Abstract
Background
Analgesia and sedation are cornerstone therapies for mechanically ventilated patients. Despite data showing that early deep sedation in the ICU influences outcome, this has not been investigated in the ED. Therefore, ED-based sedation practices, and their influence on outcome, remain incompletely defined. This study’s objectives were to describe ED sedation practices in mechanically ventilated patients and to test the hypothesis that ED sedation depth is associated with worse outcomes.
Methods
This was a cohort study of a prospectively compiled ED registry of adult mechanically ventilated patients at a single academic medical center. Hospital mortality was the primary outcome and hospital-, ICU-, and ventilator-free days were secondary outcomes. A backward stepwise multivariable logistic regression model evaluated the primary outcome as a function of ED sedation depth. Sedation depth was assessed with the Richmond Agitation-Sedation Scale (RASS).
Results
Four hundred fourteen patients were studied. In the ED, 354 patients (85.5%) received fentanyl, 254 (61.3%) received midazolam, and 194 (46.9%) received propofol. Deep sedation was observed in 244 patients (64.0%). After adjusting for confounders, a deeper ED RASS was associated with mortality (adjusted OR, 0.77; 95% CI, 0.63-0.94).
Conclusions
Early deep sedation is common in mechanically ventilated ED patients and is associated with worse mortality. These data suggest that ED-based sedation is a modifiable variable that could be targeted to improve outcome.
Key Words: ED, mechanical ventilation, sedation depth
Abbreviations: RASS, Richmond Agitation-Sedation Scale; SOFA, Sequential Organ Failure Assessment
Analgesia and sedation are cornerstone therapies for mechanically ventilated patients. Although critical to treat pain, relieve anxiety, and facilitate ventilator synchrony, medications used to achieve analgosedation influence duration of ventilation and lengths of stay.1 Data strongly support optimizing sedation early to improve outcome; therefore, targeting improved analgosedation in the immediate postintubation period could be a high-impact intervention.2
Despite frequent endotracheal intubation in the ED, little data exist regarding postintubation analgosedation or sedation depth in mechanically ventilated ED patients. The available data are up to a decade old and limited by study design, as well as lacking detail on medication administration, including dose and sedation depth.3, 4, 5, 6 No studies have examined the impact of ED-based sedation on clinically relevant outcomes.
The majority of clinical trials evaluating sedation enrolled patients after 48 hours of mechanical ventilation, leaving significant knowledge gaps regarding the impact of early sedation on clinical outcomes.1, 7, 8 Most relevant to the ED and from the limited data that do exist, early (ie, within 48 hours of admission) deep sedation in the ICU is associated with longer mechanical ventilation duration and increased mortality.7, 8 However, it is unknown whether sedation practices immediately following endotracheal intubation influence outcome. As a result, modern ED sedation practices are incompletely characterized, and the impact on clinical outcome is poorly understood.
This study sought to (1) characterize modern ED analgosedation practices and (2) assess the relationship between ED sedation depth and clinical outcomes, including mortality, duration of mechanical ventilation, and length of stay. We hypothesized that deeper sedation in the ED would be associated with significant differences in clinical outcomes.
Methods
Study Design, Setting, and Population
This study was a secondary analysis of a prospective observational cohort of patients with acute initiation of mechanical ventilation in the ED. It was conducted at a single tertiary academic medical center (October 2014-March 2016).
An automated electronic pager system identified consecutive mechanically ventilated patients. This system was activated in one of three ways: (1) an order for ventilator settings, (2) an order for neuromuscular blockade, or (3) documentation of an endotracheal intubation procedure. After cases were identified, data collection occurred prospectively. This approach has been used for identifying all mechanically ventilated patients in our ED for almost 5 years.9, 10 As the original study was not specifically designed to evaluate sedation practices, additional sedation-pertinent data were obtained retrospectively from the electronic medical record.
Mechanically ventilated ED patients were assessed for inclusion. Inclusion criteria included (1) age ≥ 18 years and (2) mechanical ventilation through an endotracheal tube. Exclusion criteria included (1) death or discontinuation of mechanical ventilation within 24 hours, (2) chronic mechanical ventilation, (3) presence of a tracheostomy, (4) transfer to another hospital, and (5) neurologic injury or sudden cardiac arrest as the reason for initiation of mechanical ventilation. Patients were identified as having neurologic injury or cardiac arrest if they were admitted to a neurologic ICU or had a primary diagnosis of cardiac arrest as the reason for mechanical ventilation and admission; these two patient groups were excluded, as patients with neurologic injury and cardiac arrest could have depressed levels of consciousness independent of sedative and analgesic medications administered, confounding the association between documented sedation depth and clinical outcome. The Washington University institutional review board approved this study under waiver of informed consent.
Measurements
Baseline demographics, comorbid conditions, vital signs, laboratory variables, illness severity (ie, Sequential Organ Failure Assessment [SOFA]), reason for mechanical ventilation, ED length of stay, and location of intubation were collected.11, 12, 13 ED process of care variables included IV fluid, blood products, central venous catheters, antibiotics, and vasopressor infusion.
Retrospectively, data on neuromuscular blockers and induction agents administered to facilitate endotracheal intubation and subsequent medications (analgesics, sedatives, and neuromuscular blockers) given to achieve analgosedation in the ED were collected. Agents administered for the management of analgosedation during the first 48 hours of ICU admission were also collected. In addition to those medications used in the ED, other agents commonly used in the ICU for the management of analgosedation were recorded (dexmedetomidine and antipsychotic agents [quetiapine, haloperidol]).
Sedation depth was assessed with the Richmond Agitation-Sedation Scale (RASS), ranging from –5 (unarousable) to +4 (combative). When more than one RASS assessment per patient was documented in the ED, the median RASS value was used. Deep sedation was defined as a median RASS of –3 to –5.7 In patients for whom no RASS was documented in the ED, the first ICU RASS served as a surrogate for the ED RASS provided that it was measured within the first 3 hours of ICU admission. Use of an early ICU RASS score as a reliable surrogate for ED RASS is supported by previous data demonstrating that sedation depth remains relatively static during the first 24 hours of ICU admission.7
Patients were followed until hospital discharge or death. The primary outcome was hospital mortality. Secondary outcomes included ventilator-, hospital-, and ICU-free days. Outcomes were assessed as a function of ED sedation depth, with the a priori hypothesis being that deep sedation in the ED is associated with increased mortality, longer lengths of stay, and greater ventilator duration.
Data Analysis
Descriptive statistics and frequency distributions were used to assess patient characteristics. Categorical characteristics were compared using the χ2 test. Continuous characteristics were compared using the independent samples t test or the Mann-Whitney U test. Normality of the data was assessed by inspection of histograms and examining skewness and kurtosis.
To test the relationship between ED sedation depth and survival, an explanatory logistic regression model was constructed to adjust for potentially confounding covariates. A priori baseline characteristics with known prognostic significance for the primary outcome were selected for model inclusion: age, indication for mechanical ventilation, vasopressor use, and illness severity.14, 15 Other clinically relevant and biologically plausible variables, without missing data and statistically significant in univariate analysis at a P < .05 level, were also included in the model (malignancy and dexmedetomidine use in the ICU). The model was a backward stepwise multivariable logistic regression model that selected variables for inclusion or exclusion in a sequential fashion based on a significance level of 0.10 for entry and 0.10 for removal, with the goal of achieving parsimony. Statistical interactions were assessed. Collinearity diagnostics (eg, variance inflation factor) were assessed to test the assumption of no multicollinearity. The model used variables that were statistically independent of other variables in the model. The model’s goodness of fit was assessed with the Hosmer-Lemeshow test and by examining residuals. Adjusted ORs (aORs) and corresponding 95% CIs are reported for variables in the multivariable model, adjusted for all variables in the model. All tests were two-tailed, and a P value < .05 was considered statistically significant.
To further examine the association between ED sedation depth and outcome, multiple a priori subgroup analyses were conducted. To better control for the influence of the indication for mechanical ventilation, patients with trauma and medical indications for mechanical ventilation were examined separately. Furthermore, in patients not receiving analgosedation in the ED, it is possible that sedation was withheld for clinically indicated reasons (ie, mental status). To control for potential confounding by indication, a subgroup analysis excluding patients that did not receive any sedation in the ED was performed. Three post hoc analyses were also conducted. Since ED sedation depth could be influenced by medications received prior to ED arrival, the first was an analysis on only those patients intubated in the ED. Second, as dexmedetomidine use in the ICU was associated with lower mortality in the primary analysis, a subgroup analysis was performed that excluded patients who received dexmedetomidine in the ICU. Finally, to investigate whether the administration of midazolam or propofol influenced outcome, several exploratory logistic regression models were run that analyzed these agents with the variables included in the original multivariable model.
Results
Population Description
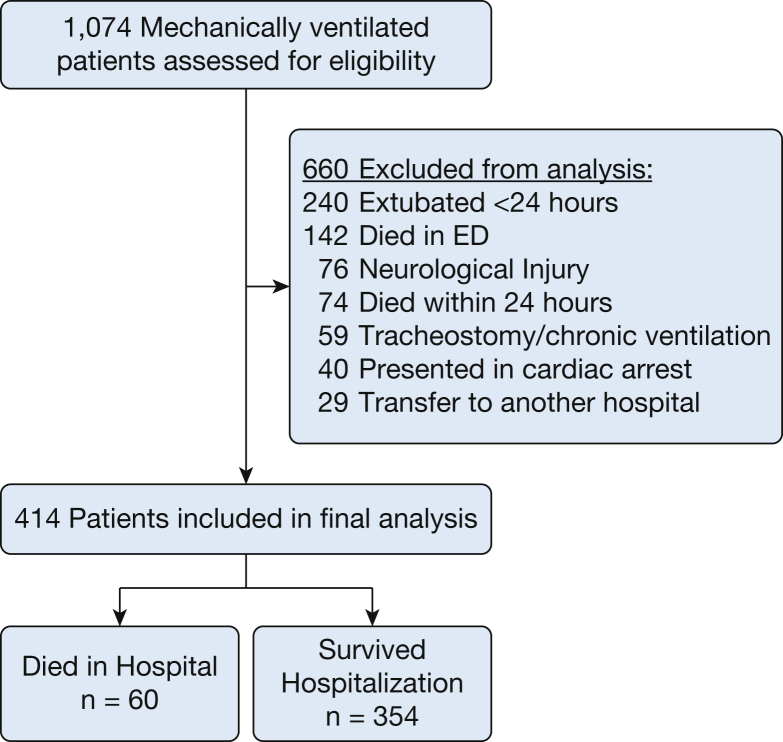
A total of 1,074 patients were assessed for inclusion and 414 were included in the final study population (Fig 1). Baseline characteristics are shown in Table 1.
Figure 1.
Patient inclusion flow diagram.
Table 1.
Characteristics of Mechanically Ventilated ED Patients
| Baseline Characteristics | All Subjects N = 414 |
Mortality Status |
||
|---|---|---|---|---|
| Nonsurvivors (n = 60) |
Survivors (n = 354) |
P Value | ||
| Age, y | 54.6 (18.5) | 59.8 (19.0) | 53.7 (18.3) | .019 |
| Male, No. (%) | 247 (59.7) | 38 (63.3) | 209 (59.0) | .531 |
| Race, No. (%) | ||||
| African American | 234 (56.5) | 32 (53.3) | 202 (57.1) | .590 |
| White | 180 (43.5) | 28 (46.7) | 152 (42.9) | |
| Comorbidities, No. (%) | ||||
| Diabetes | 131 (31.6) | 29 (33.3) | 111 (31.4) | .761 |
| COPD | 104 (25.1) | 9 (15.0) | 95 (26.8) | .051 |
| CHF | 95 (22.9) | 13 (21.7) | 82 (23.2) | .799 |
| Alcohol abuse | 61 (14.7) | 9 (15.0) | 52 (14.7) | .950 |
| Malignancy | 54 (13.0) | 13 (21.7) | 41 (11.6) | .032 |
| Immunosuppression | 54 (13.0) | 11 (18.3) | 43 (12.1) | .188 |
| Dialysis | 38 (9.2) | 7 (11.7) | 31 (8.8) | .470 |
| Cirrhosis | 37 (8.9) | 9 (15.0) | 28 (7.9) | .075 |
| HIV/AIDS | 5 (1.4) | 0 (0.0) | 5 (1.2) | .354 |
| BMI | 28.8 (9.7) | 27.9 (10.9) | 28.9 (9.5) | .449 |
| Temperature (°C) | 36.6 (36.1-37.1) | 36.5 (35.3-37.3) | 36.6 (36.1-37.1) | .206 |
| MAP | 75 (56-115) | 64 (47-110) | 77 (57-116) | .052 |
| Lactate (n = 367) | 2.8 (1.5 - 5.1) | 5.0 (2.8 - 7.9) | 2.5 (1.5 - 4.6) | < .001 |
| Creatinine | 1.2 (0.9-2.0) | 1.3 (0.9-2.5) | 1.2 (0.9-2.0) | .234 |
| Total bilirubin (n = 365) | 0.4 (0.3-0.8) | 0.6 (0.3-2.1) | 0.4 (0.3-0.7) | .007 |
| Albumin (n = 365) | 3.4 (2.9-3.7) | 3.0 (2.4-3.5) | 3.4 (2.9-3.8) | .002 |
| APACHE IIa | 18 (12-25) | 20 (13-29) | 17.0 (12-24) | .020 |
| SOFAa | 5 (2-7) | 7 (4-10) | 4 (2-10) | < .001 |
| Reason for mechanical ventilation, No. (%) | ||||
| Sepsis | 154 (37.2) | 27 (45.0) | 127 (35.9) | .176 |
| Trauma | 128 (30.9) | 19 (31.7) | 109 (30.8) | .892 |
| COPD | 29 (7.0) | 3 (5.0) | 26 (7.3) | .511 |
| Drug overdose | 21 (5.1) | 1 (1.7) | 20 (5.6) | .194 |
| CHF/pulmonary edema | 17 (4.1) | 2 (3.3) | 15 (4.2) | .744 |
| Asthma | 9 (2.2) | 0 (0) | 9 (2.5) | .212 |
| Other | 56 (13.5) | 8 (13.3) | 48 (13.6) | .962 |
| Sepsis, No. (%) | 180 (43.5) | 32 (53.3) | 148 (41.8) | .096 |
| ED length of stay, h | 4.8 (3.2-6.8) | 4.8 (3.2-6.8) | 5.0 (3.4-6.9) | .809 |
| Location of intubation | ||||
| ED | 317 (76.6) | 46 (76.7) | 271 (76.6) | .985 |
| Prehospital | 45 (10.9) | 7 (11.7) | 38 (10.7) | .830 |
| Outside hospital/other facility | 52 (12.6) | 7 (11.7) | 45 (12.7) | .821 |
| Process of care variables | ||||
| IV fluids in ED | 1 (0.5-2.5) | 1 (0-2.9) | 1 (0.5-2.5) | .647 |
| Blood products, No. (%) | 77 (18.6) | 23 (38.3) | 54 (15.3) | < .001 |
| Central venous catheter, No. (%) | 143 (34.5) | 31 (51.7) | 112 (31.6) | .003 |
| Antibiotics, No. (%) | 221 (53.4) | 35 (58.3) | 186 (52.5) | .406 |
| Vasopressor infusion, No. (%) | 129 (31.2) | 37 (61.7) | 92 (26.0) | < .001 |
Continuous variables are reported as mean (SD) and median (interquartile range). For measurements in which more than 1 value was present in the ED (ie, vital signs), the initial ED value is presented.
APACHE = Acute Physiology and Chronic Health Evaluation; CHF = congestive heart failure; MAP = mean arterial pressure; SOFA=Sequential Organ Failure Assessment.
Modified score, which excludes Glasgow Coma Scale.
Medications Administered
Three hundred seventeen patients were intubated in the ED. The majority underwent paralysis for rapid-sequence intubation with succinylcholine or rocuronium and induction with etomidate or ketamine (e-Table 1).
Analgesic and sedative drugs administered in the ED are presented in Table 2. Three hundred fifty-four patients (85.5%) received fentanyl, 254 (61.4%) received midazolam, and 194 (46.9%) received propofol. Excluding induction for intubation, ketamine was administered to 68 patients (16.4%) and etomidate to 15 (3.6%). Postintubation, an additional dose of neuromuscular blockade with rocuronium was given to 20 patients (4.8%). Fifty-nine patients (14.3%) received no analgesia and 63 (15.2%) received no sedation while in the ED.
Table 2.
Sedation Variables in the ED
| Drug | All Subjects (N = 414) |
Mortality |
||
|---|---|---|---|---|
| Nonsurvivors (n = 60) |
Survivors (n = 354) |
P Value | ||
| Fentanyl, No. (%) | 354 (85.5) | 41 (68.3) | 313 (88.4) | < .001 |
| Infusion only, No. (%) | 19 (4.6) | 4 (6.7) | 15 (4.2) | .406 |
| Bolus only, No. (%) | 202 (48.8) | 19 (31.7) | 183 (51.7) | .004 |
| Both, No. (%) | 133 (32.1) | 18 (30.0) | 115 (32.5) | .703 |
| Cumulative dose (μg) | 287 (135-450) | 200 (117-400) | 300 (150-452) | .128 |
| Weight-based dose (μg/kg) | 3.3 (1.6-5.6) | 2.4 (1.5-5.0) | 3.4 (1.7-5.6) | .207 |
| Midazolam, No. (%) | 254 (61.4) | 33 (55.0) | 221 (62.4) | .274 |
| Infusion only, No. (%) | 9 (2.2) | 3 (5.0) | 6 (1.7) | .105 |
| Bolus only, No. (%) | 190 (45.9) | 24 (40.0) | 166 (46.9) | .322 |
| Both, No. (%) | 55 (13.3) | 6 (10.0) | 49 (13.8) | .418 |
| Cumulative dose (mg) | 6.0 (3.0-10.0) | 5.0 (2.4-8.0) | 6.0 (3.0-10.0) | .257 |
| Weight-based dose (mg/kg) | 0.32 (0.17-0.54) | 0.23 (0.17-0.57) | 0.32 (0.17-0.53) | .880 |
| Propofol, No. (%) | 194 (46.9) | 18 (30.0) | 176 (49.7) | .005 |
| Infusion only, No. (%) | 84 (20.3) | 8 (13.3) | 76 (21.5) | .147 |
| Bolus only, No. (%) | 20 (4.8) | 3 (5.0) | 17 (4.8) | .947 |
| Both, No. (%) | 90 (21.7) | 7 (11.7) | 83 (23.4) | .041 |
| Cumulative dose (mg) | 279 (96-586) | 156 (55-529) | 297 (98-591) | .219 |
| Weight-based dose (mg/kg) | 3.2 (1.2-7.1) | 1.9 (0.7-7.1) | 3.3 (1.2-7.1) | .155 |
| Ketamine, No. (%) | 68 (16.4) | 5 (8.3) | 63 (17.8) | .067 |
| Cumulative dose (mg) | 150 (100-200) | 100 (80-165) | 150 (100-200) | .358 |
| Weight-based dose (mg/kg) | 1.7 (1.0-2.6) | 1.5 (0.7-1.6) | 1.7 (1.0-2.6) | .179 |
| Lorazepam, No. (%) | 40 (9.7) | 4 (6.7) | 36 (10.2) | .396 |
| Cumulative dose (mg) | 2.5 (1.3-5.8) | 3.5 (2.3-5.5) | 2.0 (1.0-5.8) | .528 |
| Weight-based dose (mg/kg) | 0.03 (0.02-0.07) | 0.04 (0.03-0.06) | 0.03 (0.02-0.07) | .586 |
| Etomidate, No. (%) | 15 (3.6) | 2 (3.3) | 13 (3.7) | .897 |
| Cumulative dose (mg) | 40.0 (20.0-40.0) | 60.0 (20.0-NA) | 40.0 (20.0-40.0) | .686 |
| Weight-based dose (mg/kg) | 0.38 (0.28-0.52) | 0.86 (0.28-NA) | 0.38 (0.28-0.52) | .686 |
| Morphine, No. (%) | 11 (2.7) | 2 (3.3) | 9 (2.5) | .725 |
| Cumulative dose (mg) | 4.0 (4.0-8.0) | 5.0 (4.0-NA) | 4.0 (4.0-10.0) | 1.0 |
| Weight-based dose (mg/kg) | 0.05 (0.04-0.06) | 0.03 (0.02-NA) | 0.05 (0.04-0.09) | .327 |
| Hydromorphone, No. (%) | 3 (0.7) | 0 (0) | 3 (0.8) | .474 |
| Cumulative dose (mg) | 1.7 (1.2) | NA | 1.7 (1.2) | NA |
| Weight-based dose (mg/kg) | 0.02 (0.01) | NA | 0.02 (0.01) | NA |
| Diazepam, No. (%) | 1 (0.2) | 0 (0) | 1 (0.3) | .680 |
| Cumulative dose (mg) | 15.0 (NA) | NA | 15.0 | NA |
| Weight-based dose (mg/kg) | 0.17 (NA) | NA | 0.17 | NA |
| No analgesia in ED, No. (%) | 59 (14.3) | 19 (31.7) | 40 (11.3) | < .001 |
| No sedation in ED, No. (%) | 63 (15.2) | 17 (28.3) | 46 (13.0) | .002 |
| Neuromuscular blocker (rocuronium), No. (%) | 20 (4.8) | 2 (3.3) | 18 (5.1) | .559 |
| Time elapsed from intubation to sedation, min | 16 (8-29) | 24 (10-43) | 15 (8-27) | .102 |
| Time elapsed from intubation to analgesia, min | 20 (10-50) | 27 (9-51) | 20 (10-49) | .551 |
| ED sedation depth (RASS) | –3 (–4 to –2) | –4.0 (–4 to –3) | –3 (–4 to –2) | < .001 |
| Deep sedation in ED, No. (%)a | 244 (64.0) | 47 (82.5) | 197 (60.8) | .002 |
NA = not available; RASS=Richmond Agitation-Sedation Scale.
Medians and interquartile ranges were determined excluding patients who did not receive that medication.
Early sedation depth data were available for 381 patients. A total of 571 RASS observations were used to determine ED RASS level and sedation depth.
During the first 48 hours of ICU care, 370 patients (89.4%) received fentanyl, and 259 patients (62.6%) and 212 (51.2%) received propofol and midazolam, respectively. Dexmedetomidine was used in 131 patients (31.6%). The remainder of the drugs given for sedation and analgesia in the ICU are presented in e-Table 2.
Exposures and Outcomes
The median ED RASS level was –3.0 (–4.0 to –2.0), and deep sedation was observed in 244 patients (64.0%) (Table 2). The median RASS in the ICU was –3 (–4 to –2) at 24 hours and –2 (–3 to –1) at 48 hours (e-Table 2).
The primary outcome of hospital mortality occurred in 60 patients (14.5%). The ED RASS was deeper in patients who died (–4 [–4 to –3]) compared with those who survived to hospital discharge (–3 [–4 to –2]) (P < .001) (Table 2).
Table 3 displays the multivariable logistic regression model with hospital mortality as the dependent variable. After adjusting for all identified significant confounders, a deeper ED RASS was associated with mortality (aOR, 0.77; 95% CI, 0.54-0.94).
Table 3.
Multivariable Logistic Regression Analysis With Hospital Mortality as the Dependent Variable
| Variable | aOR | 95% CI | SE | P Value |
|---|---|---|---|---|
| Age | 1.02 | 0.99-1.04 | 0.01 | .067 |
| Vasopressor infusion | 2.6 | 1.14-5.80 | 0.42 | .023 |
| Malignancy | 2.46 | 1.06-5.70 | 0.43 | .036 |
| ED SOFA | 1.16 | 1.02-1.33 | 0.07 | .027 |
| Reason for mechanical ventilation | ||||
| COPD | 2.22 | 0.54-9.18 | 0.72 | .270 |
| Sepsis | 0.75 | 0.31-1.81 | 0.45 | .523 |
| Trauma | 2.73 | 1.17-6.40 | 0.43 | .020 |
| ED RASS level | 0.77 | 0.63-0.94 | 0.10 | .010 |
| ICU dexmedetomidine use | 0.17 | 0.06-0.49 | 0.55 | .001 |
aOR=adjusted OR. See Table 2 legend for expansion of other abbreviations.
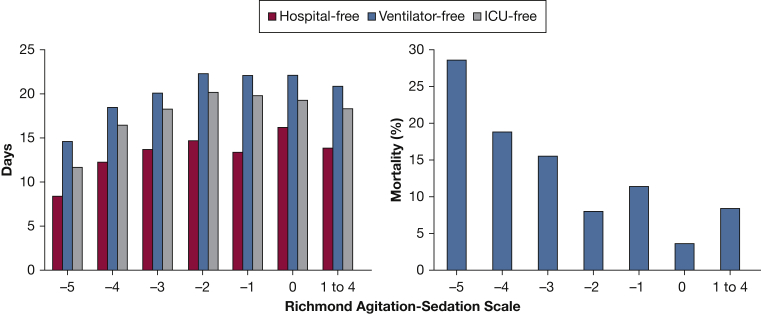
Compared with patients who were not deeply sedated in the ED, those who were deeply sedated experienced fewer ventilator-, ICU-, and hospital-free days (e-Table 3). There also appeared to be improved clinical outcomes associated with incrementally lighter ED sedation depth (Fig 2).
Figure 2.
ED Richmond Agitation-Sedation Scale and clinical outcomes. There were improved clinical outcomes associated with incrementally lighter ED sedation.
When analysis was restricted to patients mechanically ventilated for trauma (aOR, 0.39; 95% CI, 0.20-0.78), and medical indications (aOR, 0.79; 95% CI, 0.63-0.99), deeper ED sedation remained associated with higher mortality. In the subgroup analysis excluding patients who did not receive analgosedation in the ED (aOR, 0.77; 95% CI, 0.62-0.95), deeper ED sedation was again associated with higher mortality. In the post hoc subgroup analyses, deeper ED sedation remained associated with higher mortality in the following groups: (1) excluding patients given dexmedetomidine in the ICU and (2) patients intubated in the ED (e-Table 4). Finally, in the exploratory logistic regression models examining the association between midazolam and propofol with mortality, deeper ED sedation was associated with higher mortality in all models; neither midazolam or propofol was associated with outcome (e-Table 5).
Discussion
Mechanically ventilated patients commonly experience pain and anxiety; analgesia and sedation are therefore necessary and patient-centered interventions.1 Data suggest that optimizing analgosedation delivery and achieving a goal-oriented sedation depth improves outcome.1, 2 Given this fact, postintubation analgesia and sedation management is fundamental for clinicians initiating mechanical ventilation. Although significant clinical data exist regarding analgosedation in the ICU, limited data are devoted to the immediate postintubation period in the ED. This investigation sought to characterize ED sedation practices and assess relationships between ED sedation depth and clinical outcomes. This study’s results extend the topic of analgosedation to the ED and have several implications.
Our descriptive data highlight several important aspects of analgosedation in the ED. Mechanically ventilated ED patients are sedated primarily with fentanyl, midazolam, and propofol. This is consistent with ICU-based data, demonstrating that these medications are the most frequent medications used for sedation.7, 16 The most striking difference between ED and ICU sedation in the current study was the fact that 16.4% of patients received ketamine in the ED, and 31.6% received dexmedetomidine in the ICU. This ketamine use rate is higher than that historically reported in the ICU, and we are unaware of any direct comparative studies involving ketamine vs other agents to improve outcome.1 Mounting data suggest that dexmedetomidine use is associated with a lower incidence of delirium and a shorter duration of ventilator use compared with benzodiazepines and propofol.17, 18, 19 In our study, it was also associated with a lower mortality, and its early use in the ICU is currently being investigated in a multicenter randomized trial.20 However, to our knowledge, there are no ED-based studies examining this agent.
The limited data on ED sedation for mechanically ventilated patients has focused on inadequate postintubation analgosedation, showing that up to 50% of mechanically ventilated ED patients received inadequate analgesia or sedation.4, 5, 6 In the current study, although the rate was much lower, a significant minority of patients received no analgesia (14.3%) or sedation (15.2%) in the ED. However, there are multiple clinical factors that may influence the decision to withhold or minimize analgosedation; it is possible that patients were sedated prior to ED arrival or medications were withheld for clinical reasons (ie, neurologic examination). It is also possible that these patients did not show signs of discomfort clinically; therefore, withholding analgesia and sedation could have been the appropriate approach.
Contrary to previous literature, our data highlight the frequency with which patients are deeply sedated in the ED (64.0%), with a median RASS of –3.0 (–4.0 to –2.0). Prior work has shown that early deep sedation during the first 48 hours of ICU care is common and is a predictor of death and mechanical ventilation duration.7 Also, there is clear evidence of an excess of oversedation in the ICU that can extend for days; our data suggest that the ED may be the genesis of potential sedation overshoot.21 Although we cannot comment on whether this sedation depth was clinically warranted in the ED, it seems unlikely that two-thirds of mechanically ventilated ED patients would require deep sedation.
Our most significant finding was the association between deep sedation in the ED and worse clinical outcomes. Deep sedation in the ED was associated with worse mortality, greater time on the ventilator, and increased lengths of stay. This agrees with data on deep sedation within the first 48 hours of ICU admission but is the first to investigate the impact of deep sedation in the immediate postintubation period. We observed what appears to be incremental improvement in outcome as sedation depth was lightened (Fig 2), up to a RASS of –2. Deep sedation also remained associated with mortality across multiple subgroup analyses. Together, these data suggest that targeted sedation in the ED should be investigated further as a means to improve outcome.
Limitations
This is a single-center study and therefore may only reflect local ED sedation practices. Sedation and medication data were obtained retrospectively, leaving the possibility of potential inaccuracies during routine clinical documentation. This may be especially true for the cohort of patients intubated outside our facility. As RASS is highly reproducible during routine care and has been part of local institutional protocols for years, we are assured of some face validity in its accuracy. Also, our subgroup analysis excluding patients not intubated in our ED was consistent with the primary analysis, suggesting against any potential confounding due to medications delivered prior to ED arrival. As a reflection of a lack of standardization in ED analgosedation practices, sedation depth was inconsistently recorded in the ED, necessitating the use of the first ICU RASS as a surrogate. Given the data showing the frequency with which deep sedation can persist for days, we believe it unlikely that sedation depth changed much during the first 3 hours of ICU admission.21 Also, there is little literature regarding whether ED sedation depth is static or dynamic in nature. It is possible that the documented sedation levels did not comprehensively capture the full spectrum of ED sedation levels. Future prospective studies should rigorously track ED sedation over time. The study design documents associations and cannot establish causality. Deep sedation potentially was a marker of illness severity, clinically warranted or driven by non-sedation-related factors, such as neurologic status. Given our exclusion of patients admitted to a neurologic ICU and those with a primary diagnosis of cardiac arrest, and our subgroup analysis excluding patients not administered sedation in the ED, this seems less likely. Although not always statistically significant, the more deeply sedated nonsurviving patients received lower doses of some sedative and analgesic drugs. This suggests the presence of potential confounders, such as important patient-level variables (eg, pre-existing delirium or dementia), or medication-level variables (eg, drug-drug interactions) that could not be accounted for in the analysis. As an exploratory cohort study, these results should be viewed as hypothesis generating for future prospective investigations.
Conclusions
Deep sedation is common in mechanically ventilated ED patients and is associated with worse outcome. These data highlight the importance of the early period of mechanical ventilation and suggest that ED-based sedation is a modifiable variable that could be targeted to improve clinical outcomes.
Acknowledgments
Author Contributions: R. J. S. takes full responsibility for the content of the manuscript, including data and analysis, and is the guarantor of this paper. R. J. S. contributed to study conception and design, acquisition of data, interpretation and analysis of data, and drafting the manuscript. E. A., A. M. D., C. P., B. T.W., B. W. R., S. Y. L., M. H. K., and N. M. M contributed to study conception and design and drafting the manuscript. B. M. F. contributed to study conception and design, interpretation and analysis of data, and drafting the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. J. S. received funding from Washington University Institute of Clinical and Translational Sciences and from the National Center for Advancing Translational Sciences of the National Institutes of Health. E. A. was supported by the Washington University School of Medicine Faculty Scholars Grant and the Foundation for Barnes-Jewish Hospital. N. M. M. was supported by grant funds from the Health Resources and Services Administration. B. W. R. was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute. B. M. F. and A. M. D. were funded by the KL2 Career Development Award, and this research was supported by the Washington University Institute of Clinical and Translational Sciences grants from the National Center for Advancing Translational Sciences. B. M. F. was also funded by the Foundation for Barnes-Jewish Hospital Clinical and Translational Sciences Research Program. A. M. D. was also funded by a grant from the Division of Clinical and Translational Research of the Department of Anesthesiology at Washington University School of Medicine. S. Y. L. was supported by the KM1 Comparative Effectiveness Research Career Development Award [Grant No. KM1CA156708-01], the Clinical and Translational Science Award program [Grant No. UL1RR024992] of the National Center for Advancing Translational Sciences, and the Barnes-Jewish Patient Safety & Quality Career Development Program, which is funded by the Foundation for Barnes-Jewish Hospital. M. H. K. was supported by the Foundation for Barnes-Jewish Hospital. None declared (C. P., B. T. W.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: R. J. S. received funding from Washington University Institute of Clinical and Translational Sciences Grant UL1TR000448, subaward TL1TR000449 and from the National Center for Advancing Translational Sciences of the National Institutes of Health. B. W. R. was supported by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (K23HL126979). B. M. F. and A. M. D. were funded by the KL2 Career Development Award, and this research was supported by the Washington University Institute of Clinical and Translational Sciences [Grants UL1 TR000448 and KL2 TR000450] from the National Center for Advancing Translational Sciences. B. M. F. was also funded by the Foundation for Barnes-Jewish Hospital Clinical and Translational Sciences Research Program [Grant No. 8041-88].
Some of the data and analyses described in this paper were described in an abstract presented at the annual meeting of the Society for Academic Emergency Medicine May 16-19, 2017, Orlando, FL.
Supplementary Data
References
- 1.Barr J., Fraser G.L., Puntillo K. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Jackson D.L., Proudfoot C.W., Cann K.F., Walsh T. A systematic review of the impact of sedation practice in the ICU on resource use, costs and patient safety. Crit Care. 2010;14(2):1. doi: 10.1186/cc8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat R., Goyal M., Graf S. Impact of post-intubation interventions on mortality in patients boarding in the emergency department. West J Emerg Med. 2014;15(6):708. doi: 10.5811/westjem.2014.7.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonomo J.B., Butler A.S., Lindsell C.J., Venkat A. Inadequate provision of postintubation anxiolysis and analgesia in the ED. Am J. Emerg Med. 2008;26(4):469–472. doi: 10.1016/j.ajem.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Chong I.D., Sandefur B.J., Rimmelin D.E. Long-acting neuromuscular paralysis without concurrent sedation in emergency care. Am J Emerg Med. 2014;32(5):452–456. doi: 10.1016/j.ajem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Weingart G.S., Carlson J.N., Callaway C.W., Frank R., Wang H.E. Estimates of sedation in patients undergoing endotracheal intubation in US EDs. Am J Emerg Med. 2013;31(1):222–226. doi: 10.1016/j.ajem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Shehabi Y., Bellomo R., Reade M.C. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka L.M.S., Azevedo L.C.P., Park M. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Crit Care. 2014;18(4):1. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller BM, Ferguson IT, Mohr NM, et al. Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): a quasi-experimental, before-after trial [published online ahead of print March 1, 2017]. Ann Emerg Med.http://dx.doi.org/10.1016/j.annemergmed.2017.01.013. [DOI] [PMC free article] [PubMed]
- 10.Fuller B.M., Ferguson I., Mohr N.M. Lung-protective ventilation initiated in the emergency department (LOV-ED): a study protocol for a quasi-experimental, before-after trial aimed at reducing pulmonary complications. BMJ Open. 2016;6(4):e010991. doi: 10.1136/bmjopen-2015-010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent J., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 12.Vincent J., De Mendonça A., Cantraine F. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Vincent J., Angus D., Artigas A. Effects of drotrecogin alfa (activated) on organ dysfunction in the PROWESS trial. Crit Care Med. 2003;31(3):834–840. doi: 10.1097/01.CCM.0000051515.56179.E1. [DOI] [PubMed] [Google Scholar]
- 14.Esteban A., Anzueto A., Frutos F. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 15.Fuller B.M., Mohr N.M., Dettmer M. Mechanical ventilation and acute lung injury in emergency department patients with severe sepsis and septic shock: an observational study. Acad Emerg Med. 2013;20(7):659–669. doi: 10.1111/acem.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payen J.-F., Chanques G., Mantz J. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106(4):687–695. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande P.P., Pun B.T., Herr D.L. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 18.Riker R., Shehabi Y., Bokesch P. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;3015:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 19.Jakob S.M., Ruokonen E., Grounds R.M., Dexmedetomidine for Long-Term Sedation Investigators Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation. JAMA. 2012;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 20.Centre AaNZICR. Early goal-directed sedation compared with standard care in mechanically ventilated critically ill patients: a prospective multicentre randomised controlled trial. 2017. https://clinicaltrials.gov/ct2/show/NCT01728558. Accessed January 17, 2017.
- 21.Jackson D.L., Proudfoot C.W., Cann K.F., Walsh T.S. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. 2009;13(6):1. doi: 10.1186/cc8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.