Abstract
Environmental stressors, such as pollutants, can increase disease risk in wildlife. For example, the herbicide atrazine affects host defenses (e.g. resistance and tolerance) of the amphibian chytrid fungus Batrachochytrium dendrobatidis (Bd), but the mechanisms for these associations are not always clear. Given that pollutants can alter the gut microbiota of hosts, which in turn can affect their health and immune systems, one potential mechanism by which pollutants could increase infection risk is by influencing host-associated microbiota.
Here, we test whether early-life exposure to the estimated environmental concentration (EEC; 200 μg/L) of atrazine affects the gut bacterial composition of Cuban tree frog (Osteopilus septentrionalis) tadpoles and adults and whether any atrazine-induced change in community composition might affect host defenses against Bd. We also determine whether early-life changes in the stress hormone corticosterone affect gut microbiota by experimentally inhibiting corticosterone synthesis with metyrapone.
With the exception of changing the relative abundances of two bacterial genera in adulthood, atrazine did not affect gut bacterial diversity or community composition of tadpoles (in vivo or in vitro) or adults. Metyrapone did not significantly affect bacterial diversity of tadpoles, but significantly increased bacterial diversity of adults.
Gut bacterial diversity during Bd exposure did not predict host tolerance or resistance to Bd intensity in tadpoles or adults. However, early-life bacterial diversity negatively predicted Bd intensity as adult frogs. Specifically, Bd intensity as adults was associated negatively with the relative abundance of phylum Fusobacteria in the guts of tadpoles.
Our results suggest that the effect of atrazine on Bd infection risk is not mediated by host-associated microbiota because atrazine does not affect microbiota of tadpoles or adults. However, host-associated microbes seem important in host resistance to Bd because the early-life microbiota, during immune system development, predicted later-life infection risk with Bd. Overall, our study suggests that increasing gut bacterial diversity and relative abundances of Fusobacteria might have lasting positive effects on amphibian health.
Keywords: atrazine, bacteria, Batrachochytrium dendrobatidis, chytrid fungus, corticosterone, Fusobacteria, Osteopilus septentrionalis, stress
Introduction
Anthropogenic factors, such as pollutants, can dramatically affect the health of organisms (Newman 1979; Vitousek et al. 1997; Martin et al. 2010). Pollutants directly affect the development, reproductive output, and survival of organisms and indirectly affect fitness by increasing disease risk (Fig. 1, paths a-b) (Rohr et al. 2008, 2013b; Martin et al. 2010). For example, pollutants can decrease immune function and thus decrease resistance to infection (Arkoosh et al. 1998; Rowe et al. 2006; Rohr et al. 2008; Koprivnikar 2010; reviewed in Martin et al. 2010); host resistance reduces parasite damage by reducing parasite fitness (Read, Graham & Råberg 2008; Schmid-Hempel 2011). Additionally, exposure to pollutants, such as the herbicide atrazine, can reduce amphibian tolerance of the fungal pathogen, Batrachochytrium dendrobatidis (Bd) (Rohr et al. 2013); host tolerance minimizes damage caused by parasites without affecting parasite fitness (Miller, White & Boots 2006; Råberg, Sim & Read 2007; Read et al. 2008; Medzhitov, Schneider & Soares 2012). Bd is an important pathogen because it has contributed, in part, to the global decline of amphibians (Wake & Vredenburg 2008), which are the most threatened class of vertebrates in the world (Stuart et al. 2004). Therefore, it is important to understand the mechanisms by which pollutants affect diseases like Bd in order to mitigate the impact of these factors on hosts. Despite mounting evidence that pollutants increase infection risk, mechanisms mediating these increases remain relatively untested.
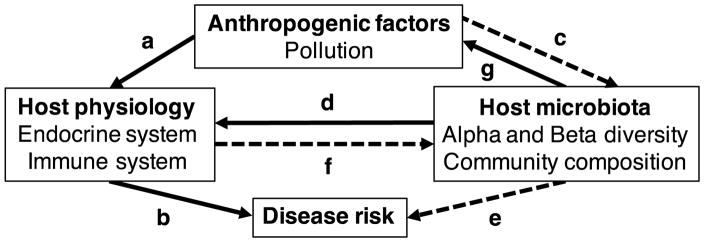
Fig. 1.
Potential interactions among anthropogenic factors, such as pollution, host-associated microbiota and physiology, and disease risk. Pathways are designated with letters and described in the main text. Dotted lines indicate the pathways for which we addressed in this study.
One potential mechanism for how pollutants increase disease risk is by altering the symbiotic microbiota of the host (Fig. 1, e.g. paths c-e, g-a-b, a-f-e) (Claus, Guillou & Ellero-Simatos 2016). Many microbes are instrumental in breaking down pollutants in the environment (Fig. 1, path g) (Horvath 1972; Häggblom 1992; Bansal 2012; Staley, Harwood & Rohr 2015) and thus the contaminants can serve as a resource for microbiota, potentially increasing some of their abundances. In contrast, many contaminants can be directly toxic to microbiota (Fig. 1 path c) (Staley et al. 2015). In hosts, exposure to pollutants, especially during formative stages of life, can induce immediate and lasting changes to gut bacterial communities (Fig. 1, path c) (Shehata et al. 2013; Kohl et al. 2015). Changes to the normal gut and skin microbiota of hosts just before parasite exposure have been shown to decrease host resistance (Fig. 1, path e) (Koch & Schmid-Hempel 2011; Theriot et al. 2014; Schuijt et al. 2016; Schwarz, Moran & Evans 2016; Woodhams et al. 2016). For example, the gut bacterial community can protect their host from infections through direct competition with the parasite (Fig. 1, path e) (Dethlefsen, McFall-Ngai & Relman 2007; Costello et al. 2012). In contrast to this direct effect, host-associated microbiota may indirectly affect infections by influencing the maintenance or development of the immune system (Macpherson & Harris 2004; Round & Mazmanian 2009; Hooper, Littman & Macpherson 2012), which in turn, can increase later-life infection risk (Fig. 1, paths d-b) (Knutie et al. 2017b).
Amphibians may be at high risk of alterations to their early-life microbiota via pollutants because most amphibians spend their formative stages in water bodies that frequently receive run-off containing chemical contaminants (Schwarzenbach et al. 2006). Several studies have found that amphibian skin and gut microbiota is important in determining infection risk. For example, Woodhams et al. (2016) found that several bacterial taxa, such as Pseudomonas sp., Janthinobacterium lividum, and Rhodococcus fascians, produce volatile antifungal compounds that directly reduce the growth of Bd, which suggests a direct interaction between the microbiota and infection risk. Additionally, Knutie et al. (2017) found that an early-life disruption of the gut microbiota affects later-life resistance to a parasitic gut worm infection; these results suggests that there is an indirect interaction between the microbiota and infection risk, which is likely mediated by the immune system (Fig. 1, paths d-b). If pollutants interact with the early-life microbiota of amphibians during immune development and disruptions in microbiota affect later-life infection risk, then the microbiota may be mediating the effect of early-life exposure to pollutants on later-life infection risk.
Host-associated microbiota may not be the only factor mediating the effect of pollutants on disease risk. Pollutants can affect host physiology, such as their endocrine and immune systems (Fig. 1, path a), which can alter their ability to resist and tolerate infections (Fig. 1, paths a-b) (Martin et al. 2010). For example, pollutants can cause dysregulation of the stress hormone corticosterone (Laws et al. 2009; McMahon et al. 2011) and can decrease host immunity (Hopkins, Mendonça & Congdon 1999; Bellinger, Lubahn & Lorton 2008). Interestingly, corticosterone can also interact with host-associated microbiota (Fig. 1, paths d-f) (Clarke et al. 2014). For example, corticosterone levels in hosts have been shown to be negatively correlated with bacterial diversity (Stothart et al. 2016), which is likely mediated by the immune system (O’Mahony et al. 2009, Bailey et al. 2011).
Gabor et al. (2017) recently conducted a study related to our experiment to determine whether the effect of atrazine on Bd infection risk in Cuban tree frog (Osteopilus septentrionalis) tadpoles and post-metamorphic frogs (from here on we refer to them as adults) was mediated by corticosterone. In this study, Gabor et al. (2017) exposed tadpoles to metyrapone, a corticosterone synthesis inhibitor, and atrazine (in a fully crossed design) and then inoculated tadpoles and adults with Bd. While metyrapone countered atrazine-induced corticosterone elevations, atrazine exposure reduced Bd abundance across life stages in the absence of metyrapone but increased abundance in the presence of metyrapone. Although atrazine affected host tolerance of Bd (also shown in Rohr et al. 2013), Gabor et al. (2017) found that metyrapone did not mediate this relationship.
In the present paper, we explore whether another mechanism, i.e. host-associated gut microbiota, mediates the effect of atrazine on Bd infections (Fig. 1, paths c-e; Fig. 2). Skin bacterial taxa (e.g. Janthinobacterium lividum, Pseudomonas sp., and Rhodococcus fascians) of frogs can release metabolites that can inhibit Bd (Woodhams et al. 2014, 2015, 2016), however we are unaware of any studies linking skin bacteria to immune development in any vertebrate. In contrast, gut bacteria, the focus of our study, has been associated with effects on parasite fitness through direct (Koch & Schmid-Hempel 2011; Theriot et al. 2014; Schuijt et al. 2016; Schwarz et al. 2016) and indirect pathways, such as by affecting immune system development (Round & Mazmanian 2009; Hooper et al. 2012). For example, an early-life disruption of frog gut microbiota affected later-life resistance to parasites, a result attributed to the effect of gut microbiota on amphibian immune development (Knutie et al. 2017b). Thus, the present study will explore how the microbiota of the gut affects parasites on the skin (probably via the immune system), which has received little attention.
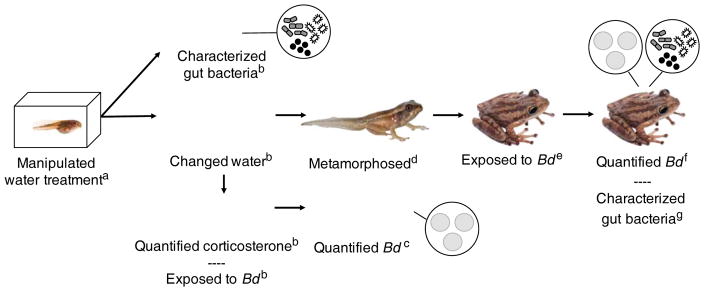
Fig. 2.
Methods to determine the relationships among atrazine exposure, corticosterone levels, host-associated gut microbiota, and Bd infection risk in Cuban tree frogs. Superscript letters represent the timing of treatment exposure and sampling effort: aOn day one, tadpoles were placed in tanks with water that was treated experimentally with atrazine and/or metryapone using a 2×2 factorial design (8 tadpoles per tank). bOn day six, tadpoles from each tank: 1) were euthanized and their gut bacterial community was characterized (n = 1 tadpole per tank), 2) remained in their respective tanks after the pond water was changed to remove residual chemicals from the water treatment (n = ≤ 3 tadpoles per tank), or 3) were used to quantify their corticosterone levels in response to water treatment and then exposed to Bd or a solvent control; four tadpoles from each original tank were either exposed to Bd (n = 2) or the solvent control (n = 2) in new tanks. cOn day 27, Bd load was quantified from the skin of tadpoles that were exposed to Bd or the solvent control. dTadpoles that remained in their respective tanks, after the experimental water treatment of atrazine and/or metyrapone, were allowed to metamorphose, which occurred, on average, on day 50. eOn day 84, post-metamorphic (adult) frogs were exposed to Bd or the solvent control and then fBd was quantified on approximately day 100 from the skin of adults (n = ≤ 2 adults per treatment per tank). gOn day 119, adult frogs were euthanized and their gut bacterial community was characterized. Photos by Mark Yokoyama.
First, we test whether an early-life exposure to atrazine and metyrapone (in a fully crossed design) affects the gut bacteria of Cuban tree frog tadpoles and whether there are lasting effects on gut bacteria into adulthood (Fig. 1, path c). We also conduct an in vitro experiment to determine the direct effects of atrazine on the gut bacteria of tadpoles (Fig. 1, path c). Then, we sought to determine whether any changes in these bacteria affect early- and later-life resistance and tolerance of Bd. We hypothesize that exposure to atrazine changes gut bacterial communities of hosts because this pattern has been observed with other pollutants (Fig. 1, path c) (Shehata et al. 2013; Theriot et al. 2014; Kohl et al. 2015) and atrazine interacts with bacteria in the environment (Newcombe & Crowley 1999). If atrazine does alter the microbiota, we hypothesize that these changes in microbiota will be associated with changes in defenses against Bd (Fig. 1, paths c-e). Specifically, we determined whether the abundance of phylum Fusobacteria, which has been shown to affect infectious and non-infectious disease risk (McCoy et al. 2013; Scher et al. 2013; Burns et al. 2015; Morton et al. 2015; Knutie et al. 2017b), affects Bd infection risk. We also hypothesize that the effects of atrazine on microbiota is mediated by corticosterone (Fig. 1, paths a-f). If so, then the effect of atrazine on microbiota should be counteracted by the corticosterone synthesis inhibitor metyrapone.
Materials and Methods
TADPOLE COLLECTION AND HUSBANDRY
We collected multiple clutches of tadpoles of Osteopilus septentrionalis in August 2014 from the Botanical Gardens of the University of South Florida (N 28°03.537’ W 82°25.410’). We maintained them in the lab for at least a week until the majority reached Gosner stage 35 (Gosner, 1960). All tadpoles were fed a mixture of fish food and spirulina suspended in agar ad libitum and were maintained at 21°C with a 12:12 h light:dark cycle. Survival was noted daily.
ATRAZINE AND METYRAPONE EXPOSURE IN TADPOLES
We filled forty 12-liter tanks with 8-liters of water from a pond at Trout Creek Park, FL (N 28°092250’, W 082°348083’), which was not exposed to agricultural runoff (i.e., no measurable level of atrazine; see below). We assigned 16 O. septentrionalis tadpoles haphazardly to each tank. We randomly assigned each tank to one of four exposure treatments: (1) the estimated environmental concentration (EEC) of atrazine (200 μg/L based on US Environmental Protection Agency GENEEC v2 software; Chemservice, West Chester, PA; technical grade, purity more than 98%) by dissolving atrazine in 120 μL of ethanol (n = 10), (2) 110 μM of metyrapone (Sigma Chemical Co. # M2696; St. Louis, MO) dissolved in 120 μL of ethanol, (n = 10), (3) the EEC of atrazine and 110 μM of metyrapone jointly dissolved in 120 μL of ethanol (n = 11), and (4) only 120 μL of ethanol (n = 10) as a control. We used 110 μM of metyrapone because this level reduced whole body corticosterone in tadpoles by >50% (but does not block it entirely) and exposure is non-toxic (Glennemeier & Denver 2002). Previous work did not detect effects of ethanol on any measured trait, and thus a water control was not included (reviewed by Rohr et al., 2013). Tadpoles were exposed to these treatments for six days. See Fig. 2 for experimental design.
Water samples were collected from each of the 40 tanks one hour after dosing and atrazine was quantified in these samples using the Abraxis ELISA microtiter plate kit (Abraxis LLC, Warminster, PA). Mean ± SE (standard error) atrazine concentration was 178.2 ± 7.8 μg/L. All atrazine values for the non-atrazine exposed tanks were below the detection limit of 0.06 μg/L (this was the level in the pond water). We re-dosed each tank with 110 μM of metyrapone every third day (following Hossie et al. 2010). We did not re-dose with atrazine because its half-life is on the order of weeks and Rohr et al. (2004) found no detectable breakdown of atrazine over seven days under similar conditions.
After six days in the treated water, we measured the snout vent length (SVL; mm) and body mass (to the 0.001g) of one tadpole from each tank (41 total). We removed the digesta (guts) of each tadpole using sterile technique and guts were frozen at −80°C until DNA extraction. We also obtained water-borne corticosterone from two tadpoles per replicate (80 total). Briefly, we placed tadpoles individually in 250mL beakers filled with 75mL of water for one hour then measured their body mass and SVL. Water samples were frozen at −20°C immediately after collection until hormone extraction.
We also tested the direct effect of atrazine on gut bacteria of tadpoles in vitro. Ten tadpoles (that were not used in the in vivo experiment) were staged and measured before they were euthanized; their whole guts were then placed in a sterile 200 mL Nalgene glass bottle containing either atrazine in an ethanol solvent (n = 5) or solvent only (n = 5). Bottles from the atrazine treatment contained 50 mL sterile deionized water and atrazine (200 μg/L) dissolved in 0.1mL of ethanol and bottles from the control treatment contained 50mL sterile deionized water with only 0.1 mL of ethanol. We swabbed the solution after the guts were added to the bottles but immediately (<1 min) before the atrazine was added and 24 and 144 hours (six days) after the atrazine was added to collect a subsample of the bacteria. Swabs were swirled in the solution for three seconds then placed in a 1.5 mL tube and immediately frozen at −80°C until the DNA extraction.
BD EXPOSURE IN TADPOLES
A subset of eight tadpoles from each replicate was exposed to either Bd (SRS812 isolate; McMahon, Romansic & Rohr 2013) or a solvent control. Briefly, tadpoles were removed from each tank and divided between two 6-liter plastic shoeboxes with 2-liters of fresh pond water (n = 4 tadpoles per tank and 80 total tanks); one of the pairs of shoeboxes received a 6 mL inoculum containing 7×104 Bd zoospores per mL in deionized (DI) water and the other received an inoculum that was identical to the Bd inoculum but was free of Bd (i.e., we washed clean agar plates with DI water). We re-exposed all tadpoles to Bd (2 mL of 3×105 zoospores per mL) or DI water three days later and maintained the tadpoles in these boxes for a total of 21 days. We then euthanized tadpoles with an overdose of MS-222 and measured their mass and SVL. We used the quantitative PCR procedure described by Boyle et al. (2004) to quantify Bd samples taken from up to two tadpoles per Bd-exposed tank (depending on survival, n = 69 total), and a total of 10 tadpoles (each from separate tanks) that were not exposed to Bd.
BD EXPOSURE IN ADULTS
The remaining subset of tadpoles was reared through metamorphosis. Their water was changed after the six-days chemical treatment and water was subsequently changed every two weeks until all tadpoles metamorphosed. When frogs had all four limbs, individuals were removed from the tanks and placed in cups (6 cm high × 12 cm diameter) with moist Sphagnum sp. moss. The post-metamorphic frogs were maintained in the laboratory (12 h light cycle, 22°C) and fed ad libitum vitamin- and mineral-dusted crickets twice per week. Eighty-four days after the start of the experiment and approximately one month after most of the tadpoles metamorphosed, adult frogs were randomly assigned to receive an inoculum of either Bd (isolate SRS812) or solvent control (each tank had 1–2 frogs exposed to each treatment depending on survival). Adult frogs were weighed then exposed to Bd by pipetting 1 mL of 6 × 104 zoospores per mL onto the frog’s dorsal side. Excess inoculum remained in each frog’s plastic container, which contained moist sterile Sphagnum moss. Control frogs received the inoculum without Bd. Survival was monitored daily for 5 weeks. Frogs were also weighed weekly and swabbed at 2 and 3 weeks after Bd exposure. Bd from the swabs was quantified using the qPCR methods described above. Frogs were then euthanized using an overdose of Anbesol®, which was applied to the dorsal side of the frog. Frogs were then weighed, their SVL was measured, and their guts were removed using sterile technique. The guts were then frozen at −80°C until DNA extraction.
HORMONE EXTRACTION AND VALIDATION
We extracted water-borne hormones following (Gabor et al. 2017). We re-suspended the dried hormone residue in 260 μL enzyme-immunoassay (EIA) buffer (provided by Cayman Chemicals Inc., Ann Arbor, MI, USA) and we further diluted all samples to 1:2. We measured corticosterone in duplicate using a corticosterone EIA kit (Cayman Chemicals Inc.) on a spectrophotometer plate reader set to 405 nm (BioTek ELX800). We ran 4 plates, and based on our control samples our intra-plate variation ranged from 0.09 – 4.01% and the inter-plate variation was 6.02%. We previously validated the use of water-borne corticosterone collection method from O. septentrionalis on EIA plates (Gabor et al. 2017).
BACTERIAL DNA EXTRACTION AND SEQUENCING
We isolated total DNA from frog guts using a MoBio PowerFecal DNA Isolation Kit; DNA extracts were then sent to Argonne National Labs for sequencing. Microbial inventories were conducted by amplifying the V4 region of the 16S rRNA gene using primers 515F and 806R and paired end sequencing on an Illumina MiSeq platform (Caporaso et al. 2012). Sequences were analyzed using QIIME version 1.9.1 (Caporaso et al. 2010b). We applied standard quality control settings and split sequences into libraries using default parameters in QIIME. Sequences were grouped into operational taxonomic units (OTUs) using pick_open_reference_otus.py with a minimum sequence identity of 97%. The most abundant sequences within each OTU were designated as a “representative sequence” and aligned against the Greengenes core set (DeSantis et al. 2006) using PyNAST (Caporaso et al. 2010a) with default parameters set by QIIME. A PH Lane mask supplied by QIIME was used to remove hypervariable regions from aligned sequences. A phylogenetic tree of representative sequences was built using FastTree (Price, Dehal & Arkin 2009). OTUs were classified using UCLUST (Edgar 2010) against the Greengenes database (DeSantis et al. 2006). Singleton OTUs and sequences identified as chloroplasts or mitochondria were removed from the analysis. Additionally, any OTUs present in the ‘blank samples’ were considered contaminants and were removed from all other samples.
Several measurements of alpha diversity were calculated. We calculated the number of observed OTUs (species richness), equitability (species evenness), the Shannon index, and Faith’s phylogenetic diversity (Faith 1992), the latter of which measures the cumulative branch length on the phylogenetic tree of all representative sequences. For these measurements, we calculated the mean of 20 iterations for a random subsampling of 6800 sequences for in vivo tadpole and adult samples and 570 sequences for in vitro samples (the minimum number of sequences returned from each sample). We calculated unweighted and weighted UniFrac distances between samples in QIIME for bacterial community composition analyses.
STATISTICAL ANALYSES
We used general linear models (GLM) to determine the effect of water treatments (atrazine and metyrapone) on gut bacterial diversity of tadpoles (in vivo). We used a generalized linear mixed model (GLMM) to determine the direct effect of atrazine on gut bacterial diversity of tadpoles (in vitro) with bottle (i.e. replicate) as a random effect. We also used GLMs to determine the relationship between bacterial diversity and log Bd infection intensity in tadpoles and adults (measure of resistance), corticosterone release rates in tadpoles and bacterial diversity of tadpoles and adults, and bacterial diversity of tadpoles and bacterial diversity of adults. We also determined the effect of bacterial diversity on host tolerance by testing for interactions between Bd intensity and the treatments on mass loss using GLMs. We did not include atrazine treatment as a fixed effect in our analyses of the relationship between bacterial diversity and infection intensity because we did not find evidence that atrazine affected the gut microbiota. Tadpole and adult samples were collected from different individuals within the same replicate (tank) because tadpole sampling required destructive sampling.
We used generalized linear mixed models (GLMM) with Gaussian errors and tank as a random effect to determine the effect of tadpole water treatment on gut bacterial diversity of adults. We only used tank as a random effect for analyses with adults because we often had more than one individual per tank, whereas for tadpoles, we only sampled one individual per tank. We present Faith’s phylogenetic diversity as our measure of alpha diversity in the main text because it accounts for phylogenetic differences among taxa. Results based on other alpha diversity measurements can be found in the Supplemental Tables. Gaussian analyses without and with random effects were conducted using the glm (GLM) and lmer (GLMM) functions in the lme4 package. We generated ANOVA tables using the Anova function in the car package in RStudio (version 0.98.1062).
We determined the effect of tadpole water treatment on gut bacterial community membership (unweighted) and structure (weighted) with PERMANOVAs (with 999 permutations) using the PERMANOVA add-on to the software PRIMER. For adults, tank was included as a random effect. Unweighted scores represent bacterial community membership, which is based on the presence or absence of bacterial taxa, whereas weighted scores represent bacterial community structure, which also takes into account relative abundance of bacterial taxa.
To compare relative abundances of microbial taxa across treatments, we first removed any phyla that were present in less than 25% of samples (White, Nagarajan & Pop 2009). Given that the gut microbial community is largely restructured over the course of metamorphosis (Kohl et al. 2013), we compared relative abundances of bacteria in tadpoles and adult frogs separately. We determined the effect of tadpole water treatment (atrazine and metyrapone) on relative abundances (arcsine square root transformed) of bacterial phyla in tadpoles and adults using ANOVAs in JMP (version 12) with water treatment as an independent variable and for adults, tank as a random effect. For these analyses, P-values were corrected using the Benjamini-Hochberg False Discovery Rate for multiple comparisons. See Supplemental Table 1 for individual- and tank-level sample sizes for analyses on the effect of treatment on bacterial communities.
Results
EFFECT OF WATER TREATMENT ON MICROBIOTA OF TADPOLES
Tadpole water treatment did not significantly affect bacterial alpha diversity (Fig. 3a; Table S1–2), community structure (PERMANOVA, atrazine: F1,36 = 0.79, P = 0.59; metyrapone: F1,36 = 2.00, P = 0.07; interaction: F1,36 = 0.77, P = 0.62), or community membership in tadpoles in vivo (atrazine: F1,36 = 0.90, P = 0.69; metyrapone: F1,36 = 1.10, P = 0.21; interaction: F1,36 = 0.99, P = 0.46). Similarly, tadpole corticosterone release rates were not related significantly to bacterial diversity in tadpoles (Table S5; GLM, χ2 = 0.05, df = 1, P = 0.82). Metyrapone exposure significantly increased relative abundance of phyla Actinobacteria (one-way ANOVA, F = 10.66, P = 0.04) and Verrucomicrobia (F = 12.08, P = 0.04) in tadpoles. More specifically, metyrapone exposure significantly increased the relative abundance of genus Mycobacterium from phylum Actinobacteria (F = 14.39, P = 0.02) and genus Candidatus Xiphinematobacter from phylum Verrucomictrobia (F = 17.17, P = 0.007). However, atrazine and the interaction between atrazine and metyrapone did not significantly affect abundances of microbial taxa in tadpoles (all P > 0.05).
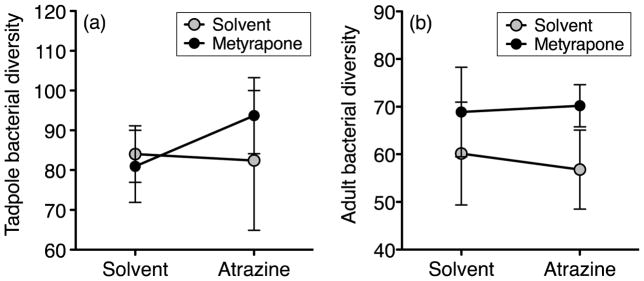
Fig. 3.
Mean (± 95% CI) alpha Faith’s bacterial diversity (phylogenetic diversity metric) across water treatments for samples from the guts of (a) tadpoles and (b) adults. Water treatment did not significantly affect bacterial diversity of tadpoles (GLM, atrazine: χ2 = 1.85, df = 1, P = 0.17, metyrapone: χ2 = 0.66, df = 1, P = 0.42, interaction: χ2 = 2.19, df = 1, P = 0.14), but adults exposed to metyrapone as tadpoles had significantly higher bacterial diversity compared to adults that were not exposed to metyrapone (GLMM, atrazine: χ2 = 0.20, df = 1, P = 0.65, metyrapone: χ2 = 4.56, df = 1, P = 0.03, interaction: χ2 = 0.54, df = 1, P = 0.47).
For the in vitro tadpole experiment, bacterial phylogenetic diversity decreased overtime (Table S3–S4; GLMM, χ2 = 5.91, df = 1, P = 0.02). However, there was no effect of atrazine on bacterial diversity (Table S3–S4; χ2 = 0.37, df = 1, P = 0.54) or an effect of the interaction between time and treatment on diversity (χ2 = 0.09, df = 1, P = 0.76). Atrazine also did not affect abundances of microbial taxa (one-way ANOVA, all P > 0.05), community structure (PERMANOVA, F1,8 = 1.40, P = 0.19), or community membership (F1,8 = 1.05, P = 0.36) after 6 days of atrazine treatment.
EFFECT OF WATER TREATMENT ON MICROBIOTA OF ADULTS
Gut bacterial diversity of tadpoles did not predict bacterial diversity of adults (Table S6). Adults exposed to metyrapone as tadpoles had significantly higher bacterial diversity (n = 9, 69.47 ± 1.89; Fig. 3b; Table S1 and S7) compared to adults not exposed to metyrapone (n = 15, 58.37 ± 2.71). Interestingly, however, bacterial diversity of adults was not significantly related to corticosterone release rates in tadpoles from the same tanks (Table S5; GLM, χ2 = 0.97, df = 1, P = 0.32). Metyrapone exposure did not significantly affect abundances of microbial taxa (one-way ANOVA, all P > 0.05), community structure (PERMANOVA, F1,49 = 0.87, P = 0.48), or community membership in adults (F1,49 =0.97, P = 0.52). Atrazine and the interaction between metyrapone and atrazine also did not significantly affect bacterial alpha diversity (Fig. 3b; Tables S1 and S6), community structure (atrazine: F1,49 = 0.81, P = 0.52; interaction: F1,49 = 0.98, P = 0.38), or community membership (atrazine: F1,49 = 0.89, P = 0.79; interaction: F1,49 = 1.16, P = 0.12). However, exposure to atrazine as tadpoles significantly increased the relative abundance of the genus Desulfovibrio (one-way ANOVA, F =12.99, P = 0.008) and decreased the abundance of Delftia (F = 11.51, P = 0.01) in adults.
HOST-ASSOCIATED MICROBIOTA AND DEFENSES AGAINST BD
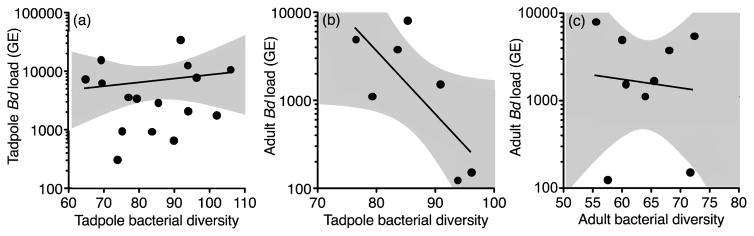
Gut bacterial diversity of tadpoles did not significantly predict Bd intensity of tadpoles nor did bacterial diversity of adults significantly predict Bd intensity of adults (Fig. 4a and c; Table S8). However, bacterial diversity of tadpoles negatively predicted Bd intensity in adults raised in the same tanks (Fig. 4b; Table S8). More specifically, the relative abundance of phylum Fusobacteria in tadpoles negatively predicted Bd intensity in adults (Fig. 5; Table S8). The gut bacterial diversity did not significantly affect host tolerance (measured using the reaction norm between Bd intensity and change in mass during infection) of Bd intensity in tadpoles (n = 18) (Table S9) or adults (n = 9 tanks) (Table S10).
Fig. 4.
Relationship between bacterial phylogenetic diversity in the guts and Bd intensity (zoospore genetic equivalent (GE)). Bacterial phylogenetic diversity in tadpoles did not predict Bd intensity in tadpoles (n = 16 tanks; GLM, χ2 = 0.22, df = 1, P = 0.64), but significantly predicted Bd intensity in adults (n = 7 tanks; χ2 = 6.22, df = 1, P = 0.01). Bacterial diversity in adults did not predict Bd intensity in adults (n = 9 tanks; χ2 = 0.05, df = 1, P = 0.83).
Fig. 5.
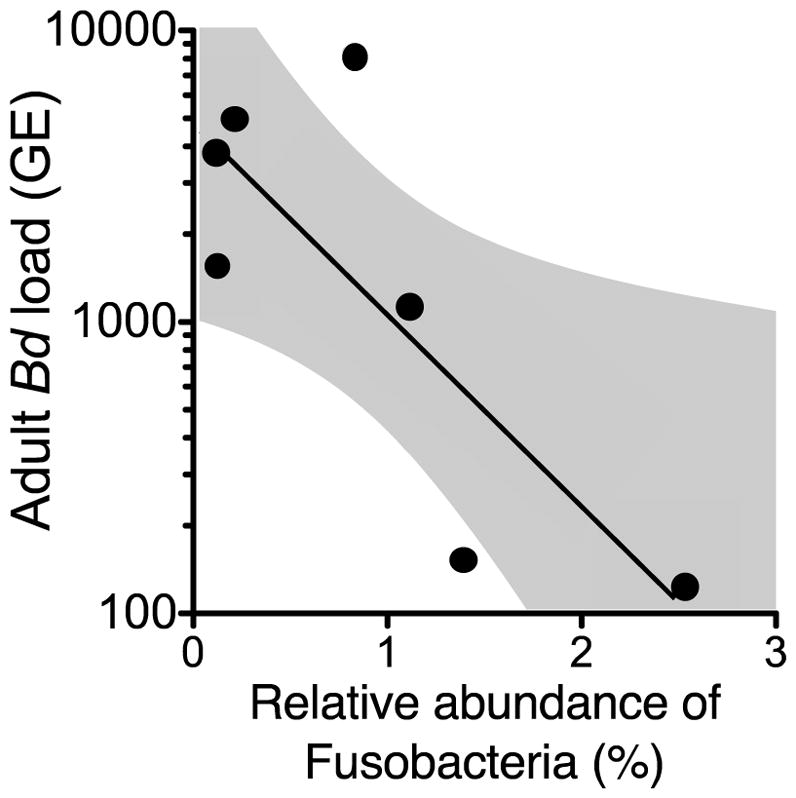
Relative abundance of phylum Fusobacteria in tadpoles significantly predicted Bd intensity (zoospore genetic equivalent (GE)) later in life (n = 7 tanks; GLM, χ2 = 8.48, df = 1, P = 0.004).
Discussion
Our study found that early-life exposure to atrazine did not generally affect gut microbiota of tadpoles or adults (Fig. 1, path c). Exposure to metyrapone also did not affect microbiota of tadpoles, but significantly increased gut bacterial diversity of adults, which was not driven by corticosterone release rates in tadpoles (Fig. 1, path f). Host-associated microbiota at the time of Bd exposure did not affect Bd intensity in tadpoles or adults. However, early-life gut bacterial diversity of tadpoles negatively predicted Bd intensity in adult frogs (Fig. 1, path e). Specifically, higher relative abundances of phylum Fusobacteria in tadpoles were associated with decreases in Bd intensity in adults. These results suggest that host-associated microbiota do not mediate the effect of atrazine and/or corticosterone on Bd infection risk (Fig. 1, paths c-e and c-d-b), but instead, the early-life microbiota itself likely predicts later-life resistance to infection (path e).
With the exception of changing the relative abundances of two bacterial genera in adulthood, atrazine did not affect gut bacterial diversity or community composition of tadpoles (in vivo or in vitro) or adults (Fig. 1, path c). Other studies have found an effect of pollutants, such as PCBs and antibiotics, on microbial communities (Shehata et al. 2013; Theriot et al. 2014; Kohl et al. 2015; Schwarz et al. 2016), but in several cases, the concentrations of these chemicals were quite high. There are several potential reasons why atrazine exposure did not affect the microbiota of tadpoles or adults. First, atrazine might not directly (e.g. via toxicity in soil microbes, DeLorenzo, Scott & Ross 2001) or indirectly (e.g. via host physiology) affect bacterial communities in hosts. Second, the concentration of atrazine that we used in our study might not have been effective at altering gut bacterial communities in the frogs. In the environment, tadpoles could be exposed to atrazine concentrations up to 1000 μg/L (Graymore, Stagnitti & Allinson 2001) and perhaps the EEC concentration that we used was too low to affect bacterial communities. Alternatively, the microbiota might have a non-monotonic dose response to atrazine, where only low and high concentrations affect microbiota. Non-monotonic dose response patterns in relation to the fitness and physiology of frogs have been observed in response to other agrochemicals (Storrs & Kiesecker 2004; Shelley et al. 2009; McMahon et al. 2011) but the mechanism underlying this response remains unknown. A future study could look at the dose response of host-associated microbiota in vivo and in vitro to atrazine to determine if there is an effect of atrazine on microbiota at different concentrations. Additionally, although we did not find an effect of atrazine on bacterial diversity metrics, we did find that bacterial phylogenetic diversity decreased by approximately 30% over the 6-day treatment period, which suggests that the in vitro experiment itself affected bacteria survival. We also did not account for the degradation of tissue in the experiment, which may have influenced bacterial communities. Future in vitro experiments should attempt to exclude the gut tissue in the experiment and determine if there are more desirable conditions for the bacteria (e.g. temperature and light conditions).
Like atrazine, metyrapone did not have a significant effect on the microbiota of tadpoles. In contrast, exposure to metyrapone as tadpoles had a lasting effect on the microbiota of adults, but this effect was not related to corticosterone release rates in tadpoles. These results suggest that metyrapone did not solely affect corticosterone production but also had non-target effects, which in turn, had lasting effects on gut bacterial communities. One example of a non-target effect of metyrapone is that it can decrease aldosterone production (Tucci et al. 1967), which is a hormone responsible for the reabsorption of sodium and water reabsorption in the gut, and the regulation of extracellular potassium and blood pressure (Randall, Burggren & French 2002). In turn, these physiological changes may be responsible for the increase in gut bacterial diversity of adults in response to metyrapone, but this hypothesis requires further investigation.
Host-associated microbiota do not appear to have a direct effect, or mediate the effect of atrazine or corticosterone, on host tolerance against Bd (Fig. 1, paths c-e, a-f-e). Instead, the effect of atrazine on host tolerance of Bd is likely caused by direct effects of atrazine, such as energy lost to atrazine detoxification or repair from damage caused by atrazine (Fig. 1, paths a-b) (Nieves-Puigdoller, Bjornsson & McCormick 2007). Alternatively, these effects could be from indirect effects of atrazine on unmeasured hormones, such as thyroxine. For example, atrazine exposure in salamanders can increase their thyroxine levels, a hormone associated with amphibian condition (Larson et al. 1998), which in turn, could affect the ability of hosts to withstand the effects of Bd. These factors serve as candidate mechanisms that could mediate the effect of atrazine on host tolerance to Bd and should be tested in the future.
Gut bacterial diversity at the time of Bd exposure did not affect host resistance or tolerance in tadpoles or adult frogs (Fig. 1, path e). In contrast, several other studies have found that the symbiotic microbiota of hosts can increase resistance to infection (Koch & Schmid-Hempel 2011; Theriot et al. 2014; Schuijt et al. 2016). These conflicting findings may be explained by the mechanism by which microbiota affect infection risk. Previous studies mostly examined the relationship between microbiota and pathogens in the gut of the host, which suggests that the microbiota may help the host resist the pathogen by either directly competing with it (Fig. 1, paths e) or locally upregulating the immune system (Fig. 1, paths d-b). It is possible that the gut microbiota at the time of Bd exposure does not affect resistance to Bd on the skin. Instead, during Bd exposure, the symbiotic microbiota on the skin, particularly Janthinobacterium lividum, Pseudomonas sp., and Rhodococcus fascians, likely promote resistance to Bd (Woodhams et al. 2016). These results suggest that skin and gut microbiota have different modes of action at different life stages to protect frogs against Bd.
Gut bacterial phylogenetic diversity in tadpoles was negatively correlated with Bd intensity in adults, which suggests that the microbiota might be priming immune system development. Interestingly, this pattern was not significant with regards to the other diversity metrics (i.e. Shannon index, species richness, species evenness) suggesting that the phylogenetic relatedness of bacterial taxa influences later-life infection risk. Similarly, Knutie et al. (2017a) found that bacterial diversity of tadpoles was positively related to later-life resistance to parasitic gut nematodes. This suggests that either: 1) the microbiota of tadpoles primes multiple components of the immune system that affect resistance to a diversity of parasites in different regions of the body, or 2) the microbiota primes a specific immune response that is effective against both Bd and parasitic worms. Previous studies support the latter hypothesis by showing that frogs produce an IgY antibody response to both parasitic worms (Knutie et al. 2017a; c) and Bd (Ramsey et al. 2010). Germ-free mice devoid of bacteria exhibit lower analogous IgG antibody production to pathogens when compared to conventional mice (Slack et al. 2009). Thus, the IgY antibody response could provide a candidate immune mechanism for our results that could be explored in future studies.
Particular bacterial taxa can affect the immune system of hosts (Fulde & Hornef 2014; Kabat, Srinivasan & Maloy 2014; Rakoff-Nahoum et al. 2015); thus, our findings might provide insight into which bacterial taxa could have driven the long-term changes in host resistance to infections (Fig. 1, paths d-b). In our study, the relative abundance of Fusobacteria in tadpoles was negatively correlated with Bd intensity in adults. In previous studies, higher relative abundance of Fusobacteria was related to lower prevalence of infectious and non-infectious diseases in both human and non-human vertebrate hosts (McCoy et al. 2013; Scher et al. 2013; Burns et al. 2015; Morton et al. 2015; Knutie et al. 2017b). This suggests that Fusobacteria are important in predicting immunity and disease risk. However, all previous studies are correlational and require experimental tests to determine the causal link between phylum Fusobacteria and the immune system, including what mechanism (e.g. butyrate production; Furusawa et al. 2013) is driving the relationship between Fusobacteria and immunity.
The fungal pathogen Bd is responsible for the decline and extinction of many amphibians worldwide (Wake & Vredenburg 2008). Determining which factors affect Bd risk might help mitigate the effect of Bd-driven population declines. Even though we did not find that host-associated microbiota mediate the effect of atrazine on Bd infections, our work suggests that there are critical windows in development where the loss of microbiota can have adverse persistent effects on infection risk. Specifically, increasing the presence of Fusobacteria during formative times of development may decrease infection risk later in life. Overall, our study suggests that increasing gut bacterial diversity and relative abundance of Fusobacteria might have lasting positive effects on amphibian health.
Supplementary Material
Acknowledgments
We thank Neal Halstead, Betsy Roznik, Christina Wilkinson, Virginia Caponera, Alyssa Obermayer, Sahara Peters, Shaan Sehgal, and Tiara Da Silva for lab support and Morgan Turney and Alexis Marquess for help with data entry. We also thank Cory Merow for input on the manuscript. This research was supported by a British Ecological Society Large Research Grant (5599-6643) to S.A.K., grants from the National Science Foundation (EF-1241889), National Institutes of Health (R01GM109499, R01TW010286), US Department of Agriculture (NRI 2006-01370, 2009-35102-0543), and US Environmental Protection Agency (CAREER 83518801) to J.R.R, and a Texas State University Research Enhancement Grant to C.R.G. This project was approved by IACUC#IS00001610. The 16S recombinant DNA sequences have been deposited in the BioProject database under accession code PRJNA312587. The authors declare no conflict of interest.
Footnotes
Authors’ contribution statement
SAK, CG, and JRR conceived the ideas and designed methodology; SAK and CG collected the data; SAK, and KDK analyzed the data; SAK led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Data accessibility
The authors declare that all other relevant data supporting the findings of the study can be found on FigShare doi: 10.6084/m9.figshare.5417629 (Knutie et al. 2017d).
Supporting information
Tables S1–S10. Results of statistical analyses.
References
- Arkoosh MR, Casillas E, Clemons E, Kagley AN, Olson R, Reno P, Stein JE. Effect of pollution on fish diseases: Potential impacts on salmonid populations. Journal of Aquatic Animal Health. 1998;10:182–190. [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain, Behavior, and Immunity. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal OP. Pesticides: Evaluation of Environmental Pollution. CRC Press; New York: 2012. Degradation of pesticides; pp. 47–77. [Google Scholar]
- Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: Relevance to immunotoxicology. Journal of Immunotoxicology. 2008;5:419–444. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Burns MB, Lynch J, Starr TK, Knights D, Blekhman R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Medicine. 2015;7:55. doi: 10.1186/s13073-015-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010a;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010b;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens S, Betley J, Fraser L, Bauer M, Gormley N, Gilbert J, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Gut microbiota: the neglected endocrine organ. Molecular Endocrinology. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? npj Biofilms and Microbiomes. 2016;2:16003. doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–1262. doi: 10.1126/science.1224203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo ME, Scott GI, Ross PE. Toxicity of pesticides to aquatic microorganisms: A review. Environmental Toxicology and Chemistry. 2001;20:84–98. doi: 10.1897/1551-5028(2001)020<0084:toptam>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Faith DP. Conservation evaluation and phylogenetic diversity. Biological Conservation. 1992;61:1–10. [Google Scholar]
- Fulde M, Hornef MW. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunological Reviews. 2014;260:21–34. doi: 10.1111/imr.12190. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gabor CR, Roznik EA, Knutie SA, Rohr JR. Are the adverse effects of stressors on amphibians mediated by their effects on stress hormones? bioRxiv. 2017:165282. doi: 10.1007/s00442-017-4020-3. [DOI] [PubMed] [Google Scholar]
- Glennemeier KA, Denver RJ. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. Journal of Experimental Zoology. 2002;292:32–40. doi: 10.1002/jez.1140. [DOI] [PubMed] [Google Scholar]
- Graymore M, Stagnitti F, Allinson G. Impacts of atrazine in aquatic ecosystems. Environment International. 2001;26:483–495. doi: 10.1016/s0160-4120(01)00031-9. [DOI] [PubMed] [Google Scholar]
- Häggblom MM. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiology Letters. 1992;103:29–71. doi: 10.1111/j.1574-6968.1992.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WA, Mendonça MT, Congdon JD. Responsiveness of the hypothalamo-pituitary-interrenal axis in an amphibian (Bufo terrestris) exposed to coal combustion wastes. Comparative Biochemistry and Physiology - C Pharmacology Toxicology and Endocrinology. 1999;122:191–196. doi: 10.1016/s0742-8413(98)10104-4. [DOI] [PubMed] [Google Scholar]
- Horvath RS. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriological Reviews. 1972;36:146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossie TJ, Ferland-Raymond B, Burness G, Murray DL. Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Ecoscience. 2010;17:100–108. [Google Scholar]
- Kabat AM, Srinivasan N, Maloy KJ. Modulation of immune development and function by intestinal microbiota. Trends in Immunology. 2014;35:507–517. doi: 10.1016/j.it.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutie SA, Shea LA, Kupselaitis M, Wilkinson CL, Kohl KD, Rohr JR. Early-life diet affects host microbiota and later-life defenses against parasites in frogs. Integrative and Comparative Biology. 2017a doi: 10.1093/icb/icx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutie SA, Wilkinson CL, Kohl KD, Rohr JR. Early-life disruption of amphibian microbiota decreases later-life resistance to parasites. Nature Communications. 2017b;8:86. doi: 10.1038/s41467-017-00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutie SA, Wilkinson CL, Wu QC, Ortega CN, Rohr JR. Host resistance and tolerance of parasitic gut worms depend on resource availability. Oecologia. 2017c;183:1031–1040. doi: 10.1007/s00442-017-3822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutie SA, Gabor CR, Kohl KD, Rohr JR. Do host-associated gut microbiota mediate the effect of an herbicide on disease risk in frogs? Figshare. 2017d doi: 10.1111/1365-2656.12769. https://doi.org/10.6084/m9.figshare.5417629.v1. [DOI] [PMC free article] [PubMed]
- Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19288–19292. doi: 10.1073/pnas.1110474108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl KD, Cary TL, Karasov WH, Dearing MD. Restructuring of the amphibian gut microbiota through metamorphosis. Environmental Microbiology Reports. 2013;5:899–903. doi: 10.1111/1758-2229.12092. [DOI] [PubMed] [Google Scholar]
- Kohl KD, Cary TL, Karasov WH, Dearing MD. Larval exposure to polychlorinated biphenyl 126 (PCB-126) causes persistent alteration of the amphibian gut microbiota. Environmental Toxicology and Chemistry. 2015;34:1113–1118. doi: 10.1002/etc.2905. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J. Interactions of environmental stressors impact survival and development of parasitized larval amphibians. Ecological Applications. 2010;20:2263–2272. doi: 10.1890/09-1558.1. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Mason MR, Brooker MR, O’Brien K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. Journal of Clinical Periodontology. 2012;39:425–433. doi: 10.1111/j.1600-051X.2012.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson DL, McDonald S, Fivizzani AJ, Newton WE, Hamilton SJ. Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: duration, larval growth, and hormonal response. Physiological Zoology. 1998;71:671–679. doi: 10.1086/515999. [DOI] [PubMed] [Google Scholar]
- Laws SC, Hotchkiss M, Ferrell J, Jayaraman S, Mills L, Modic W, Tinfo N, Fraites M, Stoker T, Cooper R. Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male wistar rats. Toxicological Sciences. 2009;112:78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nature Reviews Immunology. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD, Rohr JR. The effects of anthropogenic global changes on immune functions and disease resistance. Annals of the New York Academy of Sciences. 2010;1195:129–148. doi: 10.1111/j.1749-6632.2010.05454.x. [DOI] [PubMed] [Google Scholar]
- McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8:e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Halstead NT, Johnson S, Raffel TR, Romansic JM, Crumrine PW, Boughton RK, Martin LB, Rohr JR. The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environmental Health Perspectives. 2011;119:1098–1103. doi: 10.1289/ehp.1002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Romansic JM, Rohr JR. Nonmonotonic and monotonic effects of pesticides on the pathogenic fungus Batrachochytrium dendrobatidis in culture and on tadpoles. Environmental Science and Technology. 2013;47:7958–7964. doi: 10.1021/es401725s. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider D, Soares M. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. The evolution of parasites in response to tolerance in their hosts: the good, the bad, and apparent commensalism. Evolution. 2006;60:945–956. [PubMed] [Google Scholar]
- Morton ER, Lynch J, Froment A, Lafosse S, Heyer E, Przeworski M, Blekhman R, Ségurel L. Variation in rural African gut microbiota is strongly correlated with colonization by entamoeba and subsistence. PLoS Genetics. 2015;11:e1005658. doi: 10.1371/journal.pgen.1005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe DA, Crowley DE. Bioremediation of atrazine-contaminated soil by repeated applications of atrazine-degrading bacteria. Applied Microbiology and Biotechnology. 1999;51:877–882. doi: 10.1007/s002530051477. [DOI] [PubMed] [Google Scholar]
- Newman JR. Effects of industrial air pollution on wildlife. Biological Conservation. 1979;15:181–190. [Google Scholar]
- Nieves-Puigdoller K, Bjornsson BT, McCormick SD. Effects of hexazinone and atrazine on the physiology and endocrinology of smolt development in Atlantic salmon. Aquatic Toxicology. 2007;84:27–37. doi: 10.1016/j.aquatox.2007.05.011. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EMM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biological Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Kong Y, Kleinstein SH, Subramanian S, Ahern PP, Gordon JI, Medzhitov R. Analysis of gene–environment interactions in postnatal development of the mammalian intestine. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:1929–1936. doi: 10.1073/pnas.1424886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infection and Immunity. 2010;78:3981–3992. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall D, Burggren W, French K. Eckert Animal Physiology: Mechanisms and Adaptations. W.H. Freeman and Company; New York, NY, USA: 2002. [Google Scholar]
- Read AF, Graham AL, Råberg L. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biology. 2008;6:2638–2641. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH, Sager T, Sih A, Palmer BD. Multiple stressors and salamanders: Effects of an herbicide, food limitation, and hydroperiod. Ecological Applications. 2004;14:1028–1040. [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B: Biological Sciences. 2013a;280:20131502–20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proceedings of the Royal Society B: Biological Sciences. 2013b;280:20131502–20131502. doi: 10.1098/rspb.2013.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Schotthoefer AM, Raffel TR, Carrick HJ, Halstead N, Hoverman JT, Johnson CM, Johnson LB, Lieske C, Piwoni MD, Schoff PK, Beasley VR. Agrochemicals increase trematode infections in a declining amphibian species. Nature. 2008;455:1235–1239. doi: 10.1038/nature07281. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009;9:313–324. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe AM, Brundage KM, Schafer R, Barnett JB. Age-dependent decrease in BALB/c mouse immune function following early life exposure to the herbicide atrazine. Journal of Immunology. 2006;176:S165–S165. [Google Scholar]
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics. Oxford University Press; Oxford: 2011. [Google Scholar]
- Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz RS, Moran NA, Evans JD. Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:9345–9350. doi: 10.1073/pnas.1606631113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B. The challenge of micropollutants in aquatic systems. Science. 2006;313:1072–7. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- Shchipkova AY, Nagaraja HN, Kumar PS. Subgingival microbial profiles of smokers with periodontitis. Journal of Dental Research. 2010;89:1247–1253. doi: 10.1177/0022034510377203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Current Microbiology. 2013;66:350–358. doi: 10.1007/s00284-012-0277-2. [DOI] [PubMed] [Google Scholar]
- Shelley LK, Balfry SK, Ross PS, Kennedy CJ. Immunotoxicological effects of a sub-chronic exposure to selected current-use pesticides in rainbow trout (Oncorhynchus mykiss) Aquatic Toxicology. 2009;92:95–103. doi: 10.1016/j.aquatox.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MA, Geuking MB, Beutler B, Tedder TF, Hardt WD, Bercik P, Verdu EF, McCoy KD, Macpherson AJ. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley ZR, Harwood VJ, Rohr JR. A synthesis of the effects of pesticides on microbial persistence in aquatic ecosystems. Critical Reviews in Toxicology. 2015;8444:1–24. doi: 10.3109/10408444.2015.1065471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrs SI, Kiesecker JM. Survivorship patterns of larval amphibians exposed to low concentrations of atrazine. Environmental Health Perspectives. 2004;112:1054–1057. doi: 10.1289/ehp.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothart MR, Bobbie CB, Schulte-hostedde AI, Boonstra R, Palme R, Mykytczuk NCS, Newman AEM. Stress and the microbiome: linking glucocorticoids to bacterial community dynamics in wild red squirrels. Biology Letters. 2016;12:14–17. doi: 10.1098/rsbl.2015.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart SN, Chanson JS, Cox Na, Young BE, Rodrigues ASL, Fischman DL, Waller RW. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Theriot CM, Koenigsknecht MJ, Carlson PE, Hatton GE, Nelson AM, Li B, Huffnagle GB, Li JZ, Young VB. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nature communications. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci JR, Espiner EA, Jagger PI, Lauler DP. The effect of metyrapone on aldosterone secretion in man. Acta Endocrinologica. 1967;56:376–384. doi: 10.1530/acta.0.0560376. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Computational Biology. 2009:5. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams DC, Alford RA, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz M, Daskin JH, Davis LR, Flechas SV, Lauer A, Gonzalez A, Harris RN, Holden WM, Hughey MC, Ibáñez R, Knight R, Kueneman J, Rabemananjara F, Reinert LK, Rollins-Smith LA, Roman-Rodriguez F, Shaw SD, Walke JB, McKenzie V. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology. 2015;96:595–595. [Google Scholar]
- Woodhams DC, Bletz M, Kueneman J, McKenzie V. Managing amphibian disease with skin microbiota. Trends in Microbiology. 2016;24:161–164. doi: 10.1016/j.tim.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Brandt H, Baumgartner S, Kielgast J, Küpfer E, Tobler U, Davis LR, Schmidt BR, Bel C, Hodel S, Knight R, McKenzie V. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0096375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.