Abstract
Limited information exists regarding the longitudinal association between the left ventricular structure and fucntion and future cognitive impairment and dementia in a large population without clinically recognized cardiovascular disease at baseline. The aim of the present study was to investigate the association between cardiac structure and function and risk of dementia and cognitive impairment in the Multi-Ethnic Study of Atherosclerosis cohort. Measures of left ventricular structure and function were determined using magnetic resonance imaging at baseline in 4,999 participants free of clinically diagnosed cardiovascular disease and dementia. Probable incident clinical dementia was ascertained from hospitalization discharge records. Cognitive function was evaluated using tests addressing global cognitive function, processing speed, and memory. Associations of measures of left ventricular structure and function with the incidence of clinically diagnosed dementia and cognitive performance were evaluated using Cox proportional hazard regression models adjusted for demographics, cardiovascular risk factors, and cardiovascular events. During a median follow-up of 12 years, 130 probable incident dementia cases were documented. Higher left ventricular mass index (HR 1.01, 95%CI 1.00–1.02), and LV mass to volume ratio (HR 2.37, 95%CI 1.25–4.43) were independently associated with incident dementia, as well as impaired cognitive function. Measures of left ventricular function were not associated with risk of dementia or cognitive impairment. In conclusion, in a multiethnic cohort of participants without clinically detected cardiovascular disease and dementia at baseline, left ventricular hypertrophy and concentric remodeling were independently associated with incident dementia and cognitive impairment.
Keywords: Left ventricule, cognitive impairment, dementia
Introduction
Heart failure and dementia are two of the major geriatric public health problems worldwide. Heart failure affects over 5 million adults in the United States and is responsible for 36% of cardiovascular deaths1. Dementia is a leading cause of morbidity and disability that substantially shortens life expectancy2. Heart failure and dementia often occur together, causing substantial financial burden on health and social network systems3, 4. Both conditions share common risk factors, such as hypertension, obesity, and diabetes5, 6, and population-based studies have shown that heart failure is independently associated with dementia7, 8.
While numerous studies have shown associations between clinical heart failure and dementia7, 9, 10, in the general population and in the absence of heart disease, the association between subclinical cardiac dysfunction and dementia has not been fully established. Findings from the Framingham Heart Study suggested that a non-linear association exists between measures of systolic function and accelerated cognitive aging11. Recently, using echocardiography it was shown that diastolic dysfunction (calculated using the Doppler peak E velocity divided by Doppler peak A velocity), was associated with dementia in the Rotterdam Study12. LV hypertrophy has also been associated with poor cognitive performance in older subjects13–15.
Cardiac magnetic resonance (CMR) imaging is a precise and reproducible tool for assessment of cardiac structure and function16. In this regard, there are no studies investigating the longitudinal association of systolic and diastolic function and structural measures of CMR imaging with incident dementia in a large population of men and women without clinically recognized cardiovascular disease at baseline. Therefore, in the present study, we aim to investigate the relationship between CMR-derived measures of cardiac structure and function at baseline MESA exam with cognitive impairment and incident dementia in a large multi-ethnic population of both sexes free of cardiovascular disease at baseline. We hypothesize that structural parameters including LV mass index and mass-to-volume ratio, but not functional (systolic or diastolic) parameters are associated with future cognitive impairment/dementia.
Methods
Study population
The data that support the findings of this study are available and can be accessed at https://www.mesa-nhlbi.org The design of the MESA (Multi-Ethnic Study of Atherosclerosis) has been described previously17. Briefly, between July 2000 and August 2002, a total of 6,814 men and women aged 45 to 84 year and free of clinically apparent cardiovascular disease were recruited from 6 US communities representing four racial/ethnic groups (Caucasian, African-American, Hispanic, and Chinese-American). Subjects with prior clinical diagnosis of CVD at baseline were not included. To evaluate cardiac structure and function, a total of 4,999 individuals underwent CMR at baseline, with 1,502 participants agreeing to MRI tagging as a part of the imaging protocol. The study protocol was approved by the institutional review board of each MESA field center, and all participants gave informed consent.
Magnetic resonance imaging
CMR imaging was performed using 1.5-T MRI scanners (Avanto and Espree, Siemens Medical Systems; Signa LX, GE Healthcare) with a 6-channel anterior phased array torso coil and corresponding posterior coil elements18. The detailed MR protocol has been previously described (supplemental material)18.
Stroke volume was defined as the difference between LV end-diastolic volume and LV end-systolic volumes and the LV ejection fraction was calculated as LV stroke volume divided by LV end-diastolic volume. LV mass was indexed to body surface area. LV mass-to-volume ratio was obtained with the papillary muscle mass being included in the left ventricular cavity and excluded from the left ventricular mass. LV shape indices were calculated both at end-diastole and end-systole as previously described19.
Tagged images were analyzed using harmonic phase imaging (Diagnosoft, Palo Alto, California)20. Regional myocardial systolic function was assessed using myocardial circumferential midwall shortening in tagged images as quantified by circumferential strain. Circumferential strain (Ecc) is a negative value, with lower values representing greater LV systolic function. Indices of diastolic function including diastolic strain rate, torsion recoil rate and strain relaxation index were also calculated from tagged images as previously described21. Lower diastolic strain rate and strain relaxation index and higher torsion recoil rate indicate greater diastolic function.
Follow up and end points
A telephone interview was conducted every 9 to 12 months with each participant to identify interim hospitalizations, outpatient cardiovascular diagnoses and deaths (Supplemental Figure S1). For all events, medical records were obtained, including discharge diagnoses. Probable dementia cases included vascular dementia, Alzheimer’s and Pick’s disease and were classified from the coded discharge diagnoses using the International Statistical Classification of Diseases Medical Diagnosis Codes, 9th Revision. The candidate dementia cases were identified using the following diagnosis codes: ICD9: 290, 294, 331.0, 331.1, 331.2, 331.82, 331.83, 331.9, 438.0, and 780.93; ICD10: F00, F01, F03, F04, G30, G31 (excluding G31.2), I69.91, and R41 (supplemental Table S1). In addition, a physician ascertained all potential cases of dementia by reviewing the medical records of each case while blinded to ICD codes, looking for phrases that would indicate, or contradict the diagnosis of dementia. For cases where medical records were indeterminate, the records were sent to the MESA adjudication committee. The use of hospital discharge ICD Codes for probable dementia diagnosis has been used in prior publications from MESA22, 23.
All cognitive measurements were examined during 5th MESA follow up exam taking place between April 2010 and February 2012 (Table 1). The diagnosis of cardiovascular diseases were adjudicated and classified by 2 physicians from the MESA mortality and morbidity review committee.
Table 1.
Cognitive function tests used in the present study.
| Test | Cognitive area measured | Description |
|---|---|---|
| CASI | Global cognitive function | 25 items testing attention, concentration, orientation, short-term memory; long-term memory, language, visual construction, verbal fluency and abstraction/judgment |
| DSC | Processing speed | measuring how quickly simple perceptual or mental operations can be performed |
| Digit span | Working memory | administered in two parts requiring the participant to repeat gradually increasing spans of numbers (e.g., 2-7-4) first forwards and then backwards |
CASI: Cognitive Abilities Screening Instrument, DSC: Digit Symbol Coding,
Statistical Analysis
Categorical variables were presented as percent proportions and compared between groups using chi-square tests. Continuous variables were presented as mean ± SD and compared between groups using two-sided t-tests. Natural logarithmic transformation was applied to early diastolic strain rate, and strain relaxation index given that these variables had skewed distributions.
Multivariable competing-risks Cox proportional hazard regression models were constructed to assess the association of LV structure and functional parameters with newly-identified probable dementia24. Death before the diagnosis of dementia was considered as a competing event (Fine and Gray method24) as it hindered the observation of the dementia. Hazard ratios (HRs) were calculated along with the corresponding 95% confidence intervals to make statistical inference on the covariate effects. The multivariable models used in the present study were based on prior literature13–15 and were constructed as following:
Model 1 was adjusted for demographics, educational level and income level.
Model 2 included all variables in model 1 in addition to traditional CVD risk factors at baseline MESA exam including systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, and coronary artery calcium score.
Model 3 was adjusted for all variables in model 2 and also interim cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke as time-varying covariates.
Model 4 included all variables in model 3 and also LV mass index.
Sensitivity analyses were performed with the exclusion of participants who had interim stroke, patients with suboptimal CMR imaging and patients receiving medications that prevent cardiac remodeling. Cumulative event rate plots were calculated for tertiles of LV structure and functional parameters. General linear models were used to examine the associations between cognitive tests and the LV structure and functional indices adjusted for age, gender, race/ethnicity, level of education, income level, systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, coronary artery calcium score, cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke during the follow-up period. The CASI variable was log-transformed in order to better approximate a normal distribution.
LV sphericity was measured and divided into quintiles as previously described19. Briefly, the LV shape was divided into quintiles of the sphericity volume index (SVI), with the first and last quintile representing low sphericity (or high conicity) and high sphericity, respectively. Quintiles 2–4 were combined to form the reference group.
Two-tailed P-values < 0.05 were used for significance testing. All statistical analysis was done using Stata 14.0 (StataCorp LP, College Station, TX, USA).
Results
In total, 4999 MESA participants who underwent CMR at MESA baseline exam were included in this study. Data from tagged images were available for 1502 of the included participants. A total of 130 incident probable dementia cases were ascertained over a median of 12 years of follow up, with 47 cases of probable dementia among individuals with available MRI tagging data. Table 2 describes the baseline characteristics of those who developed dementia and those who did not. Participants with probable dementia were older and had lower income. They had higher systolic blood pressure, pulse pressure, resting heart rate, and higher use of anti-hypertensive medications, and were more likely to have non-zero coronary artery calcium (CAC) score. Furthermore, participants who developed probable dementia had higher unadjusted LV mass index, mass-to-volume ratio, and strain relaxation index, and lower unadjusted stroke volume and early diastolic strain rate.
Table 2.
Baseline characteristics of study participants stratified by dementia (N=4999)
| Characteristics | No dementia (4,869) | Dementia (130) | P value |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD | 61 ± 9 | 73 ± 7 | <0.001 |
| Males, n (%) | 2.308 (47.4) | 71 (54.6) | 0.11 |
| Race, n (%) | 0.58 | ||
| Caucasian | 1,893 (39) | 62 (47.6) | |
| Chinese | 644 (13.2) | 9 (7.1) | |
| African-American | 1,247 (25.6) | 36 (27.7) | |
| Hispanic | 1,085 (22.2) | 23 (17.6) | |
| Income, $ | <0.001 | ||
| <$20 000 | 1,052 (22.3) | 46 (37.4) | |
| $20 000 to $49 999 | 1,708 (36.2) | 56 (45.5) | |
| ≥$50 000 | 1,954 (41.5) | 21 (17.1) | |
| Cardiovascular risk factors | |||
| Systolic blood pressure, (mmHg), mean ± SD | 125 ± 21 | 135 ± 23 | <0.001 |
| Diastolic blood pressure, (mmHg), mean ± SD | 71 ± 10 | 72 ± 10 | 0.41 |
| Pulse pressure, (mmHg) mean ± SD | 53 ± 16 | 62 ± 18 | <0.001 |
| Hypertension medication use, n (%) | 1,697 (34.8) | 66 (50.7) | <0.001 |
| Beta blocker use, n (%) | 580 (11.9) | 29 (22.3) | 0.01 |
| Angiotensin converting enzyme inhibitor, n (%) | 770 (15.8) | 36 (27.6) | 0.003 |
| Angiotensin receptor blocker use, n (%) | 223 (4.5) | 9 (6.9) | 0.23 |
| Heart rate, mean ± SD | 62 ± 9 | 64 ± 10 | 0.030 |
| Body mass index, (kg/m2), mean ± SD | 27 ± 4 | 27 ± 4 | 0.175 |
| HDL cholesterol (mg/dl), mean ± SD | 51 ± 14 | 51 ± 14 | 0.68 |
| Total cholesterol (mg/dl), mean ± SD | 194 ± 35 | 193 ± 33 | 0.87 |
| Diabetes, n (%) | 558 (11.49) | 20 (15.38) | 0.37 |
| Smoking status, n (%) | 0.81 | ||
| Former | 1,738 (35.8) | 46 (35.38) | |
| Current | 620 (12.77) | 14 (10.77) | |
| Alcohol use, n (%) | 2,723 (69.34) | 69 (70.41) | 0.92 |
| Coronary artery calcium score, n (%) | <0.001 | ||
| 0 | 2,538 (52.1) | 34 (26.2) | |
| 1–99 | 1,296 (26.6) | 27 (20.7) | |
| 100–399 | 616 (12.6) | 40 (30.7) | |
| >399 | 420 (8.7) | 29 (22.4) | |
| CMR indexes (N=4,999) | |||
| LV mass index, (g/m2) mean ± SD | 77 ± 16 | 81 ± 15 | 0.007 |
| Mass-to-volume ratio, (g/ml) mean ± SD | 1.16 ± 0.24 | 1.30 ± 0.28 | <0.001 |
| LV ejection fraction, (%), mean ± SD | 69 ± 7 | 68 ± 8 | 0.81 |
| Stroke volume, ml, mean ± SD | 46 ± 8 | 43 ± 8 | <0.001 |
| CMR indexes (N= 1,502) | |||
| LV circumferential strain, % mean ± SD | −15.07 ± 2.86 | −14.79 ± 2.87 | 0.51 |
| LV torsion recoil, (deg/cm/ms), mean ± SD | −19.39 ± 11.24 | −22.13 ± 14.38 | 0.15 |
| Strain relaxation index, (ms/%) ± SD | 0.74 ± 0.01 | 0.97 ± 0.07 | 0.017 |
| Early diastolic strain rate, (%/ms) mean ± SD | −2.22 ± 0.012 | −2.38 ± 0.05 | 0.019 |
LV: Left ventricular,
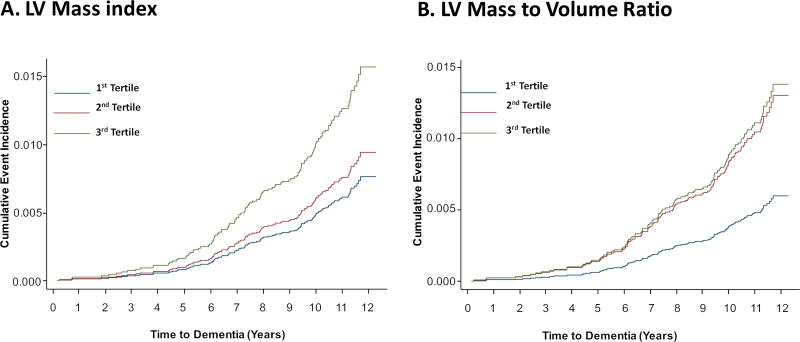
Table 3 describes the adjusted hazard ratios for probable dementia in relation to LV functional and structural parameters. All LV functional parameters showed linear associations with probable dementia. None of the indices of systolic function including LV ejection fraction, stroke volume or LV circumferential strain index were associated with dementia in multivariable analysis (Table 3). In contrast, the markers of diastolic dysfunction and LV remodeling were associated with the risk of probable dementia, including LV mass index, mass-to-volume ratio, LV global function index, strain relaxation index and early diastolic strain rate. The hazard ratio remained significant after adjustment for sociodemographics, cardiovascular risk factors, and cardiovascular events during follow-up (Table 3). However, inclusion of the LV mass index into the regression model abolished the significant association between diastolic measures of strain relaxation index and early diastolic strain rate with risk of probable dementia (Model 4, Table 3). Nevertheless, adjustment for LV mass index did not attenuate the significant relationship between structural parameters of mass-to-volume ratio with risk of dementia (Table 3). The relationship remained significant even after a sensitivity analysis was performed including only patients receiving medications angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or beta blockers that prevent left ventricular remodeling (HR 5.50 (1.30–23.22), P=0.020). The findings were similar when participants who developed clinically recognized stroke during follow-up were excluded. Excluding patients who had suboptimal CMR images (22 patients) did not alter any of the observed associations. Figure 1 shows the cumulative incidence curves for probable dementia.
Table 3.
Hazard ratio for association between left ventricular functional parameters and risk of dementia.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | ||||
|
|
||||
| Systolic function | ||||
| LV ejection fraction, (%) | 0.98 (0.96–1.00) | 0.98 (0.95–1.02) | 0.99 (0.96–1.01) | 0.99 (0.96–1.00) |
| Stroke volume, (ml) | 0.99 (0.97–1.00) | 0.99 (0.96–1.00) | 0.98 (0.96–1.01) | 0.98 (0.96–1.01) |
| LV circumferential strain, (%) | 1.00 (0.90–1.12) | 1.05 (0.93–1.16) | 1.05 (0.92–1.18) | 1.05 (0.93–1.18) |
| Diastolic function | ||||
| LV torsion recoil, (deg/cm/ms) | 0.98 (0.95–1.01) | 0.98 (0.96–1.01) | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) |
| Log (Strain Relaxation Index), (ms/%) | 1.51 (1.01–2.24) | 1.61 (1.05–2.45) | 1.60 (1.05–2.42) | 1.53 (0.99–2.33) |
| Log (Early Diastolic Strain Rate), (%/ms) | 0.60 (0-37–0.94) | 0.57 (0.35–0.95) | 0.58 (0.34–0.95) | 0.60 (0.36–1.01) |
| LV structural indices | ||||
| LV mass index, (g/m2) | 1.01 (1.00–1.02) | 1.01 (1.00–1.03) | 1.01 (1.00–1.02) | |
| LV mass-to-volume ratio, (g/ml) | 2.20 (1.21–3.99) | 2.28 (1.23–4.25) | 2.31 (1.23–4.29) | 2.37 (1.25–4.43) |
HR: Hazard Ratio, LV: Left ventricular, Significant HR and their confidence intervals are presented in bold. Model 1 included covariates for demographics including age, gender, race/ethnicity, level of education and income level. Model 2 included all variables in model 1 in addition to risk factors including systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, fasting glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, and coronary artery calcium score. Model 3 included all model 2 variables, with the addition of cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke during the follow-up period. Model 4 included all variables in model 3, with the addition of LV mass index.
Figure 1.
Cumulative event rates for probable dementia events by tertiles for LV mass index (A), LV mass-to-volume ratio (B), controlled for age, gender, race/ethnicity, level of education and income level, systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, fasting glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, and coronary artery calcium score, and cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke during the follow-up period (Model 3).
Table 4 demonstrates the hazard ratios for the prediction of dementia using SVI. Low sphericity at end-diastole was a predictor of dementia independent of conventional risk factors. This relationship remained significant even after adjustment for LV mass index (Table 4).
Table 4.
Hazard ratio for association between sphericity indices at end-diastole and end-systole and risk of dementia.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | ||||
|
|
||||
| SVIED | ||||
| Low sphericity/high conicity | 7.31 (1.67–52.66) | 8.15 (1.92–60.46) | 8.19 (2.1–59.60) | 8.07 (1.11–58.73) |
| High sphericity | 1.37 (0.59–3.20) | 1.34 (0.59–3.05) | 1.42 (0.62–3.26) | 1.46 (0.62–3.43) |
| SVIES | ||||
| Low sphericity/high conicity | 1.62 (0.74–3.55) | 1.49 (0.58–3.78) | 1.48 (0.58–3.73) | 1.40 (0.55–3.54) |
| High sphericity | 1.33 (0.48–3.69) | 1.71 (0.57–5.05) | 1.71 (0.58–5.03) | 1.57 (0.52–4.71) |
HR: Hazard Ratio, SVIED, sphericity volume index at end-diastole; SVIES, sphericity volume index at end-systole, Significant HR and their confidence intervals are presented in bold. Model 1 included covariates for demographics including age, gender, race/ethnicity, level of education and income level. Model 2 included all variables in model 1 in addition to risk factors including systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, fasting glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, and coronary artery calcium score. Model 3 included all model 2 variables, with the addition of cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke during the follow-up period. Model 4 included all variables in model 3, with the addition of LV mass index.
The association between cognitive test scores and LV functional and structural parameters are shown in Table 4. Increases in LV mass index and mass-to-volume ratio (all of which are indicative of increased cardiac remodeling) were associated with decreased cognitive performance (Table 5). None of the LV systolic or diastolic function parameters were associated with measures of cognitive performance.
Table 5.
Coefficients for Multivariable Linear Regression of the associations between cognitive test scores and left ventricular functional parameters
| Digit Span | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| CASI | DSC | Forward | Backward | |||||
|
|
||||||||
| Systolic function | ||||||||
| LV ejection fraction, (%) | −0.099 (−2.345–1.751) | 0.79 | 0.009 (−1.281–0.127) | 0.24 | 0.039 (−0.172–0.251) | 0.49 | 0.012 (−0.151–0.014) | 0.81 |
| Stroke volume, (ml) | −0.064 (−0.121–0.672) | 0.89 | 0.010 (−0.721–1.281) | 0.33 | 0.044 (−0.172–0.239) | 0.46 | −0.014 (−0.412–0.011) | 0.82 |
| LV circumferential strain, (%) | −0.027 (−1.231–1.145) | 0.92 | −0.015 (−0.192–0.123) | 0.12 | 0.08 (−0.152–0.381) | 0.61 | 0.023 (−0.126–0.131) | 0.56 |
| Diastolic function | ||||||||
| LV torsion recoil, (deg/cm/ms) | −1.839 (−4.261–1.189) | 0.23 | −0.006 (−0.716–0.912) | 0.82 | −0.09 (−0.712–0.012) | 0.54 | −0.130 (−0.812–0.182) | 0.44 |
| Log (Strain Relaxation Index), (ms/%) | 0.046 (−.1.124–0.812) | 0.71 | 0.046 (−0.012–0.329) | 0.48 | −0.007 (−0.192–0.015) | 0.34 | −0.008 (−0.182–0.081) | 0.35 |
| Log (Early Diastolic Strain Rate), (%/ms) | −0.010 (−1.241–0.871) | 0.22 | 0.001 (−0.012–0.001) | 0.21 | −0.001 (−0.193− 0.028) | 0.34 | 0.009 (−0.124–0.301) | 0.19 |
| LV structural indices | ||||||||
| LV mass index, (g/m2) | −2.367 (−4.110– −1.231) | 0.002 | −0.105 (−0.691– −0.012) | 0.001 | −0.23 (−1.231– −0.001) | 0.01 | −0.449 (−1.192– −0.012) | 0.001 |
| LV mass-to-volume ratio, (g/ml) | −0.030 (−0.078– −0.001) | 0.013 | −0.001 (−0612– −0.000) | 0.001 | −0.003 (−0.191– −0.000) | 0.009 | −0.005 (−0.017– −0.000) | 0.001 |
Coefficients and 95% confidence intervals (in brackets) for multivariable linear regression model to assess the associations between CMR variables with different cognitive performance domains. Models adjusted for demographics including age, gender, race/ethnicity, level of education and income level, risk factors including systolic blood pressure, diastolic blood pressure, pulse pressure, use of antihypertensives, resting heart rate, high-density lipoprotein, low-density lipoprotein, fasting glucose level, body mass index, diabetes, CRP levels, cigarette smoking, alcohol use, coronary artery calcium score and cardiovascular events including coronary events (myocardial infarction and resuscitated cardiac arrest), heart failure, coronary revascularization (percutaneous coronary intervention or coronary artery bypass surgery) and clinical stroke during the follow-up period.
CASI: Cognitive Abilities Screening Instrument, DSC: Digit Symbol Coding
Discussion
The findings of the present study indicate that in a large multiethnic cohort, free of clinically recognized cardiovascular disease at enrollment, measures of LV hypertrophy and concentric remodeling were strongly associated with a higher risk of cognitive impairment and probable dementia. These findings were independent of demographic confounders, cardiovascular risk factors, and cardiovascular events. Diastolic dysfunction was originally associated with probable dementia and cognitive impairment, but this relationship was eliminated after adjusting for LV mass index, suggesting that LV hypertrophy mediates the association of diastolic dysfunction and dementia. There was no association between systolic function and either probable dementia or cognitive impairment.
In the present study, greater LV mass index and concentric remodeling (defined by elevated LV mass to volume ratio) were inversely associated with measures of cognitive function. Additionally, both parameters were also associated with increased risk of probable dementia. These associations remained significant after adjustments for multiple covariates, including blood pressure and use of anti-hypertensive medications. Previous studies have also shown the association between LV mass and cognitive performance, after adjusting for age and sex13–15. While the association was found to be attenuated with adjustment for mean systolic blood pressure in earlier studies14, 15, in a more recent study performed on 400 elderly subjects, similar associations were found between increased LV mass index and worse cognitive performance, regardless of adjustment for blood pressure values13. While the study population was older (mean age 79 years old) with a higher prevalence of hypertension (70%) compared to the present study, the results showed that the association between increased LV mass index and higher likelihood of having dementia was independent of blood pressure levels and/or large artery stiffness.
While high blood pressure is a key risk factor in the development of increased LV mass and LV hypertrophy and remodeling25, one possible explanation for the observed association between measures of LV structure and cognition, independent of blood pressure levels, could be ascribed to the fact that both markers represent sensitive indicators of lifelong exposure to higher BP levels26. Results from the British birth cohort study showed that elevated blood pressure from early midlife predicted higher LV mass index and LV remodeling in later life, even after accounting for current blood pressure levels27. The authors also found that subjects receiving antihypertensive medications had higher LV mass index and prevalence of LV hypertrophy, suggesting that effective treatment of hypertension may not achieve complete reversal of cardiac target organ damage27.
The results of the present study showed that low sphericity or increased conicity at end-diastole was associated with increased risk of probable future dementia. The relationship was independent of sociodemographics, cardiovascular risk factors, cardiovascular events during follow-up and LV mass index. It has previously been shown that patients exhibiting low sphericity on CMR imaging have greater concentric remodelling and ventricular hypertrophy, leading to increased cardiomyocyte stress as evidenced by higher levels of NT-proBNP found in this group of patients19. Low sphericity has been indicative of a stiffer and more fibrotic ventricle, and has been shown to be a predictor of incident cardiovascular disease28. These findings suggest that the LV geometry is a predictor of future dementia independent of LV size or conventional risk factors.
Previous reports have indicated an increased risk of dementia among patients with overt cardiac disease including heart failure7, 29 atrial fibrillation30–32 and coronary heart disease33, 34. However, limited information exists among asymptomatic populations on systolic and diastolic function related to dementia. Recently, findings from the Rotterdam Study showed that worse diastolic function is related to an increased risk of dementia through echocardiography measures in people without clinical cardiac disease12. In the present study, measures of impaired diastolic function were associated with increased risk of probable dementia after adjusting for patients’ demographics, cardiovascular risk factors and cardiovascular events. However, when LV mass index was added to the covariate set, the relation between diastolic function and probable dementia was rendered nonsignificant. These findings suggest that LV hypertrophy plays an important role in the relation between diastolic function and probable dementia.
The association between systolic function and dementia in the general population without cardiovascular disease has not been consistent among all studies. While the Rotterdam Study did not find any association between measures of systolic function and risk of dementia12, findings from the Framingham Heart Study demonstrated a U-shaped association between left ventricular systolic function and cognitive performance11. Similarly, other studies have shown that diminished systolic function is related to lower brain volume35 and silent brain infarcts36, both of which are important markers of brain aging. However, these studies used echocardiography for systolic function measurement12, 36 or were cross-sectional by design11, 35. In the present study, both global and regional systolic function was assessed using CMR which has proven to be the most accurate method to assess cardiac systolic function16. Our results indicate that measures of systolic function at baseline are not related to impaired cognitive function or dementia in individuals without overt cardiovascular disease.
The present study has a number of limitations. Probable incident dementia cases were ascertained from hospitalization records using ICD-9 codes. This method of dementia diagnosis likely underestimates the incidence of disease, since it only identifies subjects who were hospitalized and dementia was included among their discharge diagnoses. In addition, the presence of multiple diagnosis in hospitalized patients creates a downward bias in the association by decreasing the probability of the dementia diagnosis being recorded among the discharge codes. However, this method of identifying dementia cases has been shown to have adequate positive predictive value in a recent validation study on MESA participants with the diagnosis of dementia22. In addition, the strong association between both poor cognitive scores and probable dementia cases and LV structural measures, suggests that the present study is identifying true dementia cases. Another limitation of the present study is the notion that while many known risk factors for cardiovascular and brain disease were adjusted for in the multivariable analyses, the possibility that unmeasured confounders might be involved in the observed associations cannot be excluded. Therefore, the results of the present study should be interpreted in the context of its limitations.
Perspectives
The results of the present study indicate that in a large diverse population-based study with long follow-up periods among individuals free of symptomatic cardiovascular disease at enrollment, measures of increased LV mass and LV remodeling and lower sphericity but not LV function were associated with the risk of cognitive impairment and probable clinical dementia. These findings highlight the possibility of early involvement of the heart in patients with dementia and may be useful in identification of individuals at risk of cognitive impairment. Whether normalization of LV mass and reversal of LV remodeling through improved blood pressure control and other measures could prevent future cognitive impairment and dementia remains to be established.
Supplementary Material
Novelty and Significance.
1) What Is New
Studies investigating the longitudinal association of systolic and diastolic function and structural measures with cognitive performance and incident dementia in a large population of men and women without clinically recognized cardiovascular disease at baseline are sparse.
2) What Is Relevant?
CMR measures of increased left ventricular remodeling were independently associated with increased risk of cognitive impairment and probable dementia. This association remained significant after controlling for sociodemographics, cardiovascular risk factors, and cardiovascular events during follow-up.
Summary.
In a large multiethnic cohort, free of clinically recognized cardiovascular disease at enrollment, CMR measures of LV hypertrophy and concentric remodeling were strongly associated with a higher risk of future cognitive impairment and dementia.
Acknowledgments
The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Sources of Funding:
This work was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Footnotes
Disclosures:
None
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Executive summary: Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: A review. International journal of geriatric psychiatry. 2013;28:1109–1124. doi: 10.1002/gps.3946. [DOI] [PubMed] [Google Scholar]
- 3.Dayer M, Cowie MR. Heart failure: Diagnosis and healthcare burden. Clinical medicine. 2004;4:13–18. doi: 10.7861/clinmedicine.4-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. BioMed research international. 2014;2014:908915. doi: 10.1155/2014/908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Archives of internal medicine. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the kungsholmen project: A 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 7.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and alzheimer disease: A population-based cohort study. Archives of internal medicine. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 8.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. European journal of heart failure. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Ampadu J, Morley JE. Heart failure and cognitive dysfunction. International journal of cardiology. 2015;178:12–23. doi: 10.1016/j.ijcard.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 10.Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart failure reviews. 2016;21:661–673. doi: 10.1007/s10741-016-9568-1. [DOI] [PubMed] [Google Scholar]
- 11.Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O'Donnell CJ, Wolf PA, Manning WJ, Beiser AS, Benjamin EJ. Relation of left ventricular ejection fraction to cognitive aging (from the framingham heart study) The American journal of cardiology. 2011;108:1346–1351. doi: 10.1016/j.amjcard.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bruijn RF, Portegies ML, Leening MJ, Bos MJ, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW, Franco OH, Koudstaal PJ, Ikram MA. Subclinical cardiac dysfunction increases the risk of stroke and dementia: The rotterdam study. Neurology. 2015;84:833–840. doi: 10.1212/WNL.0000000000001289. [DOI] [PubMed] [Google Scholar]
- 13.Scuteri A, Coluccia R, Castello L, Nevola E, Brancati AM, Volpe M. Left ventricular mass increase is associated with cognitive decline and dementia in the elderly independently of blood pressure. European heart journal. 2009;30:1525–1529. doi: 10.1093/eurheartj/ehp133. [DOI] [PubMed] [Google Scholar]
- 14.Elias MF, Sullivan LM, Elias PK, D'Agostino RB, Wolf PA, Seshadri S, Au R, Benjamin EJ, Vasan RS. Left ventricular mass, blood pressure, and lowered cognitive performance in the framingham offspring. Hypertension. 2007;49:439–445. doi: 10.1161/01.HYP.0000256361.68158.24. [DOI] [PubMed] [Google Scholar]
- 15.Kahonen-Vare M, Brunni-Hakala S, Lindroos M, Pitkala K, Strandberg T, Tilvis R. Left ventricular hypertrophy and blood pressure as predictors of cognitive decline in old age. Aging clinical and experimental research. 2004;16:147–152. doi: 10.1007/BF03324544. [DOI] [PubMed] [Google Scholar]
- 16.Sechtem U, Mahrholdt H, Vogelsberg H. Cardiac magnetic resonance in myocardial disease. Heart. 2007;93:1520–1527. doi: 10.1136/hrt.2005.067355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: Objectives and design. American journal of epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR. American journal of roentgenology. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 19.Ambale-Venkatesh B, Yoneyama K, Sharma RK, Ohyama Y, Wu CO, Burke GL, Shea S, Gomes AS, Young AA, Bluemke DA, Lima JA. Left ventricular shape predicts different types of cardiovascular events in the general population. Heart. 2017;103:499–507. doi: 10.1136/heartjnl-2016-310052. [DOI] [PubMed] [Google Scholar]
- 20.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA. Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase mri. Circulation. 2000;101:981–988. doi: 10.1161/01.cir.101.9.981. [DOI] [PubMed] [Google Scholar]
- 21.Ambale-Venkatesh B, Armstrong AC, Liu CY, Donekal S, Yoneyama K, Wu CO, Gomes AS, Hundley GW, Bluemke DA, Lima JA. Diastolic function assessed from tagged mri predicts heart failure and atrial fibrillation over an 8-year follow-up period: The multi-ethnic study of atherosclerosis. European heart journal cardiovascular Imaging. 2014;15:442–449. doi: 10.1093/ehjci/jet189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiyoshi A, Jacobs DR, Jr, Alonso A, Luchsinger JA, Rapp SR, Duprez DA. Validity of death certificate and hospital discharge icd codes for dementia diagnosis: The multi-ethnic study of atherosclerosis. Alzheimer disease and associated disorders. 2017;31:168–172. doi: 10.1097/WAD.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiyoshi A, Jacobs DR, Jr, Fitzpatrick AL, Alonso A, Duprez DA, Sharrett AR, Seeman T, Blaha MJ, Luchsinger JA, Rapp SR. Coronary artery calcium and risk of dementia in mesa (multi-ethnic study of atherosclerosis) Circulation. Cardiovascular imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JPGR. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. Journal of the American College of Cardiology. 1988;12:996–1004. doi: 10.1016/0735-1097(88)90467-6. [DOI] [PubMed] [Google Scholar]
- 26.Verdecchia P, Carini G, Circo A, Dovellini E, Giovannini E, Lombardo M, Solinas P, Gorini M, Maggioni AP, Group MS. Left ventricular mass and cardiovascular morbidity in essential hypertension: The mavi study. Journal of the American College of Cardiology. 2001;38:1829–1835. doi: 10.1016/s0735-1097(01)01663-1. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh AK, Hardy RJ, Francis DP, Chaturvedi N, Pellerin D, Deanfield J, Kuh D, Mayet J, Hughes AD. Medical Research Council National Survey of H, Development S, Data Collection T. Midlife blood pressure change and left ventricular mass and remodelling in older age in the 1946 british birth cohort study. European heart journal. 2014;35:3287–3295. doi: 10.1093/eurheartj/ehu389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohyama Y, Ambale-Venkatesh B, Noda C, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: The multi-ethnic study of atherosclerosis. Circulation. Cardiovascular imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haeusler KG, Laufs U, Endres M. Chronic heart failure and ischemic stroke. Stroke. 2011;42:2977–2982. doi: 10.1161/STROKEAHA.111.628479. [DOI] [PubMed] [Google Scholar]
- 30.Medi C, Hankey GJ, Freedman SB. Stroke risk and antithrombotic strategies in atrial fibrillation. Stroke. 2010;41:2705–2713. doi: 10.1161/STROKEAHA.110.589218. [DOI] [PubMed] [Google Scholar]
- 31.Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The rotterdam study. Stroke. 1997;28:316–321. doi: 10.1161/01.str.28.2.316. [DOI] [PubMed] [Google Scholar]
- 32.Thacker EL, McKnight B, Psaty BM, Longstreth WT, Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: A longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta M, Hanna E, Das P, Steinhubl SR. Incidence and prevention of ischemic stroke following myocardial infarction: Review of current literature. Cerebrovascular diseases. 2006;22:331–339. doi: 10.1159/000094847. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and alzheimer's disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. Journal of the American Geriatrics Society. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 35.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O'Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: The framingham heart study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: The cardiovascular abnormalities and brain lesions (cabl) study. Circulation. 2013;128:1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.