Abstract
Despite continuous efforts of regional governmental agencies, air pollution remains a major threat to public health worldwide. In January 2017, a severe episode of smog similar to the Great Smog of 1952 occurred in London. The longest episode of Chinese haze also developed in Beijing, during which levels of particulate matter < 2.5 μm rose to 500 μg/m3. European smog and Chinese haze are associated with large numbers of premature deaths each year, at 400,000 and 1.2 million, respectively, primarily from respiratory diseases, cerebrovascular diseases, and ischemic heart diseases. In addition to air pollution, some are exposed to other harmful environmental factors, such as secondhand smoke. For countries with large populations of smokers, such as China, India, the United States, and Russia, surviving both smog and smoke is a serious problem. With novel genomic and epigenomic studies revealing air pollution- and smoking-induced mutational signatures and epigenetic editing in diseases such as lung cancer, it has become feasible to develop precision strategies for early intervention in the disease-causing pathways driven by the specific mutations or epigenetic regulations, or both. New therapies guided by gene-drug interactions and genomic biomarkers may also be developed. We discuss both perspectives regarding the urgent need to manage the toxic effects of smog and smoke for the benefit of global health and the novel concept of precision intervention to protect the exposed individuals when exposure to smog and secondhand smoke cannot be voluntarily avoided or easily modified.
Key Words: genomics, precision medicine, respiratory diseases, secondhand smoke, smog
Abbreviations: GWAS, genome-wide association studies; PM2.5, particulate matter < 2.5 μm; PM10, particulate matter < 10 μm; ROS, reactive oxygen species; SNP, single nucleotide polymorphism; WHO, World Health Organization
Despite continuous efforts of regional governmental agencies, air pollution remains a major threat to public health worldwide.1 Notably, the damage from the London fog (or the Great Smog of 1952) is still in effect,2 after causing 12,000 deaths in 1 week.3 Mortality rates for the smog episode from December 1952 to February 1953 were 50% to 300% higher than in the previous year.3 The latest data indicate that exposure to the Great Smog in the first year of life increased the likelihood of childhood and adulthood asthma by 19.9% and 9.5%, respectively.2 It is telling that earlier in January of 2017, a severe smog episode similar to the Great Smog of 1952, occurred in London.4 The air pollution in London is at present causing approximately 10,000 deaths each year.5 This episode was considered a consequence of a push for diesel fuel and increased open use of wood-burning fires in the winter months.5 For the whole of Europe, it is estimated that exposure to excessive particulate matter < 2.5 μm (PM2.5) causes more than 400,000 premature deaths per year.6
In China, air pollution has become increasingly worse in recent years, and it has gained a new term as Chinese haze.7, 8, 9 A haze event is defined by the China Meteorological Administration as a pollution phenomenon that cuts atmospheric visibility to < 10 km. For 8 days beginning December 30, 2016, residents of Beijing and surrounding cities suffered from the longest haze episode on record.10 The PM2.5 concentrations were at approximately 500 μg/m3.10 Of note, the World Health Organization (WHO) guideline for a healthy index of PM2.5 is < 10 μg/m3 for an annual mean, or < 25 μg/m3 for a 24-hour mean (http://www.who.int/mediacentre/factsheets/fs313/en/). For particulate matter < 10 μm (PM10), the WHO guideline requires a level < 20 μg/m3 for an annual mean or 50 μg/m3 for a 24-hour mean. In the United States, the real-time PM2.5 reporting system indicates that the levels of PM2.5 in most of US cities monitored are < 50-100 μg/m3 (https://airnow.gov/index.cfm?action=airnow.pollutant_summary&pollutant=pm25).
The development of haze in Beijing is affected by climate factors such as humidity and airflow.11 Of note, it has been shown that excessive PM2.5 caused more than 1.2 million premature deaths in China in 2010, 42% higher than the level in 2000.12 Recent evidence has indicated that outdoor air pollution directly affects lung function in both children and adults, and triggers exacerbations of COPD symptoms.13 The Chinese Ministry of Environmental Protection has recently started to tackle these problems by using a robust approach for extensive data collection and analyses and making the information publicly available.14 In the United States, the government started to publish PM2.5 levels in 1997 (United States Environmental Protection Agency; https://www.epa.gov/green-book/green-book-pm-25-1997-area-information) following the Air Pollution Control Act of 1955 that marked the first action by the federal government to address air pollution problems (https://www.epa.gov/clean-air-act-overview/evolution-clean-air-act). The overall efforts in China now include satellite collection of pollution data, drone monitoring of pollution discharges and air quality and effectiveness of protection programs, and use of chemical modeling for early warning of potentially severe haze episodes.14
Of note, smog can be separated into two types: industrial smog and photochemical smog. Industrial smog occurs in foggy cool weather, typical of that in London. Photochemical smog develops in hot dry climates such as Los Angeles and Mexico City.8 Although ozone is the primary pollutant for photochemical smog,15 industrial smog (including Chinese haze) contains particulate matter, sulfur dioxide, nitrogen dioxide, and carbon monoxide, and sometimes ozone.9, 16 Nonetheless, for the January 2017 smog in London, it was reported that nitrogen dioxide was the main toxic component.5
There is no doubt that exposure to smog or haze can cause respiratory diseases (COPD, asthma, lung cancer, and lower respiratory infections), cerebrovascular diseases, ischemic heart diseases, and other medical conditions.1 These account for the associated morbidity and mortality. In addition to smog or haze that cannot be easily modified to reduce injuries to individuals exposed to it, some experience additional layers of harmful environmental exposures such as secondhand smoke (also called environmental tobacco smoke). Nonsmokers who breathe in secondhand smoke take in nicotine and toxic chemicals the same way that smokers do (American Cancer Society [https://www.cancer.org/cancer/cancer-causes/tobacco-and-cancer/secondhand-smoke.html]). Although smoking cessation can be championed as an intervention, exposure to secondhand smoke is nonelective and unavoidable, similar to air pollution. It is a major form of indoor air pollution. Of note, China has the largest population of smokers in the world, at 3 of 10 billion people.17 There are an estimated 7.4 billion people in China who are exposed to secondhand smoke every day, 1.8 billion of whom are children.18 Therefore, in China, there is a serious condition of surviving smog and smoke at the same time. People living in other countries with large populations of smokers, such as India, the United States, and Russia,19 face the same problem.
The potential health damage from smog and smoke may be additive or synergistic, resulting in more morbidity and mortality. Smoking shares similarities with smog in generating the same pollutants of particulate matter, sulfur dioxide, nitrogen dioxide, and carbon monoxide.20, 21, 22, 23 Recent studies indicate that both smog and smoke can increase oxidative stress in cells of the airways.24 Elevated levels of PM2.5 induce oxidative stress and inflammation in cultured macrophages.25, 26 In the Framingham Heart Study, even a short-term exposure to increased PM2.5 levels resulted in corresponding increases in oxidative stress biomarkers.27 Of note, it has been established that smoking induces oxidative stress in vascular endothelial cells.28 The disease-causing effects of oxidative stress are attributed to elevated levels of reactive oxygen species (ROS) within a certain range and in a dose-dependent manner. Furthermore, initial production of ROS is able to provoke ROS-induced ROS release, resulting in sustained oxidative stress.29, 30, 31, 32, 33, 34 One of the major consequences of oxidative stress is DNA damage and mutations.35, 36 Importantly, the latest studies have revealed mutational signatures and epigenetic footprints in response to air pollution, smoking, and smoking condensates, as discussed further on.
Since both environmental factors of smog and secondhand smoke cannot be voluntarily avoided, perhaps it is time to consider precision interventions to protect affected individuals, for example, through personalized alteration of the disease-causing pathways. Using whole genome and targeted exome sequencing, a recent study by Yu et al37 reported that somatic mutations in air pollution induced lung cancer (164 patients with non-small cell lung cancer: 79 in highly polluted regions vs 85 in control regions). The subject population was from regions in China where exposure to smoky coal generates high concentrations of PM2.5 and PM10. It was previously shown that when PM2.5 increases by 5 μg/m3, the risk of lung cancer increases by 18%; and when PM10 increases by 10 μg/m3, the lung cancer risk increases by 22%.38 In this study, a total of 80 somatic mutations of TP53 were identified (46 in highly polluted regions vs 34 in control regions), with only nine common mutations.37 The different 37 mutations of TP53 can be further validated functionally for oncogenic effects.37, 39 In addition, Alexandrov et al40 recently identified mutational signatures for 17 types of smoking-induced cancers, one of which is lung cancer. This study also used whole genome and exome sequencing to analyze 3,553 samples (2,490 from tobacco smokers and 1,063 from never smokers). A total of 21 mutational signatures have been identified for lung cancer, including subtypes of small cell lung cancer, lung squamous cell cancer, and lung adenocarcinoma.40
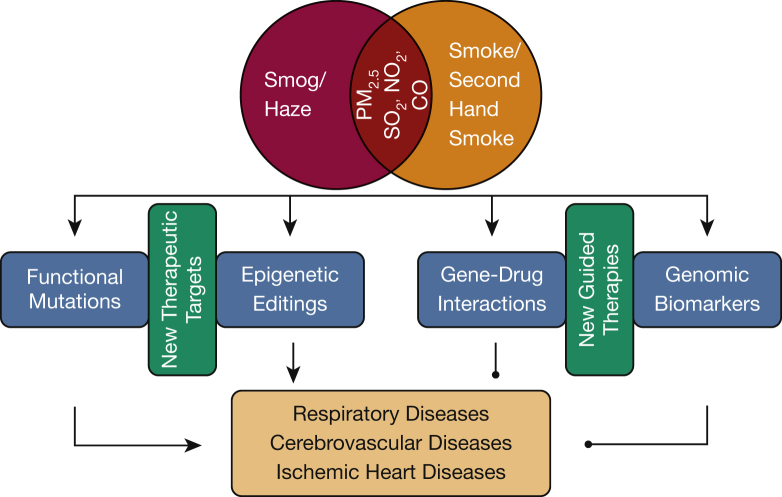
Of note, another study by Joehanes et al41 reported epigenetic editing in response to cigarette smoking. A meta-analysis of genome-wide DNA methylation responses, which were assessed using the Illumina BeadChip 450K array on 15,907 blood-derived DNA samples from participants in 16 cohorts, was carried out. A large number of cytosine-phosphate-guanine site annotations were identified in 1,405 genes, which were found to be associated with pulmonary function, cancers, inflammatory diseases, and heart disease.41 Furthermore, exposure to smoking condensates has been shown to cause DNA damage and injuries to DNA repair mechanisms,42 although detailed mutational profiling needs to be further elucidated. This shares similarities with previous studies in which 11 different smoking condensates were found to be mutagenic.43 Collectively, these emerging discovery data will promote subsequent mechanistic investigations of mutation/epigenetic regulation-dependent pathogenic pathways, manipulation of which might be of therapeutic or preventive potential. Besides revealing new therapeutic targets, these novel mutations and epigenetic footprints may also be relevant to specific implementation of pharmacogenomics and biomarker-guided treatments.44, 45, 46 Pharmacogenomics to identify gene-drug interactions would be beneficial in precision intervention using existing or new drugs. Validated oncogenic somatic mutations can also be screened to use as a guide for targeted therapies. In addition, genome-wide association studies (GWAS) are expected to identify germ line polymorphisms that put individuals at higher risk for the development of a specific disease.47, 48 Although the counseling value to patients of these types of genomic biomarkers has been controversial, the latest studies have shown that GWAS-evaluated single nucleotide polymorphisms (SNPs) are functionally important for the development of childhood asthma in response to air pollution exposure.49 Likewise, SNPs indicative of lung cancer risk have been discovered in coal-exposed populations.50 These genomic biomarkers are of potential benefit in guiding early interventions in subjects who are genetically predisposed to related diseases. The overall strategies of precision intervention for subjects exposed to smog and smoke are summarized in Figure 1.
Figure 1.
Precision interventions for individuals exposed to smog and smoke. Smog/haze and smoke/secondhand smoke are primary causes of air pollution that remain a major threat to public health worldwide. Particulate matter, sulfur dioxide, nitrogen dioxide, and carbon monoxide are common pollutants shared by smog/haze and smoke/secondhand smoke. Elevated levels of particulate matter < 2.5 μm induce oxidative stress, which is known to cause mutations and DNA damage. Recent genomic and epigenomic studies have revealed novel mutational signatures and epigenetic footprints in response to air pollution and smoking exposure. Manipulations of the downstream pathways of these regulatory pathways might be of preventive or therapeutic value. In addition, new information collected on gene-drug interactions and disease-predicting single nucleotide polymorphisms can be used to develop guided therapies, primarily through treating patients more effectively, given the precise genetic background influencing the efficacy of the particular drug, and by treating subjects early when they happen to carry predisposing genomic biomarkers. CO = carbon monoxide; NO2 = nitrogen dioxide; SO2 = sulfur dioxide.
With the concept of precision medicine rapidly developing into practice,51, 52 it has become feasible to perform “omics” analyses of individual subjects to generate an integrated genomic and epigenomic atlas that can potentially be instrumental to early intervention in diseases caused by exposure to smog and smoke.53 This information can be used for precision interventions by promoting individualized application of novel therapies that are developed based on the newly identified therapeutic targets and by promoting individualized application of new therapies that are guided by newly identified gene-drug interactions and disease-predicting SNPs (as summarized in Fig 1). In addition, some generic approaches to counteract oxidative stress may also prove to be helpful and valuable for immediate use before personalized protection is optimized. For example, a recent study has shown that B vitamins attenuated air pollution-induced epigenetic regulation in healthy subjects, presumably by attenuation of systematic oxidative stress.54 It is postulated that other antioxidants might have similar effects through epigenomic modulations. These include dietary phytochemicals,55 the active component of green tea—epigallocatechin gallate,56 and vitamin C.57 In the B-vitamin cocktail used, folic acid has been shown to exert potent vascular protective effects in addition to its antioxidative action.58, 59
Surviving smog and smoke represents a major public health concern that needs urgent attention. Personalized precision intervention may be a feasible approach to achieve rapid progress in effectively protecting exposed individuals from smog- and smoke-induced injuries, while history has shown that it could take decades to half a century to solve the severe air pollution problems such as those currently presented by Chinese haze. Implementation of precision medicine is evolving quickly worldwide60, 61; efforts in this direction to control damage to health caused by smog and smoke may prove to be fruitful explorations in generating novel therapies for the prevention and treatment of related diseases.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Footnotes
FINANCIAL SUPPORT: This study was supported by the National Key Research and Development Program of China [Grants 2016YFC1303900 (C. W.), 2016YFC0901102 (C. W.)], National Institute of Health National Heart, Lung and Blood Institute (NHLBI) [Grants HL077440 (H. C.), HL088975 (H. C.), HL108701 (H. C.), HL119968 (H. C.)], and an American Heart Association Established Investigator Award [Grant 12EIA8990025 (H. C.)].
References
- 1.Cohen A.J., Brauer M., Burnett R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;10:30505–30506. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharadwaj P., Zivin J.G., Mullins J.T., Neidell M. Early-life exposure to the great smog of 1952 and the development of asthma. Am J Respir Crit Care Med. 2016;194:1475–1482. doi: 10.1164/rccm.201603-0451OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell M.L., Davis D.L. Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution. Environ Health Perspect. 2001;109:389–394. doi: 10.1289/ehp.01109s3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates J. The Great Smog of 2017 should be a wake-up call to government. Nurs Stand. 2017;31:31. doi: 10.7748/ns.31.25.31.s37. [DOI] [PubMed] [Google Scholar]
- 5.Millar L. London air pollution kills about 10,000 people annually. ABC News. January 9, 2017 [Google Scholar]
- 6.Badyda A.J., Grellier J., Dabrowiecki P. Ambient PM2.5 exposure and mortality due to lung cancer and cardiopulmonary diseases in Polish cities. Adv Exp Med Biol. 2017;944:9–17. doi: 10.1007/5584_2016_55. [DOI] [PubMed] [Google Scholar]
- 7.Gao J., Woodward A., Vardoulakis S. Haze, public health and mitigation measures in China: a review of the current evidence for further policy response. Sci Total Environ. 2017;578:148–157. doi: 10.1016/j.scitotenv.2016.10.231. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J.J., Samet J.M. Chinese haze versus Western smog: lessons learned. J Thorac Dis. 2015;7:3–13. doi: 10.3978/j.issn.2072-1439.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G., Zhang R., Gomez M.E. Persistent sulfate formation from London fog to Chinese haze. Proc Natl Acad Sci U S A. 2016;113:13630–13635. doi: 10.1073/pnas.1616540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smog levels in Beijing off the charts. South China Morning Post. January 1, 2017.
- 11.Miao Y., Liu S., Zheng Y. Numerical study of the effects of local atmospheric circulations on a pollution event over Beijing-Tianjin-Hebei, China. J Environ Sci (China) 2015;30:9–20. doi: 10.1016/j.jes.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Xie R., Sabel C.E., Lu X. Long-term trend and spatial pattern of PM2.5 induced premature mortality in China. Environ Int. 2016;97:180–186. doi: 10.1016/j.envint.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Hu G., Zhong N., Ran P. Air pollution and COPD in China. J Thorac Dis. 2015;7:59–66. doi: 10.3978/j.issn.2072-1439.2014.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B., Hughes R.M. Environment: China deploys big data to clear smog. Nature. 2017;542:31. doi: 10.1038/542031a. [DOI] [PubMed] [Google Scholar]
- 15.Mustafa M.G. Biochemical basis of ozone toxicity. Free Radic Biol Med. 1990;9:245–265. doi: 10.1016/0891-5849(90)90035-h. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y., Wang L., Wang Y., Quan L., Zirui L. In situ measurements of SO2, NOx, NOy, and O3 in Beijing, China during August 2008. Sci Total Environ. 2011;409:933–940. doi: 10.1016/j.scitotenv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Liu S., Zhang M., Yang L. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health. 2017;71:154–161. doi: 10.1136/jech-2016-207805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.740 million second-hand smokers in China. China Daily. July 2, 2013.
- 19.GBD 2015 Tobacco Collaborators Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;5:30819–30840. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silveira Fleck A., Carneiro M.F., Barbosa F., Jr., Thiesen F.V., Amantea S.L., Rhoden C.R. Monitoring an outdoor smoking area by means of PM2.5 measurement and vegetal biomonitoring. Environ Sci Pollut Res Int. 2016;23:21187–21194. doi: 10.1007/s11356-015-5878-4. [DOI] [PubMed] [Google Scholar]
- 21.Rodgman A., Perfetti T.A. 2nd ed. CRC Press; Boca Raton: 2013. The Chemical Components of Tobacco and Tobacco Smoke. [Google Scholar]
- 22.Shorter J.H., Nelson D.D., Zahniser M.S., Parrish M.E., Crawford D.R., Gee D.L. Measurement of nitrogen dioxide in cigarette smoke using quantum cascade tunable infrared laser differential absorption spectroscopy (TILDAS) Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:994–1001. doi: 10.1016/j.saa.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Maga M., Janik M.K., Wachsmann A. Influence of air pollution on exhaled carbon monoxide levels in smokers and non-smokers. A prospective cross-sectional study. Environ Res. 2017;152:496–502. doi: 10.1016/j.envres.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Gilmour M.I., Jaakkola M.S., London S.J., Nel A.E., Rogers C.A. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006;114:627–633. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekki K., Ito T., Yoshida Y. PM2.5 collected in China causes inflammatory and oxidative stress responses in macrophages through the multiple pathways. Environ Toxicol Pharmacol. 2016;45:362–369. doi: 10.1016/j.etap.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Su R., Jin X., Zhang W., Li Z., Liu X., Ren J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere. 2017;167:444–453. doi: 10.1016/j.chemosphere.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Li W., Wilker E.H., Dorans K.S. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc. 2016;5(5):e002742. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 29.Chalupsky K., Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai H. Hydrogen peroxide regulation of endothelial function: mechanisms, consequences and origins. Cardiovasc Res. 2005;68(1):26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 32.Zinkevich N.S., Gutterman D.D. ROS-induced ROS release in vascular biology: redox-redox signaling. Am J Physiol Heart Circ Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn J.Y., Siu K.L., Li Q., Harrison D.G., Cai H. Oxidase interaction in cardiovascular disease. In: Laher I., editor. System Biology of Oxidative Stress and Antioxidants. Springer; Berlin, Germany: 2013. pp. 849–876. [Google Scholar]
- 34.Siu K.L., Li Q., Zhang Y. NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol. 2017;11:118–125. doi: 10.1016/j.redox.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadet J., Davies K.J.A. Oxidative DNA damage and repair: an introduction. Free Radic Biol Med. 2017;28 doi: 10.1016/j.freeradbiomed.2017.03.030. 30184-30183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38(7):592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Yu X.J., Yang M.J., Zhou B. Characterization of somatic mutations in air pollution-related lung cancer. EBioMedicine. 2015;2:583–590. doi: 10.1016/j.ebiom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raaschou-Nielsen O., Andersen Z.J., Beelen R. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 39.Watson I.R., Takahashi K., Futreal P.A., Chin L. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandrov L.B., Ju Y.S., Haase K. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joehanes R., Just A.C., Marioni R.E. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet. 2016;9:436–447. doi: 10.1161/CIRCGENETICS.116.001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holcomb N., Goswami M., Han S.G. Exposure of human lung cells to tobacco smoke condensate inhibits the nucleotide excision repair pathway. PLoS One. 2016;11:e0158858. doi: 10.1371/journal.pone.0158858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X., Verkler T.L., Chen Y. Mutagenicity of 11 cigarette smoke condensates in two versions of the mouse lymphoma assay. Mutagenesis. 2011;26:273–281. doi: 10.1093/mutage/geq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Francia R, De Monaco A, Saggese M, et al. Pharmacological profile and Pharmacogenomics of anti-cancer drugs used for targeted therapy [published online ahead of print February 8, 2017]. Curr Cancer Drug Targets. http://dx.doi.org/10.2174/1568009617666170208162841. [DOI] [PubMed]
- 45.Dong O.M., Wiltshire T. Advancing precision medicine in healthcare. Physiol Genomics. 2017;49(7):346–354. doi: 10.1152/physiolgenomics.00029.2017. [DOI] [PubMed] [Google Scholar]
- 46.Liu D., Vokes N.I., Van Allen E.M. Toward molecularly driven precision medicine in lung adenocarcinoma. Cancer Discov. 2017;7:555–557. doi: 10.1158/2159-8290.CD-17-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon R. Genomic biomarkers in predictive medicine: an interim analysis. EMBO Mol Med. 2011;3:429–435. doi: 10.1002/emmm.201100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosse Y, Amos CI. A decade of GWAS results in lung cancer [Published online ahead of print June 14, 2017]. Cancer Epidemiol Biomarkers Prev. http://dx.doi.org/10.1158/1055-9965.EPI-16-0794. [DOI] [PMC free article] [PubMed]
- 49.Gref A., Merid S.K., Gruzieva O. Genome-wide interaction analysis of air pollution exposure and childhood asthma with functional follow-up. Am J Respir Crit Care Med. 2017;195:1373–1383. doi: 10.1164/rccm.201605-1026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosgood H.D., III, Song M., Hsiung C.A. Interactions between household air pollution and GWAS-identified lung cancer susceptibility markers in the Female Lung Cancer Consortium in Asia (FLCCA) Hum Genet. 2015;134:333–341. doi: 10.1007/s00439-014-1528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.AACR Project GENIE: Powering precision medicine through an international consortium. Cancer Discov. 2017;7(8):1–14. doi: 10.1158/2159-8290.CD-17-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coyne G.O., Takebe N., Chen A.P. Defining precision: the precision medicine initiative trials NCI-MPACT and NCI-MATCH. Curr Probl Cancer. 2017;11:30016–30018. doi: 10.1016/j.currproblcancer.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Moreira A.L., Eng J. Personalized therapy for lung cancer. Chest. 2014;146:1649–1657. doi: 10.1378/chest.14-0713. [DOI] [PubMed] [Google Scholar]
- 54.Zhong J., Karlsson O., Wang G. B vitamins attenuate the epigenetic effects of ambient fine particles in a pilot human intervention trial. Proc Natl Acad Sci U S A. 2017;114:3503–3508. doi: 10.1073/pnas.1618545114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shankar E., Kanwal R., Candamo M., Gupta S. Dietary phytochemicals as epigenetic modifiers in cancer: promise and challenges. Semin Cancer Biol. 2016;40-41:82–99. doi: 10.1016/j.semcancer.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D., Perkins J.T., Hennig B. EGCG prevents PCB-126-induced endothelial cell inflammation via epigenetic modifications of NF-kappaB target genes in human endothelial cells. J Nutr Biochem. 2016;28:164–170. doi: 10.1016/j.jnutbio.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camarena V., Wang G. The epigenetic role of vitamin C in health and disease. Cell Mol Life Sci. 2016;73:1645–1658. doi: 10.1007/s00018-016-2145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao L., Chalupsky K., Stefani E., Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice. A Novel HPLC-based fluorescent assay for DHFR activity. J Mol Cell Cardiol. 2009;47:752–760. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao L., Siu K.L., Chalupsky K. Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation: treatment with folic acid. Hypertension. 2012;59:158–166. doi: 10.1161/HYPERTENSIONAHA.111.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Precision Medicine in China. Science Washington, DC: Science/AAAS. December 23, 2016.
- 61.Jensen MA, Ferretti V, Grossman RL, Staudt LM. The NCI Genomic Data Commons as an engine for precision medicine [published online ahead of print June 9, 2017]. Blood.http://dx.doi.org/10.1182/blood-2017-03-735654. [DOI] [PMC free article] [PubMed]