Abstract
Pulmonary disease remains a primary source of morbidity and mortality in persons living with HIV (PLWH), although the advent of potent combination antiretroviral therapy has resulted in a shift from predominantly infectious to noninfectious pulmonary complications. PLWH are at high risk for COPD, pulmonary hypertension, and lung cancer even in the era of combination antiretroviral therapy. The underlying mechanisms of this are incompletely understood, but recent research in both human and animal models suggests that oxidative stress, expression of matrix metalloproteinases, and genetic instability may result in lung damage, which predisposes PLWH to these conditions. Some of the factors that drive these processes include tobacco and other substance use, direct HIV infection and expression of specific HIV proteins, inflammation, and shifts in the microbiome toward pathogenic and opportunistic organisms. Further studies are needed to understand the relative importance of these factors to the development of lung disease in PLWH.
Key Words: COPD, HIV, inflammation, lung cancer, pulmonary hypertension
Abbreviations: AM, alveolar macrophage; cART, combination antiretroviral therapy; HIV-PAH, HIV-associated pulmonary arterial hypertension; INHALD, Investigating HIV-Associated Lung Disease Network; PD-1, programmed death 1; PLWH, persons living with HIV
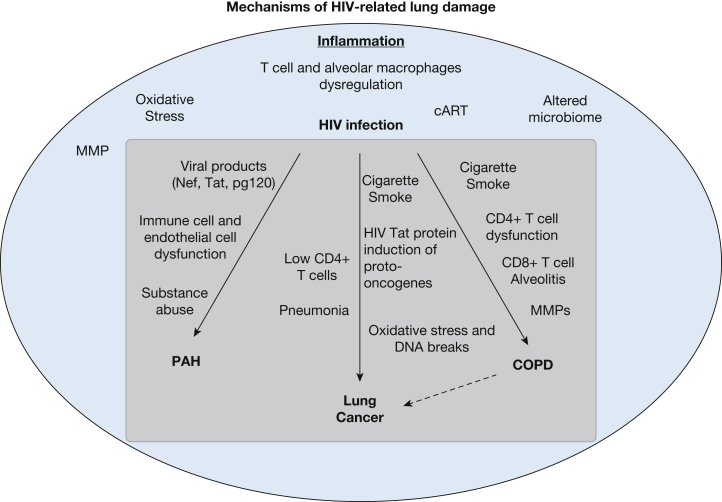
Pulmonary complications remain a significant source of morbidity and mortality in patients infected with HIV. Prior to the advent of combination antiretroviral therapy (cART), pulmonary infections, especially Pneumocystis jirovecii, tuberculosis, and community-acquired pneumonia, were among the leading causes of death.1 With the advent of cART, noninfectious pulmonary complications have become more prevalent in persons living with HIV (PLWH), including COPD, HIV-related pulmonary artery hypertension (HIV-PAH), and lung cancer.2, 3, 4 The prevalence of COPD in PLWH in the modern cART era varies by study and by definition, but by spirometry 7% to 9% of PLWH have clinical obstruction, while one-third have respiratory symptoms.5, 6 The prevalence of PAH is 2,500 times higher than in the general population, with 0.5% of PLWH estimated to have HIV-PAH, despite effective cART.7 The incidence of lung cancer is 2.7 times higher in PLWH than in the general population.8, 9 The mechanisms underlying the increased rates of noninfectious lung disease are multifactorial. High rates of tobacco use and intravenous drug use likely contribute, in addition to chronic lung inflammation and subsequent oxidative stress and tissue damage. In this review, we discuss what is currently known about the pathogenesis of COPD, PAH, and lung cancer in PLWH, describe other potential relevant mechanisms of lung damage that may contribute to these diseases (Fig 1), and outline priorities for better understanding the underlying mechanisms in the current cART era.
Figure 1.
Mechanisms of HIV-related lung damage. cART = combination antiretroviral therapy; MMP = matrix metalloproteinase; PAH = pulmonary arterial hypertension.
HIV and COPD
COPD is highly prevalent among cigarette smokers, and incidence increases with age.10 As PLWH are living longer, high smoking rates contribute to the increasing incidence of COPD.3 Rates of hospitalization for obstructive lung disease have increased for PLWH in the cART era, and are expected to increase further as the population ages.2 Several studies have demonstrated that PLWH have higher rates of dyspnea and alterations in pulmonary function test results, particularly low diffusing capacity of the lung for carbon monoxide, as well as imaging findings of emphysema.4, 5, 6 PLWH also appear to have more respiratory symptoms, including dyspnea and cough, than smokers with similar disease burden by pulmonary function test criteria.6
Inflammation plays an important role in the pathogenesis of COPD in PLWH. Reduced frequencies and absolute numbers of CD4+ T cells are seen in BAL of PLWH with COPD.11 These CD4+ T cells, but not CD8+ T cells, demonstrate impaired lung mucosal immunity to HIV, and express high levels of programmed cell death 1 (PD-1), a marker of immune activation and exhaustion. They also express high levels of the Fas death receptor, CD95, and demonstrate increased Fas-dependent activation-induced cell death.11 All of these findings result in progressive loss of CD4+ T cells in the BAL, leading to a profound imbalance in CD4:CD8 ratio and persistent CD8+ T-cell alveolitis in HIV-associated COPD. This inflammation leads to the expression of inflammatory cytokines and release of matrix metalloproteinases associated with COPD.12, 13
HIV-PAH
The association between HIV infection and pulmonary hypertension was recognized early on in the AIDS epidemic.14 Although cART may have decreased the incidence of HIV-PAH and has been shown to partially reverse pulmonary hypertension in a small number of PLWH,7 PAH remains a significant clinical complication in PLWH, particularly as life expectancy increases. Symptoms and signs usually present late, and one-half of patients with HIV-PAH die during a median follow-up period of 8 months,15 although a more recent study demonstrated that 3-year survival is 72% in patients treated with both cART and specific PAH therapy.16 Specific therapy for PAH appears to improve hemodynamic parameters more than cART, as cART has minimal effect even if it is instituted early in disease.17 Approximately two-thirds of deaths are attributed to pulmonary hypertension rather than complications of immune deficiency.14, 15 The CD4+ T-cell count is the only independent predictor of survival, and patients with HIV-PAH have poorer survival rates when compared with uninfected patients with PAH.14, 15
Direct HIV infection, substance abuse, and chronic inflammation are particularly important in the development of HIV-PAH.18 Chronic exposure to HIV viral proteins in the lung (eg, Nef, Tat, gp120), as well as HIV-induced immune dysregulation, contribute to pulmonary vascular disease, particularly through an impact on pulmonary endothelial cells. HIV Nef protein co-localizes with endothelial cells in PAH-like plexiform lesions in animal models.19 Substance abuse also plays a significant role. In animal models, cocaine increases proliferation of pulmonary vascular endothelial cells, and morphine contributes to vascular disease and oxidant stress.20, 21
HIV and Lung Cancer
Lung cancer is the primary cause of cancer-related death among PLWH.8 Low CD4+ T-cell counts and prior pneumonia are associated with lung cancer risk.22, 23 Cigarette smoke, HIV infection, and chronic inflammation can increase oxidative stress, leading to oxidative DNA lesions and DNA double-strand breaks. Incorporation of the HIV genome into infected cells is dependent on host cell DNA repair proteins, and HIV-induced alterations in DNA repair proteins have been implicated in augmenting genomic integration and replication in host cells.24 HIV Tat protein in vitro can induce expression of proto-oncogenes (c-myc, c-fos, c-jun) and down-regulate the p53 tumor suppressor gene.25 Although cART can have genotoxic effects, no association has been found between cART and lung cancer risk, and early cART is associated with decreased risk.26
Common Mechanisms of Lung Disease in PLWH
The cause of higher rates of noninfectious lung complications in PLWH is multifactorial. PLWH demonstrate evidence of chronic lung inflammation from a variety of causes, including direct effects of HIV, cART, illicit drug use, immunodeficiency, opportunistic infections, and alterations in the lung microbiome. The potential mechanisms by which these factors contribute both specifically and generally to HIV-PAH, COPD, and lung cancer are outlined in Figure 1.
Smoking and HIV
The prevalence of smoking in PLWH ranges from similar to twice that of the general population, depending on the comparison group. The largest study demonstrated that 42% of PLWH are current smokers, compared with 21% of the general population.27 In contrast, when the comparison group consists of uninfected subjects with a high smoking prevalence, such as those in the Veterans’ Aging Cohort Study or Multicenter AIDS Cohort Study, which match patients with similar risk profiles, smoking rates are more similar in the two groups, although PLWH still have higher rates.28, 29 Compared with nonsmokers, PLWH who smoke have higher levels of inflammatory markers, including soluble CD14 and expression of HLA-DR on both CD8+ and CD4+ T cells.30, 31 Cigarette smoking has been linked to increased rates of mortality due to cardiovascular disease, COPD, pneumonia, and lung cancer.28 If HIV is treated, modeling studies suggest that PLWH who smoke lose more than 6 years of life expectancy, more than that lost to HIV infection.32
PLWH are less likely to quit than the general population (32% vs 52%).27 Multiple factors are associated with continued smoking in PLWH, including higher rates of other substance abuse, psychiatric disorders, low socioeconomic status, and poor access to care.33 Perhaps because of multiple competing interests and limited time to address medical issues, counseling on smoking cessation happens less frequently for PLWH than for the general population.34
Despite clear evidence that cigarette smoking contributes to significant disease in PLWH, most studies of smoking-related illness are poorly controlled for the impact of cumulative exposure to cigarette smoke, and control only for smoking status, that is, current, past, or nonsmoking status. One study clearly demonstrated that both cumulative pack-year smoking history and time since smoking cessation are more strongly associated with lung and heart disease than smoking status,35 suggesting that a true understanding of the role of cigarette smoke as a pathogenic factor in HIV-associated lung disease will require better collection of data on total tobacco exposure.
HIV Infection
HIV pathology in the lung is driven by infection of CD4+ T cells and alveolar macrophages, which play an important role in the development of pulmonary disease.36, 37 Animal models demonstrate that the lungs and intestines harbor the highest levels of simian immunodeficiency virus among nonlymphoid tissues,38 and early in infection, CD4+ T cells are rapidly depleted from mucosal sites.36, 39 Recent data suggest that lung epithelium can also be directly infected with HIV, especially CXCR4-tropic strains associated with advanced disease. HIV infection results in integration of the viral genetic material into the cellular genome. This integration may change gene expression and immune response.40 Expression of viral proteins, such as Tat, increases inflammation and oxidative stress in animal models.41 HIV infection alters the function of airway epithelial cells by impairing cell-cell adhesion and increasing the expression of inflammatory mediators.42 Thus HIV infection contributes to lung disease by both direct effects of infection and through modulation of systemic inflammation and immunodeficiency.
Combination Antiretroviral Therapy
Treatment of HIV-1 infection by cART has generally been associated with improved outcomes,28 especially in reduction of infectious pulmonary complications. Its influence on noninfectious pulmonary complications has been more controversial. Protease inhibitor use has been linked to an increased incidence of malignancy, although not lung cancer specifically.26 Older antiretroviral drugs may have had genotoxic effects that contribute to this increased risk. Although one study demonstrated that cART increased the risk of COPD,43 the pulmonary substudy embedded in the large Strategic Timing of Antiretroviral Therapy (START) trial demonstrated that the timing of ART had no effect on COPD progression,44 and poor HIV control in a predominant smoking population is associated with the development of HIV-associated COPD and accelerated annual lung decline.45 Antiretroviral drug use improves hemodynamics and survival in HIV-PAH, although not as dramatically as PAH therapy.17, 46
Inflammation in the Lung
From early in the epidemic, it was noted that HIV infection is associated with a CD8+ T-cell alveolitis that occurs in both asymptomatic patients and those with respiratory symptoms and HIV disease progression.47 Many of these cytotoxic T cells are directed against HIV-infected cells or other opportunistic pathogens (eg, cytomegalovirus, Pneumocystis).48, 49 Lung CD8+ T cells appear to be dysfunctional, expressing high levels of the exhaustion marker, PD-1, in the absence of antiviral therapy.48 Lymphocytes expressing exhaustion markers such as PD-1 and CD57 are thought to be terminally differentiated senescent cells. However, CD8+ T cells maintain the capacity to secrete proinflammatory effector cytokines in response to HIV antigens under conditions of poor viral control as well as following viral suppression.11, 48, 50 Thus, the presence of terminally differentiated effector lymphocytes in the lungs contributes to local inflammation in response to HIV itself, as well as other pathogens. HIV infection can cause lung inflammation in other ways as well. For example, alveolar macrophages (AMs) are infected with HIV, even in healthy, nonsmoking PLWH, and infected cells have impaired phagocytic function as well as abnormal oxidative burst and cytokine secretion.51, 52 Untreated PLWH who smoke showed significant lung CD4+ T-cell dysfunction and depletion, along with high susceptibility to apoptosis, which improved following cART.50
Thus, HIV-associated lung disease in PLWH likely is driven by multiple inflammatory mechanisms: (1) HIV replication in lung CD4+ T cells and AMs, (2) CD8+ T-cell alveolitis and an imbalance of the physiologic lung CD4:CD8 ratio, (3) progressive CD4+ T-cell depletion/dysfunction, (4) dysregulation of AMs, and (5) impaired immune function in response to other pathogens, which predisposes to infection and parenchymal damage. Together with tobacco exposure, this combination of cellular activation and immune dysfunction contributes to the pathogenesis of lung disease.
HIV-Associated Pulmonary Infections and the Microbiome
Studies have revolutionized our understanding of the role the microbiome plays in health and disease.53 Although the microbial community is smaller in the lung than in the gut, it is clear that it is diverse and modulated by disease.54, 55 Pulmonary flora is modulated by inhaled corticosteroids or bronchodilator use,55 while smoking has minimal effects on the lung microbiome.55, 56 In general, most inflammatory disease states, such as COPD, are associated with a decline in microbiome diversity, and increased abundance of inflammatory proteobacterial species such as Pseudomonas, Moraxella, and Haemophilus influenzae.56, 57
Changes in the bacterial microbiome of the lung have been described in HIV infection, and may account for increased pathology at both of these sites, particularly in the context of advanced immunodeficiency. The first study of the lung microbiome in HIV infection demonstrated an increased prevalence of Tropheryma whipplei in untreated subjects, although a broader comprehensive analysis of upper and lower bacterial microbiomes, using 16S rRNA methods, did not demonstrate a significant difference in bacterial communities between healthy PLWH and uninfected individuals.57, 58 In contrast, PLWH with low CD4+ T-cell counts have fewer numbers of species (decreased α diversity), although a greater number of unique species are present (increased β diversity).58, 59 The HIV lung microbiome contains increased amounts of Veillonella and Prevotella, bacteria previously shown to be associated with inflammation.60 An increase in detection of Pneumocystis jirovecii is associated with both HIV infection and COPD.61
HIV pathogenesis is characterized by gastrointestinal CD4+ T-cell depletion and a compromised mucosal barrier, leading to microbial translocation, endotoxemia, and systemic immune activation.39 Shifts in the gut microbiome have also been seen in PLWH,62, 63 although the extent to which this is due to HIV infection vs other factors, such as sexual preference, is still unclear.64 In addition to systemic immune activation, which appears to be related to gastrointestinal pathology, changes in the gut microbiome may have a particular influence on pulmonary disease. Several epidemiologic studies, as well as studies in murine models, have demonstrated a correlation between susceptibility to pulmonary infections, allergic airway disease, and an altered fecal microbiome.65 There is an epidemiologic correlation between COPD and inflammatory bowel disease,66 which may be smoking related, but may also be due to shifts in the microbiome, inflammation, or modulation of matrix metalloproteinases.67
Oxidative Stress
The lung is at particular risk of damage due to excessive oxidative stress, because it is directly exposed to oxygen, inhaled pollutants, and microbes that produce pro-oxidant reactive oxygen and nitrogen species. Higher levels of markers of oxidative stress and lower levels of antioxidant glutathione are found in smokers, as well as in patients with COPD and/or HIV.68 Oxidative stress is worsened during acute exacerbations of COPD.69 HIV infection is associated with high rates of oxidative stress,68 which may be due to the direct effects of the viral proteins gp120 and Tat on lung epithelium.41 Alcohol and tobacco use by patients infected with HIV contribute to oxidative stress, potentially through the involvement of antioxidant pathways and the cytochrome P450 system.70
Matrix Metalloproteinases
Matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases that can cleave the majority of structural components of the extracellular matrix, including elastin. Studies have identified increased proteolytic activity in PLWH who smoke and have emphysema,12 and examination of their BAL confirmed elevated expression of MMP mRNA in alveolar macrophages, and of MMP protein and activity in supernatant. Recent studies have demonstrated that high levels of inflammatory cytokines, such as IL-23, are seen in the BAL of these patients. Infection of AMs results in expression of IL-23, which can lead to up-regulation of MMP-9 in AMs in an AM/lymphocyte coculture model.13 Studies of human macrophages have consistently located MMP-12 and MMP-9 in emphysematous lung.71 Murine models have demonstrated that macrophage overexpression of MMP-9 or MMP-1 can spontaneously induce emphysema, while MMP-12 knockdown is protective from cigarette smoke-induced emphysema.72 MMP expression is additionally up-regulated and has been demonstrated to play an important role in the development of PAH in animal models.73 It is notable that MMP-12 is quite responsive to smoking cessation, and if this protease has an exaggerated role in HIV-mediated lung disease, smoking cessation may result in an even more significant impact in this cohort.74
Addressing Molecular Mechanisms of Lung Disease in HIV
We currently are at a critical point in investigating lung disease in PLWH. The continued development of less toxic cART has resulted in more persistent and robust recovery of immune function. Nonetheless, one study has highlighted both significant underprescription of cART and ineffective viral suppression in PLWH.75 The institution of more generalized screening of individuals at risk for HIV has resulted in fewer opportunistic infections and specialized care earlier in disease. Although issues such as smoking and intravenous drug use are difficult to treat, the epidemiology from several large cohorts has made the potential size of our future problem quite clear. At this time a major knowledge gap remains concerning whether lung disease is occurring at a very rapid rate prior to treatment or whether there is continued accelerated decline with disease even among patients receiving cART. In addition, cohorts composed of subjects over age 40 years are needed to longitudinally assess lung T-cell subsets, antigen-specific immunity including HIV-specific responses, macrophage activation, and Pneumocystis colonization to determine which of these predict progression of lung disease. Further, there is a need to study patients who have not had significant immune suppression and opportunistic infections to evaluate whether they are at high risk of lung disease in the absence of advanced immunodeficiency.
Another unmet need is the execution of well-designed mechanistic studies on the pathogenesis of lung disease. To understand the mechanisms of HIV-associated inflammation, we must define the relative importance of a variety of potential causes of inflammation: HIV itself; tobacco, alcohol, and illicit drug use; opportunistic infections; shifts in the microbiome to organisms capable of causing chronic inflammation (bacteria such as Prevotella, persistent latent virus infection, persistent fungi such as Pneumocystis), current antiretroviral drugs, or other as yet unidentified mechanisms. Understanding these factors may be critical to our understanding of other inflammatory disorders in PLWH, and may allow us to develop therapeutic interventions to alter the progression of disease.
The Investigating HIV-Associated Lung Disease (INHALD) Network (https://statepiaps7.jhsph.edu/inhaldpub/content/welcome-inhald-public-website) is a collaborative effort designed to evaluate and understand the mechanisms of lung disease in PLWH. The INHALD cohort will provide invaluable data to assist in our understanding of lung disease in patients during the modern era of cART, and will generate information about specific downstream mechanisms. Sites are conducting assessments including pulmonary function testing, imaging, and echocardiography. Biologic samples, including blood, peripheral blood mononuclear cells, and BAL, are being collected and banked. Sites are collaborating on projects to evaluate the microbiome (with a particular focus on virome and shotgun metagenomics), inflammation, and oxidative stress, and welcome outside collaborations. As the HIV-infected population ages, lung disease is likely to become an increasingly common comorbidity given the high rates of smoking in this population. Appropriate management will require an understanding of modifiable mechanisms of disease to define best practices.
Acknowledgments
Financial/Nonfinancial Disclosures: None declared.
Role of Sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Collaborators: INHALD sites and principal investigators: Indiana University (Homer Twigg, Kenneth Knox, George Weinstock); Johns Hopkins University (Gregory Kirk, John McDyer); Massachusetts General Hospital (Benjamin Medoff, Douglas Kwon); University of Colorado, Denver—PAH (Sonia Flores, Todd Bull, Priscilla Hsue); University of Colorado, Denver—COPD (Andrew Fontenot, Thomas Campbell, Brent Palmer); University of Louisville (Julio Ramirez, Jesse Roman); Washington University, St. Louis (Jeff Atkinson, Rachel Presti); Weill Medical College of Cornell University; Data Coordinating Center—LAMDACC (Bryan Lau).
Other Contributions: The authors thank the INHALD participants.
Footnotes
FUNDING/SUPPORT: 1U01HL121831-01 (H. L. T.), 1U01HL121814-01 (J. F. M.), 1U01HL121812-01 (B. L., C. R. L.), 1U01HL121819-01 (S. C. F.), 1U01HL121816-01 (A. P. F., B. E. P.), 1U01HL121807-01 (J. R.), and 1U01HL121804-01 (R. M. P., J. J. A.).
References
- 1.Rothenberg R., Woelfel M., Stoneburner R. Survival with the acquired immunodeficiency syndrome: experience with 5833 cases in New York City. N Engl J Med. 1987;317(21):1297–1302. doi: 10.1056/NEJM198711193172101. [DOI] [PubMed] [Google Scholar]
- 2.Grubb J.R., Moorman A.C., Baker R.K. The changing spectrum of pulmonary disease in patients with HIV infection on antiretroviral therapy. AIDS. 2006;20(8):1095–1107. doi: 10.1097/01.aids.0000226949.64600.f9. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick M., Brooks J.T., Kaplan J.E. Epidemiology of HIV-associated lung disease in the United States. Semin Respir Crit Care Med. 2016;37(2):181–198. doi: 10.1055/s-0036-1572556. [DOI] [PubMed] [Google Scholar]
- 4.Crothers K., Thompson B.W., Burkhardt K. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8(3):275–281. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.George M.P., Kannass M., Huang L. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4(7):e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campo M., Oursler K.K., Huang L. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr. 2014;65(5):557–563. doi: 10.1097/QAI.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitbon O., Lascoux-Combe C., Delfraissy J.F. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg M.J., Lau B., Achenbach C.J. Cumulative incidence of cancer among persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507–518. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grulich A.E., van Leeuwen M.T., Falster M.O. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 10.Lokke A., Lange P., Scharling H. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popescu I., Drummond M.B., Gama L. Activation-induced cell death drives profound lung CD4+ T-cell depletion in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(7):744–755. doi: 10.1164/rccm.201407-1226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaner R.J., Santiago F., Crystal R.G. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1+ smokers with early emphysema. J Leukoc Biol. 2009;86(4):913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barjaktarevic I.Z., Crystal R.G., Kaner R.J. The role of interleukin-23 in the early development of emphysema in HIV1+ smokers. J Immunol Res. 2016;2016:3463104. doi: 10.1155/2016/3463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Speich R., Jenni R., Opravil M. Primary pulmonary hypertension in HIV infection. Chest. 1991;100(5):1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 15.Mehta N.J., Khan I.A., Mehta R.N. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest. 2000;118(4):1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 16.Degano B., Guillaume M., Savale L. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24(1):67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 17.Pal J., Sen K., Sarkar G. Effect of antiretroviral therapy on pulmonary hypertension in HIV patients. J Indian Med Assoc. 2013;111(12):845–846. 849. [PubMed] [Google Scholar]
- 18.Hassoun P.M., Mouthon L., Barbera J.A. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 suppl):S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Marecki J.C., Cool C.D., Parr J.E. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174(4):437–445. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhillon N.K., Li F., Xue B. Effect of cocaine on human immunodeficiency virus-mediated pulmonary endothelial and smooth muscle dysfunction. Am J Respir Cell Mol Biol. 2011;45(1):40–52. doi: 10.1165/rcmb.2010-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spikes L., Dalvi P., Tawfik O. Enhanced pulmonary arteriopathy in simian immunodeficiency virus-infected macaques exposed to morphine. Am J Respir Crit Care Med. 2012;185(11):1235–1243. doi: 10.1164/rccm.201110-1909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigel K., Makinson A., Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS. 2017;12(1):31–38. doi: 10.1097/COH.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shebl F.M., Engels E.A., Goedert J.J. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr. 2010;55(3):375–379. doi: 10.1097/QAI.0b013e3181eef4f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper A., Garcia M., Petrovas C. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013;498(7454):376–379. doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- 25.el-Solh A., Kumar N.M., Nair M.P. An RGD containing peptide from HIV-1 Tat-(65-80) modulates protooncogene expression in human bronchoalveolar carcinoma cell line, A549. Immunol Invest. 1997;26(3):351–370. doi: 10.3109/08820139709022692. [DOI] [PubMed] [Google Scholar]
- 26.Bruyand M., Ryom L., Shepherd L. Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D: A:D study. J Acquir Immune Defic Syndr. 2015;68(5):568–577. doi: 10.1097/QAI.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 27.Mdodo R., Frazier E.L., Dube S.R. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–344. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 28.Crothers K., Goulet J.L., Rodriguez-Barradas M.C. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(3 suppl):40–53. doi: 10.1521/aeap.2009.21.3_supp.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhtar-Khaleel W.Z., Cook R.L., Shoptaw S. Long-term cigarette smoking trajectories among HIV-seropositive and seronegative MSM in the Multicenter AIDS Cohort Study. AIDS Behav. 2016;20(8):1713–1721. doi: 10.1007/s10461-016-1343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cioe P.A., Baker J., Kojic E.M. Elevated soluble CD14 and lower D-dimer are associated with cigarette smoking and heavy episodic alcohol use in persons living with HIV. J Acquir Immune Defic Syndr. 2015;70(4):400–405. doi: 10.1097/QAI.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grubb J.R., Overton E.T., Presti R. Reply to Ganesan, et al. J Infect Dis. 2012;205(3):518–519. doi: 10.1093/infdis/jir758. [DOI] [PubMed] [Google Scholar]
- 32.Reddy K.P., Parker R.A., Losina E. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: a US-based modeling study. J Infect Dis. 2016;214(11):1672–1681. doi: 10.1093/infdis/jiw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirley D.K., Kesari R.K., Glesby M.J. Factors associated with smoking in HIV-infected patients and potential barriers to cessation. AIDS Patient Care STDS. 2013;27(11):604–612. doi: 10.1089/apc.2013.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crothers K., Goulet J.L., Rodriguez-Barradas M.C. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007;22(6):749–754. doi: 10.1007/s11606-007-0158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guaraldi G., Raggi P., Gomes A. Lung and heart diseases are better predicted by pack-years than by smoking status or duration of smoking cessation in HIV patients. PLoS One. 2015;10(12):e0143700. doi: 10.1371/journal.pone.0143700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douek D.C., Brenchley J.M., Betts M.R. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 37.Le Douce V., Herbein G., Rohr O. Molecular mechanisms of HIV-1 persistence in the monocyte-macrophage lineage. Retrovirology. 2010;7:32. doi: 10.1186/1742-4690-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horiike M., Iwami S., Kodama M. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423(2):107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Brenchley J.M., Price D.A., Douek D.C. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7(3):235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 40.Sherrill-Mix S., Ocwieja K.E., Bushman F.D. Gene activity in primary T cells infected with HIV89.6: intron retention and induction of genomic repeats. Retrovirology. 2015;12:79. doi: 10.1186/s12977-015-0205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota-Gomez A., Flores A.C., Ling X.F. HIV-1 Tat increases oxidant burden in the lungs of transgenic mice. Free Radic Biol Med. 2011;51(9):1697–1707. doi: 10.1016/j.freeradbiomed.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brune K.A., Ferreira F., Mandke P. HIV impairs lung epithelial integrity and enters the epithelium to promote chronic lung inflammation. PLoS One. 2016;11(3):e0149679. doi: 10.1371/journal.pone.0149679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gingo M.R., George M.P., Kessinger C.J. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182(6):790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunisaki K.M., Niewoehner D.E., Collins G. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med. 2016;4(12):980–989. doi: 10.1016/S2213-2600(16)30319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drummond M.B., Merlo C.A., Astemborski J. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS. 2013;27(8):1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuber J.P., Calmy A., Evison J.M. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clin Infect Dis. 2004;38(8):1178–1185. doi: 10.1086/383037. [DOI] [PubMed] [Google Scholar]
- 47.Plata F., Autran B., Martins L.P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- 48.Neff C.P., Chain J.L., MaWhinney S. Lymphocytic alveolitis is associated with the accumulation of functionally impaired HIV-specific T cells in the lung of antiretroviral therapy-naive subjects. Am J Respir Crit Care Med. 2015;191(4):464–473. doi: 10.1164/rccm.201408-1521OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Twigg H.L., Soliman D.M., Day R.B. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1439–1444. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 50.Popescu I., Drummond M.B., Gama L. HIV suppression restores the lung mucosal CD4+ T-cell viral immune response and resolves CD8+ T-cell alveolitis in patients at risk for HIV-associated chronic obstructive pulmonary disease. J Infect Dis. 2016;214(10):1520–1530. doi: 10.1093/infdis/jiw422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cribbs S.K., Lennox J., Caliendo A.M. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Res Hum Retroviruses. 2015;31(1):64–70. doi: 10.1089/aid.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans M.R., Wansbrough-Jones M.H. Alveolar macrophage activation in HIV infection. J Infect. 1996;33(2):91–94. doi: 10.1016/s0163-4453(96)92967-9. [DOI] [PubMed] [Google Scholar]
- 53.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sze M.A., Dimitriu P.A., Suzuki M. The host response to the lung microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):438–445. doi: 10.1164/rccm.201502-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erb-Downward J.R., Thompson D.L., Han M.K. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morris A., Beck J.M., Schloss P.D. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lozupone C., Cota-Gomez A., Palmer B.E. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187(10):1110–1117. doi: 10.1164/rccm.201211-2145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beck J.M., Schloss P.D., Venkataraman A. Multi-center comparison of lung and oral microbiomes of HIV-infected and HIV-uninfected individuals. Am J Respir Crit Care Med. 2015;192(11):1335–1344. doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Twigg H.L., III, Knox K.S., Zhou J. Effect of advanced HIV infection on the respiratory microbiome. Am J Respir Crit Care Med. 2016;194(2):226–235. doi: 10.1164/rccm.201509-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segal L.N., Alekseyenko A.V., Clemente J.C. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):19. doi: 10.1186/2049-2618-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui L., Lucht L., Tipton L. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am J Respir Crit Care Med. 2015;191(8):932–942. doi: 10.1164/rccm.201409-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S.X., Armstrong A.J., Neff C.P. Complexities of gut microbiome dysbiosis in the context of HIV infection and antiretroviral therapy. Clin Pharmacol Ther. 2016;99(6):600–611. doi: 10.1002/cpt.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams B., Landay A., Presti R.M. Microbiome alterations in HIV infection a review. Cell Microbiol. 2016;18(5):645–651. doi: 10.1111/cmi.12588. [DOI] [PubMed] [Google Scholar]
- 64.Noguera-Julian M., Rocafort M., Guillen Y. Gut microbiota linked to sexual preference and HIV infection. EBioMedicine. 2016;5:135–146. doi: 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shreiner A., Huffnagle G.B., Noverr M.C. The “Microflora Hypothesis” of allergic disease. Adv Exp Med Biol. 2008;635:113–134. doi: 10.1007/978-0-387-09550-9_10. [DOI] [PubMed] [Google Scholar]
- 66.Ekbom A., Brandt L., Granath F. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung. 2008;186(3):167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 67.Pender S.L., Li C.K., Di Sabatino A. Role of macrophage metalloelastase in gut inflammation. Ann N Y Acad Sci. 2006;1072:386–388. doi: 10.1196/annals.1326.019. [DOI] [PubMed] [Google Scholar]
- 68.Porter K.M., Sutliff R.L. HIV-1, reactive oxygen species, and vascular complications. Free Radic Biol Med. 2012;53(1):143–159. doi: 10.1016/j.freeradbiomed.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drost E.M., Skwarski K.M., Sauleda J. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60(4):293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ande A., McArthur C., Ayuk L. Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals. PLoS One. 2015;10(4):e0122402. doi: 10.1371/journal.pone.0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atkinson J.J., Lutey B.A., Suzuki Y. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183(7):876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foronjy R., Nkyimbeng T., Wallace A. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1149–L1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 73.Chelladurai P., Seeger W., Pullamsetti S.S. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J. 2012;40(3):766–782. doi: 10.1183/09031936.00209911. [DOI] [PubMed] [Google Scholar]
- 74.Babusyte A., Stravinskaite K., Jeroch J. Patterns of airway inflammation and MMP-12 expression in smokers and ex-smokers with COPD. Respir Res. 2007;8:81. doi: 10.1186/1465-9921-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bradley H., Hall H.I., Wolitski R.J. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV—United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]