Abstract
Background
Heparin-induced thrombocytopenia (HIT) complicated by severe thrombocytopenia and thrombosis can pose significant treatment challenges. Use of alternative anticoagulants in this setting may increase bleeding risks, especially in patients who have a protracted disease course. Additional therapies are lacking in this severely affected patient population.
Methods
We describe three patients with HIT who had severe thromboembolism and prolonged thrombocytopenia refractory to standard treatment but who achieved an immediate and sustained response to IVIg therapy. The mechanism of action of IVIg was evaluated in these patients and in five additional patients with severe HIT. The impact of a common polymorphism (H/R 131) in the platelet IgG receptor FcγRIIa on IVIg-mediated inhibition of platelet activation was also examined.
Results
At levels attained in vivo, IVIg inhibits HIT antibody-mediated platelet activation. The constant domain of IgG (Fc) but not the antigen-binding portion (Fab) is required for this effect. Consistent with this finding, IVIg had no effect on HIT antibody binding in a solid-phase HIT immunoassay (platelet factor 4 enzyme-linked immunoassay). The H/R131 polymorphism in FcγRIIa influences the susceptibility of platelets to IVIg treatment, with the HH131 genotype being most susceptible to IVIg-mediated inhibition of antibody-induced activation. However, at high doses of IVIg, activation of platelets of all FcγRIIa genotypes was significantly inhibited. All three patients did well on long-term anticoagulation therapy with direct oral anticoagulants.
Conclusions
These studies suggest that IVIg treatment should be considered in patients with HIT who have severe disease that is refractory to standard therapies.
Key Words: DOAC, heparin, HIT, IVIg, thrombocytopenia, thrombosis
Abbreviations: CABG, coronary artery bypass grafting; DOAC, direct oral anticoagulant; ELISA, enzyme-linked immunosorbent assay; HIT, heparin-induced thrombocytopenia; OD, optical density; PEA, PF4-dependent P-selectin expression assay; PF4, platelet factor 4; PF4 ELISA, IgG-specific PF4/polyvinyl sulfonate ELISA; SRA, serotonin release assay; TPE, therapeutic plasma exchange
FOR EDITORIAL COMMENT SEE PAGE 453
Heparin-induced thrombocytopenia (HIT), an important cause of patient morbidity and mortality, is characterized by antibodies directed against complexes of heparin and platelet factor 4 (PF4).1 Direct thrombin inhibitors, fondaparinux (off-label), and danaparoid (not available in the United States) are the mainstays of HIT therapy in the acute setting.1 Thrombosis and severe/protracted thrombocytopenia are common in severe HIT syndromes such as “spontaneous HIT,”2 seen in patients without proximate heparin exposure and “delayed-onset HIT,”3, 4, 5, 6 in which disease occurs or worsens after heparin treatment has been discontinued. Use of alternative nonheparin anticoagulants in the setting of severe thrombocytopenia in these and other severely affected patients can be dangerous because of bleeding risks, even though the likelihood of thrombosis is paradoxically high.2, 3, 4 Mortality in HIT is high,7 as is the burden of health-care costs.8, 9 A finding that IgG can inhibit activation of platelets by a monoclonal HIT-like antibody10 suggested a rationale for IVIg treatment in HIT; however, this therapy has been used only sporadically,11, 12, 13, 14, 15, 16, 17, 18, 19, 20 and its effectiveness is not established.
We describe three patients with severe HIT complicated by thrombosis and protracted thrombocytopenia who were refractory to standard therapy but had a rapid and sustained response to IVIg treatment. Using samples from these patients and several others with severe disease, we show that the IgG Fc domain is necessary and sufficient for inhibition of platelet activation by HIT antibodies and that this effect is influenced by the H/R 131 polymorphism of the platelet Fc receptor FcγRIIa. These findings indicate that IVIg should be considered in patients with severe HIT refractory to standard therapy.
Methods
Detection of HIT Antibodies
The PF4-dependent P-selectin expression assay (PEA) for detection of platelet-activating HIT antibodies was performed as previously described.21 Briefly, pooled washed O blood group normal platelets (1 × 106) were treated with PF4 (37.5 μg/mL) for 20 min followed by patient serum for 1 h. After addition of fluorochrome-labeled anti-P-selectin (HB299 hybridoma, ATCC) and anti-GPIIb (290.5 hybridoma, BloodCenter of Wisconsin) antibodies, platelet events were gated by GPIIb positivity, and P-selectin expression (median fluorescence intensity[MFI]) was recorded. Maximum P-selectin expression (100%) was measured by treating platelets with thrombin receptor-activating peptide (TRAP; 25 μg/mL). Results were expressed as the percentage of maximum P-selectin expression corrected for background signal obtained with normal serum as follows:
In preliminary studies, it was found that acute serum samples from the three patients produced positive PEA test results when concentrations of PF4 lower than the standard 37.5 ug/mL were used to prime platelets. Therefore, additional studies were performed with platelets pretreated with 1.25 μg PF4/mL (patient 1) and 3.75 μg/mL (patients 2, 3 and HIT patients 1-5) (“PEA, low PF4”). The serotonin release assay (SRA) and PF4 ELISA were performed as described previously.22
Laboratory Studies with IVIg
A US Food and Drug Administration-approved IVIg preparation, GAMMAGARD (Shire) was used for in vitro experiments. An incremental recovery rate of 2.3 mg/dL for each mg/kg body weight of IVIg administered was used to mimic levels of IgG attained in human blood in vivo (GAMMAGARD package insert, on file). Using this standard, doses of 0.25 g/kg, 0.5 gm/kg, 1 g/kg, and 2 g/kg IVIg administered to patients corresponds with IgG concentrations of 6 mg/mL, 12 mg/mL, 23 mg/mL, and 46 mg/mL, respectively, attained in patient plasma. Studies presented in e-Figure 1 were performed with three other IVIg preparations: Octagam (Octapharma), GAMUNEX-C (Grifols), and Hizentra (CSL Behring). Purified Fc and Fab fragments (Athens Research and Technology) were used at equimolar and twice-molar amounts relative to IgG, respectively.
FcγRIIa Genotyping
DNA was extracted from whole blood or blood clots using QIAamp DNA Mini Kit (Qiagen). Polymerase chain reaction was performed using primers F 5′-CTTTCAGAATGGCTGGTGCT-3′ and R 5′- TTTGCTGCTATGGGCTTTCT-3′ specific for the FcγRIIa gene (Integrated DNA Technologies). Polymerase chain reaction products (that included the FcγRIIa H/R 131 polymorphism site) were sequenced in a 3130xl Genetic Analyzer (Applied Biosystems) using standard procedures.
Statistical Analysis and Institutional Review Board Approval
Data are expressed as mean + 1 SD. Significance was calculated by the Student t test. Statistical analyses were performed using GraphPad Prism, version 6.07 (GraphPad). Studies were approved by the institutional review board of the Medical College of Wisconsin (Protocol PRO00023318).
Results
Case Reports
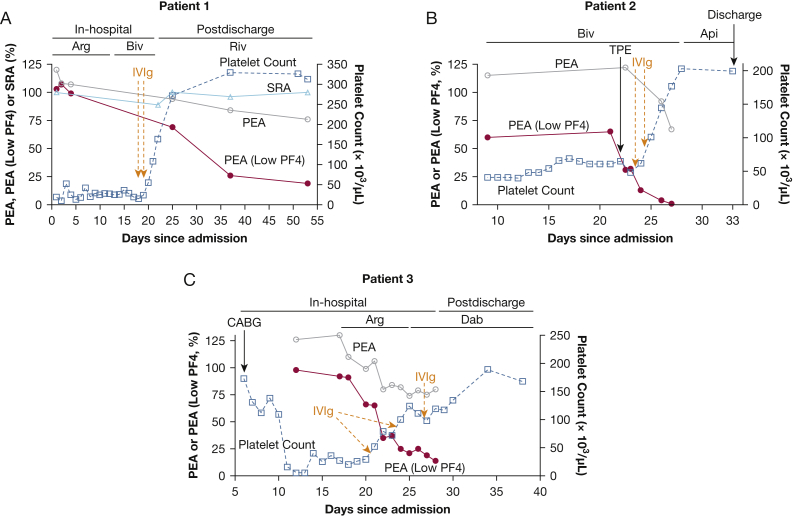
Patient 1: A 47-year-old man was readmitted for complaints of left leg numbness and weakness 13 days after heparin exposure during coronary artery bypass grafting (CABG). Imaging studies demonstrated thrombosis of veins and arteries in the left leg, right pulmonary embolism, and a right atrial thrombus, and laboratory studies showed severe thrombocytopenia (13,000/μL). IgG-specific polyvinyl sulfonate-PF4 ELISA (PF4 ELISA)22 and SRA23 were strongly positive at 2.70 optical density (OD) and 100% release, respectively. He was given the direct thrombin inhibitor argatroban, and thrombectomy and revascularization procedures were performed. Despite multiple platelet transfusions, dexamethasone treatment, and a switch from argatroban to bivalirudin to exclude the possibility of argatroban-induced thrombocytopenia, severe thrombocytopenia persisted for about 2.5 weeks (Fig 1A). He was then treated with 1 g/kg/d of IVIg on 2 consecutive days. Platelet counts rose rapidly to 55,000, 108,000, and 164,000/μL over the next 3 days. He was given rivaroxaban 15 mg twice daily at a platelet count of 164,000/uL and then transitioned to 20 mg once daily. He continues to take the DOAC 8 months postdischarge and has been free of thrombocytopenia and thrombosis during this period.
Figure 1.
IVIg treatment led to prompt recovery of platelet counts. Left ordinate denotes (A-C) PEA, PEA (low PF4), and (A) SRA test results. Right ordinate depicts platelet count. PEA (open circle) and PEA (Low PF4, closed circle) results shown are the average of duplicate measurements, and SRA (open triangles) and platelet counts (open squares) shown are single determinations. Orange arrows and dashes denote IVIg treatment (1g/kg body weight). Api = apixaban; Arg = argatroban; Biv = bivalirudin; Dab = dabigatran; PEA = PF4-dependent P-selectin expression assay; PF4 = platelet factor 4; Riv = rivaroxaban.
Patient 2: A 73-year-old man was readmitted with shortness of breath 9 days after receiving heparin during a CABG procedure. Investigations revealed severe thrombocytopenia (25,000/μL), 2.30 OD by PF4 ELISA, 100% release by the SRA, and pulmonary embolism. He was treated with bivalirudin but remained severely thrombocytopenic for about 3 weeks (Fig 1B). Therapeutic plasma exchange (TPE) was performed on the 22nd day of admission but had no effect on platelet counts. IVIg 1 g/kg/d was then administered on 2 consecutive days. Platelet counts rose sharply to 101,000/μL, 145,000/μL, and 177,000/μL over the next 3 days (Fig 1B). He was given apixaban 5 mg twice daily at a platelet count of 203,000/μL and continues on the drug approximately 7 months after discharge. He has remained free of thrombocytopenia and thrombosis during this period. He was rehospitalized twice, however, once for sepsis due to infection of a decubitus ulcer that developed during his prolonged hospitalization for HIT and a second admission due to dehydration and hypotension secondary to diarrhea.
Patient 3: A 72-year-old man was admitted for chest pain secondary to coronary artery disease. He was treated with heparin and underwent cardiac catheterization and CABG procedures. Four days after surgery, his platelet counts decreased from a baseline of 173,000/μL to 109,000/μL and further to 16,000/μL the following day (Fig 1C). HIT was confirmed with strong positive PF4 ELISA and SRA results of 2.60 OD and 100% release, respectively, and imaging revealed bilateral lower-extremity deep vein thrombosis. Despite no exposure to heparin since his CABG procedure, the platelet count was persistently low at 29,000/μL 2 weeks later (Fig 1C). Two doses of 1 g/kg IVIg were administered 2 days apart and resulted in a rapid increase in platelet counts to > 50,000/μL and 100,000/μL, respectively, within 24 h of each dose. A third IVIg dose of 0.5 g/kg was given 3 days after the second dose due to a decline in platelet count from 124,000/μL to 111,000/μL. He was given dabigatran 150 mg twice daily at a platelet count of 111,000/μL. Two weeks later, while he was still receiving the drug, he presented to the ED with severe hypotension (systolic pressures in the 60s) and shortness of breath. A pericardial effusion with tamponade physiology complicated by ischemic hepatitis and acute kidney injury was identified. The effusion, reported as hemorrhagic, was deemed “likely secondary to postpericardiotomy syndrome,” although dabigatran-related bleeding cannot be ruled out. Urgent pericardiocentesis was performed and resulted in prompt improvement of liver and kidney function. He then underwent anticoagulation with warfarin in place of dabigatran due to a concern that bilateral deep vein thrombosis may have progressed while he was taking the DOAC, although imaging results suggested that the clots were not occlusive and were likely old. The patient had normal platelet counts throughout this admission. He has remained on warfarin since that time and is free of thrombocytopenia and thrombosis 112 days postdischarge.
PEA Results Obtained With Platelets Treated With Low Concentrations of PF4 Correlated Well with Response to IVIg
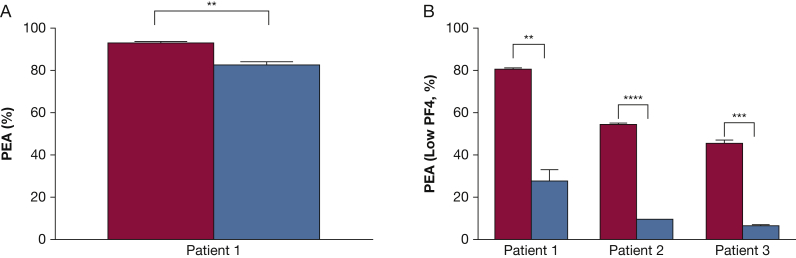
The dramatic increase in platelet counts seen in all three patients following IVIg treatment was consistent with the possibility that IgG at high plasma levels achieved after treatment competed with HIT antibody Fc domains for binding to the platelet IgG receptor FcγRIIa, thus inhibiting activation and clearance of platelets from the circulation. However, IVIg levels expected to be achieved with a dose of 2 g/kg produced only a slight inhibition (10%) of P-selectin expression induced by patient 1’s serum in the “standard” PEA21 (Fig 2A). Likewise, despite achievement of normal platelet counts after IVIg treatment, the PEA (and the SRA, tested in patient 1) continued to give strongly positive HIT test results in all three patients (Fig 1A-C). These findings seemed inconsistent with the rapid platelet recovery seen after IVIg treatment.
Figure 2.
Effect of IVIg on HIT antibody-mediated platelet activation. A and B, IVIg was added to patient serum to produce a final IgG concentration equivalent to that achieved after treatment at 2 g/kg (blue bars) or no treatment (red bars). A, PEA or (B) PEA (low PF4) was then performed and is depicted by the ordinate. Figures show mean + 1 SD of triplicate measurements. Mean values were compared with results obtained with untreated serum using the Student t test. Asterisks denote P < .01 (**), P < .001 (***), and P < .0001 (****). See Figure 1 legend for expansion of abbreviations.
A possible explanation for these findings was that strong platelet-activating HIT antibodies like those seen in patients with severe HIT might be capable of activating platelets primed with very small amounts of PF4. To test this possibility, studies were done using platelets treated with significantly lower concentrations of PF4 (“low PF4,” 1.25-3.75 μg/mL) than that used in the standard assay (PEA, 37.5 μg/mL).21 We found that under these conditions, IVIg at concentrations achieved in vivo with a 2 g/kg dose significantly inhibited P-selectin expression on PF4-treated platelets induced by all three patient samples (Fig 2B). Likewise, the PEA (low PF4) was strongly positive in all three patients before IVIg treatment but was much weaker after treatment, coinciding with the rapid increase in platelet counts (Fig 1A-C).
Fc Is Necessary and Sufficient for IVIg-Mediated Inhibition of HIT Antibody-Induced Platelet Activation
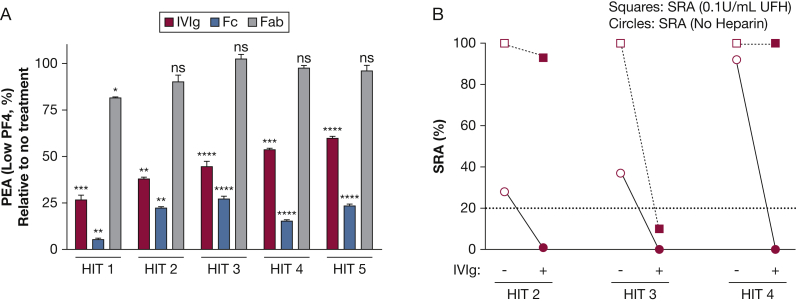
To further understand the IVIg effect, we studied five additional patients with severe HIT whose samples were available in adequate quantities (Table 1). Four of these patients had life- or limb- threatening thrombosis, whereas one had severe protracted thrombocytopenia. All patients were unresponsive to standard treatments, necessitating the use of TPE as salvage therapy. In each case, IVIg significantly inhibited antibody-mediated platelet activation in the PEA (low PF4) at concentrations achievable in vivo (Fig 3A). Studies with purified Fc and Fab fragments showed that Fc was both necessary and sufficient for the inhibitory effect, whereas Fab fragments had little or no effect (Fig 3A). Interestingly, on a molar basis, Fc fragments inhibited activation more effectively than did intact IVIg. Three of these five HIT samples activated platelets in the SRA without the addition of heparin (Fig 3B). Similar to findings made with the PEA (low PF4) (Figs 2B, 3A), IVIg was highly effective in inhibiting serotonin release in the SRA when no heparin was added compared with the “standard” SRA performed with 0.1 units/mL of heparin (Fig 3B). e-Figure 1 further demonstrates, using three additional IVIg preparations, that the inhibitory effect of IVIg was not preparation specific.
Table 1.
Serologic Characteristics and Clinical Features of Additional Patients With Severe HIT
| Patient | Age, y | Sex | SRA, % | Delayed-onset HIT | Thrombosis | Digit/Limb Amputation | Salvage TPE | Mortality |
|---|---|---|---|---|---|---|---|---|
| HIT 1 | 51 | F | 106 | – | + | + | + | – |
| HIT 2 | 51 | F | 92 | + | + | + | + | – |
| HIT 3 | 45 | M | 99 | – | + | – | + | + |
| HIT 4 | 68 | M | 109 | – | + | – | + | – |
| HIT 5 | 70 | M | 107 | + | – | – | + | – |
Delayed-onset HIT = development of heparin-induced thrombocytopenia after heparin treatment had ceased; SRA = serotonin release assay; TPE = therapeutic plasma exchange.
Figure 3.
Effect of IVIg, Fc, and Fab fragments on HIT antibody-mediated platelet activation in the PEA (low PF4) and SRA. A, IVIg equivalent to the concentration achieved in vivo following treatment with 2 g/kg (red bars), Fc fragments (blue bars), or Fab fragments (gray bars) were added to serum samples obtained from five patients who had severe HIT (Fc and Fab fragments were used at equimolar and twice-molar amounts relative to IgG, respectively). Samples were tested in the PEA (low PF4) shown on the ordinate. Figures show mean + 1 SD of triplicate measurements. Mean values were compared with results obtained with untreated serum using the Student t test. Asterisks denote P < .05 (*), P < .01 (**), P < .001, (***), and P < .0001 (****). B, IVIg equivalent to the concentration achieved in vivo following treatment with 2 g/kg (closed circles and closed squares) or buffer (open circles and open squares) was added to serum samples obtained from three patients experiencing severe HIT (HIT patients 2-4). The SRA, shown on the ordinate, was measured as a single determination in the absence of heparin (circles) or in the presence of 0.1 units/mL unfractionated heparin (squares). The dotted line (20%) indicates the cutoff for a positive result. Fab = fragment antigen-binding; Fc = fragment crystallizable; HIT = heparin-induced thrombocytopenia; SRA = serotonin release assay; UFH = unfractionated heparin. See Figure 1 legend for expansion of other abbreviations.
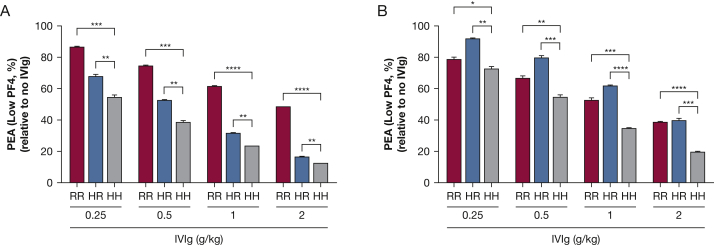
FcγRIIa HH131 Platelets Are Most Susceptible to IVIg-Mediated Inhibition of Platelet Activation
The platelet FcγRIIa receptor possesses a common H/R 131 polymorphism.24 IgG2, an isotype accounting for approximately one-third of total IgG, binds poorly to the R131 allele of FcγRIIa, whereas IgG1 binds both R131 and H131.10 Using samples from two patients with severe HIT (HIT patients 1 and 2), we found that IVIg inhibited HIT antibody-induced platelet activation in the PEA (low PF4) with all platelet genotypes (HH, HR, and RR131), but the extent of inhibition was maximal when HH131 platelets were used as targets (Figs 4A, 4B). Platelet quiescence mediated by IVIg was dose-dependent, with the greatest inhibition of P-selectin expression seen with the highest dose of IVIg used (Figs 4A, 4B). Notably, the three patients described in this report possessed the FcγRIIa RR131 (patients 1 and 2) or HR131 (patient 3) genotypes, yet all three patients responded well to IVIg treatment. As expected, IVIg treatment had no impact on PF4 ELISA results (e-Fig 2), consistent with the proposed mechanism of action through platelet FcγRIIa blockade.
Figure 4.
FcγRIIa HH131 platelets are most susceptible to inhibition by IVIg. IVIg was added to serum from two patients with severe HIT (A, HIT patient 1 and B, HIT patient 2) to produce IgG concentrations achieved after treatment with IVIg at doses shown on the abscissa (see Methods section). Samples were then tested in the PEA (low PF4) using platelets from donors with RR, HR, and HH131 FcγRIIa genotypes. P-selectin expression (percent of value obtained with untreated serum) is shown on the ordinate. Figures show mean + 1 SD of triplicate measurements. Mean values were compared using the Student t test. Asterisks denote P < .05 (*), P < .01 (**), P < .001, (***), and P < .0001 (****). See Figures 1 and 3 legends for expansion of abbreviations.
Discussion
The literature contains 10 reports describing the use of IVIg in HIT/presumed HIT, most involving only one case.11, 12, 13, 14, 15, 16, 17, 18, 19, 20 In three of these reports, no HIT testing was performed, and thus the diagnosis was unconfirmed.12, 13, 14 In three others in which only the PF4 ELISA, a relatively nonspecific test, was performed and reported as “positive” or “detected,” the lack of quantitative results (optical density) limit conclusions about whether the patients studied actually had HIT.15, 16, 17 In the largest study, involving three patients, two had low-positive PF4 ELISA ODs of 0.58 and 0.76, respectively, typically associated with a low probability of HIT.11, 25 A previous study from 1994 by Greinacher et al26 evaluated the role of IVIg in inhibiting HIT antibody-mediated platelet activation in vitro. Three of four IVIg preparations used in their study were either completely ineffective or only modestly effective in this respect, and no correlative studies in patients were carried out. Most expert reviews and evidence-based practice guidelines on HIT treatment fail to list IVIg as a treatment option,27, 28, 29, 30, 31, 32, 33, 34, 35 and the few that mention it classify IVIg as an unproven1 or possibly effective therapy.36 One consensus conference report on IVIg use in hematologic disease even lists IVIg as a contraindicated drug in HIT.37 Thus, there is no consensus on the role of IVIg in HIT treatment in both experimental reports and expert reviews due to limited, unclear, or conflicting information on the efficacy of this drug in HIT.
The three patients with severe HIT described here were refractory to standard therapy, yet they experienced rapid and sustained improvement following IVIg treatment. The timing of these responses and complementary laboratory studies showing that IVIg inhibited platelet activation in vitro leave little doubt about a cause and effect relationship between IVIg infusion and clinical improvement. Although our laboratory findings support the concept that patients with the FcγRIIa RR131 or HR131 genotype may be relatively resistant to IVIg treatment, HIT antibody-mediated activation of platelets of all FcγRIIa genotypes (HH, HR, and RR131) was inhibited at least 50% by concentrations of IgG attained with a 2 g/kg dose. This level of inhibition may be adequate to mediate a clinical response even in patients who have FcγRIIa RR131/HR131 genotypes. Further studies are needed to define whether IVIg can be used at lower doses in patients with FcγRIIa HH131. Our studies also demonstrate that the efficacy of IVIg in inhibiting HIT antibody-induced platelet activation is not specific to a particular preparation of the drug.
Two assays used to detect platelet-activating antibodies in these cases, the SRA and the “standard” PEA, continued to give strong positive results even after restoration of platelet levels to normal following IVIg treatment. Findings made with the SRA are not surprising, since it is well documented that the median time to a negative SRA (50 days)38 is much longer than the median time to platelet recovery (4 days).39 We tested the possibility that potent antibodies found in patients with severe HIT might be able to activate platelets pretreated with small amounts of PF4 (PEA, low PF4) and found that this was true of all three patients described here as well as five others (HIT patients 1-5) who had presented previously with severe disease. It is apparent that PEA results obtained with platelets primed with low doses of PF4 correlated best with each patient’s clinical course following IVIg therapy. Similarly, platelet activation induced by three sera that were tested in the SRA in the absence of heparin was significantly inhibited by IVIg, whereas platelet activation induced by only one of these samples was inhibited by IVIg in the “standard” SRA (using low-dose heparin). Further studies are under way to evaluate the diagnostic utility of the low-PF4 PEA and SRA performed in the absence of heparin.
Conclusions
HIT continues to be an important cause of morbidity and mortality in hospitalized patients.1 Findings made in IVIg-treated patients with HIT described here and with samples from other patients who had severe disease indicate that the use of IVIg in patients refractory to standard therapy deserves careful prospective study. Since IVIg treatment by itself may predispose to thromboembolism,40 treatment decisions should be made cautiously after individual risk-benefit assessment.
Acknowledgments
Author contributions: A. P. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. P., C. G. J., S. M. P., B. R. C., D. W. B., M. S. I., B. D., R. W., D. W., and R. H. A. were involved in study design and development. C. G. J. and S. M. P performed the experiments described. B. J. B, J. B. A., T. G. D., and K. P. M provided helpful suggestions and patient correlates. A. P. analyzed the data. R. H. A. provided advice on all aspects of the study. A. P. wrote the manuscript and all authors edited and approved the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. P., C. G. J., D. W. B., and R. H. A. have filed a patent application related to the PF4-dependent P-selectin expression assay (Method of Detecting Platelet-Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591). None declared (S. M. P., B. R. C., M. S. I., B. J. B., J. B. A.,T. G. D., K. P. M., B. D., R. W., D. W.).
Other contributions: We thank Amanda VanSandt, DO, from the Oregon Health & Science University for assistance in obtaining patient samples for testing. We also thank Merav Sendowski, MD (Oregon Health & Science University), David Garcia, MD (University of Washington), and Joshua Field, MD (BloodCenter of Wisconsin), for helpful suggestions and critical review of the manuscript.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
Findings contained in this manuscript were presented in part at the American Society of Hematology Annual Meeting and Exposition, December 3-6, 2016, San Diego, CA.
FUNDING/SUPPORT: This study was supported in part by funds from the American Heart Association (A. P.), CTSI of Southeastern Wisconsin (A. P.), and the National Institutes of Health [grant HL-13629 to R. H. A].
Supplementary Data
References
- 1.Greinacher A. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252–261. doi: 10.1056/NEJMcp1411910. [DOI] [PubMed] [Google Scholar]
- 2.Warkentin T.E., Basciano P.A., Knopman J., Bernstein R.A. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123(23):3651–3654. doi: 10.1182/blood-2014-01-549741. [DOI] [PubMed] [Google Scholar]
- 3.Rice L., Attisha W.K., Drexler A., Francis J.L. Delayed-onset heparin-induced thrombocytopenia. Ann Intern Med. 2002;136(3):210–215. doi: 10.7326/0003-4819-136-3-200202050-00009. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin T.E., Kelton J.G. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(7):502–506. doi: 10.7326/0003-4819-135-7-200110020-00009. [DOI] [PubMed] [Google Scholar]
- 5.Warkentin T.E. Agents for the treatment of heparin-induced thrombocytopenia. Hematol Oncol Clin North Am. 2010;24(4):755–775. doi: 10.1016/j.hoc.2010.05.009. ix. [DOI] [PubMed] [Google Scholar]
- 6.Warkentin T.E. Ischemic limb gangrene with pulses. N Engl J Med. 2015;373(7):642–655. doi: 10.1056/NEJMra1316259. [DOI] [PubMed] [Google Scholar]
- 7.Lewis B.E., Wallis D.E., Berkowitz S.D. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103(14):1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 8.Nanwa N., Mittmann N., Knowles S. The direct medical costs associated with suspected heparin-induced thrombocytopenia. Pharmacoeconomics. 2011;29(6):511–520. doi: 10.2165/11584330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.McGowan K.E., Makari J., Diamantouros A. Reducing the hospital burden of heparin-induced thrombocytopenia: impact of an avoid-heparin program. Blood. 2016;127(16):1954–1959. doi: 10.1182/blood-2015-07-660001. [DOI] [PubMed] [Google Scholar]
- 10.Rollin J., Pouplard C., Sung H.C. Increased risk of thrombosis in FcgammaRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood. 2015;125(15):2397–2404. doi: 10.1182/blood-2014-09-594515. [DOI] [PubMed] [Google Scholar]
- 11.Winder A., Shoenfeld Y., Hochman R., Keren G., Levy Y., Eldor A. High-dose intravenous gamma-globulins for heparin-induced thrombocytopenia: a prompt response. J Clin Immunol. 1998;18(5):330–334. doi: 10.1023/a:1023238915316. [DOI] [PubMed] [Google Scholar]
- 12.Frame J.N., Mulvey K.P., Phares J.C., Anderson M.J. Correction of severe heparin-associated thrombocytopenia with intravenous immunoglobulin. Ann Intern Med. 1989;111(11):946–947. doi: 10.7326/0003-4819-111-11-946. [DOI] [PubMed] [Google Scholar]
- 13.Grau E., Linares M., Olaso M.A., Ruvira J., Sanchis J. Heparin-induced thrombocytopenia—response to intravenous immunoglobulin in vivo and in vitro. Am J Hematol. 1992;39(4):312–313. doi: 10.1002/ajh.2830390417. [DOI] [PubMed] [Google Scholar]
- 14.Cho F.N., Liu C.B. Potential role of intravenous immunoglobulin in the management of peripartum maternal thrombocytopenia due to various causes. J Chin Med Assoc. 2008;71(5):267–269. doi: 10.1016/S1726-4901(08)70119-9. [DOI] [PubMed] [Google Scholar]
- 15.Betrosian A.P., Theodossiades G., Lambroulis G. Heparin-induced thrombocytopenia with pulmonary embolism and disseminated intravascular coagulation associated with low-molecular-weight heparin. Am J Med Sci. 2003;325(1):45–47. doi: 10.1097/00000441-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Betrosian A.P., Theodossiades G., Balla M., Diakalis C., Georgiades G. Heparin-induced thrombocytopenia: correction of severe bleeding complication with intravenous immune globulin. Intensive Care Med. 2004;30(11):2136. doi: 10.1007/s00134-004-2437-8. author reply 2137. [DOI] [PubMed] [Google Scholar]
- 17.Gul E.E., Abdulhalikov T., Aslan R., Aydogdu I. A rare and undesirable complication of heparin-induced thrombocytopenia: acute massive pulmonary embolism. Clin Appl Thromb Hemost. 2011;17(5):546–548. doi: 10.1177/1076029610379399. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin T.E., Sheppard J.A. Serological investigation of patients with a previous history of heparin-induced thrombocytopenia who are reexposed to heparin. Blood. 2014;123(16):2485–2493. doi: 10.1182/blood-2013-10-533083. [DOI] [PubMed] [Google Scholar]
- 19.Tvito A., Bakchoul T., Rowe J.M., Greinacher A., Ganzel C. Severe and persistent heparin-induced thrombocytopenia despite fondaparinux treatment. Am J Hematol. 2015;90(7):675–678. doi: 10.1002/ajh.23971. [DOI] [PubMed] [Google Scholar]
- 20.Prull A., Nechwatal R., Riedel H., Maurer W. The therapy of the heparin-induced thrombosis-thrombocytopenia syndrome with immunoglobulins. Dtsch Med Wochenschr. 1992;117(48):1838–1842. doi: 10.1055/s-2008-1062518. [in German] [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan A., Jones C.G., Curtis B.R. A novel PF4-dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis. Chest. 2016;150(3):506–515. doi: 10.1016/j.chest.2016.02.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarland J., Lochowicz A., Aster R., Chappell B., Curtis B. Improving the specificity of the PF4 ELISA in diagnosing heparin-induced thrombocytopenia. Am J Hematol. 2012;87(8):776–781. doi: 10.1002/ajh.23248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan D., Carter C., Kelton J.G. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 24.Burgess J.K., Lindeman R., Chesterman C.N., Chong B.H. Single amino acid mutation of Fc gamma receptor is associated with the development of heparin-induced thrombocytopenia. Br J Haematol. 1995;91(3):761–766. doi: 10.1111/j.1365-2141.1995.tb05383.x. [DOI] [PubMed] [Google Scholar]
- 25.Warkentin T.E., Sheppard J.I., Moore J.C., Sigouin C.S., Kelton J.G. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304–1312. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 26.Greinacher A., Liebenhoff U., Kiefel V., Presek P., Mueller-Eckhardt C. Heparin-associated thrombocytopenia: the effects of various intravenous IgG preparations on antibody mediated platelet activation—a possible new indication for high dose i.v. IgG. Thromb Haemost. 1994;71(5):641–645. [PubMed] [Google Scholar]
- 27.Cuker A. Management of the multiple phases of heparin-induced thrombocytopenia. Thromb Haemost. 2016;116(5):835–842. doi: 10.1160/TH16-02-0084. [DOI] [PubMed] [Google Scholar]
- 28.Warkentin T.E., Greinacher A. Management of heparin-induced thrombocytopenia. Curr Opin Hematol. 2016;23(5):462–470. doi: 10.1097/MOH.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 29.Scully M., Gates C., Neave L. How we manage patients with heparin induced thrombocytopenia. Br J Haematol. 2016;174(1):9–15. doi: 10.1111/bjh.14102. [DOI] [PubMed] [Google Scholar]
- 30.Salter B.S., Weiner M.M., Trinh M.A. Heparin-induced thrombocytopenia: a comprehensive clinical review. J Am Coll Cardiol. 2016;67(21):2519–2532. doi: 10.1016/j.jacc.2016.02.073. [DOI] [PubMed] [Google Scholar]
- 31.McKenzie S.E., Sachais B.S. Advances in the pathophysiology and treatment of heparin-induced thrombocytopenia. Curr Opin Hematol. 2014;21(5):380–387. doi: 10.1097/MOH.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuker A., Cines D.B. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209–2218. doi: 10.1182/blood-2011-11-376293. [DOI] [PubMed] [Google Scholar]
- 33.Lee G.M., Arepally G.M. Heparin-induced thrombocytopenia. Hematology Am Soc Hematol Educ Program. 2013;2013:668–674. doi: 10.1182/asheducation-2013.1.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warkentin T.E. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143–149. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 35.Linkins L.A., Dans A.L., Moores L.K. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e495S–530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warkentin T.E., Greinacher A. Treatment of heparin-induced thrombocytopenia: an overview. In: Warkentin T.E., Greinacher A., editors. Heparin-Induced Thrombocytopenia. 5th ed. Informa Healthcare; Boca Raton, FL: 2013. pp. 315–355. [Google Scholar]
- 37.Anderson D., Ali K., Blanchette V. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21(2 suppl 1):S9–S56. doi: 10.1016/j.tmrv.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Warkentin T.E., Kelton J.G. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- 39.Warkentin T.E. Think of HIT. Hematology Am Soc Hematol Educ Program. 2006:408–414. doi: 10.1182/asheducation-2006.1.408. [DOI] [PubMed] [Google Scholar]
- 40.Ammann E.M., Jones M.P., Link B.K. Intravenous immune globulin and thromboembolic adverse events in patients with hematologic malignancy. Blood. 2015;127(2):200–207. doi: 10.1182/blood-2015-05-647552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.