 | 7.40 | 17250 | -54 | 18 | 30 | 0.001 | 0.01 | |
| L.IPL/ Precuneus | 40/7 | 5.16 | 85 | -24 | -54 | 54 | 0.001 | 0.01 | |
| Pseudowords > Fixation | L.FG/ITG | 37/19 | 7.47 | 357 | -44 | -46 | -18 | 0.001 | 0.01 |
| L.IFG | 44/45/47 | 6.93 | 11810 | -48 | 10 | 26 | 0.001 | 0.01 | |
| L.IPL/SPL | 40/7 | 4.74 | 40 | -26 | -50 | 54 | 0.001 | 0.01 | |
| L.STG/SMG | 21/40 | 4.71 | 118 | -64 | -36 | 10 | 0.001 | 0.01 | |
| Words > Pseudowords | L.ITG | 19 | 4.29 | 30 | -46 | -62 | -10 | 0.001 | 0.05 |
| L.FG | 37 | 4.26 | 23 | -36 | -48 | -14 | 0.001 | 0.05 |
Note. The table includes all the peaks of the significant clusters within the inclusive mask. The inclusive mask included functional ‘words + pseudowords > fixation’ contrast activation, within the anatomical left hemisphere language network. Based on 3dClustSim, a significant (voxel threshold of p<.001 uncorrected) cluster size within this mask is k≥15 for cluster threshold of p<.01 FWE corrected, or k≥33 for cluster threshold of p<.05 FWE corrected. L- left hemisphere; FG- fusiform gyrus; ITG- inferior temporal gyrus, MTG- middle temporal gyrus; IFG-inferior frontal gyrus; IPL- inferior parietal lobule; SPL- superior parietal lobule; STG- superior temporal gyrus; SMG-supramarginal gyrus; Tri- triangularis part.
4. Discussion
In the current study, we aimed to examine the developmental specialization of the reading network by investigating word and pseudoword brain activation in children. Although comparing words to pseudowords is crucial for testing models of reading acquisition, only one study has directly examined the lexicality effect on brain mechanisms in children (van der Mark et al., 2009). However, this study incorporated a phonological lexical decision task in German, and the results of the lexicality effect were not discussed. The current study examined the lexicality effect on orthographic processing mechanisms within the left vOT cortex in English-reading children. Based on previous studies with English-speaking adults (Cattinelli et al., 2013; Jobard et al., 2003; McNorgan et al., 2015; Price & Mechelli, 2005; Taylor et al., 2013), we expected greater activation for words in anterior vOT proposed to be involved in amodal semantic processing or alternatively in visual whole-word processing. On the other hand, we expected greater activation for pseudowords in mid-posterior vOT proposed to be involved in integration with higher level language processes or alternatively in sublexical orthographic processing. We further hypothesized an age-related increase in specialization of the brain mechanisms engaged for word and pseudoword processing. The results of the current study suggest that 8- to-13-year-old children do not yet show the adult-like specialization within the left vOT and that they may use semantic information to help with spelling to sound mapping.
Consistent with findings from studies that examined the lexicality effect on different components of the reading network in adult participants (Cattinelli et al., 2013; McNorgan et al., 2015; Taylor et al., 2013), we found a greater activation for words as compared to pseudowords in the left anterior vOT (MNI: x,y,z= -36,-48,-14), a region that is sensitive to morphemes, small words and larger orthographic units (Dehaene et al., 2005). The greater activation for word as compared to pseudoword reading can be explained by two alternative interpretations. First, the anterior left vOT is associated with modality independent semantic processing (Price, 2000, 2012). Alternatively, the anterior left vOT may function as an orthographic lexicon and contain whole-word orthographic representations (Boukadi et al., 2016; Ludersdorfer et al., 2016). Hence, despite the debate on the specific role of the anterior left vOT (Taylor et al., 2013), both interpretations can explain the greater engagement of this region in word as compare to pseudoword reading for children in the current study, similar to findings from adults.
Inconsistent with findings from studies of adults, we found greater activation for words as compared to pseudowords in a more posterior part of the left vOT (MNI: x,y,z = -46,-62,-10). Some have argued that this region is associated with cross-modal integration of the low-level visual with higher-level language processes (Price & Devlin, 2011), and it usually shows greater activation for pseudowords compared to words in adults (Cattinelli et al., 2013; McNorgan et al., 2015; Taylor et al., 2013). The unexpected opposite effect of greater word as compared to pseudoword activation found for children in the current study suggests a semantic influence on orthographic processing in this region. The interactive compensatory model (Stanovich, 1980) predicts age- and skill-related decreases in reliance on semantics in word recognition, along with increases in efficiency of spelling to sound mechanisms. More recently, this model was supported by neuroimaging studies demonstrating greater semantic-related activation for children as compared to adults during rhyming and spelling tasks, despite these tasks not requiring explicit access to word meaning (Booth & Burman, 2005). The children in our study may still be using semantic information to facilitate visual word recognition in the vOT.
Consistent with the imaging results of greater activation for word as compared to pseudoword activation in the left vOT and with behavioral findings in adults, our behavioral results show that the children in the current study read words faster and more accurately as compared to pseudowords, perhaps reflecting the familiarity with the real word orthographic patterns. However, the lexicality effect was no longer significant when controlling for age, and age was negatively correlated with response times across lexical conditions. The behavioral results suggest that reading experience has an effect on word recognition performance in general, with less of an impact on the specific familiarity with the orthographic stimuli. In terms of brain activation, there were no significant age main effects or lexicality by age interactions. The lack of an age effect on brain activation suggests that the specialization in the left vOT only appears later in development, consistent with the findings that adult-like patterns of activation in this region only appear by the age of 15 years (Ben-Shachar et al., 2011; Olulade et al., 2013).
The greater activation for words as compared to pseudowords in the left anterior and mid-posterior vOT found for the English speaking children in the current study contradicts the results from van der Mark and colleagues (van der Mark et al., 2009) showing the opposite direction of the lexicality effect in a group of 7-12-year-old German speaking children. The discrepancies in the results might be due to task differences. The phonological lexical decision task in the German study may have increased demands on phonological and lexical search in the left vOT for pseudowords as compared to words. Hence, pseudowords may have increased the prediction error from higher language process (Price & Devlin, 2011; Taylor et al., 2013) resulting in greater activation in the left vOT. A recent study with 9- to-13-year-old German speaking children compared brain activation of pseudowords in the same phonological lexical decision task to real word activation in a semantic categorization task (Liebig et al., 2017). This study did not find a significant lexicality effect in the left vOT, indicating that the specific task used influences the lexicality effect in this region.
Future studies should further examine the prediction that older children would show the adult-like specialization in the left vOT (i.e., for the dissociation between word and pseudoword activation). Moreover, a connectivity approach could reveal whether this specialization results from decreased top-down semantic influence along with increased spelling to sound mapping efficiency. These age-related changes should be evident in increased connectivity between the left FG and IPL/SMG, the latter of which is implicated in phonological processing. This change may, in turn, predict decreased connectivity between the left FG and MTG, the latter of which is implicated in semantic processing. This would suggest that gains in the automaticity of grapheme-phoneme may result in less semantic influence.
5. Conclusion
The current study is the first to demonstrate a lexicality effect in left anterior vOT in English speaking children, indicating an early sensitivity to orthographic familiarity within this region. Although the greater activation for words than pseudowords in anterior vOT is consistent with adult studies, the greater activation for words than pseudowords in mid-posterior vOT is not consistent with previous studies in adults showing the reverse. The opposite pattern found for young readers in our study in the left mid-posterior vOT might suggest that this region is influenced by top-down semantic processing in young readers with less efficient spelling to sound mechanisms. However, this interpretation should be taken cautiously, since the results could be affected by the nature of the task used in the current study.
Figure 1.
Performance in the in-scanner task for words (blue) and pseudowords (red). a) Accuracy percentage by age; b) Response time by age (msec). The values represent the average accuracy percentage and response times per participant for the conditions that were included in the analysis (O+P+ and O-P-)
Highlights.
Lexicality effect was examined by comparing words to pseudowords in children
Greater activation for words was found in anterior and posterior vOT
The anterior, not posterior, effects are consistent with previous adult studies
The posterior vOT results suggest a robust semantic influence in children
Children may use semantic information to help map graphemes to phonemes
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [grant number HD042049] to James R. Booth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- Backes W, Vuurman E, Wennekes R, Spronk P, Wuisman M, van Engelshoven J, Jolles J. Atypical brain activation of reading processes in children with developmental dyslexia. Journal of Child Neurology. 2002;17(12):867–871. doi: 10.1177/08830738020170121601. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. The development of cortical sensitivity to visual word forms. Journal of Cognitive Neuroscience. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD. Using neuro-imaging to test developmental models of reading acquisition. In: Catts H, Kamhi A, editors. The connections between language and reading disabilities. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 131–154. [Google Scholar]
- Boukadi M, Potvin K, Macoir J, R, Poulin S, Brambati SM, Wilson MA. Lexical decision with pseudohomophones and reading in the semantic variant of primary progressive aphasia : A double dissociation. Neuropsychologia. 2016;86:45–56. doi: 10.1016/j.neuropsychologia.2016.04.014. https://doi.org/10.1016/j.neuropsychologia.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Brem S, Halder P, Bucher K, Summers P, Martin E, Brandeis D. Tuning of the visual word processing system: Distinct developmental ERP and fMRI effects. Human Brain Mapping. 2009;30(6):1833–1844. doi: 10.1002/hbm.20751. https://doi.org/10.1002/hbm.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno JL, Zumberge A, Manis FR, Lu ZL, Goldman JG. Sensitivity to orthographic familiarity in the occipito-temporal region. NeuroImage. 2008;39(4):1988–2001. doi: 10.1016/j.neuroimage.2007.10.044. https://doi.org/DOI:10.1016/j.neuroimage.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The Feasibility of a Common Stereotactic Space for Children and Adults in fMRI Studies of Development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. https://doi.org/10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Estévez A, Price CJ. Brain activation for lexical decision and reading aloud: two sides of the same coin? Journal of Cognitive Neuroscience. 2007;19(3):433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Cattinelli I, Borghese NA, Gallucci M, Paulesu E. Reading the reading brain: a new meta-analysis of functional imaging data on reading. Journal of Neurolinguistics. 2013;26(1):214–238. https://doi.org/10.1016/j.jneuroling.2012.08.001. [Google Scholar]
- Dehaene S, Cohen L, Morais J, Kolinsky R. Illiterate to literate: behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience. 2015;16(4) doi: 10.1038/nrn3924. https://doi.org/10.1038/nrn3924. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends in Cognitive Sciences. 2005;9(7):335–341. doi: 10.1016/j.tics.2005.05.004. https://doi.org/10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113 doi: 10.1073/pnas.1602413113. https://doi.org/10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C, Friederici A, Müller K, Cramon Dvon. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Cognitive Neuroscience, Journal of. 2002;14(1):11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Binder JR, Desai RH, Conant LL, Seidenberg MS. Neural correlates of implicit and explicit combinatorial semantic processing. NeuroImage. 2010;53(2):638–646. doi: 10.1016/j.neuroimage.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: cooperative division of labor between visual and phonological processes. Psychological Review. 2004;111(3):662. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. NeuroImage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. https://doi.org/10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. taksi: on orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19(10):1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Kronbichler M, Hutzler F, Wimmer H, Mair A. The visual word form area and the frequency with which words are encountered : Evidence from a parametric fMRI. Neuroimage. 2004;21(3):946–953. doi: 10.1016/j.neuroimage.2003.10.021. https://doi.org/10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Liebig J, Froehlich E, Morawetz C, Braun M, Jacobs AM, Heekeren HR, Ziegler JC. Neurofunctionally dissecting the developing reading system. Developmental Cognitive Neuroscience. 2017 Aug;27:45–57. doi: 10.1016/j.dcn.2017.07.002. https://doi.org/10.1016/j.dcn.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludersdorfer P, Wimmer H, Richlan F, Schurz M, Hutzler F, Kronbichler M. Left ventral occipitotemporal activation during orthographic and semantic processing of auditory words. NeuroImage. 2016;124:834–842. doi: 10.1016/j.neuroimage.2015.09.039. https://doi.org/10.1016/j.neuroimage.2015.09.039. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Chabal S, O'Young D, Lukic S, Booth JR. Task dependent lexicality effects support interactive models of reading: A meta-analytic neuroimaging review. Neuropsychologia. 2015;67:148–158. doi: 10.1016/j.neuropsychologia.2014.12.014. https://doi.org/10.1016/j.neuropsychologia.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Ralph MAL, Patterson K, et al. Price CJ. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17(11):1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging Studies of Word and Pseudoword Reading: Consistencies, Inconsistencies, and Limitations. Journal of Cognitive Neuroscience. 2003;15(2):260–271. doi: 10.1162/089892903321208196. https://doi.org/10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12(5):538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden GF. Developmental differences for word processing in the ventral stream. Brain and Language. 2013;125(2):134–145. doi: 10.1016/j.bandl.2012.04.003. https://doi.org/10.1016/j.bandl.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden GF. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage: Clinical. 2015;7:742–754. doi: 10.1016/j.nicl.2015.02.013. https://doi.org/10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattamadilok C, Chanoine V, Pallier C, Anton JL, Nazarian B, Belin P, Ziegler JC. Automaticity of phonological and semantic processing during visual word recognition. NeuroImage. 2017;149(September 2016):244–255. doi: 10.1016/j.neuroimage.2017.02.003. https://doi.org/10.1016/j.neuroimage.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Plaut DC, Booth JR. Individual and developmental differences in semantic priming: empirical and computational support for a single-mechanism account of lexical processing. Psychological Review. 2000;107(4):786–823. doi: 10.1037/0033-295x.107.4.786. https://doi.org/10.1037/0033-295X.107.4.786. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of Functional MRI Data Analysis (Cambridge) Cambridge: 2011. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011 doi: 10.1016/j.tics.2011.04.001. https://doi.org/10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed]
- Price CJ, Mechelli A. Reading and reading disturbance. Current Opinion in Neurobiology. 2005;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. https://doi.org/10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Protopapas A, Orfanidou E, Taylor JSH, Karavasilis E, Kapnoula EC, Panagiotaropoulou G, et al. Kelekis D. Evaluating cognitive models of visual word recognition using fMRI: Effects of lexical and sublexical variables. NeuroImage. 2016;128:238–341. doi: 10.1016/j.neuroimage.2016.01.013. https://doi.org/10.1016/j.neuroimage.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Frost SJSJ, Sandak R, Landi N, Moore D, Della Porta G, et al. Mencl W. Mapping the word reading circuitry in skilled and disabled readers. The Neural Basis of Reading. 2010:281–305. [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, et al. Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56(3):1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. https://doi.org/DOI:10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Schurz M, Kronbichler M, Crone J, Richlan F, Klackl J, Wimmer H. Top-down and bottom-up influences on the left ventral occipito-temporal cortex during visual word recognition: An analysis of effective connectivity. Human Brain Mapping. 2014 doi: 10.1002/hbm.22281. https://doi.org/10.1002/hbm.22281. [DOI] [PMC free article] [PubMed]
- Stanovich KE. Toward an interactive-compensatory model of individual differences in the development of reading fluency. Reading Research Quarterly. 1980:32–71. [Google Scholar]
- Taylor JSH, Rastle K, Holloway R, Davis MH, Holloway R, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin. 2013;139(4):766. doi: 10.1037/a0030266. https://doi.org/10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. TOWRE–2 test of word reading efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TAT, Eden GFG. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmüller J, et al. Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47(4):1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. https://doi.org/10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Hoeft F, Norton ES. Integrating MRI brain imaging studies of pre-reading children with current theories of developmental dyslexia: A review and quantitative meta-analysis. Current Opinion in Behavioral Sciences. 2016 doi: 10.1016/j.cobeha.2016.06.007. https://doi.org/10.1016/j.cobeha.2016.06.007. [DOI] [PMC free article] [PubMed]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, Schrank F. Woodcock-Johnson III. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Woollams AM, Silani G, Okada K, Patterson K, Price CJ. Word or word-like? Dissociating orthographic typicality from lexicality in the left occipito-temporal cortex. Journal of Cognitive Neuroscience. 2011;23(4):992–1002. doi: 10.1162/jocn.2010.21502. https://doi.org/10.1162/jocn.2010.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]