Abstract
Background
Predicting the need for intensive care among adults with community-acquired pneumonia (CAP) remains challenging.
Methods
Using a multicenter prospective cohort study of adults hospitalized with CAP, we evaluated the association of serum procalcitonin (PCT) concentration at hospital presentation with the need for invasive respiratory or vasopressor support (IRVS), or both, within 72 h. Logistic regression was used to model this association, with results reported as the estimated risk of IRVS for a given PCT concentration. We also assessed whether the addition of PCT changed the performance of established pneumonia severity scores, including the pneumonia severity index and the American Thoracic Society minor criteria, for prediction of IRVS.
Results
Of 1,770 enrolled patients, 115 required IRVS (6.5%). Using the logistic regression model, PCT concentration had a strong association with IRVS risk. Undetectable PCT (< 0.05 ng/mL) was associated with a 4% (95% CI, 3.1%-5.1%) risk of IRVS. For concentrations < 10 ng/mL, PCT had an approximate linear association with IRVS risk: for each 1 ng/mL increase in PCT, there was a 1% to 2% absolute increase in the risk of IRVS. With a PCT concentration of 10 ng/mL, the risk of IRVS was 22.4% (95% CI, 16.3%-30.1%) and remained relatively constant for all concentrations > 10 ng/mL. When added to each pneumonia severity score, PCT contributed significant additional risk information for the prediction of IRVS.
Conclusions
Serum PCT concentration was strongly associated with the risk of requiring IRVS among adults hospitalized with CAP and is potentially useful for guiding decisions about ICU admission.
Key Words: biomarkers, pneumonia, prognosis, respiratory failure, septic shock
Abbreviations: ATS, American Thoracic Society; AUC, area under the curve; CAP, community-acquired pneumonia; IQR, interquartile range; IRVS, invasive respiratory or vasopressor support; PCT, procalcitonin; PSI, pneumonia severity index; ROC, receiver operating characteristic
FOR EDITORIAL COMMENT SEE PAGE 769
Pneumonia accounts for approximately 63,000 deaths, 1.2 million hospitalizations, 2.3 million ED visits, and $10 billion in hospital costs in the United States annually.1, 2, 3 Assessment of illness severity and the risk for clinical deterioration at the time of initial diagnosis are essential for optimal pneumonia management, including selection of the best site of care (outpatient, in-patient general floor, or ICU).4, 5, 6 However, early severity assessment and risk stratification for community-acquired pneumonia (CAP) are challenging because overt clinical signs at presentation are not highly predictive of which patients will experience deterioration of their condition.7, 8
Although several guidelines and clinical scoring systems exist to assist clinicians with early severity assessment,4, 9, 10, 11, 12, 13 some of these resources are difficult to use in routine practice, and both overestimation and underestimation of CAP severity continue to result in suboptimal admission decisions.14, 15, 16 ICU admission improves outcomes for patients who require invasive respiratory or vasopressor support (IRVS) (ie, intubation for respiratory failure or vasopressors for septic shock) at any time during their hospitalization.14 However, ICU care is a costly and limited resource.17, 18 Therefore, objective easy to use measures that aid clinicians in determining a patient’s risk for subsequently requiring IRVS would be useful for guiding ICU admission decisions.11, 19, 20
Procalcitonin (PCT) is a prohormone of calcitonin that is emerging as a clinical biomarker.21, 22, 23, 24, 25 PCT concentrations tend to be higher in patients with pneumonia who have more severe infections.25, 26, 27, 28, 29, 30 However, PCT has not previously been evaluated as a marker for patients who require IRVS, a highly relevant outcome for ICU admission decision-making.11, 19, 20 Therefore, we evaluated the association of a single serum PCT measurement at hospital presentation with the need for IRVS during the subsequent 72 h among adults hospitalized with CAP. We also evaluated the additive value of PCT when used in conjunction with several existing pneumonia severity scores.
Methods
We conducted a prospective cohort study of adults hospitalized with CAP nested in the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study.31 Institutional review boards at the enrolling centers and the CDC approved the protocol (IRB No. 091422 at Vanderbilt University). Informed consent was obtained from all participants.
Patient Recruitment
The EPIC study included adults (≥ 18 years) hospitalized with CAP at three hospitals in Chicago, Illinois and two hospitals in Nashville, Tennessee between January 2010 and June 2012.31 All enrolled patients had clinical and radiographic evidence of CAP; detailed eligibility criteria have been described previously.31 Sera were obtained and banked from enrolled patients at the time of hospital presentation. For the current study, we included adult patients in the EPIC cohort who had adequate serum volume for PCT measurement (200 μL).
Procalcitonin Measurement
The primary exposure variable was serum PCT concentration. Two research laboratories—one in Chicago and one in Nashville—performed PCT measurements using the miniVIDAS instrument and VIDAS B.R.A.H.M.S. PCT immunoassay kits (BioMerieux) according to the package insert.32 The lower limit of PCT detection was 0.05 ng/mL. Laboratory personnel performing PCT measurements were blinded to all clinical information. Clinicians caring for enrolled patients were blinded to PCT results.
Outcome
The primary study outcome was IRVS, defined as intubation for respiratory failure or vasopressor administration for septic shock within 72 h of hospital presentation. IRVS was selected as the primary outcome because it provides a more objective assessment of critical illness than does ICU admission, which may be driven by factors other than illness severity.11, 19, 20 A window of 72 h was chosen to limit the outcome to manifestations most likely related to CAP and not delayed nosocomial complications.4, 33
Pneumonia Severity Scores
We calculated pneumonia severity scores indicative of the patient’s condition at the time of hospital presentation, including the American Thoracic Society minor criteria for severe CAP (ATS minor criteria),4, 34 pneumonia severity index (PSI),9 and SMART-COP.11
Statistical Analysis
PCT distributions were compared between patients who required IRVS and those who did not using the Wilcoxon rank-sum test. We also constructed a nonparametric receiver operating characteristic (ROC) curve for PCT to discriminate between patients who did and those who did not require IRVS; the area under the curve (AUC) was calculated. For comparison with PCT, an ROC curve for WBC count, a biomarker currently in widespread clinical use for CAP, was also constructed. We also performed these analyses after stratifying the population by the initial location of hospital admission: general medical floor vs ICU.
Logistic Regression Models
We used logistic regression models to assess the association of PCT concentration and the risk of IRVS. Since PCT values were skewed, we modeled PCT values using a restricted cubic spline function with four knots located at the fifth, 35th, 65th, and 95th percentile of PCT distribution.35 We then estimated the risk of IRVS according to PCT values, using the predictive probabilities from the logistic regression models.
We also evaluated whether PCT added predictive risk information to existing severity scores (ATS minor criteria, PSI, and SMART-COP). We constructed logistic regression models using each of the severity scores as the sole predictor and IRVS as the outcome. We then added PCT as a predictor to each of the severity score models and conducted likelihood ratio tests comparing the models with and those without PCT. These tests examined whether PCT had a statistically significant additive contribution to each of the severity scores for predicting IRVS.
Since current CAP management guidelines from the Infectious Disease Society of America and American Thoracic Society emphasize the ATS minor criteria (e-Table 1) as a tool to assist with ICU admission decisions,4 we used it as the primary score for detailed comparison with PCT. To determine the contribution of PCT and ATS minor criteria for the prediction of IRVS, we first compared the relative strength of the association between PCT and each of the nine individual ATS minor criteria with IRVS. We constructed a multivariable logistic regression model with IRVS as the outcome and PCT and each of the ATS minor criteria as predictors. Individual predictors were removed from the full model one at a time, and variation in the model deviance was quantified using the difference in the likelihood ratio χ2 between the full model and the model after removal of the single predictor. Percentage of the full model likelihood ratio χ2 statistic contributed by each predictor was calculated to demonstrate the relative strength of IRVS prediction for PCT and each of the ATS minor criteria.
Performance of PCT Added to Pneumonia Severity Scores
We further explored whether PCT contributed risk information to assessments based on established severity scores. In the clinical setting, CAP severity scores are often used to dichotomize patients into high- and low-risk categories.4 To resemble this real-life binary implementation of severity scores (high risk vs low risk), we evaluated PCT in three separate stratified analyses based on the following criteria: (1) ATS minor criteria, with ≥ 3 criteria denoting high risk; 4, 34 (2) PSI, with classes IV and V classified as high risk;9 and (3) SMART-COP, with ≥ 3 points denoting high risk.11 The association of PCT with IRVS was evaluated in each of these subgroups using the rank-sum test, ROC curves, and logistic regression as detailed earlier. We also calculated the number of patients with < 3 ATS minor criteria who would be reclassified as high risk if PCT at various cut points was used as an additional indicator to identify high-risk patients. Statistical analyses were performed with Stata 12 (StataCorp LP).
Results
Among 2,320 adults with CAP in the EPIC study, 1,770 (76.3%) had adequate serum for PCT measurement and were included in this analysis (Table 1). Characteristics of patients excluded because of not having a PCT measurement were similar to the included patients (e-Table 2). Overall, 115 patients (6.5%) required IRVS within 72 h of hospital presentation, including 47 patients (2.7%) with both invasive respiratory and vasopressor support, 37 patients (2.1%) with respiratory support only, and 31 patients (1.8%) with vasopressor support only. Serum PCT concentrations were higher in patients who required IRVS (median, 1.43 ng/mL; interquartile range [IQR], 0.14-8.22 ng/mL) compared with those who did not (median, 0.14 ng/mL; IQR, < 0.05-0.72 ng/mL) (P < .01) (Table 2, e-Fig 1).
Table 1.
Patient Characteristics
| Characteristic | Adults Hospitalized With CAP and PCT Results (N = 1,770) |
|---|---|
| Age, median (IQR), y | 57 (47-70) |
| Female sex, No. (%) | 905 (51.1) |
| Race and ethnicity, No. (%) | |
| Non-Hispanic white | 783 (44.2) |
| Non-Hispanic black | 693 (39.2) |
| Hispanic | 215 (12.2) |
| Other | 79 (4.5) |
| Age groups, No. (%) | |
| 18-44 y | 396 (22.4) |
| 45-64 y | 766 (43.3) |
| 65-79 y | 373 (21.1) |
| ≥ 80 y | 235 (13.3) |
| Chronic medical conditions, No. (%) | |
| Asthma | 459 (25.9) |
| Chronic obstructive lung disease | 367 (20.7) |
| Cancer | 320 (18.1) |
| Chronic heart failure | 318 (18.0) |
| Diabetes mellitus | 438 (24.8) |
| Chronic kidney disease | 271 (15.3) |
| Chronic liver disease | 93 (5.3) |
| Immunosuppression | 294 (16.6) |
| HIV infection | 47 (2.7) |
| Current smoker | 463 (26.2) |
| PSI risk class | |
| I | 339 (19.2) |
| II | 474 (26.8) |
| III | 345 (19.5) |
| IV | 462 (26.1) |
| V | 150 (8.5) |
| Cause of pneumonia, No. (%) | |
| Bacterial | 192 (10.9) |
| Viral | 412 (23.3) |
| Bacterial-viral mixed | 51 (2.9) |
| Fungal/mycobacterial | 15 (0.9) |
| Unknown | 1,100 (62.2) |
| Antibiotic administration before hospital presentation | 325 (18.4) |
| IRVS within 72 h, No. (%) | 115 (6.5) |
| ICU admission as initial disposition, No. (%) | 280 (15.8) |
| Delayed ICU transfer from medical floor,a No. (%) | 117 (6.6) |
| Hospital length of stay, median (IQR), d | 3 (2-6) |
| In-hospital death, No. (%) | 37 (2.1) |
CAP = community-acquired pneumonia; IQR = interquartile range; IRVS = invasive respiratory or vasopressor support; PCT = procalcitonin; PSI = pneumonia severity index.
Delayed ICU transfer from medical floor was defined as initial hospital admission on the medical floor and then later transfer to an ICU at any time during the index hospitalization for pneumonia.
Table 2.
Serum PCT Concentrations for Patients Who Did and Those Who Did Not Require IRVS Within 72 ha
| Population | No. | IRVS, No. (%) | Serum PCT, Median (IQR) [ng/mL] |
AUC (95% CI) | |
|---|---|---|---|---|---|
| IRVS Present, Range | IRVS Absent, Range | ||||
| Full study population | 1,170 | 115 (6.5) | 1.43 (0.14-8.22) | 0.14 (< 0.05-0.72) | 0.69 (0.67-0.71) |
| Location of initial admission | |||||
| General floor | 1,490 | 39 (2.6) | 1.29 (0.14-6.92) | 0.13 (0.04-0.6) | 0.70 (0.68-0.73) |
| ICU | 280 | 76 (27.1) | 1.47 (0.13-8.88) | 0.47 (0.05-2.75) | 0.60 (0.54-0.65) |
| ATS minor criteria | |||||
| < 3 criteria | 1,642 | 77 (4.7) | 0.47 (0.05-4.07) | 0.13 (< 0.05-0.63) | 0.63 (0.61-0.65) |
| ≥ 3 criteria | 128 | 38 (29.7) | 4.82 (1.10-25.9) | 0.75 (0.09-5.68) | 0.68 (0.59-0.76) |
| PSI risk class | |||||
| I-III | 1,158 | 42 (3.6) | 0.35 (0.05-5.62) | 0.10 (< 0.05-0.48) | 0.64 (0.62-0.67) |
| IV-V | 612 | 73 (11.9) | 2.30 (0.19-9.54) | 0.28 (0.06-1.39) | 0.67 (0.63-0.71) |
| SMART-COP score | |||||
| < 3 points | 1,440 | 50 (3.5) | 0.46 (0.06-5.42) | 0.12 (< 0.05-0.57) | 0.65 (0.62-0.67) |
| ≥ 3 points | 330 | 65 (19.7) | 2.38 (0.19-11.76) | 0.32 (0.07-2.72) | 0.65 (0.59-0.70) |
| Specific subgroups | |||||
| Viral cause | 412 | 21 (5.1) | 0.46 (0.05-2.59) | 0.09 (< 0.05-0.52) | 0.65 (0.60-0.70) |
| Bacterial cause | 192 | 33 (17.2) | 5.62 (1.10-27.26) | 0.73 (0.14-6.22) | 0.68 (0.61-0.75) |
| COPD | 367 | 32 (8.7) | 2.69 (0.19-16.09) | 0.10 (< 0.05-0.51) | 0.76 (0.67-0.85) |
| Immunosuppression | 294 | 21 (7.1) | 1.51 (0.41-11.01) | 0.16 (< 0.05-1.08) | 0.76 (0.66-0.86) |
| Antibiotic administration before hospital presentation | 325 | 23 (7.1) | 0.47 (0.05-5.42) | 0.10 (< 0.05-0.38) | 0.70 (0.57-0.82) |
ATS = American Thoracic Society; AUC = area under the curve; ROC = receiver operating characteristic. See Table 1 legend for expansion of other abbreviations.
AUC for ROC curves demonstrate performance of PCT for discriminating patients who required IRVS from those who did not.
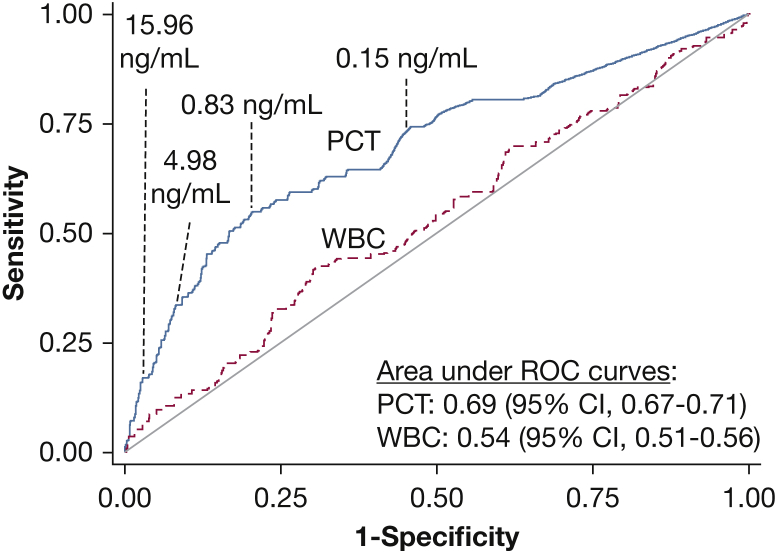
Area under the ROC curve for PCT to discriminate between patients with and those without IRVS was 0.69 (95% CI, 0.67-0.71) and was significantly higher than the area for WBC count (0.54; 95% CI, 0.51-0.56) (Fig 1). To illustrate sensitivity, specificity, and the proportion of patients who experienced IRVS at varying PCT cut points, four points representing the 50th, 75th, 90th, and 95th percentile of PCT concentration in the study population were highlighted on the ROC curve (Fig 1). For example, using the 75th percentile of PCT concentration (0.83 ng/mL) as a cut point, sensitivity and specificity for IRVS were 0.55 (95% CI, 0.45-0.64) and 0.77 (95% CI, 0.75-0.79), respectively; 3.9% of patients with a PCT concentration < 0.83 ng/mL experienced IRVS, whereas 14.2% with a concentration greater than this cut point experienced IRVS (e-Table 3).
Figure 1.
Nonparametric ROC curves for PCT and WBC count to identify patients who needed IRVS within 72 h. Selected PCT cut points at the 50th, 75th, 90th, and 95th percentiles of PCT concentration in the population are noted on the PCT curve. IRVS = invasive respiratory or vasopressor support; PCT = procalcitonin; ROC = receiver operating characteristic.
Association Between PCT and Risk of IRVS
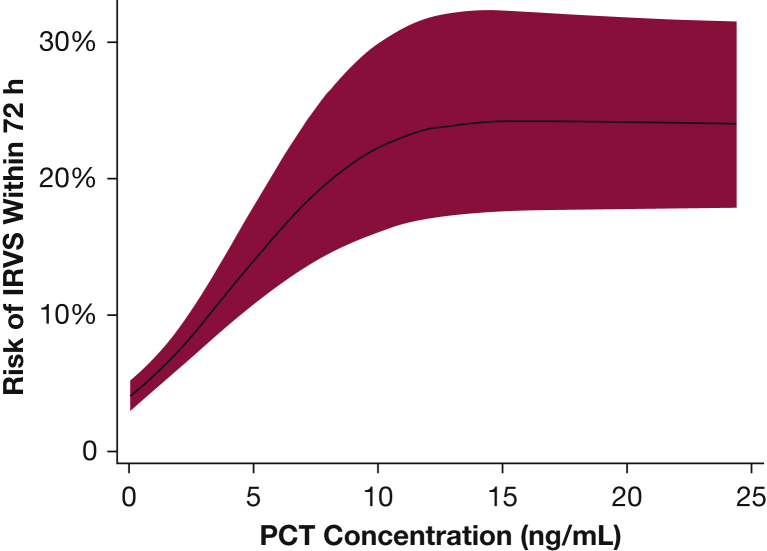
In logistic regression models, PCT concentration had a strong association with the risk of IRVS (Fig 2). Undetectable PCT using this assay (< 0.05 ng/mL) corresponded to a 4% (95% CI, 3.1%-5.1%) IRVS risk. Between 0.05 ng/mL and 10 ng/mL, PCT concentration had an approximate linear association with IRVS risk, with each incremental increase in PCT concentration of 1 ng/mL corresponding to a 1% to 2% absolute increase in IRVS risk (Fig 2). PCT concentrations of 5 ng/mL and 10 ng/mL were associated with IRVS risks of 14.2% (95% CI, 11.0%-18.1%) and 22.4% (95% CI, 16.3%-30.1%), respectively. IRVS risk plateaued at PCT concentrations > 10 ng/mL.
Figure 2.
Risk of IRVS within 72 h of hospital presentation according to initial serum PCT concentration. The plot was truncated at a PCT concentration of 25 ng/mL because of the small number of patients with PCT concentrations > 25 ng/mL. The 95% CI band is denoted with red shading. See Figure 1 legend for expansion of abbreviations.
Performance of PCT Added to Pneumonia Severity Scores
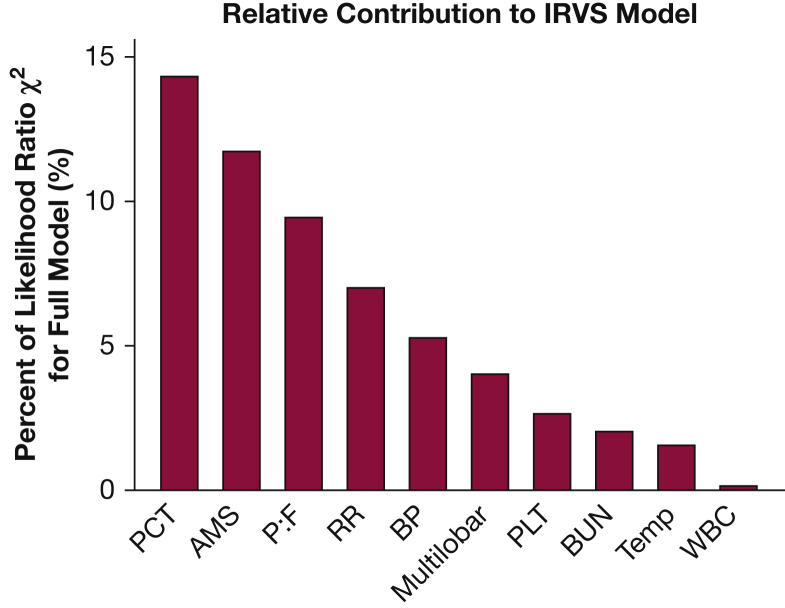
Higher PCT concentration correlated with increasing pneumonia severity at presentation as measured by the number of ATS minor criteria present, PSI score, and SMART-COP score (e-Fig 2). The addition of PCT to each of these pneumonia severity score models increased the area under the ROC curve. For example, area under the ROC curve for the ATS minor criteria alone was 0.75 and improved to 0.78 when PCT was added. Accordingly, the addition of PCT represented a significant improvement in model fit for IRVS for each severity score (likelihood ratio test P < .01 for each model). We also found that PCT concentration had a larger contribution to predicting IRVS than any of the individual ATS minor criteria (Fig 3).
Figure 3.
Relative contribution of PCT concentration and each of the American Thoracic Society (ATS) minor criteria to the prediction model for IRVS. The ATS minor criteria include AMS, partial pressure of oxygen to fraction of inspired oxygen ratio ≤ 250 (P:F), RR ≥ 30/min (RR), systolic BP < 90 mm Hg (BP), multilobar pulmonary infiltrates (Multilobar), platelets < 100,000 cells/mm3 (PLT); BUN ≥ 20 mg/dL (BUN); temperature < 36° C (Temp); WBC count < 4,000 cells/mm3 (WBC). AMS = altered mental status; RR = respiratory rate. See Figure 1 legend for expansion of other abbreviations.
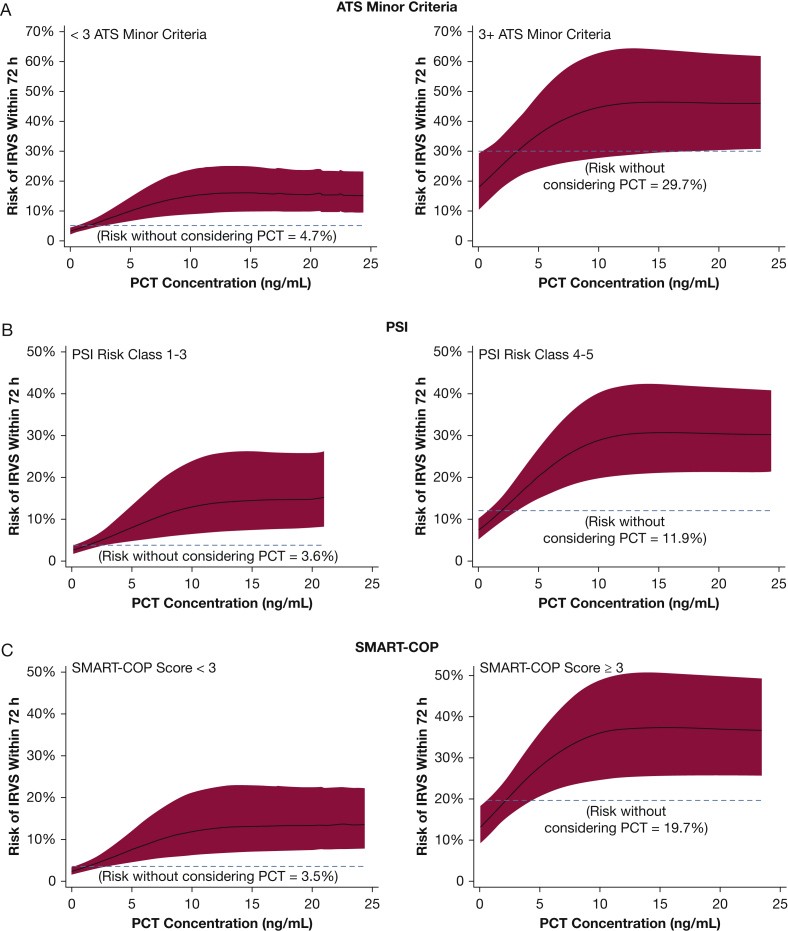
After stratification of the study population by each of the scoring systems into low- and high-risk subgroups, higher PCT was associated with greater risk of IRVS in all subgroups (Fig 4, e-Fig 3). For example, without considering PCT, patients classified as low risk by the ATS minor criteria (< 3 criteria present) had a 4.7% (95% CI, 3.7%-5.7%) risk of IRVS. After considering PCT in the low-risk ATS minor criteria subgroup, PCT < 0.05 ng/mL corresponded to a 2.4% (95% CI, 1.7%-3.4%) IRVS risk, whereas a PCT concentration of 10 ng/mL corresponded to a 12.0% (95% CI, 6.4%-21.3%) risk (Fig 4A). Without considering PCT, patients classified as high risk by the ATS minor criteria (≥ 3 criteria present) had a 29.7% (95% CI, 21.7%-37.6%) risk of IRVS. Within this high-risk subgroup by ATS minor criteria, PCT < 0.05 ng/mL was associated with a 13.2% (95% CI, 9.3%-18.5%) IRVS risk, whereas a PCT concentration of 10 ng/mL corresponded to a 36.2% (95% CI, 25.0%-49.1%) risk. Similar results were found with PSI and SMART-COP (Figs 4B, 4C). PCT values in patients with CAP caused by viruses and bacteria are reported separately in e-Appendix 1 and e-Table 4.
Figure 4.
Risk of IRVS within 72 h of hospital presentation according to initial serum PCT concentration with the study population stratified into low- and high-risk subgroups by (A) ATS minor criteria, (B) PSI, and (C) SMART-COP. Plots were truncated at a PCT concentration of 25 ng/mL because of the small number of patients with PCT concentrations > 25 ng/mL. The 95% CI band is denoted with red shading. Dashed lines represent IRVS risk in a subgroup alone without considering PCT concentration. ATS = American Thoracic Society; PSI = pneumonia severity index. See Figure 1 legend for expansion of other abbreviations.
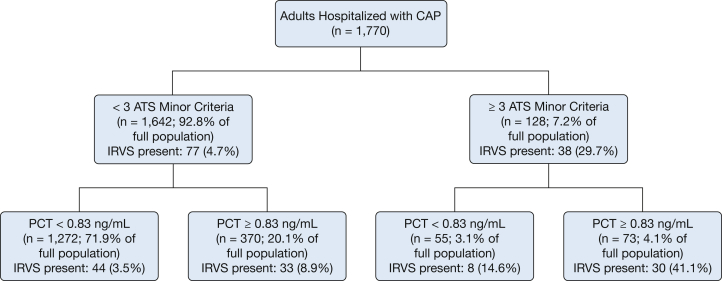
To illustrate how using PCT with a specific binary cut point could augment risk stratification by the ATS minor criteria, we developed a classification tree using either ≥ 3 ATS minor criteria or PCT ≥ 0.83 ng/mL to indicate high risk (Fig 5). Using ≥ 3 ATS minor criteria alone to indicate high risk, 77 of the 1,770 total patients (4.4%) were misclassified as low risk and experienced IRVS. Including PCT ≥ 0.83 ng/mL in addition to ≥ 3 ATS minor criteria as a high-risk indicator reduced the number of patients with IRVS misclassified as low risk to 44 (2.49%). Adding PCT ≥ 0.83 ng/mL as a high-risk indicator resulted in 370 additional patients being classified as high risk, with 33 of them correctly classified as having IRVS.
Figure 5.
Classification tree using a combination of ≥ 3 ATS minor criteria or PCT ≥ 0.83 ng/mL (which was the 75th percentile of PCT concentration in the study population) as high-risk indicators for IRVS among adults hospitalized with CAP. CAP = community-acquired pneumonia. See Figure 1 and 4 legends for expansion of other abbreviations.
Discussion
In this multicenter study of adults hospitalized with CAP, serum PCT concentrations at hospital presentation were strongly associated with the risk of IRVS during the following 72 h. Patients with PCT concentrations of 5 ng/mL and 10 ng/mL were approximately three and five times more likely to require IRVS than patients with PCT < 0.05ng/mL, respectively, suggesting that PCT is potentially a useful test to help guide ICU admission decisions. Incorporation of PCT with clinical gestalt and clinical scoring systems is likely to improve identification of patients needing intensive care; however, the accuracy of PCT for IRVS is not strong enough to base clinical decisions solely on PCT results.
Our results suggest that PCT complements other tools clinicians use to guide ICU admission decisions. As expected, higher scores for several pneumonia severity scoring systems were associated with IRVS. However, adding PCT to each of these scores improved the ability to identify patients who required IRVS. The presence of ≥ 3 ATS minor criteria is a high-risk situation that clinicians often consider as a warning sign for impending respiratory failure or shock.4 However, in this study, 67% of the 115 patients who required IRVS had < 3 ATS minor criteria. An elevated PCT level may help identify these patients without overt clinical signs of impending respiratory failure or shock but who would benefit from early ICU admission. For example, in this study, a PCT concentration of 10 ng/mL in patients with < 3 ATS minor criteria corresponded to a 12% risk of IRVS, a risk level that may warrant consideration for admission to an ICU or another setting that can ensure close monitoring.
Similar to our findings, prior studies have demonstrated an association between higher PCT levels at the time of acute presentation and adverse outcomes in patients with CAP.26, 27, 28, 29, 30, 36, 37 Huang et al27 found that adding PCT to PSI scores improved prognostic accuracy for 30-day mortality among patients with CAP in PSI risk classes IV-V. Ramirez et al30 found that combining PCT with the ATS minor criteria helped identify patients admitted to an ICU, including those with delayed ICU transfer. In a recent meta-analysis, Kutz et al36 found that PCT levels in the ED, but not in primary care clinics or in the ICU, correlated with treatment failure and mortality, suggesting that PCT measurement may be most useful for risk stratification of undifferentiated patients in the ED setting.
In most prior studies,27, 28, 30 PCT concentrations were analyzed after categorizing them into groups using various cut points (eg, 0.25 ng/mL). An advantage of our study was the use of PCT as a continuous variable to demonstrate a strong association with IRVS across a broad range of PCT concentrations. As demonstrated in Figure 2, increasing PCT concentrations up to 10 ng/mL correlated with increasing IRVS risk. Using PCT concentrations on a continuous scale without introducing binary cut points to define “positive” and “negative” values retained more information and provided the most predictive power.
Limitations
PCT measurements were obtained only from a subset of adults enrolled in the EPIC study; however, clinical and demographic characteristics were similar between included (76.3%) and excluded (23.7%) patients (e-Table 2). IRVS was chosen as the primary outcome because it is regarded as an objective measure of CAP-related critical illness,11, 19, 20 but we acknowledge that other factors are also important when considering an ICU admission. We did not evaluate mortality as an outcome in this study. Only two patients died within 72 h of hospital presentation who did not receive IRVS; excluding these two patients from the analysis had no appreciable effect on the results. Although this study demonstrates an association between PCT and IRVS, effectiveness studies with real-time use of PCT determinations are required to evaluate the impact of PCT on clinical decision-making and patient outcomes.
Conclusions
Serum PCT concentration at the time of hospital presentation was significantly associated with the risk of patients with CAP requiring IRVS within 72 h. This association remained strong after adjustment for other tools clinicians use to evaluate pneumonia severity, highlighting that PCT may be a useful marker to assist with ICU admission decisions.
Acknowledgments
Author contributions: W. H. S. had full access to the data and takes responsibility for the integrity of the data and accuracy of the analysis. W. H. S., C. G. G., S. J., K. M. E., and R. G. W. were responsible for the study concept and design. W. H. S., D. J. W., A. W., R. A. B., S. F., D. M. C., J. C., E. J. A., C. Q., C. T., K. M. E., and R. G. W. were responsible for acquisition of data. W. H. S., C. G. G., Y. Z., and A. M. B. were responsible for statistical analysis. W. H. S., C. G. G., D. J. W., A. W., Y. Z., D. M. C., E. J. A., G. W. W., S. J., K. M. D., and R. G. W. were responsible for interpretation of data. W. H. S. was responsible for drafting the initial manuscript. All authors were responsible for critical revision of the manuscript. K. M. E. and R. G. W. obtained funding. W. H. S., C. G. G., A. W., D. J. W., R. A. B., S. F., D. M. C., C. T., S. J., K. M. E., and R. G. W. were responsible for study supervision.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: W. H. S. reports grants from Centers for Disease Control (CDC); funds to conduct clinical research from BioMerieux, Affinium Pharmaceuticals, Astute Medical, BRAHMS GmbH, Pfizer, Rapid Pathogen Screening, and Venaxis and personal fees from BioFire Diagnostics and Venaxis. D. J. W. reports grants from CDC. A. W. reports funds to perform clinical research from Biomerieux. Y. Z. reports grants from CDC. J. C. reports grants from CDC, funds to conduct clinical research from BioMerieux, and patents US 8,293, 498 B2 licensed to Vanderbilt University and pending patent 13/639564. E. J. A. reports grants from MedImmune and nonfinancial support from Roche. C. T. reports personal fees from Saint Thomas Research Institute. K. M. E. reports grants from CDC and Novartis and other funding from Novartis. R. G. W. reports grants from CDC, funds to conduct clinical research from BioMerieux and Vertex Pharmaceuticals, and personal fees from BioMerieux and Visterra Inc. None declared (C. G. G., R. A. B., S. F., D. M. C., C. Q., G. W. W., A. M. B., S. J.).
Role of sponsors: BioMerieux, Inc had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. BioMerieux, Inc did review the final manuscript before submission.
Other contributions: We thank the following for their dedication in enrolling patients and testing specimens in this study: Adrienne Baughman, Kelly Moser, Shanda Phillips, Markia Ward, Karen Miller, Charity Graves, Rabon Lee Smalling, Sandy Alvarez, Rendi McHenry, Helen Donnelly, Mike Malczynski, Julie Wilkens, Alison Chevrier, Jill Sears, Amy Melvin, Rosie Lyles, Bharat Reddy Dhanireddy, Pinal Modi, and Joyce Brown.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by a cooperative agreement with the Centers for Disease Control and Prevention (U18 IP000299). Investigators from the Centers for Disease Control and Prevention participated in the study as authors. W.H.S. was supported in part by K23GM110469 from the National Institute of General Medical Sciences. Materials and funds to perform procalcitonin measurements were provided by BioMerieux, Inc.
Supplementary Data
References
- 1.Kung H.C., Hoyert D.L., Xu J., Murphy S.L. Deaths: Final date for 2005. Natl Vital Stat Rep. 2008;56(10):1–20. [PubMed] [Google Scholar]
- 2.Pfuntner A, Wier LM, Stocks C. Most frequent conditions in U.S. hospitals, 2010. HCUP Statistical Brief #148. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb148.pdf. Accessed June 15, 2016.
- 3.Self W.H., Grijalva C.G., Zhu Y. Rates of emergency department visits due to pneumonia in the United States, July 2006-June 2009. Acad Emerg Med. 2013;20(9):957–960. doi: 10.1111/acem.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell L.A., Wunderink R.G., Anzueto A. Infectious Diseases Society of American/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S42. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewig S., Torres A., Woodhead M. Assessment of pneumonia severity: A European perspective. Eur Respir J. 2006;27(1):6–8. doi: 10.1183/09031936.06.00130205. [DOI] [PubMed] [Google Scholar]
- 6.Niederman M.S., Feldman C., Richards G.A. Combining information from prognostic scoring tools for CAP: an American view on how to get the best of all worlds. Eur Respir J. 2006;27(1):9–11. doi: 10.1183/09031936.06.00130305. [DOI] [PubMed] [Google Scholar]
- 7.Fine M.J., Smith M.A., Carson C.A. Prognosis and outcomes of patient with community-acquired pneumonia: a meta-analysis. JAMA. 1996;275(2):134–141. [PubMed] [Google Scholar]
- 8.Dremsizov T., Clermont G., Kellum J.A. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129(4):968–978. doi: 10.1378/chest.129.4.968. [DOI] [PubMed] [Google Scholar]
- 9.Fine M.J., Auble T.E., Yealy D.M. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 10.Lim W.S., van der Eerden M.M., Laing R. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charles P.G.P., Wolfe R., Whitby M. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384. doi: 10.1086/589754. [DOI] [PubMed] [Google Scholar]
- 12.Renaud B., Labarere J., Coma E. Risk stratification of early admission to the intensive care unit of patients with no major criteria of severe community-acquired pneumonia: development of an international prediction rule. Crit Care. 2009;13(2):R54. doi: 10.1186/cc7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espana P.P., Capelastegui A., Gorordo I. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256. doi: 10.1164/rccm.200602-177OC. [DOI] [PubMed] [Google Scholar]
- 14.Phua J., Ngerng W.J., Lim T.K. The impact of a delay in intensive care unit admission for community-acquired pneumonia. Eur Respir J. 2010;36(4):826–833. doi: 10.1183/09031936.00154209. [DOI] [PubMed] [Google Scholar]
- 15.Dean N.C., Jones J.P., Aronsky D. Hospital admission decision for patients with community-acquired pneumonia: variability among physicians in an emergency department. Ann Emerg Med. 2012;59(1):35–41. doi: 10.1016/j.annemergmed.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L.M., Render M., Sales A. Intensive care unit admitting patterns in the Veterans Affairs health care system. Arch Intern Med. 2012;172(16):1220–1226. doi: 10.1001/archinternmed.2012.2606. [DOI] [PubMed] [Google Scholar]
- 17.Dasta J.F., McLaughlin T.P., Mody S.H., Piech C.T. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 18.Orsini J., Blaak C., Yeh A. Triage of patients consulted for ICU admission during times of ICU-bed shortage. J Clin Med Res. 2014;6(6):463–468. doi: 10.14740/jocmr1939w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalmers J.D. ICU admission and severity assessment in community-acquired pneumonia. Crit Care. 2009;13(3):156. doi: 10.1186/cc7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renaud B., Santin A. Severe community acquired pneumonia: what should we predict? Crit Care. 2009;13(5):421. doi: 10.1186/cc8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assicot M., Gendrel D., Carsin H. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuetz P., Briel M., Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA. 2013;309(7):717–718. doi: 10.1001/jama.2013.697. [DOI] [PubMed] [Google Scholar]
- 23.Ch Yo, Hsieh P.S., Lee Sh. Comparison of the test characteristics of procalcitonin to C-reactive protein and leukocytosis for the detection of serious bacterial infections in children presenting with fever without a source: a systematic review and meta-analysis. Ann Emerg Med. 2012;60(5):591–600. doi: 10.1016/j.annemergmed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Musher D.M., Bebko S.P., Roig I.L. Serum procalcitonin level, viral polymerase chain reaction analysis, and lower respiratory tract infection. J Infect Dis. 2014;209(4):631–633. doi: 10.1093/infdis/jit579. [DOI] [PubMed] [Google Scholar]
- 25.Kruger S., Ewig S., Papassotiriou J. Inflammatory parameters predict etiologic patterns but do not allow for individual prediction of etiology in patients with CAP: results from the German competence network CAPNETZ. Respir Res. 2009;10:65. doi: 10.1186/1465-9921-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masia M., Gutierrez F., Shum C. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patient’s outcome research team pneumonia severity index. Chest. 2005;128(4):2223–2229. doi: 10.1378/chest.128.4.2223. [DOI] [PubMed] [Google Scholar]
- 27.Huang D.T., Weissfeld L.A., Kellum J.A. Risk prediction with procalcitonin and clinical rules in community-acquired pneumonia. Ann Emerg Med. 2008;52(1):48–58. doi: 10.1016/j.annemergmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuetz P., Suter-Widmer I., Chaudri A. Prognostic value of procalcitonin in community-acquired pneumonia. Eur Respir J. 2011;37(2):384–392. doi: 10.1183/09031936.00035610. [DOI] [PubMed] [Google Scholar]
- 29.Kruger S., Ewing S., Marre R. Procalcitonin predicts patients at low risk of death from community-acquired pneumonia across all CRB-65 classes. Eur Respir J. 2008;31(2):349–355. doi: 10.1183/09031936.00054507. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez P., Ferrer M., Marti V. Inflammatory biomarkers and prediction for intensive care unit admission in severe community-acquired pneumonia. Crit Care Med. 2011;39(10):2211–2217. doi: 10.1097/CCM.0b013e3182257445. [DOI] [PubMed] [Google Scholar]
- 31.Jain S., Self W.H., Wunderink R.G. Community-acquired pneumonia requiring hospitalization in US adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BioMerieux, Inc. VIDAS B.R.A.H.M.S. PCT. BioMerieux, Inc. website. http://www.biomerieux-diagnostics.com/vidas-brahms-pct. Accessed July 15, 2015.
- 33.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 34.Ewig S., Ruiz M., Mensa J. Severe community-acquired pneumonia: assessment of severity criteria. Am J Respir Crit Care Med. 1998;158(4):1102–1108. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 35.Harrell F.E., Jr. Springer; New York, New York: 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression and Survival Analysis. [Google Scholar]
- 36.Kutz A., Briel M., Christ-Crain M. Prognostic value of procalcitonin in respiratory tract infections across clinical settings. Crit Care. 2015;19:74. doi: 10.1186/s13054-015-0792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuetz P., Amin D.N., Greenwald J.L. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest. 2012;141(4):1063–1073. doi: 10.1378/chest.11-2430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.