Abstract
Background
Sepsis refers to the dysregulated host immune response elicited by microbial infections resulting in life-threatening organ dysfunction. Sepsis represents a medical challenge, since it is associated with a rate of death as high as 60%. Septic shock is strongly associated with vascular dysfunction and elevated pulmonary capillary permeability. We recently reported that the combination of hydrocortisone (HC), ascorbic acid (vitC), and thiamine dramatically improves outcomes and reduces mortality in patients with sepsis. In the present study, we provide experimental evidence in support of the hypothesis that the combination of HC and vitC enhances endothelial barrier function.
Methods
Human lung microvascular endothelial cells were exposed to lipopolysaccharide (LPS) in the absence or presence of HC and vitC.
Results
LPS alone induced profound hyperpermeability, as reflected in decreased values of transendothelial electrical resistance. vitC alone did not exhibit barrier enhancement properties nor did it affect the LPS-induced hyperpermeability. Similarly, HC alone exhibited only a minor barrier-enhancing and protective effect. Conversely, the combination of HC and vitC, either as before or after treatment, dramatically reversed the LPS-induced barrier dysfunction. The barrier-protective effects of HC and vitC were associated with reversal of LPS-induced p53 and phosphorylated cofilin downregulation and LPS-induced RhoA activation and myosin light chain phosphorylation.
Conclusions
These data provide a novel mechanism of endothelial barrier protection and suggest one possible pathway that may contribute to the therapeutic effects of HC and vitC in patients with sepsis.
Key Words: barrier function, endothelial permeability, endothelium
Abbreviations: GC, glucocorticosteroid; HC, hydrocortisone; HLMVEC, human lung microvascular endothelial cell; IL, interleukin; LPS, lipopolysaccharide; MLC2, myosin light chain 2; PBS, phosphate-buffered saline; TEER, transendothelial electrical resistance; vitC, ascorbic acid
Deviations from normal endothelial barrier function can lead to or be caused by various internal or external stresses and pathologic conditions.1 Sepsis and septic shock, as recently redefined,2 are associated with pulmonary edema caused by increased permeability to proteins across pulmonary endothelial and epithelial barriers,3 and recovery from septic shock is associated with a reduction in edema, consistent with restoration of vascular function.4
The regulation of vascular permeability is tightly coordinated by a variety of intracellular factors and external stimuli.5 It is increased by inflammatory factors, such as histamine, bradykinin, platelet-activating factor, growth factors, glycation products, cytokines, reactive oxygen species, and activated leukocytes.6, 7 These mediators trigger signaling cascades that modulate the expression of junctional proteins and adhesion molecules, as well as the reorganization of the cytoskeleton and focal adhesion complexes,8 all leading to compromised barrier integrity.7
Sepsis is an insidious pathologic condition that despite years of research and numerous clinical trials levies a death rate of approximately 40%.9, 10 We recently reported on the strikingly beneficial effects of combined hydrocortisone (HC) and ascorbic acid (vitC) therapy in patients with sepsis.3 Compared with patients receiving standard-care therapy, those receiving HC and vitC exhibited dramatically reduced mortality (40% vs 8%), a reduced requirement for pressor agent support, and decreasing Sequential Organ Failure Assessment scores and procalcitonin blood levels. We speculated that at least part of these actions of HC and vitC might involve restoration of endothelial barrier function.3
Thus, the current study examines the effect of HC and vitC on lipopolysaccharide (LPS)-induced vascular dysfunction. HC is a corticosteroid that exhibits anti-inflammatory effects, increases systemic vascular resistance, and potentiates the vasoconstrictive responses of catecholamines and angiotensin II.11 It is effective against ischemia/reperfusion injury by mediating the nontranscriptional activation of endothelial nitric oxide synthase.12 It prevents the migration of inflammatory cells from the circulation to tissues by blocking the synthesis of various chemokines and cytokines.13
vitC is a potent antioxidant that has long been known to participate in several important vascular endothelial functions, including increasing the synthesis and deposition of type IV collagen in the basement membrane, stimulating endothelial proliferation, inhibiting apoptosis, scavenging radical species, and increasing the bioavailability of nitric oxide to help modulate blood flow.14
Both drugs inhibit nuclear factor-κB activation and downregulate the production of proinflammatory mediators. Furthermore, they increase tight junctions between endothelial and epithelial cells, which preserves endothelial function and microcirculatory flow. In addition, both are required for the synthesis of catecholamines, and both increase vascular vasopressor sensitivity.3
LPS is the major component of the outer wall of gram-negative bacteria. It activates macrophages, neutrophils, and dendritic and other cells that induce inflammation, oxidative stress, and endothelial damage.15 Exposure to LPS leads to endothelial barrier dysfunction, a hallmark of acute lung injury, ARDS, and sepsis.16, 17
Our observations reveal that pretreatment of human lung microvascular endothelial cells (HLMVECS) with both compounds together, but not separately, reverses the LPS-induced endothelial barrier dysfunction. Furthermore, we show that this protective effect occurs through the induction of p53 and phosphorylated cofilin and the downregulation of the RhoA/myosin light chain 2 (MLC2) pathway.16
Methods
The p53 (Catalog No. 9282s), p-MLC2 (Catalog No. 3674s), MLC2 (Catalog No. 8505), phospho-cofilin (Catalog No. 3313) and cofilin (Catalog No. 3318) antibodies were from Cell Signaling. β-actin antibody (Catalog No. P8999), CelyticM Lysis Reagent (Catalog No. C2978), L-Ascorbic acid (Catalog No. A4544), and HC (Catalog No. H6909) were from Sigma-Aldrich. Secondary mouse and rabbit antibodies were from LI-COR. Pierce BCA protein assay and nitrocellulose membranes were from Fisher Scientific. RhoA activation was detected by the Cell Signaling kit (Catalog No. 8820).
In house, HLMVECs were harvested and maintained as previously described.18 Briefly, subpleural lung tissue was cut into small fragments with scissors. After the removal of debris and erythrocytes through a 40-μm nylon net, the tissue collected in the net was treated with dispase, filtered through a 100-μm nylon net (2 times) and then a 40-μm net, centrifuged, and resuspended in Medium 199 (Gibco; Catalog No. 31100-019) with 20% fetal bovine serum. Positive selection of HLMVECs was achieved by adding magnetic beads coated with Ulex europaeus I to the cell suspension.
The barrier function of endothelial cell monolayers was estimated by the electric cell-substrate impedance sensing method, as previously described.15 Experiments were conducted on wells that achieved at least 800 Ω baseline steady-state resistance. Proteins were isolated from cells, and samples (40 μg per lane) were separated by electrophoresis, as previously described.16 Band density was visualized in a LI-COR Odyssey CLx imaging system.
Data are expressed as means ± SEM. Two-way analysis of variance with Bonferroni correction (Figure 1, Figure 2, Figure 3) or one-way analysis of variance with Dunnett’s post hoc test (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8) was performed to determine differences among groups. GraphPad Prism (GraphPad Software) was used for data analysis, and n = the number of repeats.
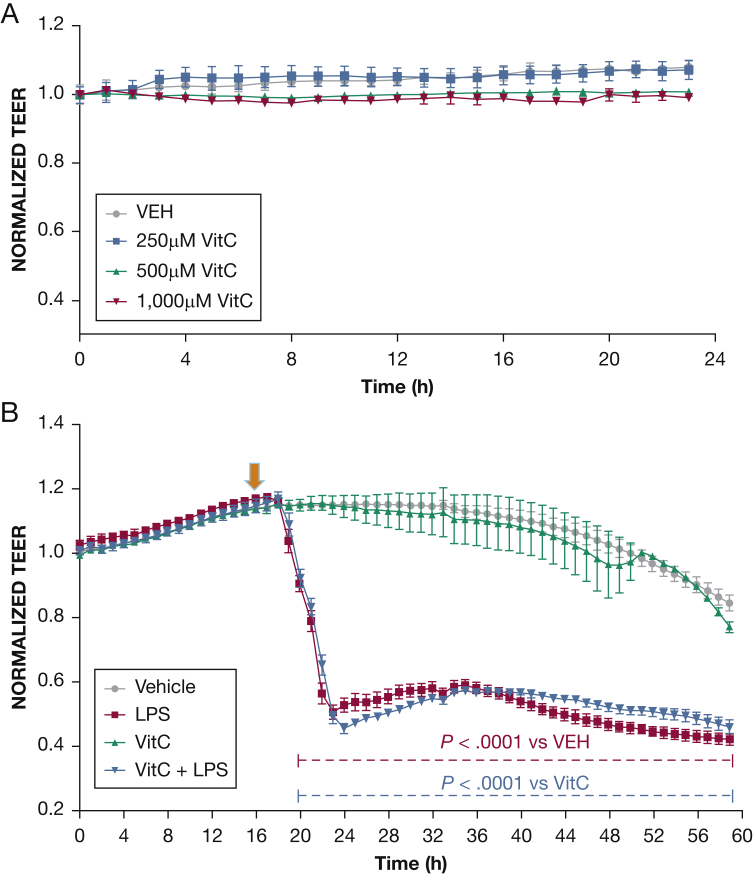
Figure 1.
Effect of vitC on endothelial barrier function. Vehicle (phosphate-buffered saline [PBS]) or vitC (250, 500, 1,000 μM) was added to the media of confluent HLMVEC monolayers at 0 hours. A, vitC did not cause a biologically significantly change in the TEER of the endothelial monolayers. B, Cells were pretreated for 16 hours with either vehicle (PBS) or vitC (1,000 μM) and were consequently exposed to LPS (0.5 endotoxin units/mL [arrow]). A gradual increase in endothelial permeability (reduced TEER) was observed in the LPS-treated cells of both groups (n = 3 per group; means ± SEM). HLMVEC = human lung microvascular endothelial cell; LPS = lipopolysaccharide; TEER = transendothelial resistance; VEH = vehicle; vitC = ascorbic acid.
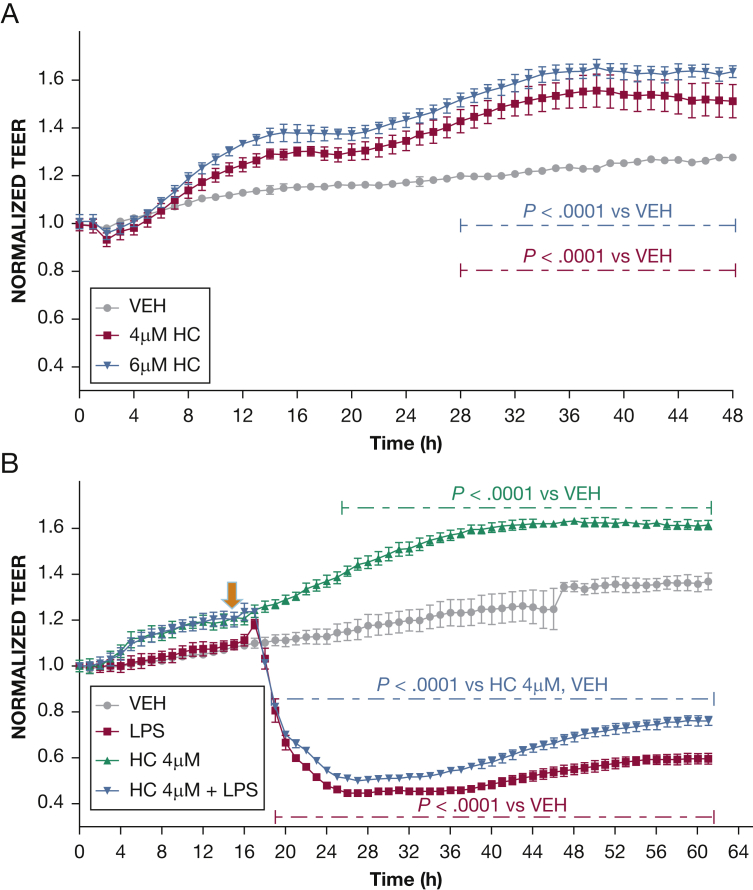
Figure 2.
Effect of HC on endothelial barrier function. A, Vehicle (phosphate-buffered saline [PBS]) or HC (4 μM, 6 μM) was added to the media of confluent HLMVEC monolayers at 0 hours. HC in both concentrations caused a dose-dependent induction of TEER (n = 3 per group; means ± SEM). B, Cells were pretreated for 16 hours with either vehicle (PBS) or HC (4 μM) and were exposed to LPS (0.5 endotoxin units/mL). A gradual increase in endothelial permeability (reduced TEER) was observed in both groups of the LPS-treated cells (n = 4 per group; means ± SEM). HC = hydrocortisone. See Figure 1 legend for expansion of other abbreviations.
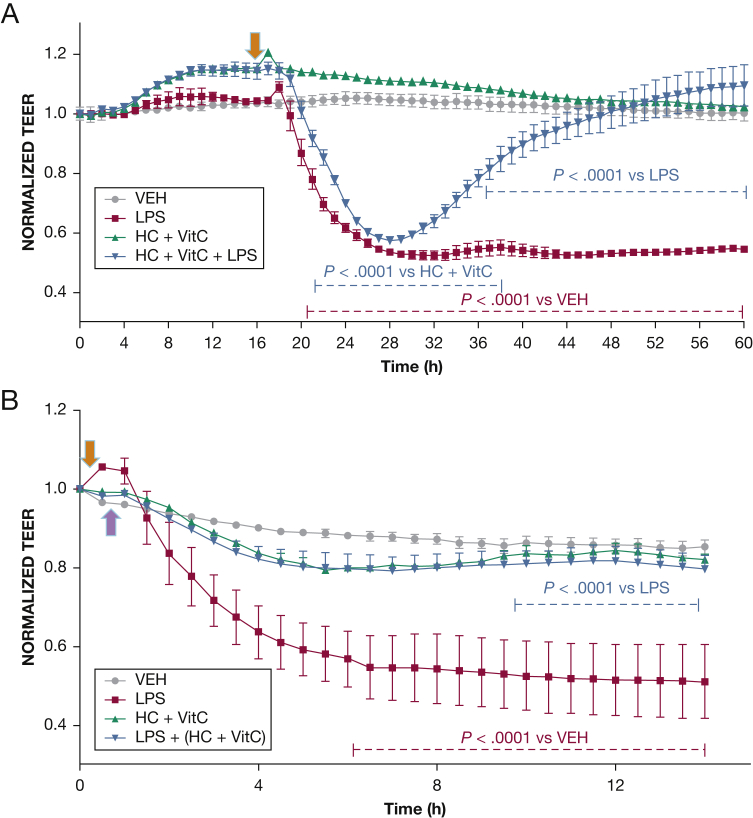
Figure 3.
The effect of the combination of HC and vitC on LPS-induced hyperpermeability. HLMVECs were seeded on gold electrodes and allowed to reach confluence (time = 0). A, Cells were pretreated for 16 hours with either vehicle or a combination of vitC (1,000 μM) and HC (6 μM) and then exposed to LPS (0.5 endotoxin units/mL [arrow]). A gradual increase in endothelial permeability (reduced TEER) was observed in all groups receiving LPS. However, HLMVECs pretreated with vitC and HC experienced a complete restoration of barrier function 24 hours after LPS treatment. B, Confluent HLMVECs received LPS (0.5 endotoxin units/mL down arrow]) and 15 min later were treated with vehicle or a combination of vitC (1,000 μM) and HC (6,μM) (up arrow). HC + vitC completely prevented the LPS-induced decrease in TEER (n = 3 per group; means ± SEM). See Figure 1 and 2 legends for expansion of abbreviations.
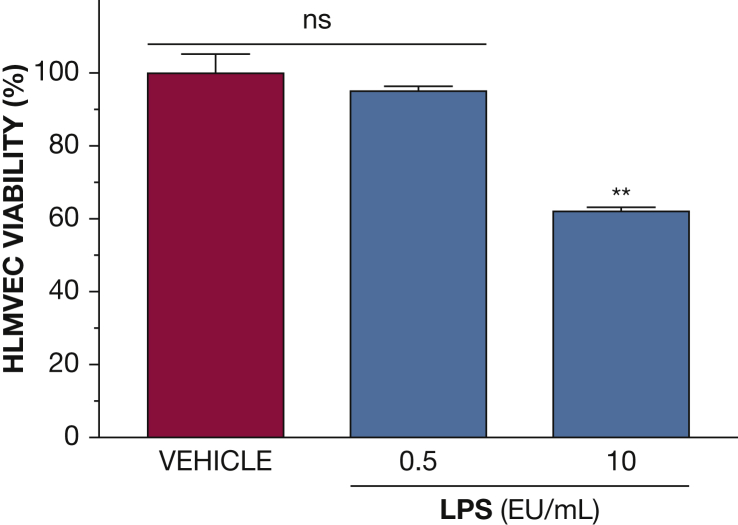
Figure 4.
Effects of LPS on HLMVEC viability. Confluent HLMVECs were exposed to LPS (0.5 or 10 EU/mL) for 48 hours. Cell viability at that time was examined by the trypan blue exclusion method. At the concentration used throughout the study (0.5 EU/mL), LPS did not affect HLMVEC viability. (**P < .01 from VEH; n = 3 per group; means ± SEM). EU = endotoxin units. See Figure 1 legend for expansion of other abbreviations.
Figure 5.
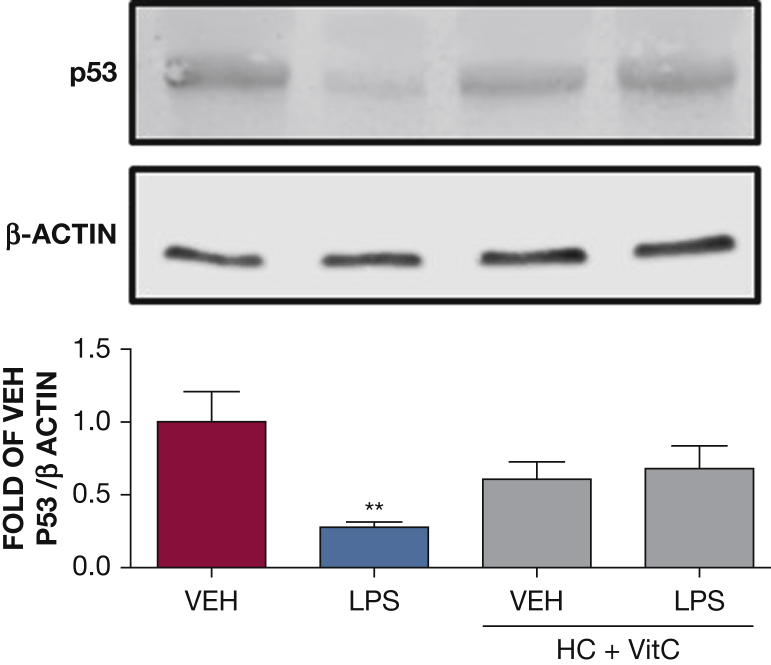
The effect of HC and vitC on LPS-induced p53 downregulation. Western blot analysis of p53 in HLMVECs pretreated with either vehicle (PBS) or vitC (1,000 μM) + HC (6 μM) prior to vehicle (PBS) or LPS treatment (0.5 endotoxin units/mL). The blot shown is representative of four independent experiments. Signal intensity of p53 was analyzed by densitometry. Protein levels were normalized to β-actin. (**P < .01 vs vehicle; means ± SEM). See Figure 1 and 2 legends for expansion of abbreviations.
Figure 6.
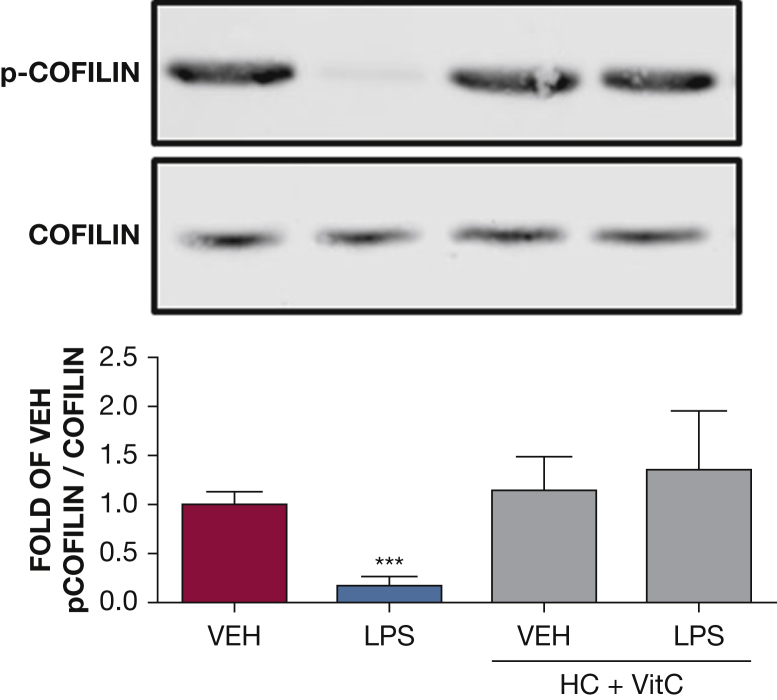
The effect of HC and vitC on LPS-induced cofilin phosphorylation. Western blot analysis of phosphorylated cofilin in HLMVECs pretreated with either vehicle (phosphate-buffered saline [PBS]), or vitC (1,000 μM) + HC (6 μM) prior to vehicle (PBS) or LPS treatment (0.5 endotoxin units/mL). The blot shown is representative of four independent experiments. Signal intensity of phosphorylated cofilin was analyzed by densitometry. Protein levels were normalized to total cofilin. (***P < .0001 vs vehicle; means ± SEM). p-cofilin = phosphorylated cofilin. See Figure 1 and 2 legends for expansion of other abbreviations.
Figure 7.
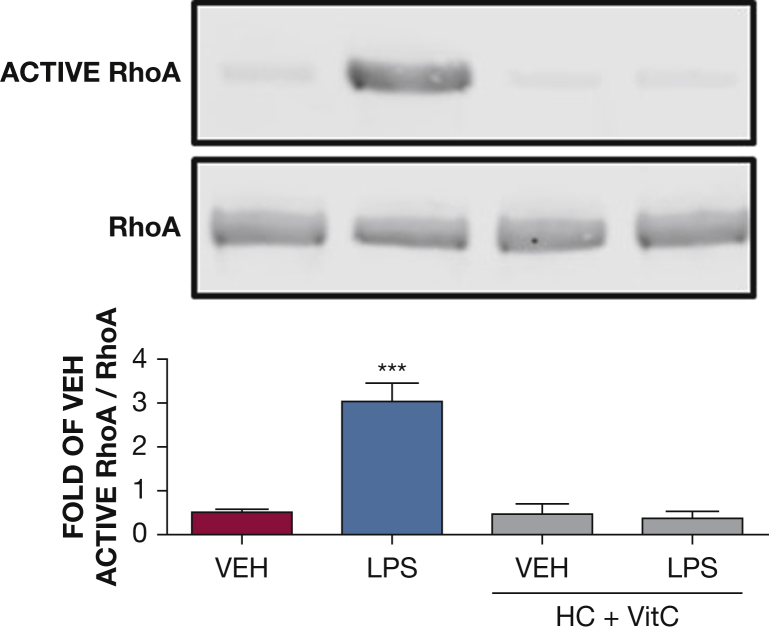
The effect of HC and vitC on LPS-induced RhoA activation. Western blot analysis of active RhoA in HLMVECs pretreated with either vehicle (phosphate-buffered saline [PBS]), or vitC (1,000 μM) + HC (6 μM) prior to vehicle (PBS) or LPS treatment (0.5 endotoxin units/mL). The blot shown is representative of four independent experiments. Signal intensity of active RhoA was analyzed by densitometry. Protein levels were normalized to total RhoA. (***P < .0001 vs vehicle; means ± SEM). See Figure 1 and 2 legends for expansion of abbreviations.
Figure 8.
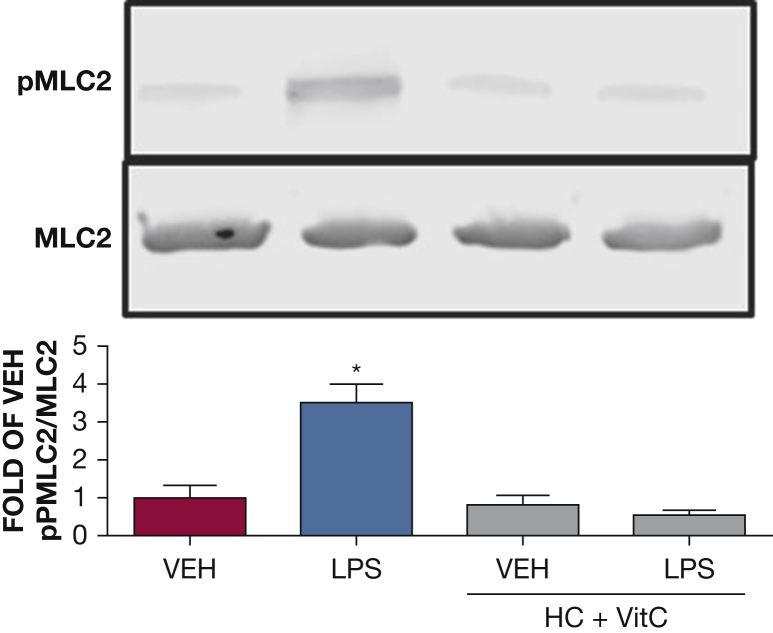
The effect of HC and vitC on LPS-induced MLC2 activation. Western blot analysis of pMLC2 in HLMVECs pretreated with either vehicle (phosphate-buffered saline [PBS]) or vitC (1,000 μM) + HC (6 μM) prior to vehicle (PBS) or LPS treatment (0.5 endotoxin units/mL). The blot shown is representative of four independent experiments. Signal intensity of pMLC2 was analyzed by densitometry. Protein levels were normalized to total MLC2. (*P < .05 vs vehicle; means ± SEM). MLC2 = myosin light chain 2; pMLC2 = phosphorylated MLC2. See Figure 1 and 2 legends for expansion of abbreviations.
Results
vitC Does Not Affect Endothelial Barrier Function
HLMVECs seeded on gold electrode arrays were exposed to phosphate-buffered saline (PBS) (vehicle) 250 μM, 500 μM or 1,000 μM vitC. vitC did not induce a biologically significant effect on transendothelial electrical resistance (TEER) (Fig 1A). In additional experiments, HLMVECs were exposed to either vehicle (PBS) or 1, 000 μM vitC before treatment with PBS or LPS (0.5 endotoxin units [EU]/mL). LPS decreased TEER values of both vehicle-treated and vitC-treated HLMVECs. There was no difference in TEER values between the two LPS-treated groups (Fig 1B).
Effects of HC on Endothelial Barrier Function
HLMVECs were exposed to PBS and 4 μM or 6 μM HC. HC induced a concentration-dependent but biologically miniscule enhancement of barrier function (Fig 2A). In additional experiments, HLMVECs were exposed to PBS or HC (4 μM) for 16 hours prior to PBS or LPS (0.5 EU/mL) treatment. Cells treated with PBS maintained stable TEER values, whereas cells treated with HC exhibited moderately increased barrier function. LPS caused a sharp decrease in TEER, which was only partially and moderately ameliorated by pretreatment with HC (Fig 2B).
The Combination of HC and vitC Prevents and Repairs the LPS-Induced Endothelial Barrier Dysfunction
HLMVECs were pretreated (Fig 3A) or posttreated (Fig 3B) with either vehicle or a combination of HC (4 μM) and vitC (1,000 μM) 16 hours prior to or 15 min after challenge with LPS (0.5 EU/mL) or PBS. PBS alone did not influence TEER values, which remained steady throughout the course of the study. As expected, LPS induced a robust decrease in TEER. However, cells pretreated or posttreated with HC and vitC exhibited complete restoration of barrier function. To investigate whether the effect of LPS was at least in part due to cell death, we evaluated HLMVEC viability after LPS by the trypan blue dye exclusion method. At 0.5 EU/mL (the concentration used throughout this study), LPS did not affect cell viability; however, cell death was observed at 20 times higher concentrations (Fig 4).
Since it appeared that HC and vitC together, but not separately, were able to prevent LPS-induced barrier dysfunction, we then investigated possible mechanisms responsible for these effects.
The Combination of HC and vitC Prevents LPS-Induced p53 Downregulation in HLMVECs
We have reported that LPS reduces p53 levels and that p53 overexpression is associated with endothelial barrier enhancement.16 We thus investigated the ability of HC plus vitC to affect p53 expression. HLMVECs were exposed to PBS, HC (4 μM), and vitC (1,000 μM) prior to LPS (0.5 EU/mL) or vehicle treatment. p53 expression was examined by Western blotting 1 hour after exposure to LPS. LPS induced profound p53 downregulation, in line with our previous reports.16, 19 HC plus vitC-treated cells exhibited normal p53 levels (Fig 5).
The Combination of HC and vitC Prevents the LPS-Induced Cofilin Activation (Dephosphorylation) in HLMVECs
Cofilin is a β-actin interacting protein that promotes stress fiber formation. HLMVECs were exposed to PBS, HC (4 μM), and vitC (1,000 μM) before LPS (0.5 EU/mL) or vehicle treatment. Active (dephosphorylated) and inactive (phosphorylated) cofilin expression was examined by Western blotting 1 hour after LPS. LPS induced significant activation of cofilin consistent with the known formation of stress fibers. HC plus vitC-treated cells exhibited normal inactive cofilin levels (Fig 6).
The Combination of HC and vitC Prevents LPS-Induced RhoA Activation in HLMVECs
The RhoA pathway is a well-known mediator of endothelial barrier dysfunction. HLMVECs were exposed to PBS, HC (4 μM), and vitC (1,000 μM) before LPS (0.5 EU/mL) or vehicle treatment. Active and total RhoA levels were examined 1 hour after LPS. LPS induced strong RhoA activation, in line with our previous reports.16, 19 HC plus vitC-treated cells exhibited normally low active RhoA levels (Fig 7).
The Combination of HC and vitC Prevents the LPS-Induced Activation (Phosphorylation) of MLC2 in HLMVECs
MLC2 activation by myosin light chain kinase or Rho kinase is responsible for actin stress fiber formation. HLMVECs were exposed to PBS, HC (4 μM), and vitC (1,000 μM) before LPS (0.5 EU/mL) or vehicle treatment. Phosphorylated and nonphosphorylated MLC2 levels were measured by Western blotting 1 hour after LPS exposure. LPS induced significant MLC2 phosphorylation, consistent with our previous reports.16, 19 HC plus vitC-treated cells exhibited baseline nonphosphorylated MLC2 levels (Fig 8).
Discussion
Sepsis is a systemic inflammatory response to infection from multiple causes. Severe sepsis describes instances in which sepsis is complicated by acute organ dysfunction and the provision of supportive therapy, such as mechanical ventilation, is required.20 In the United States, severe sepsis is recorded in 750,000 patients per year. That number represents 2% of all patients admitted to the hospital.
Sepsis is associated with microvascular thrombosis and impairment of anticoagulation mechanisms. Tissue oxygenation is further impaired by the loss of the endothelial barrier function.20 Oxygen use is impaired at the subcellular level because of damage to mitochondria from oxidative stress.21
vitC is an antioxidant that can improve the endothelium-dependent response in circumstances such as chronic smoking, diabetes mellitus, hypercholesterolemia, and hypertension.22, 23, 24 vitC protects the endothelium by scavenging superoxide, which in turn prevents nitric oxide scavenging, lipid peroxidation, platelet and neutrophil activation, and adhesion molecule upregulation.25 It scavenges peroxidase-generated reactive nitrogen species and inhibits low-density lipoprotein oxidation.26 It further acts as a lipid-soluble antioxidant, scavenging hydroperoxyl radicals in lipid milieu.27
vitC alone fails to protect endothelial function against toxic insults. Administration of oral ascorbate (1,500 mg/d) in combination with other antioxidants failed to decrease mortality in critically ill adults with multiorgan failure.28 Similar negative results were observed in a mouse model of sepsis.29, 30
The use of glucocorticosteroids (GCs) such as HC in the treatment of sepsis remains controversial. Short treatment of gram-negative bacteria with high-dose GCs in healthy volunteers was ineffective in the majority of studies.31 GCs failed to attenuate the LPS-induced coagulation cascade in humans32 and failed to inhibit LPS-induced nuclear factor-κB translocation in human monocytes.33 In contrast, stratification of mice according to levels of circulating interleukin (IL)-6 predicted mortality as well as the efficacy of GC treatment; mice with high levels of IL-6 responded to GCs.34
A recent study by Azari et al35 evaluated the protective effects of HC, vitC, and vitamin E alone or in combination against renal ischemia/reperfusion injury in rats. The inevitable injuries may occur after infarction, sepsis, and organ transplantation, and this phenomenon exacerbates tissue damage by initiating an inflammatory cascade including reactive oxygen species, cytokines, chemokines, and leukocyte activation.36 Combined administration of vitC, vitamin E, and HC before restoration of blood flow to the ischemic tissue had a synergistic protective effect against the deleterious effects of ischemia/reperfusion injury to the kidney.35
In a recent retrospective before/after clinical study, we compared the outcome and clinical course of patients with sepsis treated with IV vitC, HC, and thiamine during a 7-month period (treatment group) compared with a control group receiving standard-care therapy during the preceding 7 months. There were 47 patients in each group with no significant differences in baseline characteristics. Hospital mortality was 8.5% (four of 47 patients) in the treatment group compared with 40.4% (19 of 47 patients) in the control group. Sequential Organ Failure Assessment scores decreased in all patients in the treatment group, with none experiencing progressive organ failure. These results suggested that the early use of IV vitC together with corticosteroids and thiamine is effective in preventing progressive organ dysfunction, including acute kidney injury, and reducing the mortality of patients with severe sepsis and septic shock.3
We recently demonstrated that silencing of p53 in HLVMECs profoundly disrupts their barrier function.16 To evaluate whether p53 is involved in the “rescue” of the LPS-induced vascular dysfunction by the combination of vitC and HC, we examined the effect of these compounds in HLMVECs. In line with previous observations,16 a significant decrease in p53 expression was observed after 1 hour of LPS treatment, which was absent in cells pretreated with vitC and HC, reflecting their protective effect against vascular injury.
Cofilin is an actin-severing protein that is tightly associated with the regulation of cellular motion and vascular permeability. Rac1 activation leads to phosphorylation (deactivation) of cofilin.37 Furthermore, the small guanosine triphosphate RhoA and its downstream target Rho kinase also regulate cellular adherence through control of the actin-cytoskeletal assembly and cell contraction. RhoA directly phosphorylates MLC2, which in turn can trigger contraction, resulting in endothelial cell membrane retraction, intercellular gap formation, and barrier compromise.38 In the current study, we observed that LPS resulted in reduced cofilin phosphorylation, ie, activation and increased RhoA activation and myosin light chain phosphorylation. This agrees with other observations and suggests a similar association between LPS and cofilin or RhoA-induced hyperpermeability.39 Pretreatment of HLMVECs with HC and vitC prevented the LPS-mediated cofilin and RhoA activation, which is in agreement with the observed reversal of the LPS-induced decrease in TEER.
Conclusions
We introduce for the first time, to our knowledge, a mechanism that is responsible, at least in part, for the protective effect of the synergistic action of HC and vitC on LPS-induced hyperpermeability. The aforementioned findings should be seen in conjunction with a recent report3 demonstrating that early use of IV vitC, together with HC and thiamine, are effective in preventing progressive organ dysfunction due to severe sepsis and septic shock. Collectively, these findings provide a new and exciting approach to the management of severe sepsis.
Acknowledgements
Author contributions: J. D. C. is the guarantor of this manuscript and takes responsibility for all data presented in this manuscript. He also conceived the experimental design and edited the manuscript. N. B. contributed to the experimental design, performed experiments, analyzed data, and wrote the manuscript. V. K. contributed to the experimental design, performed experiments, and analyzed data. P. E. M. contributed to the experimental design and edited the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was supported by National Heart, Lung and Blood Institute [Grant HL101902].
Drs Barabutis and Khangoora contributed equally to this manuscript.
References
- 1.Kasa A., Csortos C., Verin A.D. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers. 2015;3(1-2):e974448. doi: 10.4161/21688370.2014.974448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, Vitamin C and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2016 doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Lee W.L., Slutsky A.S. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 5.Barabutis N., Verin A., Catravas J.D. Regulation of pulmonary endothelial barrier function by kinases. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L832–L845. doi: 10.1152/ajplung.00233.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta D., Ravindran K., Kuebler W.M. Novel regulators of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2014;307(12):L924–L935. doi: 10.1152/ajplung.00318.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukriti S., Tauseef M., Yazbeck P., Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4(4):535–551. doi: 10.1086/677356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenbroucke E., Mehta D., Minshall R., Malik A.B. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 9.Epstein L., Dantes R., Magill S., Fiore A. Varying Estimates of Sepsis Mortality Using Death Certificates and Administrative Codes–United States, 1999-2014. MMWR Morb Mortal Wkly Rep. 2016;65(13):342–345. doi: 10.15585/mmwr.mm6513a2. [DOI] [PubMed] [Google Scholar]
- 10.Kadri S.S., Rhee C., Strich J.R. Estimating Ten-Year Trends in Septic Shock Incidence and Mortality in United States Academic Medical Centers Using Clinical Data. Chest. 2017;151(2):278–285. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunfeld J.P., Eloy L. Glucocorticoids modulate vascular reactivity in the rat. Hypertension. 1987;10(6):608–618. doi: 10.1161/01.hyp.10.6.608. [DOI] [PubMed] [Google Scholar]
- 12.Hafezi-Moghadam A., Simoncini T., Yang Z. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8(5):473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukaida N., Zachariae C.C., Gusella G.L., Matsushima K. Dexamethasone inhibits the induction of monocyte chemotactic-activating factor production by IL-1 or tumor necrosis factor. J Immunol. 1991;146(4):1212–1215. [PubMed] [Google Scholar]
- 14.May J.M., Harrison F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid Redox Signal. 2013;19(17):2068–2083. doi: 10.1089/ars.2013.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barabutis N., Handa V., Dimitropoulou C. LPS induces pp60c-src-mediated tyrosine phosphorylation of Hsp90 in lung vascular endothelial cells and mouse lung. Am J Physiol Lung Cell Mol Physiol. 2013;304(12):L883–L893. doi: 10.1152/ajplung.00419.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barabutis N., Dimitropoulou C., Birmpas C., Joshi A., Thangjam G., Catravas J.D. p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308(8):L776–L787. doi: 10.1152/ajplung.00334.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi A.D., Dimitropoulou C., Thangjam G. Heat shock protein 90 inhibitors prevent LPS-induced endothelial barrier dysfunction by disrupting RhoA signaling. Am J Respir Cell Mol Biol. 2014;50(1):170–179. doi: 10.1165/rcmb.2012-0496OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Catravas J.D., Snead C., Dimitropoulou C. Harvesting, identification and barrier function of human lung microvascular endothelial cells. Vascul Pharmacol. 2010;52(5-6):175–181. doi: 10.1016/j.vph.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barabutis N., Catravas J.D. P53: “The Wall Watcher”. Med Surg Urol. 2015;4(4) [Google Scholar]
- 20.Angus D.C., van der Poll T. Severe sepsis and septic shock. The New England journal of medicine. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 21.Birukov K.G., Zebda N., Birukova A.A. Barrier enhancing signals in pulmonary edema. Comprehensive Physiology. 2013;3(1):429–484. doi: 10.1002/cphy.c100066. [DOI] [PubMed] [Google Scholar]
- 22.Heitzer T., Just H., Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94(1):6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Ting H.H., Timimi F.K., Boles K.S., Creager S.J., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. The Journal of clinical investigation. 1996;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ting H.H., Timimi F.K., Haley E.A., Roddy M.A., Ganz P., Creager M.A. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95(12):2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T., D'Uscio L.V., Eguchi D., Akiyama M., Smith L.A., Katusic Z.S. Protective effect of chronic vitamin C treatment on endothelial function of apolipoprotein E-deficient mouse carotid artery. The Journal of pharmacology and experimental therapeutics. 2003;306(1):103–108. doi: 10.1124/jpet.103.049163. [DOI] [PubMed] [Google Scholar]
- 26.Carr A.C., McCall M.R., Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arteriosclerosis, thrombosis, and vascular biology. 2000;20(7):1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 27.Traber M.G., Stevens J.F. Vitamins C and E: beneficial effects from a mechanistic perspective. Free radical biology & medicine. 2011;51(5):1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heyland D., Muscedere J., Wischmeyer P.E. A randomized trial of glutamine and antioxidants in critically ill patients. The New England journal of medicine. 2013;368(16):1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 29.Wilson J.X. Evaluation of vitamin C for adjuvant sepsis therapy. Antioxidants & redox signaling. 2013;19(17):2129–2140. doi: 10.1089/ars.2013.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P., Raghavan S.A., Saini R., Dikshit M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: modulatory effect of nitric oxide. Journal of leukocyte biology. 2004;75(6):1070–1078. doi: 10.1189/jlb.0903415. [DOI] [PubMed] [Google Scholar]
- 31.Annane D. Glucocorticoids in the treatment of severe sepsis and septic shock. Current opinion in critical care. 2005;11(5):449–453. doi: 10.1097/01.ccx.0000176691.95562.43. [DOI] [PubMed] [Google Scholar]
- 32.de Kruif M.D., Lemaire L.C., Giebelen I.A. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. Journal of immunology. 2007;178(3):1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 33.Schafer S.T., Gessner S., Scherag A. Hydrocortisone fails to abolish NF-kappaB1 protein nuclear translocation in deletion allele carriers of the NFKB1 promoter polymorphism (-94ins/delATTG) and is associated with increased 30-day mortality in septic shock. PloS one. 2014;9(8):e104953. doi: 10.1371/journal.pone.0104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuchowski M.F., Connett J., Welch K., Granger J., Remick D.G. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Critical care medicine. 2009;37(5):1567–1573. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azari O., Kheirandish R., Azizi S., Farajli Abbasi M., Ghahramani Gareh Chaman S., Bidi M. Protective Effects of Hydrocortisone, Vitamin C and E Alone or in Combination against Renal Ischemia-Reperfusion Injury in Rat. Iranian journal of pathology. 2015;10(4):272–280. [PMC free article] [PubMed] [Google Scholar]
- 36.Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. Journal of renal injury prevention. 2015;4(2):20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh M., Song X., Mouneimne G., Sidani M., Lawrence D.S., Condeelis J.S. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304(5671):743–746. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 38.Yao L., Romero M.J., Toque H.A., Yang G., Caldwell R.B., Caldwell R.W. The role of RhoA/Rho kinase pathway in endothelial dysfunction. Journal of cardiovascular disease research. 2010;1(4):165–170. doi: 10.4103/0975-3583.74258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwok W., Clemens M.G. Rho-kinase activation contributes to Lps-induced impairment of endothelial nitric oxide synthase activation by endothelin-1 in cultured hepatic sinusoidal endothelial cells. Shock. 2014;42(6):554–561. doi: 10.1097/SHK.0000000000000252. [DOI] [PubMed] [Google Scholar]