Abstract
Air pollution is linked to increased emergency room visits for headache and migraine patients frequently cite chemicals or odors as headache triggers, but the association between air pollutants and headache is not well-understood. We previously reported that chronic environmental irritant exposure sensitizes the trigeminovascular system response to nasal administration of environmental irritants. Here, we examine whether chronic environmental irritant exposure induces migraine behavioral phenotypes. Male rats were exposed to acrolein, a TRPA1 agonist, or room air by inhalation for 4 days prior to meningeal blood flow measurements, periorbital cutaneous sensory testing or other behavioral testing. Touch-induced c-Fos expression in trigeminal nucleus caudalis was compared in animals exposed to room air or acrolein. Spontaneous behavior and olfactory discrimination was examined in open field testing. Acrolein inhalation exposure produced long-lasting potentiation of blood flow responses to a subsequent TRPA1 agonist and sensitized cutaneous responses to mechanical stimulation. C-Fos expression in response to touch was increased in trigeminal nucleus caudalis in animals exposed to acrolein compared to room air. Spontaneous activity in an open field and scent preference behavior were different in acrolein exposed compared to room-air exposed animals. Sumatriptan, an acute migraine treatment, blocked acute blood flow changes in response to TRPA1 or TRPV1 agonists. Pretreatment with valproic acid, a prophylactic migraine treatment, attenuated the enhanced blood flow responses observed after acrolein inhalation exposures. Environmental irritant exposure yields an animal model of chronic migraine in which to study mechanisms for enhanced headache susceptibility after chemical exposure.
Keywords: pain, sensitization, TRPA1, acrolein, headache model
1. Introduction
Despite extensive research, migraine remains the most undertreated neurological disorder. In particular, chronic (or transformed) migraine (defined as 15 or more days of headache per month, International Classification of Headache-3) is more debilitating and refractory to treatment compared to episodic migraine. The mechanisms underlying the progression from episodic to chronic migraine are not understood. Some factors increase the risk of migraine progression, including increases in the number of baseline attacks. In addition, migraines may be triggered by environmental stimuli such as air pollutants and odors [20]. Repeated or continuous exposure to chemicals or poor air quality has been linked to the acquired disorder, Multiple Chemical Sensitivity (MCS) wherein patient complaints include headache [11]. Furthermore, MCS is associated with increased sensitivity to a variety of chemicals, a number of which are TRPA1 agonists. TRPA1 is an excitatory ion channel expressed in trigeminal sensory neurons and has been linked to migraine [3;38].
We have hypothesized that air pollution induced headache is induced by stimulation of TRPA1 receptors in nasal epithelium and subsequent activation of the trigeminovascular system, thought to be important in headache. Activation of the trigeminovascular system can be assayed with laser Doppler flowmetry and has been shown to be predictive of clinical efficacy of headache therapeutics [1;15;16;28;33]. We reported that acute nasal administration of environmental irritants increases meningeal blood flow in a TRPA1- and CGRP-dependent manner [25]. In addition, pre-exposure to environmental irritants such as acrolein may activate and sensitize TRPA1 receptors in the trigeminovascular pathway and thus predispose subjects to headache. We chose to examine this possibility using acrolein because it is prevalent in both indoor and outdoor air pollution and its health effects well documented [9;14;22;23]. In this regard, we have shown that repeated (chronic) inhalation of subacute doses of acrolein potentiate blood flow responses to subsequent acute irritant exposure [27], with no other harmful effects. This suggests that TRPA1 receptors may play a pivotal role in the conversion of episodic to chronic migraine after environmental irritant exposures. From these observations we predict that a chronic episodic inhalation paradigm, in addition to producing long-lasting trigeminovascular sensitization, will induce additional phenotypes consistent with chronic migraine. Sufferers of chronic migraine report a constellation of symptoms including cutaneous allodynia, sensitivity to smell, light and sound and reduced physical activity and appetite. To assess such phenotypes in the inhalation model, we tested periorbital mechanical pain thresholds and other measurements of central sensitization. We additionally examined the efficacy of acute and preventative clinical treatments in this model. Finally, we examined spontaneous behaviors and smell sensitivity during open-field testing.
2. Methods
2.1 Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine and followed the ethical guidelines of International Association for the Study of Pain [60]. Experiments were performed on 130 adult male (170–250 g) Sprague-Dawley rats (Harlan Bioproducts, IN). Rats were housed in pairs in solid bottom cages with hardwood chip bedding with a standard 12 hour light and dark cycle with free access to food and water. Animals were randomly assigned to experimental groups and weighed daily during treatment. No adverse effects of treatment were observed.
2.2 Inhalation exposure
Rats were exposed to acrolein by mixing acrolein gas (Air Liquide, Plumsteadville, PA) and room air to obtain the desired concentration as described previously (5). The acrolein dosage (0.3 ppm) was chosen because it produced minimal or no detectable harmful effects in previous studies [9;14;27;31]. In addition, it is equivalent to the limit for short-term exposure recommended by the National Institute of Occupational Safety and Health. Separate inhalation chambers (Braintree Scientific, Inc; 5.5 L total volume) and tubing were used for acrolein and control groups to avoid cross-contamination. The flow rate was maintained at 1.5 L/min and temperature and humidity were monitored in the chamber. Based on previous studies [9;14;27;31], rat pairs were exposed to acrolein (0.30 ± 0.03 ppm (n = 70) 4 hours per day for 4 days while control animals were exposed to room air with the same paradigm. Cumulative acrolein exposure for each animal was determined with monitoring badges placed in the chamber (Advanced Chemical Sensors Inc, Boca Raton, FL). Laser Doppler flowmetry was conducted in separate groups of animals at either 1, 4 or 7 days after the last inhalation exposure as described below.
2.3 Laser Doppler flowmetry
Laser Doppler flowmetry was performed as previously described [25] between 10:00 and 15:00. Rats were anesthetized with ketamine/xylazine (80 and 10 mg/kg body weight, respectively), followed by additional doses of ketamine/xylazine (40 and 5 mg/kg body weight) as needed. Body temperature was maintained at 37° C with a homeothermic blanket. For the measurement of meningeal blood flow, the skull was fixed in a stereotaxic frame and a cranial window prepared [25] with the dura left intact. Dural blood flow was measured with a laser Doppler flowmeter (TSI, MN). A needle type probe was placed over a large branch of the middle meningeal artery (MMA), distant from visible cortical blood vessels and the cranial window kept moist with synthetic interstitial solution (SIF) consisting of: 135 mM NaCl, 5 mM KCl, 5 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 10 mM D-glucose (pH 7.3). Blood flow was sampled at 1Hz with a Digidata 1320 interface using Axoscope software (Axon Instruments, CA).
2.4 Blood flow drug administration
To stimulate the nasal mucosa, 25 µl of test compound or vehicle solution was applied over a 30-second period at a site 2 mm into the right nostril using a Pipetman pipette [21;25]. Solutions of TRPA1 agonists, mustard oil (MO) and acrolein (Sigma) were prepared fresh daily by diluting in SIF to the desired concentrations. Stock solutions of the TRPV1 agonist, capsaicin (10 mM; Sigma) were dissolved in ethanol and stored at −20°C and then diluted to the desired concentration with SIF prior to use. Sub-maximal concentrations of agonist were used for sensitization studies [27](i.e., 10 uM mustard oil) whereas higher doses (30 uM mustard oil) were used for sumatriptan and valproic acid testing. A thirty-minute stabilization period preceded all test drug applications to ensure steady basal blood flow measurements. Each animal in blood flow experiments was given nasal saline as a vehicle control 15 minutes before administering the TRP agonist. Saline produced less than 2% change in blood flow on average consistent with our previous published results [25;26] (data not shown). For acute drug studies, rats received a single injection of saline or sumatriptan (1mg/kg, i.p.,[37]) (Santa Cruz) two hours before blood flow drug administration. For chronic drug experiments, rats received daily injections of vehicle (saline; 200 µl injection volume, i.p.,) or valproic acid (200 mg/kg, i.p, [37] (Sigma)) diluted in vehicle for 4 days prior to inhalation chamber exposure followed by daily injections 30 minutes prior to chamber exposure (8 total injections).
2.5 Tactile sensory testing
Rats were tested during the daylight portion of their circadian cycle (0900–1200 hr) and followed the protocol described by Oshinsky and Gomonchareonsiri, 2007 [36]. Rats were placed in a plastic tube restraint (inner diameter 8 cm, length 20 cm) which was used to prevent the animals from walking away from the sensory testing. Rats were acclimated to the testing apparatus through training periods prior to testing and entered the restraint tube uncoaxed. The onset of cutaneous sensitivity was assessed at 4 time points relative to the inhalation paradigm; one day before inhalation chamber (Pre, as labeled on figures), 30 minutes after the first exposure (1) or after the fourth exposure (4) and 24 hours later (Post). The duration of cutaneous sensitivity was assessed by von Frey testing at 1, 4 or 7 days after inhalation exposure. Force-response thresholds were determined by applying von Frey monofilaments (North Coast Medical, Inc., Morgan Hill, CA) to the midline periorbital region of the rat. The von Frey stimuli were presented in a sequential ascending or descending order, as necessary to determine the threshold response, as previously described [10;19]. A positive response for the von Frey test was defined as when the rat withdrew its head away from the stimulus or stroked its face with the forepaw at least two out of three trials. The testing was recorded on video and scored twice: once by the experimenter and again by a blinded observer who scored the video playback. The two scores were averaged and the results were presented as the threshold in grams ± SEM which elicited a response.
Touch-induced c-Fos expression experiments followed the procedures of Boyer et al 2014 [5] with modifications. Twenty-four hours after the last acrolein or room air chamber exposure, rats were stimulated with an 8 gm von Frey filament on the midline periorbital region (1 Hz, for 1 minute) while restrained by the experimenter. The rats were perfused transcardially with 4% paraformaldehyde and processed for immunocytochemistry 90 minutes later. To assess whether restraining the animals during the von Frey filament touch experiments might have independent effects on c-Fos expression, a group of acrolein-exposed restrained only animals was also included.
2.6 Open field testing
Open field and scent preference testing was conducted as previously described [7]. Rats subjected to either acrolein or room air, were tested in an open field 24 hours after the last exposure. Rats were placed in the center of a 90 × 90 cm opaque box and behavior was video recorded for 5 minutes and later analyzed by an experimenter, who was blind to the treatments, using ANY-maze software (version 4.75, Stoelting, Wood Dale, IL) an automated video tracking system for behavioral experiments. Rats were placed back into their home cages for 30 min before additional testing. Scent preference was tested as above with one corner of the box containing Millipore water (neutral scent) and the opposite corner containing either a pleasant (vanilla, Kroger brand Imitation Vanilla extract, Kroger Cincinnati, OH) or unpleasant (fox urine, Maine Outdoor Solutions, Herman, ME) scent. Specifically, the scents were placed on a cotton swab and then taped to the inside of a petri dish (100 mm × 15 mm) and allowed to dissipate under a fume hood for 2 hr prior to testing. They were then covered and placed outside the testing room. Prior to each exposure the dish was brought in to the room, taped to the bottom of the apparatus and uncovered. The animals were placed into the center of the apparatus and allowed 5 mins to explore arena and scents. The animals were then placed back into their home cage and allowed 4–5 min while the apparatus was cleaned with Coverage Plus (Fisher, Hampton, NH) and the scent removed and placed outside the room. The new scent was brought in and taped to the apparatus and again the animals were placed in the center and allowed 5 mins to explore. The scents were randomized between subject, with half getting vanilla scent first and half getting predator scent first. Behavior was videotaped and analyzed as above.
2.7 Immunocytochemistry
To determine whether irritant exposure, restraint, or periorbital stimulation induced c-Fos expression in spinal trigeminal nucleus caudalis (TNC) neurons, immunocytochemistry was performed as described previously (19). Following overnight fixation, the tissue was rinsed in PBS and then cryoprotected in 10% sucrose for 2 hours followed by 20% sucrose in PBS overnight at 4°C. Frozen coronal sections (40 µm) of the brainstem and spinal cord were obtained serially through the medulla to the third cervical segment and collected free-floating in PBS. The obex was used as the most rostral point and was designated 0.0 mm. The first six serial sections from regions at 1.5, 3.0, 4.5 and 6.0 mm caudal to the obex were processed and examined in each animal. Sections were blocked in 4% normal goat serum and 0.025% Triton-X100 in PBS for 1 hour and incubated with the primary antibody (c-Fos; Santa Cruz (#sc-52); 1:2000) diluted in blocking solution overnight at 4°C. Subsequently, sections were rinsed in PBS, incubated with the secondary antibody (goat anti-rabbit IgG conjugated to HRP, Jackson ImmunoResearch; 4 µg/ml) for 1 hour at room temperature and processed for visualization using DAB. Cells were considered positive for c-Fos immunoreactivity (c-Fos-IR) if the nucleus was densely stained. No reactivity was observed in control sections in which primary antibody was omitted.
2.8 Data collection and statistics
For blood flow experiments, data was collected at 1 Hz. Basal blood flow was determined as the mean flow rate measured during a 4 minute period prior to drug application and the effects of test compounds were calculated by comparing the peak response after drug or saline administration to the basal blood flow. Changes in blood flow were calculated relative to the basal blood flow for each animal, averaged within treatment groups and expressed as percent changes. Comparison of blood flow changes were performed using a two-tailed Student’s t-test with Welch’s correction for populations which have unequal standard deviations. For graphical representation, the average percent changes of acrolein- exposed animals were normalized to the average percent changes of room-air exposed animals (Fig. 1). For touch-induced c-Fos experiments, c-Fos-IR cells in the superficial laminae (i.e. I and II) of the TNC from six brain sections were manually counted in a blinded fashion at each level with a 10× objective (100× overall magnification). Anatomical boundaries were determined by coordinates established by the Paxinos and Watson [39] rat brain atlas. Left and right side cell counts from each section/level were combined per animal and compared between treatment groups. Two-way analysis of variance (ANOVA) and Sidak’s posthoc tests were performed on periorbital allodynia and c-Fos-IR cell counts. Comparison of open field behavior and scent preference were performed using a two-tailed Student’s t-test. Data presentation and statistical analyses were performed using GraphPad Prism software (GraphPad, CA). Averaged data values are presented as means ± SEM. The significance level for all tests was set at p < 0.05.
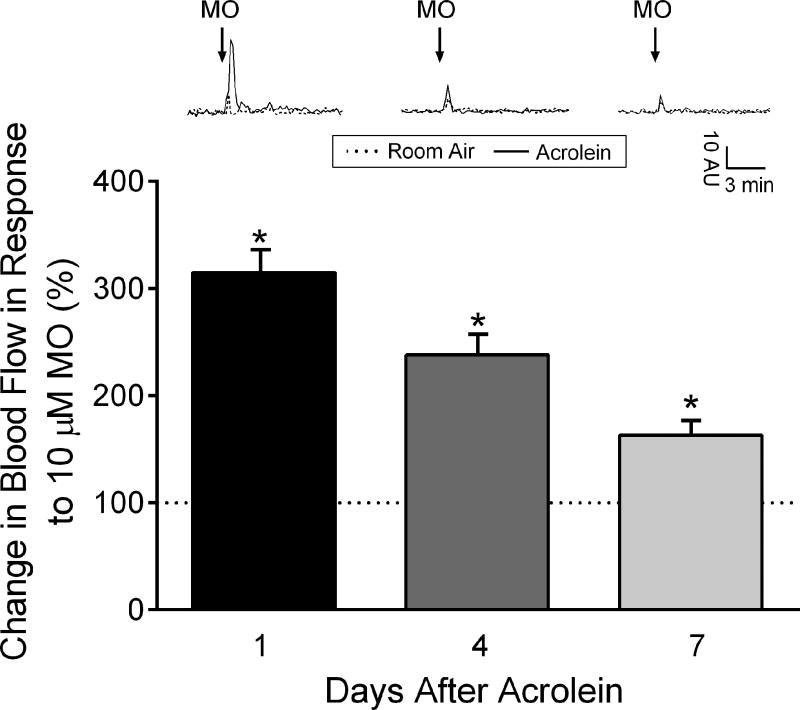
Figure 1.
Blood flow changes in the middle meningeal artery after nasal administration of mustard oil (MO) in male rats exposed to room air or acrolein. Representative traces of middle meningeal blood flow changes in response to nasally applied mustard oil after 1, 4 and 7 days following exposure to 4 × 4 acrolein or room-air. Arrows indicate nasal administration of mustard oil. Compared to room air-exposed animals, blood flow changes to nasal mustard oil (10 µM) administration were significantly increased in acrolein-exposed male rats at 1, 4 and 7 days after acrolein inhalation chamber. Values are normalized to average blood flow changes in room air-exposed animals at the same time point (depicted as a dotted line on graph). * p < 0.05 compared to changes in room air-exposed animals at the same time point. N = 5–10 animals per group.
3. Results
3.1 Acrolein inhalation produces long-lasting sensitization of meningeal blood flow
We previously reported that repeated subacute acrolein exposure significantly increased subsequent blood flow responses to TRP agonists 24 hours later [27]. To determine the duration of the sensitization, we conducted blood flow experiments at 1, 4 or 7 days after acrolein or room air inhalation in separate groups of animals. We observed sensitization of the blood flow response at all time points relative to room air-exposed animals (Fig 1). Meningeal blood flow responses to a single nasal administration of 10 µM mustard oil were significantly increased in acrolein exposed compared to room air controls at 1 day ((165 ± 15 % (n = 6) vs 33 ± 4% (n = 5), p < 0.05), 4 days ((68 ± 9% (n = 6) vs 29 ± 5% (n = 10), p < 0.05) and 7 days ((26 ± 1% (n = 8) vs 16 ± 3% (n = 7), p < 0.05) after the last exposure. As depicted in Figure 1 when normalized to changes in blood flow responses observed in room air exposed rats the acrolein exposed animals exhibited 315%, 238% and 163% higher responses at 1, 4 and 7 days after inhalation, respectively. Despite migraine being more prevalent in women than in men [51] we observed a similar sensitization in female rats exposed to acrolein 1 day after the last exposure (312%, data not shown).
3.2 Acrolein exposure induces long-lasting periorbital allodynia and potentiates touch-induced c-Fos expression in trigeminal nucleus caudalis
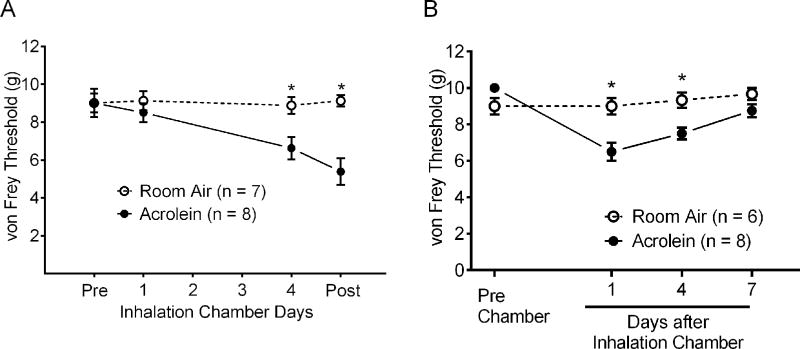
To assess whether the acrolein inhalation model produces migraine pain phenotypes, we assessed periorbital cutaneous allodynia before and after the inhalation exposures and prior to the blood flow experiments in rats (Fig. 2A). Two-way Analysis of Variance indicated significant effects for both Time F(3,42) = 7.233 and Treatment F(1,14) = 8.450. Before inhalation exposure, there were no differences in withdrawal responses to von Frey stimulation applied to the periorbital region. Similarly, no differences in withdrawal responses to cutaneous stimulation between acrolein and room air exposed animals followed one 4 hour exposure period (8.5 ± 0.5 g vs 9.1 ± 0.5 g, p = 0.894). In contrast, animals exposed to repeated daily acrolein inhalation developed periorbital allodynia which remained one day later. Following the 4×4 acrolein exposures, von Frey withdrawal thresholds were significantly reduced compared to room air only (6.6 ± 0.6 g vs 8.9 ± 0.4 g, p < 0.05, respectively). Twenty-four hours later, the withdrawal threshold response remained significantly reduced in the acrolein exposed animals compared to room air control animals (5.4 ± 0.7 g vs 9.1 ± 0.3 g, p < 0.05 respectively). To determine the duration of periorbital allodynia after 4×4 acrolein exposures, periorbital allodynia was assessed at 1, 4 and 7 days after inhalation of room air or acrolein in separate groups of rats (Fig. 2B). Two-way Analysis of Variance indicated significant effects for both Time F(3, 36) = 10.74 and Treatment F(1,12) = 9.589. No significant differences were observed between room air ((9 ± 0.5 g (n = 6)) and acrolein ((10 ± 0.0 g (n = 8)) exposed animals before inhalation exposures. Following acrolein exposures, von Frey withdrawal thresholds were significantly reduced compared to room air at 1 day (6.5 ± 0.5 g (n = 8) vs 9.0 ± 0.5 g (n = 6), p < 0.05) and 4 days (7.5 ± 0.3 g (n = 8) vs 9.3 ± 0.4 g (n = 6), p < 0.05) after inhalation exposures. Periorbital allodynia was no longer present 7 days after animals were exposed to repeated daily acrolein (8.8 ± 0.4 g (n = 8) vs 9.7 ± 0.3 g (n = 6)) inhalation. Note that blood flow sensitization (Fig. 1) and periorbital allodynia (Fig. 2B) followed a similar pattern of duration, i.e., the sensitization peaks one day after inhalation chamber and decreases over time.
Figure 2.
Development and maintenance of periorbital allodynia after repeated acrolein exposure. (A) Periorbital cutaneous responses to von Frey filament stimulation following repeated acrolein vapor or room air exposure. Cutaneous mechanical sensitivity was assessed before (Pre), 30 minutes after exposure on days 1 and 4 and 24 hours (Post) following the last chamber exposure. Von Frey thresholds were significantly reduced in acrolein exposed animals after the fourth exposure and remained lower at 24 hours after the last exposure. * indicates p < 0.05 compared to acrolein-exposed animals. (B) Maintenance of periorbital allodynia after repeated acrolein exposure. Periorbital cutaneous responses were assessed either 1, 4 or 7 days after acrolein or room air exposure. Von Frey thresholds were significantly reduced in acrolein exposed animals 1 day and 4 days but not 7 days after acrolein exposure relative to room air exposed animals. * indicates p < 0.05 compared to acrolein-exposed animals.
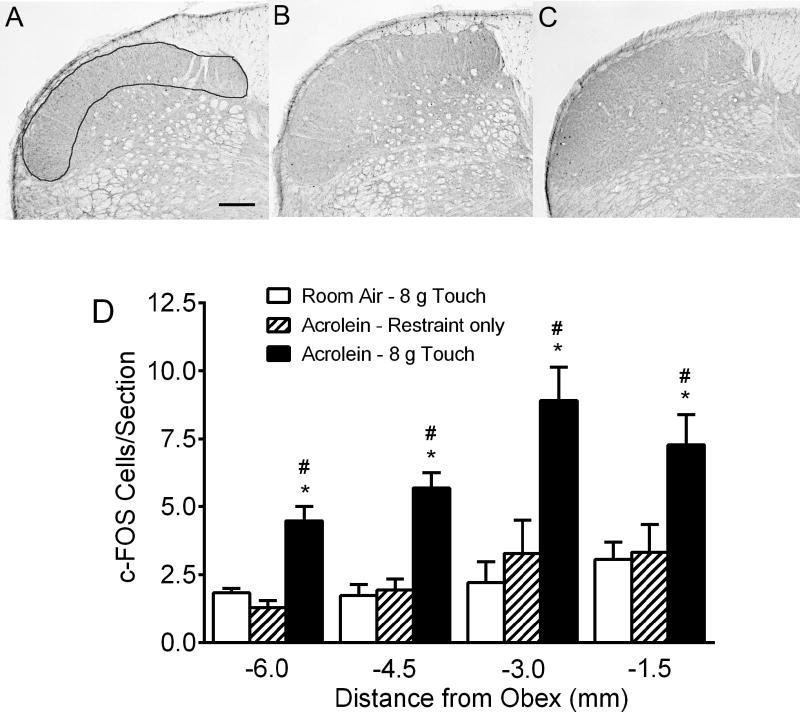
The 4×4 acrolein exposure model induces periorbital allodynia (Fig. 2A and 2B), a frequent complaint of chronic migraine patients, which is believed to be a manifestation of central sensitization. We thus sought to determine whether changes are occurring centrally in the TNC, using c-Fos expression to monitor neuronal activation. Consequently, we compared c-Fos expression in acrolein and room air exposed animals following normally non-noxious mechanical stimulation (Fig. 3). We observed very low-levels of c-Fos-IR expression in each of the brainstem and spinal cord sections of room-air exposed touch animals, consistent with the results from the withdrawal threshold studies (Fig. 2) indicating that an 8 g von Frey filament stimulation is innocuous (Fig 3A). Likewise, acrolein-exposed animals subjected to only restraint had similarly sparse c-Fos-IR expression (Fig 3B). However, two-way Analysis of Variance indicated significant effects for both Level F(3,63) = 9.37 and Treatment F(2,21) = 26.09 following acrolein-touch (Fig 3C,3D). Significant differences were observed in the number of c-Fos-IR cells between acrolein-touch and room air-touch (4.48 ± 0.53 vs 1.83 ± 0.17; p < 0.05) or acrolein-restraint only groups (1.29 ± 0.25; p < 0.05) at −6.0 mm from the obex. Likewise, an approximately two-fold increase in c-Fos-IR cells was observed in TNC spinal cord segments (−4.5, −3.0 and −1.5 mm from obex) in the acrolein-touch animals compared with either the room air-touch or acrolein-restraint only animals in the −4.5 mm (5.69 ± 0.55 vs 1.72 ± 0.38, p < 0.05 or 1.93 ± 0.4, p < 0.05), −3.0 mm (8.90 ± 1.23 vs 2.20 ± 0.72, p < 0.05 or 3.29 ± 1.2, p < 0.05) and −1.5 mm levels (7.28 ± 1.10 vs. 3.06 ± 0.6, p < 0.05 or 3.31 ± 1.01, p < 0.05, respectively). Only sporadic c-Fos-IR cells were observed in the deeper laminae of the TNC in each of the groups. Coupled with the results of the von Frey withdrawal experiments, these data indicate that repeated acrolein exposure can sensitize central pain pathways.
Figure 3.
Repeated acrolein exposure potentiates touch-induced c-Fos expression in the spinal trigeminal nucleus caudalis. (A–C) Photomicrographs of coronal sections at 3.0 mm caudal to the obex after room-air-exposure plus touch (a), acrolein-exposure only (b) and acrolein-exposure plus touch (c). Outline in (a) denotes area from which c-Fos cell counts were performed. (D) Quantification of c-Fos immunoreactive cells in laminas I and II of the spinal trigeminal nucleus. The first six serial sections from regions at 1.5, 3.0, 4.5 and 6.0 mm caudal to the obex were processed and examined in each animal. Periorbital von Frey filament stimulation significantly increased c-Fos expression in acrolein-exposed touch animals (n = 7) compared with room-air exposed touch animals (n = 8) as well as with acrolein-exposed subjected only to restraint (n = 8). Note that no differences were observed between the acrolein-exposed restraint only and the room-air touch animals. * p < 0.05 compared to room air touch animals, #, p < 0.05 compared to acrolein-exposed restraint only animals at the same level. Scale bar = 200 µm
3.3 Acrolein exposure modifies open field and scent preference behaviors
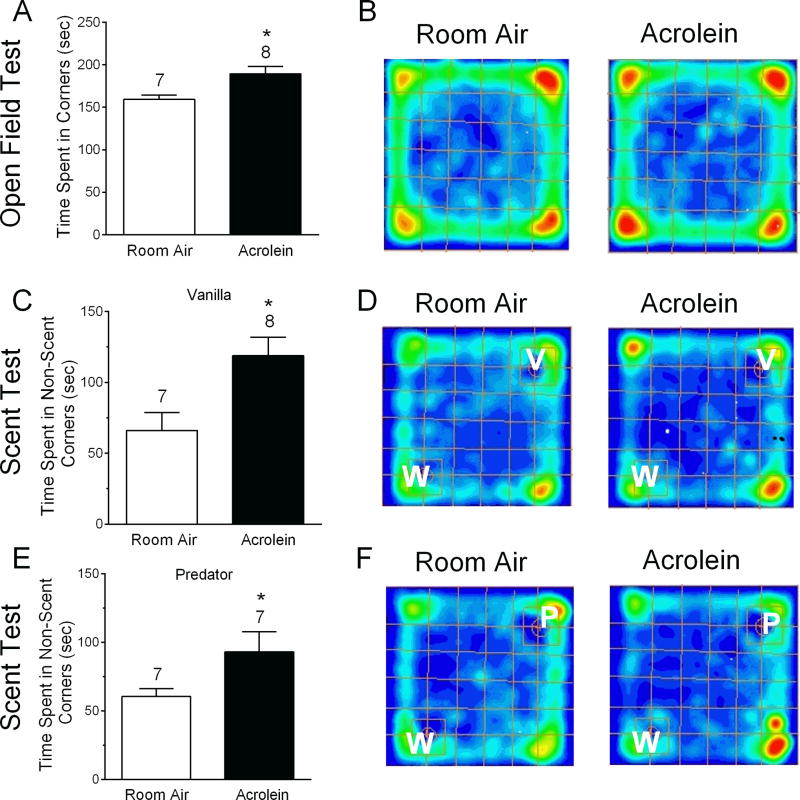
Spontaneous behavioral changes are important in validating chronic migraine models [32;43;44;52] including reduced activity levels [32]. To examine general activity levels, rats subjected to either the acrolein or room air 4×4 protocol were tested in an open field 24 hours after the last exposure (Fig. 4). Testing was conducted over 5 minutes and analyzed by video recording as previously described [7]. Representative occupancy maps (warmer color = higher occupancy) indicate where animals spent their time. Time Spent in Corners was significantly increased in acrolein exposed animals compared to room air exposed animals (189 ± 8 sec vs 159 ± 5 sec, p < 0.05; Fig. 4A and B). Interestingly, Time in Periphery, Inner Time, Distance Traveled and Mean Speed did not significantly differ between the groups (data not shown), suggesting that this phenotype did not fit a pattern reflective of anxiety.
Figure 4.
Open field behavior and scent preference changes after acrolein inhalation exposure. In open field testing (A and B), the Time Spent in Corners is increased in acrolein exposed animals compared to room air controls. Representative occupancy maps for individual animals exposed to room air (B, left) or acrolein (B, right) are displayed where warmer colors represent higher occupancy. Scent preferences with water and vanilla (C, D) or water and predator scent (E, F) were determined in separate tests. W, V or P indicate placement of water, vanilla or predator scent in the corners of the open field chamber. Summarized data indicates that Time Spent in Non-Scent Corners is increased in response to pleasant (C) or unpleasant (E) scents in acrolein exposed animals compared to room air controls. The corner is defined as the single most peripheral quadrant. * p < 0.05 compared to room air animals. Number of animals per group is indicated.
Despite the fact that odors and chemicals are known migraine triggers [47]and osmophobia is a complaint in 25–40% of chronic migraineurs [4;24;42;49] this feature has not been explored in a rodent model of migraine. Osmophobia is an aversion to either pleasant or unpleasant scents. To determine whether acrolein or room air exposed animals differ in their behavior toward scents, open field testing was performed in which one corner contained water (neutral odor) and the opposite corner contained either a pleasant scent (vanilla) or an unpleasant scent (predator). Rats pre-exposed to acrolein vs room air spent significantly more Time in Non-Scent Corners during discrimination testing for the pleasant scent (119 ± 13 sec vs. 66 ± 13 sec, p < 0.05, respectively) (Fig. 4C and D). Furthermore, rats pre-exposed to acrolein vs room air also spent significantly more Time in Non-Scent Corners during testing for unpleasant scent discrimination (93 ± 15 sec vs. 61 ± 6 sec, p < 0.05) (Fig. 4E and F). Overall, the behavior of acrolein exposed animals differs from room air exposed animals in both open field and scent preference testing.
3.4 Sumatriptan blocks acute meningeal blood flow response to nasal administration of TRP agonists
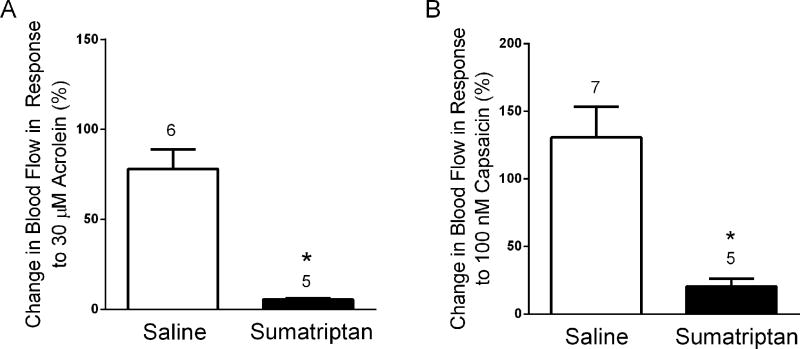
Migraine models which are predictive of clinical outcome are essential to allow comparison of currently efficacious treatments to novel potential therapeutics. Therefore, we examined the effects of sumatriptan, a drug effective in acute migraine but not generally efficacious for chronic migraine on meningeal blood flow responses to acute nasal administration of TRP agonists (Fig. 5). A single dose of sumatriptan (1 mg/kg, i.p.) administrated two hours prior to nasal application of agonist significantly attenuated the blood flow response to acrolein (Fig. 5A) compared to control animals receiving a saline injection (6 ± 1% (n = 5) vs 78 ± 11% (n = 6), p < 0.05). Sumatriptan also attenuated meningeal blood flow induced by nasally administered capsaicin (Fig. 5B). Blood flow responses were significantly reduced in animals injected with sumatriptan compared to saline controls (20 ± 6% (n = 5) vs 131 ± 23% (n = 7), p < 0.05).
Figure 5.
Sumatriptan blocks meningeal blood flow changes induced by nasal administration of (A) 30 µM acrolein or (B) 100 nM capsaicin. Sumatriptan (1mg/kg, i.p.) was administered two hours before TRP agonist stimulation in this acute study. * p < 0.05 compared to saline injected controls. Values are means ± S.E.M. Number of animals per group is indicated.
3.5 Pretreatment with valproic acid blocks acrolein-induced trigeminovascular sensitization
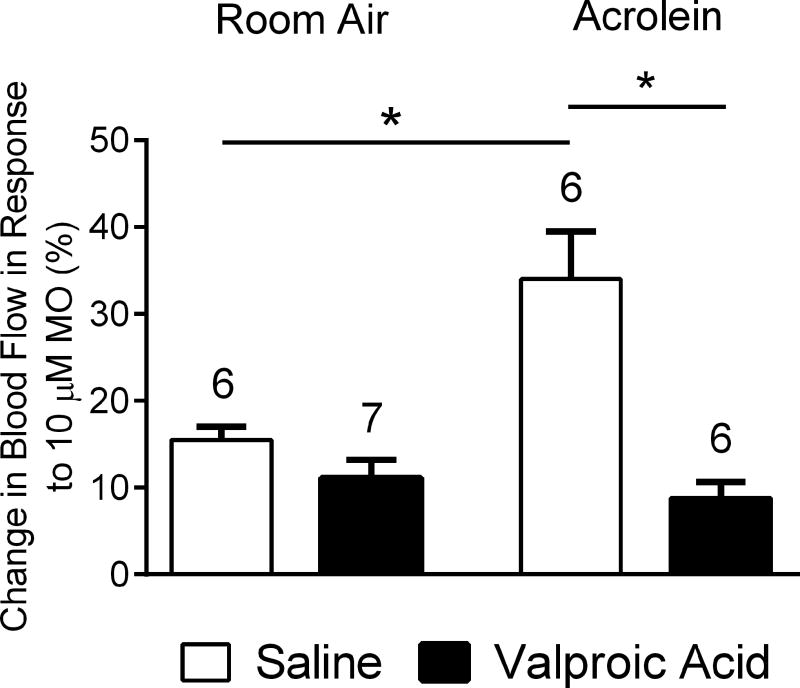
To assess the efficacy of a prophylactic migraine drug treatment in the sensitization phenomenon, we asked whether concomitant treatment with a chronic migraine drug during acrolein conditioning would block the subsequent potentiation. Valproic acid is sometimes used off-label as a prophylactic treatment for chronic migraine [50] and is efficacious in pre-clinical migraine models [30] although its mode of action is not well-understood [45]. We injected valproic acid (200 mg/kg, i.p.) into animals once per day for 4 days prior to and daily 30-minutes before placing each animal in the inhalation chamber (8 injections total). Control animals received saline injections. Blood flow responses were measured approximately 24 hours after the last inhalation period and in the absence of vehicle or valproic acid injections. Similar to our previous report [27], conditioning acrolein exposure potentiated the blood flow responses to 10 µM mustard oil relative to room air exposure (34 ± 6% (n = 6) vs 16 ± 2% (n = 6), p < 0.05) in vehicle injected animals (Fig. 6). Trigeminovascular responses were significantly blunted in those acrolein exposed animals injected with valproic acid compared to acrolein exposed animals injected with vehicle (9 ± 2% (n = 6) vs 34 ± 6% (n = 6), p < 0.05). In addition, no differences were observed between room air exposed animals pretreated with valproic acid compared with those receiving only saline (11 ± 2% (n = 7) vs 16 ± 2% (n = 6)).
Figure 6.
Effects of daily administration of valproic acid (200 mg/kg, i.p.) or saline for 4 days prior to initiating inhalation exposures and before each of the inhalation exposures (4 × 4) on blood flow changes in response to mustard oil (MO). In saline-injected animals the blood flow response to nasal mustard oil (10 µM) was significantly increased in acrolein-exposed animals compared to room-air exposed. No difference in blood flow response was observed between acrolein or room-air exposed animals injected with valproic acid, but a significant difference in blood flow response to nasal mustard oil administration was observed between saline and valproic acid injected animals exposed to acrolein. (*, p < 0.05). Values are means ± S.E.M. Number of animals per group is indicated.
4. Discussion
Environmental factors including odors and air pollution are some of the most widely reported triggers of migraine [20]. Although not extensively studied, several reports have demonstrated strong links between air borne chemicals and headache [35;54;55;58]. Recently it has been suggested that TRPA1 agonists are critically involved in air pollution-induced human headache. TRPA1 agonists, including acrolein and formaldehyde are environmental irritants found in indoor and outdoor pollution. Also of particular relevance is the “headache tree”, Umbelluria californica whose vapors can induce severe headaches in susceptible individuals [2;34]. The active component of these vapors, umbellulone, is an identified TRPA1 agonist [59], capable of activating the trigeminovascular system [34].
Headache precipitated by air pollutants is a symptom of Multiple Chemical Sensitivity (MCS), an acquired disorder linked to inhaled chemicals [12]. Other MCS symptoms include skin and respiratory ailments reflecting an increased sensitivity to subsequent exposures to a variety of chemicals. It is however, not clear how inhaled irritants induce headache and other symptoms. Due to the similarities of trigeminovascular responses in our rat model to MCS phenotypes and because air pollution as a health factor is increasing worldwide we wanted to determine whether pre-exposure to inhaled irritants sensitizes the trigeminovascular system. Utilizing the 4×4 inhalation model to expose animals to room air vs subacute acrolein (0.3 ppm) we confirmed that acrolein-exposed animals exhibit sensitized trigeminovascular responses to subsequent nasal TRP agonist challenge and we also demonstrate that the sensitization is long lasting, i.e., still present 7 days after the last exposure to a non-toxic level of acrolein.
As one of the most important risk factors for chronic migraine is frequency of headache episodes at baseline, it has been proposed that “an animal model of chronic migraine should mimic recurrent activation of the trigeminal system” [36]. It is not clear how the trigeminovascular system is activated in migraine but sensitization of peripheral neural elements may be involved. Therefore most models include repeated administration of agents to peripheral nociceptors in the meninges [32;36;56;57], or systemically, i.e., nitroglycerin [40]. Our inhalation model differs in that we repeatedly activate the trigeminovascular system via stimulation of peripheral trigeminal afferents in the nasal mucosa. This model induces some of the expected phenotypes but with the distinct advantage of employing a known exteroceptive trigger rather than an invasive method.
In addition to headache, patients with migraine uniquely exhibit other symptoms including cutaneous allodynia, photophobia, phonophobia and osmophobia, which tend to be more prevalent and severe in chronic migraine sufferers. Therefore, it is important that chronic migraine models exhibit some of these traits. Facial cutaneous allodynia is common in 60–80% of migraineurs with the prevalence and severity higher in chronic migraine [8]. After acrolein exposure we tested for periorbital allodynia and c-Fos activation in the TNC. Periorbital allodynia was induced by repetitive acrolein inhalation and persisted 24 hrs after the last exposure. This mimics the observations of Oshinsky et al, 2007 [36] who first reported periorbital allodynia after repeated administrations of inflammatory mediators (IS) to the dura. Others have also observed long lasting allodynia after repeated IS [5;18;43;53] or TRPA1 agonists [17] administered to the dura. Similar long-term facial allodynia has been described [40] after repeated systemic injections of nitroglycerin (NTG) a known migraine trigger. C-Fos expression in response to light touch was also enhanced in the TNC by repetitive acrolein inhalation, similar to results obtained in other animal models of chronic migraine [5].
Osmophobia has been cited as a unique trigger and perhaps diagnostic symptom of migraine [42;46;48]. Approximately 40% of migraineurs report osmophobia as a symptom [4;49]. As aversion to scents has not been previously examined in animal models of migraine, we compared the behavior of acrolein and room air exposed animals to pleasant and unpleasant scents in an open field test. Under testing with either pleasant or unpleasant scents, the acrolein exposed animals spent more time in corners without any scent. This is an intriguing finding as osmophobia is common in humans with migraines. Additional studies are warranted, however, to determine if these behaviors are due to general olfactory disturbances, neophobia or pain.
Spontaneous behavior changes in migraine models are increasingly important [32;52] and several laboratories have observed changes in grooming patterns, exploratory behavior and general activity levels. These altered spontaneous behaviors may be more relevant than evoked behaviors as migraineurs similarly display reduced physical activity and avoidance behaviors (ICHD-3). Consequently, we explored such behaviors via open field testing. Neither total distance traveled nor average speed was affected by repetitive acrolein exposure. This differs from some studies, which utilized other induction methods [32;53]. Unexpectedly, the acrolein exposed rats spent more Time in Corners of the open field test. This is not merely more anxiety, as our laboratories have extensively used open field to test anxiety behaviors and rats with high anxiety (e.g., post stressful stimuli) spend more time displaying thigmotaxis (i.e., near walls of arena), but do not typically show more Time Spent in Corners (usually only the center time is published, the corner data is unpublished). Collectively, these data may reflect a general avoidance behavior or pain state (e.g., migraines) after exposure to the environmental irritant, acrolein.
Another important value of validated migraine models is their potential utility in testing new migraine therapeutics. In this regard, we have begun testing our model with known efficacious drugs (e.g., triptans) to initially assess this dimension. In naïve animals (i.e. the absence of repetitive inhalation exposure) sumatriptan effectively blocks blood flow changes induced by acute nasal administration of TRPA1 or TRPV1 agonists (Fig. 5). Here, we further report that valproic acid, an anti-seizure medicine sometimes employed prophylactically for chronic migraine was effective at blocking the trigeminovascular sensitization when administered during conditioning acrolein exposure (Fig. 6). These results demonstrate that this novel model of chronic migraine induced by environmental irritant exposure may be useful in testing new therapeutics for chronic migraine, a condition which is difficult to manage with few recognized treatments.
While these studies suggest that chronic acrolein exposure induced enhanced trigeminovascular system responses, the mechanistic link between TRPA1 activation by acrolein and the observed responses remain undetermined. TRPA1 and TRPV1 are often co-expressed in nociceptive sensory neurons and have been suggested to work in concert to activate in response to TRPA1 agonists. While activation of these receptors can lead to nociceptor excitation, the depolarization induced can also result in an increase in firing threshold owing to sodium channel inactivation. While in vitro evidence [13] has suggested that acrolein may not directly excite meningeal afferents, the sequelae and interactions between nasal and meningeal afferents in vivo appears to be TRPA1 dependent and pronociceptive [17]. Nonetheless, the specific mechanism by which repetitive activation of TRPA1 induces behavioral sensitization remains a mystery.
We have previously observed changes in lipids in the trigeminal ganglion after acrolein exposure [29]. We speculate that acrolein acting on TRPA1 channels may drive changes in lipid levels, which in turn, can activate or sensitize TRP channels. This is supported by lipidomic studies which have identified novel endogenous lipids that are TRP channel activators/modulators [6;41].
In summary, our results suggest that inhalation exposure to environmental irritants induces trigeminovascular and central sensitization and reproduces some facets of chronic migraine models. Importantly our conditioning protocol represents the first model to utilize a known exteroceptive physiological and environmental trigger of human migraine. Furthermore, this novel model reinforces the hypothesis that TRPA1 receptors have important roles in episodic to chronic transformation in migraine and provides a platform to better understand the progression to chronic migraine and develop novel therapeutics.
Acknowledgments
We would like to thank Dr. Richard P. Kraig for use of the laser Doppler flowmeter and Stephanie D. Fritz who conducted the open field and scent discrimination tests.
Funding
This work was supported by a grant from the NIEHS (ES017430) to JHH and GSO.
Footnotes
Conflict of Interest Statement
The Author(s) declare that there is no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Literature Cited
- 1.Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia. 2013;33:577–592. doi: 10.1177/0333102412472071. [DOI] [PubMed] [Google Scholar]
- 2.Benemei S, Appendino G, Geppetti P. Pleasant natural scent with unpleasant effects: cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia. 2010;30:744–746. doi: 10.1111/j.1468-2982.2009.01988.x. [DOI] [PubMed] [Google Scholar]
- 3.Benemei S, De CF, Fusi C, Rossi E, Lupi C, Geppetti P. TRPA1 and other TRP channels in migraine. J Headache Pain. 2013;14:71. doi: 10.1186/1129-2377-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blau JN, Solomon F. Smell and other sensory disturbances in migraine. J Neurol. 1985;232:275–276. doi: 10.1007/BF00313864. [DOI] [PubMed] [Google Scholar]
- 5.Boyer N, Dallel R, Artola A, Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain. 2014;155:1196–1205. doi: 10.1016/j.pain.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Bradshaw HB, Raboune S, Hollis JL. Opportunistic activation of TRP receptors by endogenous lipids: exploiting lipidomics to understand TRP receptor cellular communication. Life Sci. 2013;92:404–409. doi: 10.1016/j.lfs.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, Liu N, Xiong W, Ripsch MS, Wang Y, Fehrenbacher JC, Fitz SD, Khanna M, Park CK, Schmutzler BS, Cheon BM, Due MR, Brustovetsky T, Ashpole NM, Hudmon A, Meroueh SO, Hingtgen CM, Brustovetsky N, Ji RR, Hurley JH, Jin X, Shekhar A, Xu XM, Oxford GS, Vasko MR, White FA, Khanna R. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca(2)(+) channel complex. Nat Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 9.Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundam Appl Toxicol. 1996;29:208–218. doi: 10.1006/faat.1996.0024. [DOI] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Cullen MR. The worker with multiple chemical sensitivities: an overview. Occup Med. 1987;2:655–661. [PubMed] [Google Scholar]
- 12.Cullen MR. Low-level environmental exposures. In: Rosenstock L, Cullen MR, editors. Textbook of clinical occupational and environmental medicine. Philadelphia: W.B. Saunders Company; 1994. pp. 1127–1133. [Google Scholar]
- 13.Denner AC, Vogler B, Messlinger K, de CR. Role of transient receptor potential ankyrin 1 receptors in rodent models of meningeal nociception - Experiments in vitro. Eur J Pain. 2017;21:843–854. doi: 10.1002/ejp.986. [DOI] [PubMed] [Google Scholar]
- 14.Dorman DC, Struve MF, Wong BA, Marshall MW, Gross EA, Willson GA. Respiratory tract responses in male rats following subchronic acrolein inhalation. Inhal Toxicol. 2008;20:205–216. doi: 10.1080/08958370701864151. [DOI] [PubMed] [Google Scholar]
- 15.Dux M, Will C, Eberhardt M, Fischer MJ, Messlinger K. Stimulation of rat cranial dura mater with potassium chloride causes CGRP release into the cerebrospinal fluid and increases medullary blood flow. Neuropeptides. 2017 doi: 10.1016/j.npep.2017.02.080. [DOI] [PubMed] [Google Scholar]
- 16.Dux M, Will C, Vogler B, Filipovic MR, Messlinger K. Meningeal blood flow is controlled by H2 S-NO crosstalk activating a HNO-TRPA1-CGRP signalling pathway. Br J Pharmacol. 2016;173:431–445. doi: 10.1111/bph.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, Preti D, Appendino G, Geppetti P, Dodick DW, Vanderah TW, Porreca F, Dussor G. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelmayer RM, Ossipov MH, Porreca F. An experimental model of headache-related pain. Methods Mol Biol. 2012;851:109–120. doi: 10.1007/978-1-61779-561-9_7. [DOI] [PubMed] [Google Scholar]
- 19.Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De FM, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman DI, De ver Dye T. Migraine and the environment. Headache. 2009;49:941–952. doi: 10.1111/j.1526-4610.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 21.Gottselig R, Messlinger K. Noxious chemical stimulation of rat facial mucosa increases intracranial blood flow through a trigemino-parasympathetic reflex--an experimental model for vascular dysfunctions in cluster headache. Cephalalgia. 2004;24:206–214. doi: 10.1111/j.1468-2982.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 22.Hazari MS, Griggs J, Winsett DW, Haykal-Coates N, Ledbetter A, Costa DL, Farraj AK. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovasc Toxicol. 2014;14:52–63. doi: 10.1007/s12012-013-9228-9. [DOI] [PubMed] [Google Scholar]
- 23.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache. 2004;44:1019–1023. doi: 10.1111/j.1526-4610.2004.04197.x. [DOI] [PubMed] [Google Scholar]
- 25.Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkler PE, Ballard CJ, Pellman JJ, Zhang L, Oxford GS, Hurley JH. Intraganglionic Signaling as a Novel Nasal-Meningeal Pathway for TRPA1-Dependent Trigeminovascular Activation by Inhaled Environmental Irritants. PLoS One. 2014;9:e103086. doi: 10.1371/journal.pone.0103086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunkler PE, Zhang L, Pellman JJ, Oxford GS, Hurley JH. Sensitization of the trigeminovascular system following environmental irritant exposure. Cephalalgia. 2015;35:1192–1201. doi: 10.1177/0333102415574845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosawa M, Messlinger K, Pawlak M, Schmidt RF. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br J Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leishman E, Kunkler PE, manchanda m, sangani k, Stuart JM, Oxford GS, Hurley JH, Bradshaw HB. Environmental toxin acrolein alters levels of endogenous lipids, including TRP agonists: A potential mechanism for headache driven by TRPA1 activation. Neurobiology of Pain. 2017 doi: 10.1016/j.ynpai.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Liu Y, Chen N, Zhang Y, Song G, Zhang Z. Valproate Attenuates Nitroglycerin-Induced Trigeminovascular Activation by Preserving Mitochondrial Function in a Rat Model of Migraine. Med Sci Monit. 2016;22:3229–3237. doi: 10.12659/MSM.900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyon JP, Jenkins LJ, Jr, Jones RA, Coon RA, Siegel J. Repeated and continuous exposure of laboratory animals to acrolein. Toxicol Appl Pharmacol. 1970;17:726–732. doi: 10.1016/0041-008x(70)90047-5. [DOI] [PubMed] [Google Scholar]
- 32.Melo-Carrillo A, Lopez-Avila A. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia. 2013;33:1096–1105. doi: 10.1177/0333102413486320. [DOI] [PubMed] [Google Scholar]
- 33.Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt RF. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol. 1995;73:1020–1024. doi: 10.1139/y95-143. [DOI] [PubMed] [Google Scholar]
- 34.Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De SG, la MG, Andre E, Preti D, Avonto C, Sadofsky L, Di MV, De PL, Dussor G, Porreca F, Taglialatela-Scafati O, Appendino G, Nilius B, Geppetti P. The 'headache tree' via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- 35.Nattero G, Enrico A. Outdoor pollution and headache. Headache. 1996;36:243–245. doi: 10.1046/j.1526-4610.1996.3604243.x. [DOI] [PubMed] [Google Scholar]
- 36.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oshinsky ML, Sanghvi MM, Maxwell CR, Gonzalez D, Spangenberg RJ, Cooper M, Silberstein SD. Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache. 2012;52:1336–1349. doi: 10.1111/j.1526-4610.2012.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxford GS, Hurley JH. The Role of TRP Channels in Migraine. Open Pain Journal. 2013;6:37–49. [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. London: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raboune S, Stuart JM, Leishman E, Takacs SM, Rhodes B, Basnet A, Jameyfield E, McHugh D, Widlanski T, Bradshaw HB. Novel endogenous N-acyl amides activate TRPV1–4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front Cell Neurosci. 2014;8:195. doi: 10.3389/fncel.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocha-Filho PA, Marques KS, Torres RC, Leal KN. Osmophobia and Headaches in Primary Care: Prevalence, Associated Factors, and Importance in Diagnosing Migraine. Headache. 2015;55:840–845. doi: 10.1111/head.12577. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Reyes M, Akerman S, Nguyen E, Vijjeswarapu A, Hom B, Dong HW, Charles AC. Spontaneous behavioral responses in the orofacial region: a model of trigeminal pain in mouse. Headache. 2013;53:137–151. doi: 10.1111/j.1526-4610.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero-Reyes M, Ye Y. Pearls and pitfalls in experimental in vivo models of headache: conscious behavioral research. Cephalalgia. 2013;33:566–576. doi: 10.1177/0333102412472557. [DOI] [PubMed] [Google Scholar]
- 45.Shahien R, Saleh SA, Bowirrat A. Intravenous sodium valproate aborts migraine headaches rapidly. Acta Neurol Scand. 2011;123:257–265. doi: 10.1111/j.1600-0404.2010.01394.x. [DOI] [PubMed] [Google Scholar]
- 46.Silva-Neto RP, Peres MF, Valenca MM. Accuracy of osmophobia in the differential diagnosis between migraine and tension-type headache. J Neurol Sci. 2014;339:118–122. doi: 10.1016/j.jns.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 47.Silva-Neto RP, Peres MF, Valenca MM. Odorant substances that trigger headaches in migraine patients. Cephalalgia. 2014;34:14–21. doi: 10.1177/0333102413495969. [DOI] [PubMed] [Google Scholar]
- 48.Silva-Neto RP, Rodrigues AB, Cavalcante DC, Ferreira PH, Nasi EP, Sousa KM, Peres MF, Valenca MM. May headache triggered by odors be regarded as a differentiating factor between migraine and other primary headaches? Cephalalgia. 2016 doi: 10.1177/0333102416636098. [DOI] [PubMed] [Google Scholar]
- 49.Sjostrand C, Savic I, Laudon-Meyer E, Hillert L, Lodin K, Waldenlind E. Migraine and olfactory stimuli. Curr Pain Headache Rep. 2010;14:244–251. doi: 10.1007/s11916-010-0109-7. [DOI] [PubMed] [Google Scholar]
- 50.Starling AJ, Dodick DW. Best practices for patients with chronic migraine: burden, diagnosis, and management in primary care. Mayo Clin Proc. 2015;90:408–414. doi: 10.1016/j.mayocp.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 51.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 52.Strassman AM, Burstein R. A new animal model of headache: Ongoing pain vs stimulus-evoked hypersensitivity. Cephalalgia. 2013;33:1073–1074. doi: 10.1177/0333102413491029. [DOI] [PubMed] [Google Scholar]
- 53.Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE. Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache. 2011;51:674–692. doi: 10.1111/j.1526-4610.2011.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szyszkowicz M. Ambient air pollution and daily emergency department visits for headache in Ottawa, Canada. Headache. 2008;48:1076–1081. doi: 10.1111/j.1526-4610.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 55.Szyszkowicz M, Stieb DM, Rowe BH. Air pollution and daily ED visits for migraine and headache in Edmonton, Canada. Am J Emerg Med. 2009;27:391–396. doi: 10.1016/j.ajem.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Vermeer LM, Gregory E, Winter MK, McCarson KE, Berman NE. Exposure to bisphenol A exacerbates migraine-like behaviors in a multibehavior model of rat migraine. Toxicol Sci. 2014;137:416–427. doi: 10.1093/toxsci/kft245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vermeer LM, Gregory E, Winter MK, McCarson KE, Berman NE. Behavioral effects and mechanisms of migraine pathogenesis following estradiol exposure in a multibehavioral model of migraine in rat. Exp Neurol. 2015;263:8–16. doi: 10.1016/j.expneurol.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vodonos A, Novack V, Zlotnik Y, Ifergane G. Ambient air pollution, weather and daily emergency department visits for headache. Cephalalgia. 2015;35:1085–1091. doi: 10.1177/0333102415570300. [DOI] [PubMed] [Google Scholar]
- 59.Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B. Umbellulone modulates TRP channels. Pflugers Arch. 2011;462:861–870. doi: 10.1007/s00424-011-1043-1. [DOI] [PubMed] [Google Scholar]
- 60.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]