DEAR EDITOR, olmutinib is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) for the treatment of non-small cell lung cancer (NSCLC), especially for those harboring T790M mutations, the most common reason for other EGFR-TKI resistance.1 We describe three patients who developed acquired palmoplantar keratoderma (PPK) after taking olmutinib, an adverse event which has not been reported in previous generations of EGFR-TKIs.
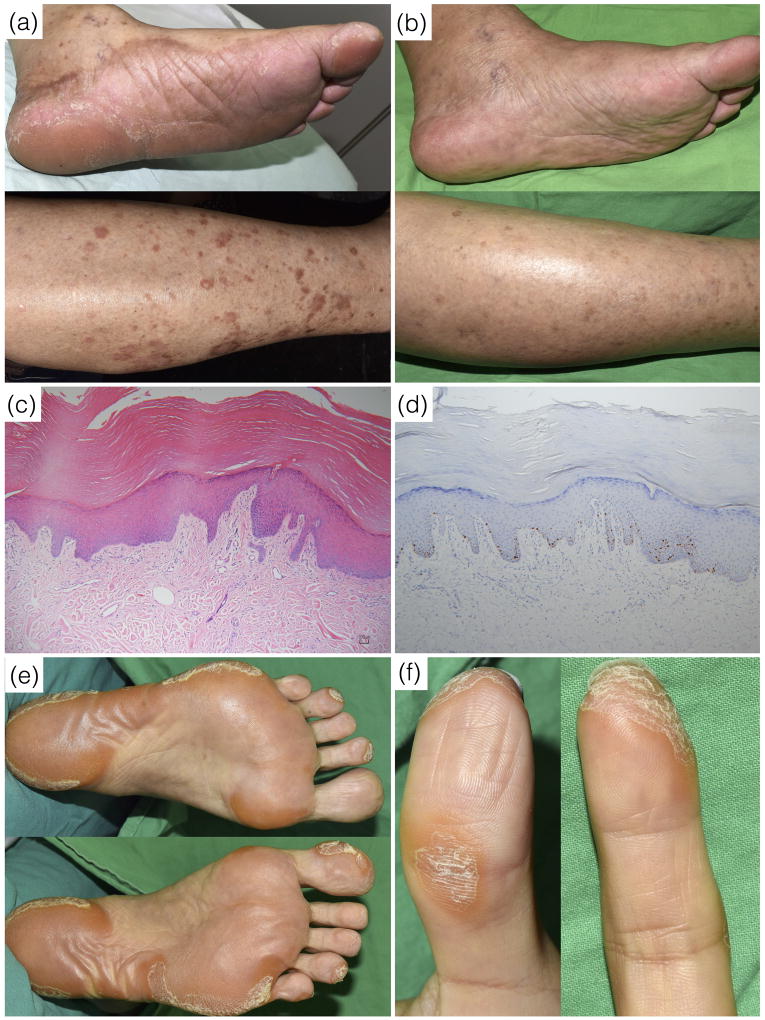
Patient 1 is a 74-year-old female with NSCLC and EGFR L858R mutations. The disease progressed despite an 11-month course of gefitinib from August 2014 to July 2015 and chemotherapy with pemetrexed and carboplatin in the following four months. She was confirmed to harbor EGFR T790M mutations and therefore commenced olmutinib 800mg daily in January 2016. One month later, asymptomatic diffuse thickening of the soles developed with discrete hyperkeratotic patches and plaques on the shins (Figure 1a). The lesions persisted during the period of treatment with olmutinib and resolved spontaneously 1 month after discontinuation of olmutinib (Figure 1b). A skin biopsy from the right sole showed acanthosis, hyperkeratosis, focal parakeratosis and papillary dermal elongation with minimal dermal inflammation, which were compatible with the pathological features of acquired PPK (Figure 1c). An immunohistochemistry study showed Ki-67 positive cells confined to the basal and suprabasal layers of the epidermis (Figure 1d).
Fig. 1.
Patient 1. (a) About one month after the initiation of olmutinib therapy, brownish asymptomatic dispersed hyperkeratotic patches and plaques developed on the bilateral soles of the feet and shins. (b) The lesions resolved spontaneously at 1 month after discontinuation of olmutinib. (c) The pathology study showed acanthosis, hyperkeratosis and papillary dermal elongation with minimal dermal inflammation (hematoxylin and eosin stain, 100X). (d) The immunohistochemistry study showed Ki-67 positive cells confined to the basal and suprabasal layers (Ki-67 stain, 100X). Patient 2. (e) Asymptomatic, focal hyperkeratotic plaques mainly on the pressure points of the soles and (f) tips of the toes and fingers, but sparing the nails.
Patient 2 is a 54-year-old female with NSCLC and EGFR exon 19 deletion mutations. The patient had received a 14-month course of therapy with erlotinib in 2013, and had received an 11-month course of afatinib in 2015. Due to disease progression and confirmation of EGFR T790M mutations, afatinib was discontinued in April 2016 and olmutinib 800mg daily was introduced in May 2016. One month later, asymptomatic and focal hyperkeratotic plaques developed on the pressure points of the soles (Figure 1e), as well as on the tips of her toes and fingers (Figure 1f). A skin biopsy from the right sole showed findings suggesting acquired PPK with positive Ki-67 staining.
Patient 3 is a 59-year-old male with NSCLC. He had experienced disease progression despite 3 months of chemotherapy with pemetrexed and carboplatin in 2014, as well as 8 months of erlotinib in 2015. Treatment with olmutinib 800mg daily was started in January 2016 due to confirmed EGFR T790M mutations. About one month after commencing olmutinib, the patient developed asymptomatic palmoplantar hyperkeratotic patches and plaques which spontaneously resolved one month after olmutinib was stopped.
PPK comprises a heterogeneous group of disorders which can be acquired or hereditary. None of our patients had a family history of PPK, nor did they have medical issues or contact with medications known to be related to acquired PPK.2 All of them developed PPK about one month after the commencing olmutinib, and patients 1 and 3 experienced normalization of PPK after discontinuing olumutinib. The wash-out period for prior EGFR-TKIs was 6 months in patient 1 and 1 month in the other two patients. In our previous 5-year retrospective study reviewing 146 patients who received gefitinib, erlotinib or afatinib, none of those patients developed PPK.3 It therefore appears acquired PPK is a specific adverse effect associated with olmutinib. Although the authors are not aware of any cases of PPK reported in clinical trials investigating olmutinib or post-approval safety surveillance, our experience that PPK developed in three out of a total of five patients treated with olmutinib in our hospital during this period would suggest this could be a relatively common adverse effect.
Patient 1 and patient 3 had diffuse PPK, whereas in patient 2, the PPK was focally distributed at the pressure points. None of them had nail changes. All three patients experienced asymptomatic thickening of the skin, which were different from painful PPK due to multi-kinase inhibitors.4
The pathogenesis of olmutinib-induced PPK remains unclear. However, activations of EGFR signaling appears to play a role in several different hereditary PPKs: (1) in Bushke-Fischer-Brauer type punctate PPK, loss-of-function mutations in AAGAB result in elevated EGFR protein expression and tyrosine phosphorylation5; (2) in tylosis with familial esophageal cancer syndrome, dominant iRHOM2 mutations increase the activity of ADAM17 in keratinocytes, which further activate EGFR signaling pathway6; and (3) in Olmsted syndrome with focal PPK, a gain-of-function mutation in TRPV3 was found to be associated with EGFR pathway activation7 and interestingly, erlotinib has been successfully used to treat a patient with Olmsted syndrome.8
In contrast to the first and second generations EGFR-TKIs, olmutinib induces irreversible inhibition of EGFR T790M mutations while sparing wild-type EGFR. Paradoxical activation of downstream signaling in the RAS-RAF-MEK-ERK pathway is a possible mechanism and warrants further investigation.
Acknowledgments
This work was supported by the National Taiwan University Hospital (NTUH 103-S2366 and 105-CGN05) to CYC. MEL is funded in part through the NIH/NCI Cancer Center Support Grant 179 P30 CA008748.
IRB approval: This study has been approved by the Research Ethics Committee of National Taiwan University Hospital (201309042RINB).
Footnotes
Financial disclosures: None to declare.
Conflict of interest: Dr. ME Lacouture is a Consultant for Boehringer Ingelheim International GmbH. Dr. C.Y. Chu received honoraria from Boehringer Ingelheim International GmbH for being a consultant and a speaker and from Novartis for being a consultant. The other authors had no conflicts of interest to declare.
Statement on prior presentation: none.
References
- 1.Kim ES. Olmutinib: First Global Approval. Drugs. 2016;76:1153–1157. doi: 10.1007/s40265-016-0606-z. [DOI] [PubMed] [Google Scholar]
- 2.Patel S, Zirwas M, English JC., 3rd Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1–11. doi: 10.2165/00128071-200708010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Chen KL, Lin CC, Cho YT, et al. Comparison of skin toxic effects associated with Gefitinib, erlotinib, or afatinib treatment for non-small cell lung cancer. JAMA Dermatol. 2016;152:340–342. doi: 10.1001/jamadermatol.2015.4448. [DOI] [PubMed] [Google Scholar]
- 4.Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy - classification and management. J Dtsch Dermatol Ges. 2010;8:652–661. doi: 10.1111/j.1610-0387.2010.07449.x. [DOI] [PubMed] [Google Scholar]
- 5.Pohler E, Mamai O, Hirst J, et al. Haploinsufficiency for AAGAB causes clinically heterogeneous forms of punctate palmoplantar keratoderma. Nat Genet. 2012;44:1272–1276. doi: 10.1038/ng.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooke MA, Etheridge SL, Kaplan N, et al. iRHOM2-dependent regulation of ADAM17 in cutaneous disease and epidermal barrier function. Hum Mol Genet. 2014;23:4064–4076. doi: 10.1093/hmg/ddu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Zeng K, Zhang X, et al. A gain-of-function mutation in TRPV3 causes focal palmoplantar keratoderma in a Chinese family. J Invest Dermatol. 2015;135:907–909. doi: 10.1038/jid.2014.429. [DOI] [PubMed] [Google Scholar]
- 8.Kenner-Bell BM, Paller AS, Lacouture ME. Epidermal growth factor receptor inhibition with erlotinib for palmoplantar keratoderma. J Am Acad Dermatol. 2010;63:e58–59. doi: 10.1016/j.jaad.2009.10.052. [DOI] [PubMed] [Google Scholar]