Abstract
Background
Attention-Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous condition for which multiple efforts to characterize brain state differences are underway. The objective of this study was to identify distinct subgroups of resting electroencephalography (EEG) profiles among children with and without ADHD and subsequently provide extensive clinical characterization of the subgroups.
Methods
Latent class analysis was used with resting state EEG recorded from a large sample of 781 children with and without ADHD (N=620 ADHD, N=161 Control), aged 6–18 years old. Behavioral and cognitive characteristics of the latent classes were derived from semi-structured diagnostic interviews, parent completed behavior rating scales, and cognitive test performance.
Results
A five-class solution was the best fit for the data, of which four classes had a defining spectral power elevation. The distribution of ADHD and control subjects was similar across classes suggesting there is no one resting state EEG profile for children with or without ADHD. Specific latent classes demonstrated distinct behavioral and cognitive profiles. Those with elevated slow-wave activity (i.e., delta and theta band) had higher levels of externalizing behaviors and cognitive deficits. Latent subgroups with elevated alpha and beta power had higher levels of internalizing behaviors, emotion dysregulation, and intact cognitive functioning.
Conclusions
There is population-level heterogeneity in resting state EEG subgroups, which are associated with distinct behavioral and cognitive profiles. EEG measures may be more useful biomarkers of ADHD outcome or treatment response rather than diagnosis.
Keywords: electrophysiology, ADHD, resting state, latent class analysis
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is a heterogeneous disorder in terms of clinical presentation, underlying neurobiology, and etiologic factors. One method for categorizing clinical presentation is ADHD subtype specifiers such as DSM 5 (APA, 2013) Inattentive, Hyperactive-Impulsive and Combined presentations, however, these behavioral subgroups are developmentally unstable and do not appear to represent distinct etiologic or neurobiologic subgroups. Different methods for identifying and characterizing heterogeneity are needed.
Electroencephalography (EEG) has been used to study the underlying neurophysiology of ADHD for nearly 100 years. Using cluster analytic statistical techniques, there have been several attempts to identify neurophysiologically-based subgroups of ADHD-affected youth. Early efforts by Chabot and Serfontein (1996) using discriminant function analyses found three subgroups among 407 children with ADHD during eyes closed resting state: one with generalized fronto-central theta/alpha excess (~46%), another with posterior alpha excess (11%), and a final group with higher frontal and/or posterior beta power (7%). Subsequent studies by this group suggested that these subgroups exhibited differential treatment response, with children demonstrating excess alpha and beta power being the most likely to exhibit improved behavioral functioning with stimulants (Chabot, di Michele, Prichep, & John, 2001).
Early studies by Clarke and colleagues reported 3 distinct subgroups that were named cortical hypoarousal (greater relative theta and lower beta power), maturational lag (elevated slow wave and deficient fast wave activity), and aberrant development, which consisted of an elevated beta group (Clarke, Barry, McCarthy, & Selikowitz, 2001). The elevated beta group was more common in boys with ADHD Combined Type who tended to be more moody and prone to temper tantrums (Clarke et al., 2001). In an expanded sample (N=155) that included children with ADHD and psychiatric comorbidities, these three clusters were replicated and an additional cluster with elevated alpha band power was reported (Clarke, Barry, Dupuy, Heckel, et al., 2011).
Thus, efforts to reduce neurophysiological heterogeneity in ADHD, primarily using cluster analysis, have thus far revealed some overlap of subtypes across studies, such as frontal slowing with decreased alpha and beta, as well as subgroups with increased alpha and beta-band power. However, behavioral and cognitive characterization of subgroups has been lacking. None of the studies have used semi-structured diagnostic interviews for assessment of ADHD and comorbid diagnoses. Furthermore, only one study has examined items from a behavior checklist to identify clinical correlates of the EEG subgroups (Clarke, Barry, Dupuy, Heckel, et al., 2011). The elevated alpha group was characterized by being ‘confused or in a fog’ and displaying a higher number of obsessive-compulsive behaviors. The elevated beta group tended to be more physically aggressive and delinquent relative to other subgroups. Thus, a preliminary analysis suggests that EEG subgroups may exhibit different behavioral profiles, however, more systematic work in behavioral and cognitive phenotyping is needed.
In addition, previous studies have had insufficient sample sizes to employ advanced statistical techniques designed to quantitatively identify subgroups such as latent class analysis (LCA). LCA is a statistically driven, model-based approach to detecting the latent structure underlying heterogeneous data by finding subgroups (or latent classes) of individuals with similar response patterns on a set of observed variables. Benefits of LCA include flexibility in modeling latent or measured variables with continuous and/or categorical variables, ability to include covariates, and empirical methods for assessing model fit (Lubke & Muthen, 2005; Masyn, 2013).
Finally, none of the EEG studies to date have included typically developing, non-ADHD children, thus failing to assess if the same subgroups exist on a population level. Several recent studies have used functional magnetic resonance imaging (fMRI), neuropsychological and temperament measures to identify subgroups within samples of children with and without ADHD (Costa Dias et al., 2015; Fair, Bathula, Nikolas, & Nigg, 2012; Gates, Molenaar, Iyer, Nigg, & Fair, 2014; Karalunas et al., 2014). These studies identified 3–5 subgroups among children with and without ADHD, suggesting that heterogeneity in brain function exists at the population level rather than solely among children with psychiatric disorders. This is consistent with newer dimensional approaches to understanding psychopathology as proposed under Research Domain Criteria (RDoC)(Insel et al., 2010).
The goal of the current study is to identify EEG-based subgroups in the largest sample to date of children with and without ADHD according to their resting state cortical activation patterns using latent class analysis. In addition, the emergent latent subgroups will be characterized by comprehensive psychiatric diagnostic interviews, behavior ratings, and cognitive assessments to understand the functional significance of each subgroup. We hypothesize that 3 to 5 subgroups will emerge and that those with elevated theta power will have a behavioral profile of higher disruptive behavioral problems whereas those with elevated alpha and beta power will have higher rates of emotional difficulties such as anxiety and depression.
Methods
Participants
The sample consists of 781 children (N=620 ADHD, N=161 Typically Developing [TD] control) aged 6–18 years-old who were recruited to participate in ADHD research studies. One study focused on the genetics of ADHD (N=483) and the other was the baseline EEG (i.e., before medications were tested) from a clinical trial of ADHD medications (N=298). Participants were recruited from the community through radio and newspaper advertisements, community organizations (i.e. CHADD), local schools and primary care physicians. After receiving verbal and written explanations of study requirements, all participants provided written informed parental permission and/or consent/assent approved by the local institutional review board.
Procedure
Diagnostic, behavioral, cognitive, and EEG data were collected for all participants in an identical manner and during a single experimental session (Loo et al., 2010). All children were evaluated for the presence of ADHD and other childhood psychiatric disorders (according to DSM-IV criteria) based on an interview with the primary caretaker (usually mother) using the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS-PL)(Kaufman et al., 1997) and a direct interview with the child if 8 years of age or older. Interviews were conducted by clinical psychologists or highly trained interviewers with extensive experience in psychiatric diagnoses. In addition, parents and teachers were asked to complete the ADHD Rating Scale (ADHD-RS)(DuPaul, Power, Anastopoulos, & Reid, 1999). ‘Best estimate’ diagnoses were determined after individual review of diagnoses, symptoms, and impairment level by senior clinicians (JJM, JTM). ADHD and other psychiatric diagnoses present at a rate greater than 3% in the total sample were used in subsequent analyses. Subjects were excluded if they were positive for any of the following: neurological disorder, head injury resulting in concussion, diagnoses of schizophrenia or autism, or estimated Full Scale IQ < 70. Subjects on stimulant medication discontinued use for 24 hours prior to their visit.
Behavior ratings
The Child Behavior Checklist (CBCL)(Achenbach, 2001) was given to assess a wide range of childhood behaviors and was completed by a caregiver (usually mother). This rating scale has 125 items and results in 8 syndrome scales, internalizing and externalizing dimensions, and total problem scores. In addition, the CBCL Dysregulation Profile was calculated and used as an index of score of emotion dysregulation (Ayer et al., 2009) All of these dependent variables were used as indicators of behavioral functioning.
Cognitive measures
Intellectual functioning (IQ) was assessed using a two-subtest estimate of IQ (Vocabulary, Block Design) from the age-appropriate version of the Wechsler scales of intelligence (Wechsler, 2003). In addition, working memory and response inhibition were assessed using two additional cognitive tasks that were administered with the EEG session. The Sternberg Spatial Working Memory (SWM) task (Glahn et al., 2002) required maintenance of a set of spatial locations over a short delay. Subjects saw a target array of 1, 3, 5, or 7 circles (12 trials per memory set size) positioned around a central fixation for 2- sec. After a fixed (3-sec) delay period during which the screen was empty, subjects were asked to indicate whether the location of a single circle (probe) matched one of the target stimuli. Dependent variables were accuracy, reaction time and reaction time variability. The Go/No-Go task (Conners, 1994) is a widely used computerized test of sustained attention or vigilance. During this 14-minute task, subjects pressed the space bar when any letter except the target letter “X” appeared. This task included more “Go” trials (75%) than “NoGo” trials (25%); stimuli were presented in 6 blocks of 60 trials (360 total); each trial included stimulus presentation (500 ms) followed by a 1s, 2s, or 4s (randomly interleaved) inter-stimulus interval. Dependent measures were omission errors reflecting inattention and commission errors reflecting impulsivity.
EEG measures
EEG recording was carried out using 40 Ag/AgCl surface electrodes that were embedded in an electrode cap in an extended International 10/20 location system. Data were referenced to linked ears. Impedance was below 10 kOhms. EEG signals were recorded using MANSCAN (Sam Technology, San Francisco, CA) hardware and software and sampled at 256 samples per second. Eye movements were monitored by electrodes placed on the outer canthus of each eye for horizontal movements (REOG, LEOG) and by electrodes above the eyes for vertical eye movements. EEG was recorded during a 5-min resting state.
Continuous EEG data were filtered using a 0.5–50 Hz bandpass filter and subjected to automatic artifact detection via algorithms designed to identify dead and bad channels, eye movements, saturation, muscle artifact, and line noise. Subsequent to this automated procedure an experienced EEG technician (who was blind to ADHD diagnostic status) visually inspected all data and identified residual contaminants. Next, continuous EEG was divided into 1-s epochs and artifact-containing epochs were removed. A minimum of 30 epochs were required to be included in the analysis (mean= 98.5 epochs, range 30–258). Spectral power estimates were computed on artifact free epochs using Fast Fourier Transformed (FFT) in MANSCAN EEG software, which uses a Welch’s Periodogram approach (Welch, 1967). We specified 1-s data segments, with 50% overlap, and a Hanning Windowing function to generate spectral content at a 1Hz resolution. Spectral data were then averaged and EEG power (µV2) was estimated and exported in the following bandwidths: Delta (1–4 Hz), Theta (4–7Hz), Alpha (8–12Hz), and Beta1 (12–16Hz), Beta 2 (16–21Hz). Relative power for each frequency band was calculated by dividing by total power and log transformed to assume a normal distribution. Electrodes were grouped together for analysis as follows: anterior frontal (AF3, AF4, Afz), frontal (F3, F4, Fz), central (C3, C4, Cz), parietal (P3, P4, Pz).
Data analytic plan
To test whether there are subgroups with distinct EEG profiles within the sample, we used LCA with spectral power from all regions (i.e., anterior frontal through parietal) and all frequency bands (delta-beta2) in Mplus (Muthen & Muthen, 1998–2011). Within the LCA, age was included as a covariate due to strong, well-documented developmental effects on EEG power. The analyses used a two-class solution as the starting place, to which an additional class was added to each successive model. The best fitting model was selected according to goodness of fit statistics: the lowest Akaike Information Criteria (AIC), Bayes Information Criterion (BIC), Adjusted Bayes Information Criterion (ABIC), entropy (ENT), and Lomendel-Rubin (LMR) p-value (Lubke & Muthen, 2005; Masyn, 2013). Statistical Package for the Social Sciences (SPSS), version 21 (IBM_Corp, 2012) was used to assess latent class characterization by behavioral and cognitive measures, either chi-square (X2) for dichotomous variables or analyses of variance (ANOVA) for continuous dependent variables. Latent class membership was the independent variable and the behavioral (CBCL scores) and cognitive measures (estimated IQ, spatial working memory and Go/NoGo scores) were used as dependent variables. Age was used as a covariate of no interest (ANCOVA); results with and without the covariate are presented. Significant effects of latent class membership were followed with post-hoc Tukey HSD to test pairwise differences between groups.
Results
Demographic characteristics of latent classes
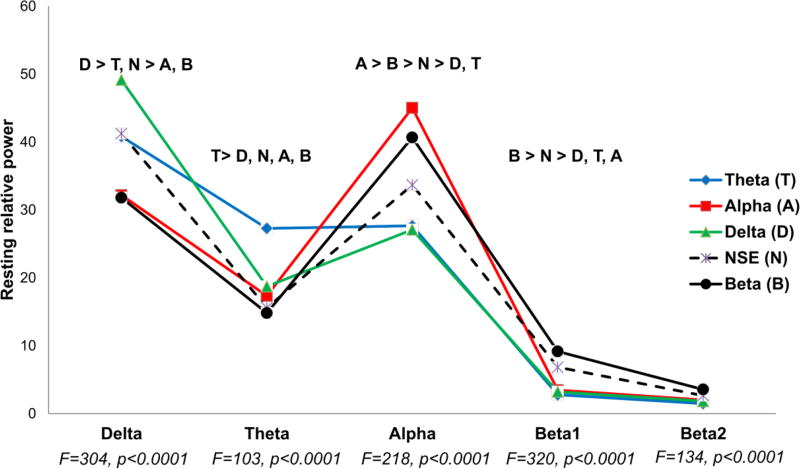
The following LCA fit statistics indicated that a 5-class solution was the best fit (2-class: AIC:108459, BIC: 108759, ENT=0.97, LMR=0.02; 3-class: AIC: 105419, BIC: 105821, ENT=0.96, LMR=0.18; 4-class: AIC:103404, BIC:103908, ENT=0.95, LMR=0.02; 5-class: AIC: 102012, BIC: 102618, ENT=0.95, LMR=0.24). LCA was re-run on samples containing only ADHD and Controls to ensure that the 5-class structure was the best fit for separate groups; the results were confirmed (data available in Supplemental Material Table S1). Within each frequency band, latent classes were significantly different in terms of spectral power; four of the classes had significantly higher spectral power than all other classes in one frequency band (see Figure 1). In contrast, none of the classes had significantly lower spectral power relative to other classes in any of the frequency bands. Thus, the latent classes were subsequently named for the defining spectral power elevation in each frequency band and are listed here with the percent of sample that was contained within each group: Delta (30%), Theta (23%), Alpha (20%), Beta (7%), and one cluster with no spectral elevation (NSE; 20%).
Figure 1. Spectral Power Profiles for EEG-based Subgroups.
Relative power in the parietal region (P3, Pz, P4) across the frequency bands for each of the five empirically defined subgroups. Each group is named for the frequency band where the power is significantly elevated above other clusters. Degrees of freedom for EEG analyses F[4,775]. Post-hoc results (p<0.05) for each frequency band are located above the top elevation.
Demographic data are presented in Table 1. Age differed significantly across classes with the Theta group being the youngest (mean age 8.7 years) and the Beta group was the oldest (mean age 13.9 years). This occurred despite the use of age as a covariate in the LCA, which uses multinomial logistic regression to regress each latent class on the covariate (see Supplemental Material Figure S1. for select age × frequency band power plots). The betas from the multinomial logistic regression suggest that the covariate age accounted for 13% of the variance in latent class membership, ranging from 6% in the Alpha and Beta classes to 40% in the Theta class. Thus, although there are significant effects of age on latent class membership, there are clearly other (i.e., neurobiological) factors influencing the latent model structure. A significant gender effect also emerged with the Delta and Theta classes having a higher percentage of males (66–67%) than the other classes (42%–52%). Estimated intelligence was in the average range for all clusters and not significantly different across groups.
Table 1.
Demographics of 5 latent classes named for spectral elevation
| Delta | Theta | Alpha | NSE | Beta | F/X2 | p-value | |
|---|---|---|---|---|---|---|---|
| N (% of sample) | 236 (30%) | 183 (23%) | 157 (20%) | 153 (20%) | 52 (7%) | ||
| Age (years) | 9.9 (2.6)a | 8.7 (2)b | 12.0 (2.6)c | 11.9 (2.7)c | 13.9 (2.8)d | 76 | <0.0001 |
| Age range (years) | 6 – 18 | 6 – 16 | 6 – 18 | 6 – 18 | 7 – 18 | ||
| IQ estimate | 107 (15) | 106 (15) | 105 (15) | 108 (15) | 108 (14) | <1 | 0.55 |
| Sex (% male) | 67%a | 66%a | 50%b | 52%b | 42%b | 23.7 | <0.001 |
| ADHD dx (%) | 79.2%ab | 83.6%a | 84.1%a | 72.5%b | 71.2b | 10.6 | 0.03 |
Note. IQ=intelligence, dx= diagnosis, NSE=no spectral elevation. Columns with different superscript letters are significantly different from each other (p≤0.05).
ADHD and control distribution across clusters
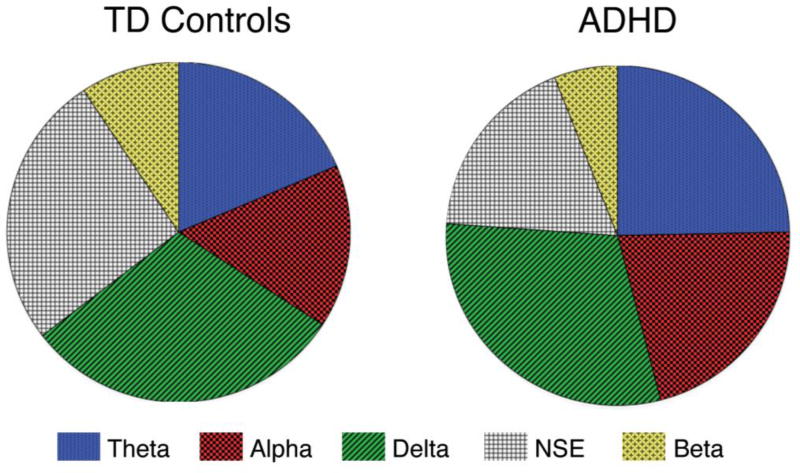
Overall, 79% of the sample had an ADHD diagnosis. While there were variations in the ADHD distribution across the 5 clusters, none of the clusters were comprised of only children with or without ADHD (see Figure 2). The Theta and Alpha groups had the highest percentage of ADHD diagnosis (both 84%) and the Beta group had the lowest percentage of ADHD diagnosis (71%), a difference that reached statistical significance (X2=10.6, p=0.03). Although ADHD diagnosis was not evenly distributed among classes, the proportion of ADHD individuals for each of the individual classes did not differ (all p’s >0.10), with the exception of NSE (X2=5.4, p=0.02). This suggests that, with the exception of the NSE group, the proportion of non-ADHD/ADHD individuals was similar within each subgroup. Within each cluster, the ADHD group did not exhibit significantly higher or lower spectral power (all p’s > 0.1). This suggests that EEG heterogeneity is apparent across the general population and that there is no one EEG metric or subgroup sufficient to characterize all children with or without ADHD.
Figure 2. Distribution of Children with and without ADHD in EEG-based subgroups.
The proportion of non-ADHD/ADHD individuals for the five empirically defined latent classes did not differ (all p’s >0.10), with the exception of the NSE subgroup (X2=5.4, p=0.02). Additionally, there is no one EEG subgroup that specifically represents ADHD or typical development. TD=typically developing, NSE=no spectral elevation
Behavioral characteristics of EEG subgroups
Behavioral differences between subgroups were tested using psychiatric diagnoses and parent-rated CBCL scores. There was significant overlap of children with and without ADHD within all latent classes on all behavioral and cognitive measures (measures that differed significantly across clusters can be seen in Supplemental Material Figure S2.). Several patterns emerged (see Table 2). The Delta and Theta group had the lowest and the Beta group the highest percentage of internalizing disorders such as mood, generalized anxiety (GAD), and obsessive-compulsive disorder (OCD). The Alpha group had the highest percentage of conduct disorder (CD) diagnosis and elevated rates of GAD, whereas NSE had the lowest percentage of ODD diagnosis.
Table 2.
Diagnostic, Behavioral and Cognitive differences among the latent classes
| Delta | Theta | Alpha | NSE | Beta | |||
|---|---|---|---|---|---|---|---|
| N (%) | 236 (30%) | 183 (23%) | 157 (20%) | 153 (20%) | 52 (7%) | F/X2 | p-value |
| Diagnostic | |||||||
| Conduct Disorder | 0.4%a | 0.6%a | 3.9%b | 1.4%a | 4.0%b | 11.1 | 0.026 |
| Oppositional Defiant Disorder | 39.0%a | 33.0%a | 35.8%a | 22.4%b | 36.0%a | 11.7 | 0.020 |
| Any mood disorder | 4.7%a,b | 2.1%a | 4.0%a,b | 8.1%b | 18.4%c | 23.1 | 0.0001 |
| Generalized Anxiety Disorder | 10.1%a,c | 8.2%a | 19.9%b,d | 15.5%b,c | 28.6%d | 21.3 | 0.0001 |
| Obsessive-Compulsive Disorder | 1.7%a | 1.1%a | 4.0%a | 2.7%a | 16.0%b | 31.3** | <0.0001 |
| Child Behavior Checklist | Age covary F(4,729) | No age covary F(4,730) | |||||
| Anxiety/Depression | 57 (8) | 57 (8) | 59 (10) | 58 (9) | 59 (10) | 1.0 | 2.3 |
| Withdrawn | 57 (8) | 56 (8)a | 59 (10)b | 56 (8)a | 56 (8)a | 3.6** | 3.5** |
| Somatic Complaints | 56 (8) | 56 (7) | 59 (9) | 57 (8) | 57 (8) | 1.8 | 2.5* |
| Social Problems | 58 (8) | 58 (8) | 60 (9) | 58 (9) | 59 (9) | 1.0 | 1.5 |
| Thought Problems | 58 (8) | 58 (8) | 60 (10) | 58 (8) | 59 (9) | 1.1 | 1.4 |
| Attention Problems | 65 (10) | 66 (10) | 67 (11)a | 64 (11)b | 64 (10)b | 2.3* | 2.3* |
| Delinquent Behavior | 58 (8) | 58 (7) | 58 (9) | 56 (7) | 56 (8) | 1.6 | 2.1 |
| Aggressive Behavior | 60 (9)a | 59 (8) | 60 (11)a | 57 (8)b | 57 (8)b | 2.6* | 2.4* |
| Internalizing | 56 (11)a | 54 (11)a | 59 (11)b | 55 (12)a | 56(12) | 2.6* | 3.3** |
| Externalizing | 57 (12) | 56 (11) | 58 (12) | 54 (11) | 54(11) | 1.8 | 2.0 |
| Total Problems | 59 (11) | 58 (11)a | 61 (12)b | 57 (12)a | 58 (10)a | 3.0* | 3.0* |
| Dysregulation profile | 182 (22) | 181(21)a | 186(26)b | 178 (23)a | 179(21) | 2.3* | 2.4* |
| Cognitive function | |||||||
| SWM % Accuracy | 72 (13) | 71 (12) | 76 (12) | 79 (13) | 79 (13) | 2.10 | 16.2*** |
| SWM Reaction Time | 1343 (343) | 1389 (291) | 1213 (298) | 1261 (281) | 1176 (329) | <1 | 7.2*** |
| SWM Reaction Time SD | 465 (110)a | 472 (91)a | 419 (113)b | 402 (96)b | 397 (89)b | 2.40* | 14.0*** |
| GNG Omission Errors | 47 (41)a | 44 (35) | 28 (26)b | 31 (31)b | 17 (16)b | 2.70* | 8.9*** |
| GNG Commission Errors | 22 (7) | 24 (6) | 20 (8) | 21 (8) | 21 (8) | 1.30 | 4.0** |
Note. SWM=spatial working memory, GNG=Go/NoGo. Columns with different superscript letters indicate significant difference (p≤0.05).
p≤0.05,
p≤0.01,
p≤0.001
On the CBCL, the Alpha group was rated as being the most behaviorally impaired. Significant differences emerged on the Withdrawn, Attention Problems, Aggressive Behavior, Internalizing and Total Problem scores. The behavioral patterns suggest that those in the elevated Alpha subgroup demonstrated the greatest level of behavioral dysfunction as reported by parents. This is somewhat at odds with the rates of psychiatric diagnoses across clusters, which suggested that the Beta group has the highest rates of mood and anxiety disorders. This discrepancy may highlight rater differences since clinicians make diagnostic determinations based on parent and child interview whereas the CBCL scores are based on parent report only. In addition, the CBCL Dysregulation Profile score was also the highest within the Alpha group. Because the Alpha group had the highest rates of CD along with elevated rates of GAD, this group may be more emotionally dysregulated in ways that don’t fit neatly into diagnostic categories.
Cognitive characteristics of EEG subgroups
Performance on the two cognitive tasks were compared across clusters and results are presented in Table 2. Using age as a covariate, significant differences emerged for the Go-NoGo (GNG) Omission Errors and spatial working memory (SWM) reaction time variability. Post-hoc comparison of means suggested that the Delta group had significantly worse performance relative to all groups on GNG omission errors and the NSE group on SWM reaction time variability. Qualitatively, inspection of group means for the cognitive control tasks suggests that the Delta and Theta groups had similar scores with generally worse performance across tasks. In contrast, the Alpha and Beta groups also tended to be more similar and have better performance compared to the Delta/Theta groups. Finally, the NSE group generally had the best performance on the cognitive control tasks.
Discussion
The purpose of the current study was to identify subgroups of children with and without ADHD according to their resting state EEG patterns using latent class analysis and subsequently provide comprehensive clinical, behavioral, and cognitive characterization of the latent subgroups. Overall, the results suggest that there are five resting state subgroups with differing patterns of associated behavioral and cognitive functioning. First, these EEG subgroups occur in both ADHD and typically developing groups and at generally the same frequencies. This suggests that there is heterogeneity in cortical activity at the population level, and there is no one subgroup that specifically represents ADHD or non-ADHD. Second, those with spectral elevations in slower frequencies (Delta and Theta bands) were more often younger and male with had higher rates of disruptive behaviors and cognitive dysfunction relative to other subgroups. In contrast, subgroups with elevated spectral power in faster frequencies (Alpha and Beta bands) tended to be older and female with have greater emotion dysregulation and internalizing behaviors but intact cognitive functioning relative to others. Finally, there was a subgroup with relatively better behavioral and cognitive functioning that had no spectral power elevations in any of the frequency bands. The current study advances the empiric literature in this area through: 1) use of a larger, more heterogeneous sample that includes typically developing controls, 2) use of sophisticated statistical techniques such as LCA to identify additional latent subgroups and 3) multilevel diagnostic, behavioral and cognitive phenotyping to facilitate comprehensive characterization and interpretation of the EEG subgroups.
Results of this study are consistent with prior studies on ADHD heterogeneity in behavioral, cognitive and neurobiological functioning. For example, neuropsychological studies (Sergeant, 2000; Sonuga-Barke, Bitsakou, & Thompson, 2010) have reported multiple cognitive pathways ending in a final common ADHD pathway, with some subgroups having executive function deficits and others having motivational deficits, which are potentially indicative of cortico-limbic dysfunction. The Delta and Theta groups appear to lie along the executive dysfunction pathway whereas the Alpha and Beta groups have greater emotion dysregulation that is associated with limbic system abnormalities. Alpha power has long been associated with mood dysregulation and, in a recent study using the CBCL Dysregulation Profile (DSP), emotion dysregulation was specifically characterized by attenuated delta and elevated alpha band power, whereas children with ADHD but no DSP elevation had the opposite profile (McGough et al., 2013). Within the EEG domain, Clarke et al (Clarke, Barry, Dupuy, Heckel, et al., 2011) found similar EEG subgroups, with elevated spectral power in slow wave, alpha and beta bands. The inclusion of systematic behavioral and cognitive phenotypes now allows functional interpretation of these EEG subgroups.
In terms of underlying neurobiological mechanisms, previous studies using concurrent EEG and fMRI have found that resting state theta (and to some extent delta) band power is negatively associated with activity in the default mode network (DMN) (Luchinger, Michels, Martin, & Brandeis, 2012), suggesting the slow wave activity subgroups may have reduced DMN activity and aberrant network interactions resulting in cortical slowing and lower neural activity during resting state. On the other hand, alpha and beta band power were positively correlated with activity in the DMN and thalamus (Jann, Kottlow, Dierks, Boesch, & Koenig, 2010; Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007) and negatively associated with attention networks as well as primary sensory regions (Luchinger et al., 2012; Nishida et al., 2015). This indicates that subgroups with elevated power in higher frequencies have higher thalamocortical arousal levels and lower sensory and cognitive processing while at rest. It is also interesting to note that attenuation of the slow frequencies (delta and theta) typically occurs with brain development and maturation (Luchinger et al, 2012). While the mean age in these groups were younger than the other latent classes, we note that there were individuals across the age range contained in the slower frequency classes. This suggests that these latent subgroups may be indexing brain maturation and other neurobiological factors more strongly than chronological age.
Extrapolated to the clinical realm, these data indicate that there is no one EEG measure or marker, such as the theta to beta ratio (TBR), that differentiates those with and without ADHD. Although early studies (pre-2010) supported the TBR as having high diagnostic validity for ADHD (Snyder & Hall, 2006), more recent (post 2010) studies have reported extremely low diagnostic accuracies ranging from 38–63% (for a review see (Lenartowicz & Loo, 2014). It is possible that early studies, particularly those with small samples may have ascertained children belonging primarily to one EEG subgroup or another, thus unduly influencing the results. Similarly, previous studies reporting elevated theta power in ADHD may have excluded certain latent subgroups with spectral elevations due to exclusion criteria that prohibited the inclusion of co-morbid diagnoses such as mood and anxiety. This would effectively eliminate many children with ADHD who had elevated alpha power. Although these results are not supportive of using resting state spectral power EEG measures as biomarkers of ADHD diagnosis, they may be more effective for identifying treatment response (Arns & Gordon, 2014) or developmental trajectory (Clarke, Barry, Dupuy, McCarthy, et al., 2011). These individual metrics may also be useful in identifying potentially problematic behaviors or co-morbidities, particularly along the internalizing spectrum. Future work with the current data will be to examine whether latent subgroups have differential medication treatment responses or long-term developmental outcomes.
These findings should be interpreted within the context of the following limitations. First, there was a much larger number of ADHD subjects compared to controls in the current study and this may have affected the structure in the latent class analysis. However, the latent model was tested separately within ADHD-only and Control-only groups and the same 5-class data structure with similar distributions among spectral elevation classes was observed. Within the latent classes, there were significant age and gender disparities, despite the use of age as a covariate in building the latent class models. Hence, even though age is a strong predictor, accounting for 6–40% of variance of latent class membership, elevated spectral power goes beyond the influence of age and likely reflects other neurobiological factors. In addition, we note that there was a wide age range within each latent class, suggesting these are not purely representing chronological age or developmental stage. It is possible that the classes are capturing maturational lags in resting state brain function that may occur differentially across individuals. And when that occurs, there appear to be specific behavioral and cognitive correlates associated with that brain state. All behavioral and cognitive characterizations were conducted using age as a covariate, to avoid the possibility of age driving latent class differences.
Conclusion
In a large, heterorgeneous sample of chldren, we detected several latent, EEG-based subgroups that are associated with differing patterns of diagnostic, behavioral, and cognitive characteristics. This variation in resting state brain activity likely represents true neurobiological differences and should be accounted for when examining group (diagnostic or otherwise) level differences in EEG spectral power. These EEG latent subgroups were not useful as classifiers of ADHD diagnosis but, with further work, may be useful in identifying the likelihood for specific psychiatric comorbidities, treatment response, or developmental outcomes.
Supplementary Material
Table S1. Separate LCAs within ADHD-only and control-only datasets.
Figure S1. Age effects within frequency bands.
Figure S2. Overlap of diagnostic group (ADHD and TD) within latent classes for behavioral, cognitive, and EEG measures
Key points.
Heterogeneity in resting state cortical activity exists at the population level, not only among children with ADHD.
There is no one EEG subgroup that specifically represents ADHD or typical development.
The subgroups have distinct behavioral and cognitive associated features but also heterogeneity with respect to age, gender, and ADHD diagnosis.
EEG measures may be more useful biomarkers of ADHD outcome or treatment response rather than diagnosis.
Acknowledgments
This work was funded by grants from the National Institutes of Health MH58277 (S.L.S.), NS054124 (S.K.L.), and MH077248 (J.T.M.). The authors would like to thank all the families that have participated in this research. J.T.M. reports consultant honoraria from Alcobra Pharmaceuticals, Think Now, Inc, research support from Psyadon Pharmaceuticals, payment for expert witness testimony for Kremers Urban Pharmaceuticals, and study drug for research from AstraZeneca. J.J.M. has received material support for investigator initiated research from NeuroSigma, DSMB honoraria from Sunovion, and has provided expert testimony for Janssen, Shire, and Tris Pharmaceuticals.
Footnotes
Additional Supporting Information may be found in the online version of this article:
The remaining authors have declared that they have no competing or potential conflicts of interest.
References
- Achenbach TM. Manual for the Revised Child Behavior Profile and Child Behavior Checklist. Burlington, VT: Author; 2001. [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-5. 4. Washington D.C: Author; 2013. [Google Scholar]
- Arns M, Gordon E. Quantitative EEG (QEEG) in psychiatry: diagnostic or prognostic use? Clinical Neurophysiology. 2014;125(8):1504–1506. doi: 10.1016/j.clinph.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Ayer L, Althoff R, Ivanova M, Rettew D, Waxler E, Sulman J, Hudziak J. Child Behavior Checklist Juvenile Bipolar Disorder (CBCL-JBD) and CBCL Posttraumatic Stress Problems (CBCL-PTSP) scales are measures of a single dysregulatory syndrome. Journal of Child Psychology & Psychiatry. 2009;50(10):1291–1300. doi: 10.1111/j.1469-7610.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- Chabot RJ, di Michele F, Prichep L, John ER. The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;13(2):171–186. doi: 10.1176/jnp.13.2.171. [DOI] [PubMed] [Google Scholar]
- Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biological Psychiatry. 1996;40(10):951–963. doi: 10.1016/0006-3223(95)00576-5. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Dupuy FE, Heckel LD, McCarthy R, Selikowitz M, Johnstone SJ. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology. 2011;122(7):1333–1341. doi: 10.1016/j.clinph.2010.12.038. doi: S1388-2457(10)00870-9 [pii] [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Dupuy FE, McCarthy R, Selikowitz M, Heaven PC. Childhood EEG as a predictor of adult attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2011;122(1):73–80. doi: 10.1016/j.clinph.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2001;112(11):2098–2105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- Conners CK. Paper presented at the American Psychological Association. Los Angeles, CA: 1994. The Continuous Performance Test (CPT): Use as a diagnostic tool and measure of treatment outcome. [Google Scholar]
- Costa Dias TG, Iyer SP, Carpenter SD, Cary RP, Wilson VB, Mitchell SH, Nigg JT, Fair DA. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Developmental Cognitive Neuroscience. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. The ADHD Rating Scale-IV: Checklists, norms, and clinical interpretation. New York: Guilford; 1999. [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proceedings of the National Academy of Science U S A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates KM, Molenaar PC, Iyer SP, Nigg JT, Fair DA. Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLoS One. 2014;9(3):e91322. doi: 10.1371/journal.pone.0091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Kim J, Cohen MS, Poutanen VP, Therman S, Bava S, Van Erp TG, Manninen M, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Cannon TD. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage. 2002;17(1):201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- IBM_Corp. IBM SPSS Statistics for Windows. Version 21. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jann K, Kottlow M, Dierks T, Boesch C, Koenig T. Topographic electrophysiological signatures of FMRI Resting State Networks. PLoS One. 2010;5(9):e12945. doi: 10.1371/journal.pone.0012945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, Nigg JT. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71(9):1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Loo SK. Use of EEG to diagnose ADHD. Curr Psychiatry Rep. 2014;16(11):498. doi: 10.1007/s11920-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Hale ST, Hanada G, Macion J, Shrestha A, McGough JJ, McCracken JT, Nelson S, Smalley SL. Familial clustering and DRD4 effects on electroencephalogram measures in multiplex families with attention deficit/hyperactivity disorder. Journal of the American Academy of Child Adolescent Psychiatry. 2010;49(4):368–377. [PMC free article] [PubMed] [Google Scholar]
- Lubke GH, Muthen B. Investigating population heterogeneity with factor mixture models. Psychol Methods. 2005;10(1):21–39. doi: 10.1037/1082-989X.10.1.21. [DOI] [PubMed] [Google Scholar]
- Luchinger R, Michels L, Martin E, Brandeis D. Brain state regulation during normal development: Intrinsic activity fluctuations in simultaneous EEG-fMRI. Neuroimage. 2012;60(2):1426–1439. doi: 10.1016/j.neuroimage.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyn KE. Latent Class Analysis and Finite Mixture Modeling. In: Little TD, editor. The Oxford Handbook of Quantitative Methods. New York: Oxford University Press; 2013. [Google Scholar]
- McGough JJ, McCracken JT, Cho AL, Castelo E, Sturm A, Cowen J, Piacentini J, Loo SK. A potential electroencephalography and cognitive biosignature for the child behavior checklist-dysregulation profile. Journal American Academy Child Adolescent Psychiatry. 2013;52(11):1173–1182. doi: 10.1016/j.jaac.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen L, Muthen B. Mplus User's Guide. Sixth. Los Angeles, CA: Muthen & Muthen; 1998–2011. [Google Scholar]
- Nishida K, Razavi N, Jann K, Yoshimura M, Dierks T, Kinoshita T, Koenig T. Integrating Different Aspects of Resting Brain Activity: A Review of Electroencephalographic Signatures in Resting State Networks Derived from Functional Magnetic Resonance Imaging. Neuropsychobiology. 2015;71(1):6–16. doi: 10.1159/000363342. [DOI] [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Bitsakou P, Thompson M. Beyond the dual pathway model: evidence for the dissociation of timing, inhibitory, and delay-related impairments in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(4):345–355. doi: 10.1016/j.jaac.2009.12.018. 00004583-201004000-00009 [pii] [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children. 4. San Antonio, Texas: The Psychological Corporation; 2003. [Google Scholar]
- Welch P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE TransAudioElectroacoust. 1967;15(2):70–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Separate LCAs within ADHD-only and control-only datasets.
Figure S1. Age effects within frequency bands.
Figure S2. Overlap of diagnostic group (ADHD and TD) within latent classes for behavioral, cognitive, and EEG measures