Abstract
There is increasing appreciation that mitochondria serve cellular functions beyond oxygen sensing and energy production. Accordingly, it has become important to explore noncanonical roles of mitochondria in normal and pathophysiological processes that influence airway structure and function in the context of diseases such as asthma and COPD. Mitochondria can sense upstream processes such as inflammation, infection, tobacco smoke, and environmental insults important in these diseases and in turn can respond to such stimuli through altered mitochondrial protein expression, structure, and resultant dysfunction. Conversely, mitochondrial dysfunction has downstream influences on cytosolic and mitochondrial calcium regulation, airway contractility, gene and protein housekeeping, responses to oxidative stress, proliferation, apoptosis, fibrosis, and certainly metabolism, which are all key aspects of airway disease pathophysiology. Indeed, mitochondrial dysfunction is thought to play a role even in normal processes such as aging and senescence and in conditions such as obesity, which impact airway diseases. Thus, understanding how mitochondrial structure and function play central roles in airway disease may be critical for the development of novel therapeutic avenues targeting dysfunctional mitochondria. In this case, it is likely that mitochondria of airway epithelium, smooth muscle, and fibroblasts play differential roles, consistent with their contributions to disease biology, underlining the challenge of targeting a ubiquitous cellular element of existential importance. This translational review summarizes the current state of understanding of mitochondrial processes that play a role in airway disease pathophysiology and identifying areas of unmet research need and opportunities for novel therapeutic strategies.
Key Words: asthma, COPD, metabolism, oxidative stress, remodeling
Abbreviations: ASM, airway smooth muscle; ATP, adenosine triphosphate; CS, cigarette smoke; DAMP, damage-associated molecular pattern; Drp1, dynamin-related protein 1; ER, endoplasmic reticulum; Fis1, fission 1; Mfn, mitofusin; mtDNA, mitochondrial DNA; Opa1, optic atrophy protein 1; PM, plasma membrane; ROS, reactive oxygen species
Alterations in bronchial airway structure and function occur throughout life and in response to functional and environmental demands. Dysfunctional excessive airway narrowing accompanied by wall thickening from increased cell proliferation and varying degrees of fibrosis occur in major lung diseases such as asthma, bronchitis, and COPD.1, 2 In these conditions, genetic, proteomic, and physiological processes within specific resident cell types (epithelial cells, airway smooth muscle [ASM], fibroblasts) and modulating effects of allergens, pollutants, cigarette smoke (CS), and inflammation all contribute to airway structural and functional changes.1, 2, 3, 4 Within this challenging framework of disease pathogenesis, there is increasing recognition that altered mitochondrial structure and function (indeed beyond the classic role as a cell’s powerhouse) occur in response to disease processes and conversely influence downstream cellular processes important in asthma5, 6, 7 and COPD.8, 9, 10, 11
Mitochondrial Bioenergetics and Airway Disease
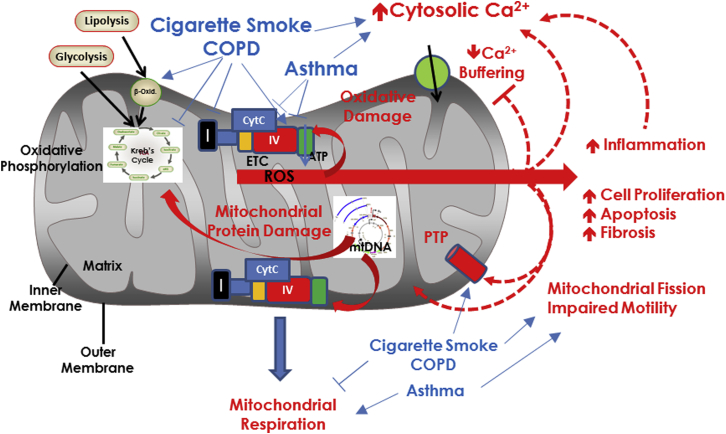
Originally derived from ancient aerobic bacteria, mitochondria have their own maternally inherited DNA (mitochondrial DNA [mtDNA]) and transcription/translational machinery. The mitochondrial outer membrane is in contact with the cellular cytosol, whereas the highly folded inner membrane contains oxidative phosphorylation enzyme complexes and the mitochondrial matrix within (Fig 1). Well known for meeting cellular energy demands through adenosine triphosphate (ATP) synthesis in the process of metabolizing carbohydrates and fatty acids using oxygen, mitochondria rapidly increase energy production under conditions of cellular stress (spare or bioenergetic respiratory capacity; critical for long-term cell survival).12
Figure 1.
Mitochondrial structure and energetic function. Mitochondria have a cytoplasm-facing outer membrane and a highly folded inner membrane that contains the enzymes of the ETC and oxidative phosphorylation, as well as mtDNA. ROS are a normal byproduct of mitochondrial respiration. Insults such as cigarette smoke and inflammation in the context of diseases such as COPD and asthma differentially influence elements of mitochondrial respiration, overall increasing ROS production, which has downstream consequences on inflammation itself, mtDNA integrity, cytosolic Ca2+ ([Ca2+]cyt) regulation, cell proliferation, and apoptosis (especially when severe stress induces the PTP), and in a feedback fashion, mitochondrial structure and function. β-Oxid = β-oxidation; CytC = cytochrome C; ETC = electron transport chain; IV = complex IV of the ETC; mtDNA = mitochondrial DNA; PTP = permeability transition pore; ROS = reactive oxygen species.
One essential aspect of mitochondrial respiration is generation of reactive oxygen species (ROS),13, 14 which serve physiological functions but are detrimental in excess (Fig 1). Altered metabolism and increased ROS adversely affect other organelles and disrupt cellular homeostasis, requiring defense measures such as the antioxidant enzymes superoxide dismutase, peroxidases, and catalase. Mitochondrial ROS can act as signal transducers to trigger expression or release (or both) of proinflammatory cytokines, activate signaling pathways, modulate transcription factors important in redox homeostasis, proliferation/survival, response to inflammation, extracellular matrix production, and Ca2+ regulation of epithelium and ASM.7, 13, 14, 15 Importantly, inflammatory mediators and ROS can in turn modulate mitochondrial structure and function (see further on), creating a dysfunctional cycle to promote disease pathophysiological mechanisms.
The relevance of mitochondrial respiration to lung disease lies in increasing recognition that factors such as inflammation in asthma and CS exposure in COPD influence bioenergetics and ROS balance (Fig 1). In human airway cells16, 17 and in mice chronically exposed to CS,16 expression of oxidative phosphorylation16 and Kreb’s cycle enzymes15, 18 is decreased, mitochondrial respiration is blunted, and respiratory reserve is suppressed.16 Lower levels of CS blunt metabolism with a shift toward β-oxidation (Fig 1).19 However, CS increases cellular demand for ATP to account for elevated cytosolic Ca2+ ([Ca2+]cyt), DNA and protein repair, cell proliferation, and other “stresses,” and thus mitochondria face a “bioenergetics failure.” Furthermore, CS enhances ROS by inhibiting respiratory complexes and overwhelming cellular scavenging systems,16 effects observed in the lungs of patients with COPD.20
Dysmorphic mitochondria have been observed in epithelium and ASM of patients with asthma and in mouse models of allergic asthma.21, 22 However, unlike in COPD, increased [Ca2+]cyt in asthmatic ASM (which contributes to airway hyperreactivity and proliferation) is associated with increased mitochondrial mass (biogenesis) and elevated baseline respiration21 and spare capacity,16 helping to match mitochondrial numbers with cell proliferation. Nonetheless, inflammation can impair other aspects of mitochondrial function. For example, TNFα, a Th1 cytokine, increases ROS generation and mitochondrial permeabilization (pore formation in mitochondrial membranes) (Fig 1), promoting loss of mitochondrial Ca2+ (thus increasing [Ca2+]cyt) and ATP production, finally triggering apoptosis.23, 24 Limited data suggest mitochondrial permeabilization in airway cells of patients with COPD.20 Conversely, TNFα and the Th2 cytokine interleukin-13 impair mitochondrial Ca2+ buffering,6 which usually protects against [Ca2+]cyt overload. In addition to cytokines, lipid metabolites of linoleic acid and lipoxygenase can induce epithelial injury and mitochondrial dysfunction.5 Conversely, antioxidant levels in serum, BAL fluid, or tissues are lower from patients with asthma.25 Furthermore, there may be a shift toward glycolysis.26
Mitochondrial Calcium and Airway Disease
Mitochondria have substantial capacity for buffering Ca2+, and mitochondrial Ca2+ ([Ca2+]mito) regulates respiration and other functions.27 Regulation of [Ca2+]mito involves multiple mechanisms, most prominently the mitochondrial calcium uniporter27 that increases [Ca2+]mito, along with mitochondrial Na+/Ca2+ exchange and Ca2+/H+ exchange that permit Ca2+ efflux, overall contributing to local control of [Ca2+]cyt itself. Under conditions of stress, the permeability transition pore leads to excessive release of [Ca2+]mito and induces cell death. Emerging data in ASM suggest that inflammation inhibits mitochondrial Ca2+ buffering, although roles of specific mechanisms are unknown.
Dysregulation of Mitochondrial Structure in Airway Disease
It is now being recognized that beyond metabolic dysfunction, mitochondrial structure is also dynamically regulated and importantly disrupted in disease.5, 7, 13, 28 Multiple mechanisms control the morphologic characteristics and networks of mitochondria (fusion), their disruption and fragmentation (fission), biogenesis (splitting and growth of existing mitochondria), and motility within cytoplasmic confines. Such dynamic spatiotemporal regulation is critical for mitochondrial interactions with organelles such as plasma membrane (PM), endoplasmic reticulum (ER), lysosomes, and nuclei, thus allowing mitochondria to influence a host of cellular functions such as ionic regulation (particularly Ca2+), protein production, turnover, proliferation, and apoptosis. In this context, understanding the relevance of specific mitochondrial mechanisms beyond metabolism that contribute to their dysfunction in airway disease is a topic of emerging importance.
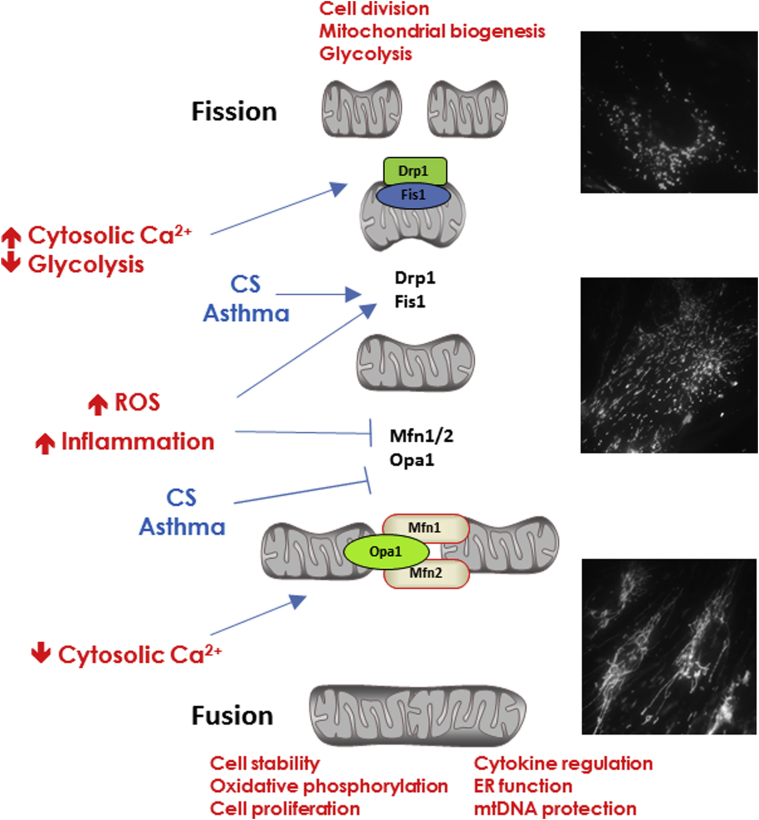
Mitochondria show cell- and context-specific heterogeneity and dynamism in morphologic features and connectivity, which are largely determined by factors that control the extent of fission vs fusion. Mitochondrial fusion is regulated by mitofusins 1 and 2 (Mfn1, Mfn2) on the outer membrane, and optic atrophy protein 1 (Opa1) on the inner membrane,7, 28 helping to fuse the two mitochondrial membranes (Fig 2) and facilitating formation of contiguous mitochondrial chains. Fission is orchestrated by factors such as fission 1 (Fis1), mitochondrial fission factor, MiD49, and MiD51, which help form focal rings around mitochondria, recruit the cytosolic dynamin-related protein 1 (Drp1) to the mitochondrial surface and thus cleave mitochondria (Fig 2). The normal functional relevance of mitochondrial fission and fusion is evident during the cell cycle, in which mitochondria fragment and rejoin constitutively during the quiescent G0 phase and are extensively networked during the stable interphase. During mitosis, mitochondria are fragmented, mixed, and distributed equally among daughter cells, in which fusion then resumes.
Figure 2.
Mitochondrial fission and fusion. Under conditions of cellular stability, mtDNA form extensive contiguous networks facilitated by the mitofusin proteins and Opa1 that promote fusion of mitochondrial membranes. Conditions of cellular stress promote mitochondrial fragmentation/fission orchestrated by multiple proteins including Drp1 and Fis1. Insults such as cigarette smoke and inflammation usually promote mitochondrial fission. CS = cigarette smoke; Drp1 = dynamin-related protein 1; ER = endoplasmic reticulum; Fis1 = fission 1; Mfn1/2 = mitofusin 1 and 2. See Figure 1 legend for expansion of other abbreviations.
The balance between fission and fusion can control cellular metabolism, linking mitochondrial structure to function. Interestingly, lung epithelial cells that rely on oxidative phosphorylation show more networked/fused mitochondria,17 whereas more glycolysis-dependent cells show fragmented mitochondria.29 Accordingly, metabolic shifts within cells, for example, under conditions of stress, could be reflected by altered mitochondrial morphologic characteristics. Indeed, mitochondrial fragmentation is activated by high [Ca2+]cyt (but is suppressed at resting levels) and oxidative stress.7, 28 Mitochondrial metabolism itself plays a role in which dissipation of mitochondrial membrane potential can induce fragmentation, whereas inhibition of glycolysis worsens fission.30 Changes in levels of fission vs fusion proteins can obviously play a role, for example, inflammation or environmental triggers promote proteasome-mediated degradation of fusion proteins.31 For example, Pink1, a mitochondrially targeted kinase, interacts with Fis1 and promotes activity of the E3 ubiquitin ligase Parkin and targets Mfn to degrade damaged mitochondria. Other quality-control processes such as ubiquitination can affect Opa1, Drp1, and Fis1 to also influence fission/fusion balance.7, 28, 29
Fission vs fusion pathways in different lung cell types or their contributions to specific airway diseases have been barely examined. In adult human epithelial cells19 and ASM,32 CS enhances fission, reflected by decreased Mfn but increased Drp1 (Fig 2), resulting in decreased oxidative phosphorylation and a shift toward glycolysis.16 Mitochondrial biogenesis is decreased in airways in COPD,8, 33 although both increased fission and hyperfusion have been observed.19, 34 In contrast, in asthma, enhanced biogenesis,21 increased Drp1, and decreased Mfn235 all suggest fission (Fig 2).
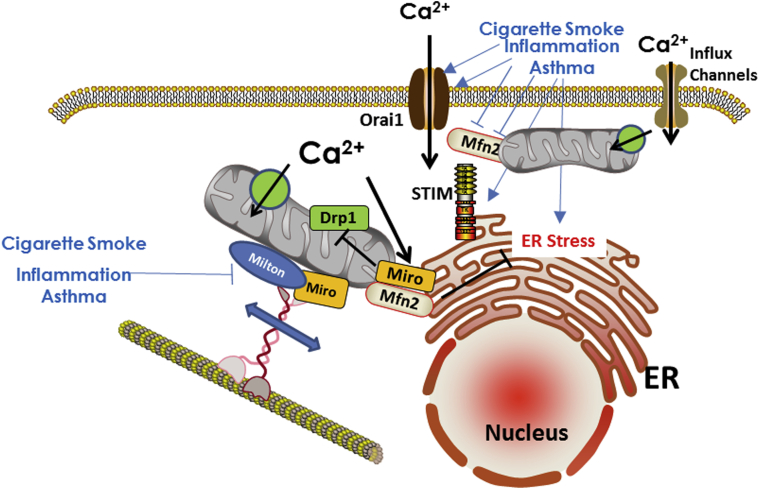
Mitochondrial localization to and interaction with other organelles, particularly ER, appear important in processes such as lipid synthesis, [Ca2+]cyt buffering, intracellular trafficking, and mitochondrial biogenesis itself.6, 7, 10, 36 Mitochondria can influence Ca2+ release through inositol trisphosphate 3 and ryanodine receptor channels, and conversely compete with ER Ca2+ ATPase for [Ca2+]cyt reuptake.6 In this case, Mfn2 acts as a physical link between ER and mitochondria, controlling mitochondrial buffering of [Ca2+]cyt in ER microdomains,6 and inversely making the mitochondria a Ca2+ reserve. Mitochondria in proximity to PM modulate Ca2+ influx by altering local gradients through Ca2+ uptake and thus prolonging influx, especially that occurring in response to intracellular Ca2+ store depletion. In this case, Mfn2 again acts as a linking protein to facilitate ER/PM interactions between proteins such as STIM1 that sense ER Ca2+ levels, and PM Ca2+ influx channels such as Orai16, 37 (Fig 3). The relationships between mitochondrial localization and regulation of other intracellular organelles in the context of airway disease have not been systematically examined.
Figure 3.
Mitochondrial motility and organelle interactions. Mitochondrial movement occurs along cytoskeletal proteins, facilitated by elements such as Miro, Milton, and Mfn. Mitochondrial proximity to the ER or the PM can help with buffering of Ca2+ fluxes and thus regulation of [Ca2+]cyt. Impairment of mitochondrial motility due to cigarette smoke or in asthma can lead to [Ca2+]cyt dysregulation and other downstream consequences. PM = plasma membrane. See Figure 2 legend for expansion of other abbreviations.
Relevance of Mitochondrial Motility
Mitochondria move within the cell,38 presumably to meet local energetic needs or to maintain [Ca2+]cyt homeostasis. Such movement involves coordinated interactions between cytoskeletal elements (actin, microtubules) and motors (kinesin, dynein), facilitated by Ca2+- dependent proteins such as Miro1 and Milton38 (Fig 3). Conditions such as increased [Ca2+]cyt dissociate mitochondrial interactions with the Miro/Milton complex and intracellular motors (Fig 3), thereby arresting mitochondrial motility and allowing mitochondria to buffer [Ca2+]cyt. Furthermore, Miro can blunt Drp1-mediated fission, and thus under conditions of high [Ca2+]cyt, which would normally trigger mitochondrial fragmentation, Miro may act to promote mitochondrial networking and stability while Ca2+ is being buffered. The relevance of mitochondrial movement and its modulation by Miro/Milton in airway diseases lies in potential disruption of these relationships by inflammation, ROS, or other factors, which already lead to increased [Ca2+]cyt and altered actin polymerization and other factors involved in mitochondrial motility, resulting overall in a mismatch between cellular demands, such as energy or the need for [Ca2+]cyt buffering, and ER function, vs proximate and dynamic availability of mitochondria. This may be particularly important in epithelial cells with high turnover rates and energetic demand and in ASM with increased contractility and proliferation in asthma or COPD.
ROS and Damage-Associated Molecular Patterns in the Airway
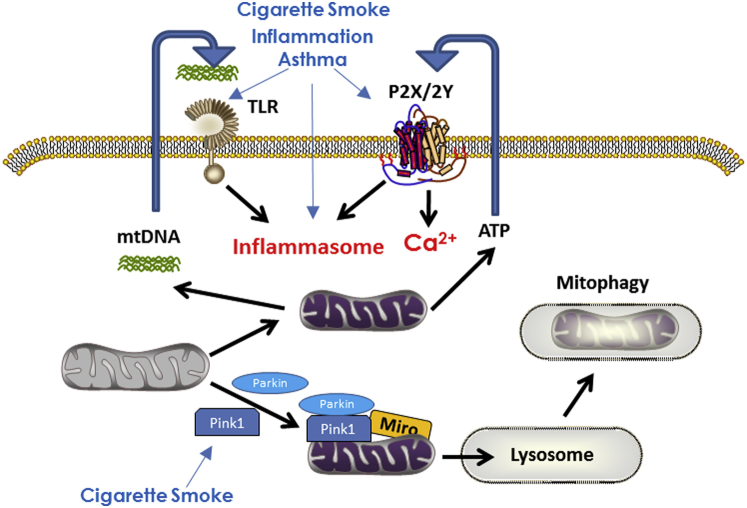
Excessive oxidant levels are known to be detrimental to airways, triggering expression and release of proinflammatory cytokines and synergistically activating signaling cascades relevant to such inflammation, promoting cell proliferation, extracellular matrix production, and Ca2+ dysregulation in asthma and COPD.14, 25, 39, 40 As discussed earlier, inflammation and ROS can in turn modulate mitochondrial morphologic characteristics and function, creating a vicious cycle of dysfunction in airway disease. Relevant to mitochondrial dysfunction in the lung is the emerging concept that damaged mitochondria—for example, in response to inflammation, oxidant stress, or other factors—release multiple mitochondrial components either into the cytosol or even extracellularly (mitochondrial damage-associated molecular patterns [DAMPs]) that normally act as signaling molecules except when produced in excess.8, 28, 41, 42 DAMPs are best known to activate pathogen recognition receptors such as toll-like receptors, inducing inflammation. One example is mtDNA fragments following unrepairable DNA damage, which can activate the inflammasome pathway, resulting in increased cytokine release by immune cells as well resident cells such as epithelium and smooth muscle.43, 44 ATP, well known to be normally released by multiple cell types in the airways, acts as a DAMP when released in excess,45 mobilizing Ca2+, activating the inflammasome, releasing mtDNA into the cytosol, and even increasing mitochondrial ROS and dysfunction.46 ATP levels are increased in BAL fluid in COPD.47 Less-studied DAMPs include succinate and cardiolipin, which in other cell systems elevate [Ca2+]cyt and activate the inflammasome.46 There is now increasing recognition for a role of the inflammasome in both asthma and COPD,43, 44, 48, 49, 50 and thus emerging data suggest a role for an autocrine effect of mitochondrial DAMPs in airway disease pathophysiology.
Autophagy/Mitophagy: Emerging Concepts in the Airway
Autophagy represents a series of cellular processes for degradation or recycling of organelles and components through channeling into lysosomes, generally considered an adaptive or protective homeostatic mechanism.11 There is now considerable interest in autophagy in lung diseases, in which autophagy may be either protective for clearing cellular components damaged due to inflammation or ROS or even deleterious due to autophagic activation of fibrosis or cell proliferation.9, 10, 11, 34 Limited data suggest a detrimental role in COPD and CS-induced epithelial cell death.34 A selective aspect of autophagy is clearance of damaged mitochondria (mitophagy) Fig 4), which, if left unchecked, will activate apoptotic signaling.8, 9, 17, 29 Mitophagy occurs in bronchial epithelial cells with CS exposure17 through stabilization of the mitophagy-associated protein Pink1. The relevance of mitophagy in COPD is suggested by increased expression of Pink1 in epithelial cells of patients with COPD and absence of Pink1 protecting against CS-induced mitophagy.17, 51 Accordingly, with CS exposure, mitophagy can induce cell death, resulting in reduced numbers of functional mitochondria. The role of mitophagy in asthma has not been examined to date. In this case, mitophagy-associated proteins such as AMPK, may be particularly relevant, given AMPK’s role as a sensor and regulator of cellular energy status and influences on glycolysis vs oxidative phosphorylation.8, 9, 17, 29 Limited data suggest altered AMPK signaling in airways in COPD,52 although the specific role of mitophagy per se is not known.
Figure 4.
Mitophagy and mitochondrial damage-associated molecular patterns (DAMPs). Insults such as cigarette smoke and inflammation promote mitochondrial destruction through autophagy mechanisms involving lysosomes. Mitochondrial dysfunction can also result from release of DAMPs such as mtDNA fragments and ATP that have autocrine/paracrine effects including activating the inflammasome, with downstream consequences on inflammation itself, cell proliferation, and apoptosis. ATP = adenosine triphosphate; TLR = toll-like receptor. See Figure 1 legend for expansion of other abbreviations.
Unanswered Questions Regarding Mitochondria in Airway Diseases
Although emerging evidence highlights mitochondrial dysfunction in airway diseases, several unanswered issues relevant to asthma and COPD pathophysiology remain. Some important questions relevant to mechanism-based diagnosis and eventual therapy include:
-
1.
Which of many metabolic, structural, localization, or functional mechanisms contribute to overall mitochondrial dysfunction in asthma vs COPD?
-
2.
Are observed changes in these mechanisms a consequence of (or incidental to) disease and upstream processes such as inflammation or CS, or are they indeed causative?
-
3.
By what mechanisms do insults such as inflammation (there likely being differential roles for various mediators) and oxidant stress affect relevant mitochondrial processes?
-
4.
Are there cell-specific differences in effects of insults on mitochondrial parameters, and what are the downstream influences of mitochondrial dysfunction on physiological parameters such as mucus production, airway contractility and dilation, remodeling and fibrosis?
-
5.
Are there age- and sex-related differences in the role of mitochondrial mechanisms in asthma or COPD? Examples include influence of mitochondrial pathways on airway development and pathophysiology of pediatric asthma or bronchopulmonary dysplasia, sex steroid influences on airway mitochondria, mitochondria-mediated senescence in aging airways, and contributions to asthma in the elderly.
-
6.
Can mitochondrial dysfunction be detected early to allow targeting in a cell- and context-specific fashion to alleviate airway disease? Linkages between mitochondrial genes and airway disease may be particularly interesting,53 as would mitochondria-specific ROS and DAMPs. Persistent insults may also lead to DAMPs, reduced mitochondrial biogenesis, inability to respond to cellular stresses, or a combination of these factors, which could reflect late manifestations of mitochondrial dysfunction.
-
7.
What are the appropriate in vitro and in vivo models to explore mitochondrial dysfunction in the airway?
Therapeutic Potential of Mitochondrial Targeting
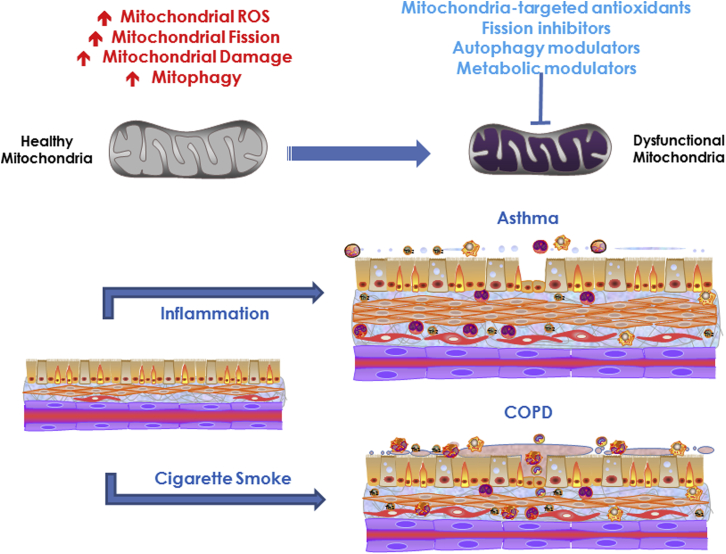
There is already interest in the idea of targeting mitochondria for therapy in airway disease5, 28, 33 (Fig 5). An obvious focus has been mitochondrial ROS using antioxidants such as vitamins C and E, although such approaches have not been successful.54 Mitochondria-targeted antioxidants such as mitoubiquinone mesylate (MitoQ) accumulate selectively within negatively charged mitochondria, suppress ROS, and protect mitochondria from oxidative damage.5 Another mitochondria-specific antioxidant mitoTEMPO has been shown to be effective in blunting fibrosis and in mouse models of asthma.55 Both MitoQ and MitoTEMPO help restore mitochondrial function following CS exposure,5 whereas MitoQ and Tiron (another mitochondria-targeted antioxidant) reverse mitochondrial dysfunction in ASM of patients with COPD and in ozone-exposure mouse models of asthma.15 Although these initial forays into targeting oxidant stress in mitochondria are exciting, a fundamental limitation may be that mitochondrial ROS are not always detrimental and at lower levels play a protective role. Thus, knowing which cell types and under what conditions to target ROS is challenging.
Figure 5.
Mitochondrial dysfunction resulting from cigarette smoke and inflammation effects on various aspects of mitochondrial structure, localization, metabolism, and other mitochondrial functions contributes to asthma and COPD. Novel therapeutic approaches for airway diseases should consider mitochondria-targeted antioxidants and methods to modulate mitochondrial fission/fusion, motility, mitophagy, and other processes. See Figure 1 legend for expansion of abbreviations.
Modulation of metabolism per se, while appealing, requires cell- and disease-specific approaches with a more thorough understanding of any unintended consequences of inhibiting or activating specific metabolic pathways. Modulation of mitochondrial fission/fusion balance and biogenesis are again appealing approaches if the conditions under which the need for greater fusion vs enhanced fission can be established. A potential limitation is that fission and fusion are dynamic even under normal conditions and furthermore are intricately linked to mitochondrial motility, metabolism, and downstream cellular processes. One overall problem is that mitochondrial damage may not always be evident in vivo, since damage does not always result in cell death, and conversely, some level of damage can in fact stimulate mitochondrial biogenesis. In the context of maintaining mitochondrial quality, an entirely novel approach being explored is transfer of mitochondria from bone marrow-derived mesenchymal stem cells into diseased tissues,56 although the efficacy of such procedures remains to be established.
Conclusions
Emerging discoveries highlight normal roles of mitochondria beyond energy production, and contributions of dysfunctional mitochondrial structure and function in airway disease pathophysiology. Although we have much to understand regarding how, where, and when mitochondria play a role in modulating the many aspects of asthma or COPD processes within specific airway cell types, the concepts that mitochondria are mechanistically important, and thus should be targeted for therapy, have already been embraced and make for an exciting era in pulmonary medicine.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by grants from the National Institutes of Health of the United States [R01 HL126451 to G. C. S. and Y. S. P., HL056470 to Y. S. P., and HL088029 to Y. S. P.].
References
- 1.Barnes P.J. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Murphy D.M., O'Byrne P.M. Recent advances in the pathophysiology of asthma. Chest. 2010;137(6):1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 3.Hirota N., Martin J.G. Mechanisms of airway remodeling. Chest. 2013;144(3):1026–1032. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 4.Prakash Y.S. Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol. 2016;311(6):L1113–L1140. doi: 10.1152/ajplung.00370.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal A., Mabalirajan U. Rejuvenating cellular respiration for optimizing respiratory function: targeting mitochondria. Am J Physiol Lung Cell Mol Physiol. 2016;310(2):L103–L113. doi: 10.1152/ajplung.00320.2015. [DOI] [PubMed] [Google Scholar]
- 6.Delmotte P., Sieck G.C. Interaction between endoplasmic/sarcoplasmic reticulum stress(ER/SR stress), mitochondrial signaling and Ca(2+) regulation in airway smooth muscle (ASM) Can J Physiol Pharmacol. 2015;93(2):97–110. doi: 10.1139/cjpp-2014-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aravamudan B., Thompson M.A., Pabelick C.M. Mitochondria in lung diseases. Expert Rev Respir Med. 2013;7(6):631–646. doi: 10.1586/17476348.2013.834252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerner C.A., Sundar I.K., Rahman I. Mitochondrial redox system, dynamics, and dysfunction in lung inflammaging and COPD. Int J Biochem Cell Biol. 2016;81(pt B):294–306. doi: 10.1016/j.biocel.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal S., Mannam P., Zhang J. Differential regulation of autophagy and mitophagy in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol. 2016;311(2):L433–L452. doi: 10.1152/ajplung.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryter S.W., Choi A.M. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol. 2015;4:215–225. doi: 10.1016/j.redox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryter S.W., Nakahira K., Haspel J.A. Autophagy in pulmonary diseases. Annu Rev Physiol. 2012;74:377–401. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls D.G. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37(pt 6):1385–1388. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- 13.Schumacker P.T., Gillespie M.N., Nakahira K. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol. 2014;306(11):L962–L974. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 15.Wiegman C.H., Michaeloudes C., Haji G. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allerg Clin Immunol. 2015;136(3):769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravamudan B., Thompson M., Sieck G.C. Functional effects of cigarette smoke-induced changes in airway smooth muscle mitochondrial morphology. J Cell Physiol. 2017;232(5):1053–1068. doi: 10.1002/jcp.25508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumura K., Cloonan S.M., Nakahira K. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124(9):3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeb R.S., Walters M.S., Strulovici-Barel Y. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am J Respir Cell Mol Biol. 2016;54(2):231–240. doi: 10.1165/rcmb.2015-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann R.F., Zarrintan S., Brandenburg S.M. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013:14–97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puente-Maestu L., Perez-Parra J., Godoy R. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40(6):746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]
- 21.Trian T., Benard G., Begueret H. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204(13):3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leishangthem G.D., Mabalirajan U., Singh V.P. Ultrastructural changes of airway in murine models of allergy and diet-induced metabolic syndrome. ISRN Allergy. 2013;2013:261297. doi: 10.1155/2013/261297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dada L.A., Sznajder J.I. Mitochondrial Ca(2)+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest. 2011;121(5):1683–1685. doi: 10.1172/JCI57748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowlands D.J., Islam M.N., Das S.R. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest. 2011;121(5):1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak J.C., Chan-Yeung M.M. Reactive oxidant species in asthma. Curr Opin Pulm Med. 2006;12(1):7–11. doi: 10.1097/01.mcp.0000198067.50457.71. [DOI] [PubMed] [Google Scholar]
- 26.Ostroukhova M., Goplen N., Karim M.Z. The role of low-level lactate production in airway inflammation in asthma. Am J Physiol Lung Cell Mol Physiol. 2012;302(3):L300–L307. doi: 10.1152/ajplung.00221.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Stefani D., Rizzuto R., Pozzan T. Enjoy the Trip: calcium in mitochondria back and forth. Annu Rev Biochem. 2016;85:161–192. doi: 10.1146/annurev-biochem-060614-034216. [DOI] [PubMed] [Google Scholar]
- 28.Cloonan S.M., Choi A.M. Mitochondria in lung disease. J Clin Invest. 2016;126(3):809–820. doi: 10.1172/JCI81113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra P., Chan D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15(10):634–646. doi: 10.1038/nrm3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillery O., Malka F., Frachon P. Modulation of mitochondrial morphology by bioenergetics defects in primary human fibroblasts. Neuromuscul Disord. 2008;18(4):319–330. doi: 10.1016/j.nmd.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Neutzner A., Benard G., Youle R.J. Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann N Y Acad Sci. 2008;1147:242–253. doi: 10.1196/annals.1427.012. [DOI] [PubMed] [Google Scholar]
- 32.Aravamudan B., Kiel A., Freeman M. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;306(9):L840–L854. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowlands D.J. Mitochondria dysfunction: a novel therapeutic target in pathological lung remodeling or bystander? Pharmacol Therapeut. 2016;166:96–105. doi: 10.1016/j.pharmthera.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Mizumura K., Cloonan S.M., Haspel J.A. The emerging importance of autophagy in pulmonary diseases. Chest. 2012;142(5):1289–1299. doi: 10.1378/chest.12-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmotte P., Dogan M., Prakash Y.S. Inflammation increases mitochondria fragmentation, mitochondria volume density and oxygen consumption rate in human airway smooth muscle. Am J Respir Crit Care Med. 2016;193:A1252. [Google Scholar]
- 36.Raffaello A., Mammucari C., Gherardi G. Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci. 2016;41(12):1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecze L., Blum W., Schwaller B. Routes of Ca2+ shuttling during Ca2+ oscillations: focus on the role of mitochondrial Ca2+ handling and cytosolic Ca2+ buffers. J Biol Chem. 2015;290(47):28214–28230. doi: 10.1074/jbc.M115.663179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz T.L. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5:6. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 40.Villegas L., Stidham T., Nozik-Grayck E. Oxidative stress and therapeutic development in lung diseases. J Pulm Respir Med. 2014;4(4):194. doi: 10.4172/2161-105X.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heijink I.H., Pouwels S.D., Leijendekker C. Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release. Am J Respir Cell Mol Biol. 2015;52(5):554–562. doi: 10.1165/rcmb.2013-0505OC. [DOI] [PubMed] [Google Scholar]
- 42.Pouwels S.D., Hesse L., Faiz A. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L881–L892. doi: 10.1152/ajplung.00135.2016. [DOI] [PubMed] [Google Scholar]
- 43.Brusselle G.G., Provoost S., Bracke K.R. Inflammasomes in respiratory disease: from bench to bedside. Chest. 2014;145(5):1121–1133. doi: 10.1378/chest.13-1885. [DOI] [PubMed] [Google Scholar]
- 44.Piantadosi C.A., Suliman H.B. Mitochondrial dysfunction in lung pathogenesis. Annu Rev Physiol. 2017;79:495–515. doi: 10.1146/annurev-physiol-022516-034322. [DOI] [PubMed] [Google Scholar]
- 45.Pelleg A., Schulman E.S., Barnes P.J. Extracellular adenosine 5′-triphosphate in obstructive airway diseases. Chest. 2016;150(4):908–915. doi: 10.1016/j.chest.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 46.Nakahira K., Hisata S., Choi A.M. The roles of mitochondrial damage-associated molecular patterns in diseases. Antioxid Redox Signal. 2015;23(17):1329–1350. doi: 10.1089/ars.2015.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lommatzsch M., Cicko S., Muller T. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(9):928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 48.Kim R.Y., Pinkerton J.W., Gibson P.G. Inflammasomes in COPD and neutrophilic asthma. Thorax. 2015;70(12):1199–1201. doi: 10.1136/thoraxjnl-2014-206736. [DOI] [PubMed] [Google Scholar]
- 49.Hosseinian N., Cho Y., Lockey R.F., Kolliputi N. The role of the NLRP3 inflammasome in pulmonary diseases. Ther Adv Respir Dis. 2015;9(4):188–197. doi: 10.1177/1753465815586335. [DOI] [PubMed] [Google Scholar]
- 50.dos Santos G., Kutuzov M.A., Ridge K.M. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L627–L633. doi: 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmad T., Sundar I.K., Lerner C.A. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29(7):2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Cheng X, Yue L, et al. Molecular pathogenesis in chronic obstructive pulmonary disease and therapeutic potential by targeting AMP-activated protein linase [published online ahead of print February 4, 2017. J Cell Physiol.http://dx.doi.org/10.1002/jcp.25844. [DOI] [PubMed]
- 53.Flaquer A., Heinzmann A., Rospleszcz S. Association study of mitochondrial genetic polymorphisms in asthmatic children. Mitochondrion. 2014;14(1):49–53. doi: 10.1016/j.mito.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Rahman I., Biswas S.K., Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1-3):222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 55.Jaffer O.A., Carter A.B., Sanders P.N. Mitochondrial-targeted antioxidant therapy decreases transforming growth factor-beta-mediated collagen production in a murine asthma model. Am J Respir Cell Mol Biol. 2015;52(1):106–115. doi: 10.1165/rcmb.2013-0519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Islam M.N., Das S.R., Emin M.T. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]