Abstract
Introduction
Urate has been identified as a predictor of ALS survival in some, but not all studies. Here we leverage the recent expansion of the PRO-ACT database to study the association between urate levels and ALS survival.
Methods
Pooled data of 1,736 ALS participants from the PRO-ACT database were analyzed. Cox proportional hazards regression models were used to evaluate associations between urate levels at trial entry and survival.
Results
After adjustment for potential confounders (i.e., creatinine and body mass index), there was an 11% reduction in risk of reaching a survival endpoint during the study with each 1 mg/dl increase in uric acid levels (adjusted HR: 0.89, 95% CI 0.82–0.97, p < 0.01).
Discussion
Our pooled analysis provides further support to urate as a prognostic factor for survival in ALS and confirms the utility of the PRO-ACT database as a powerful resource for ALS epidemiological research.
Keywords: Uric acid, survival, PRO-ACT, predictor, prognostic factor, outcomes
Introduction
The pathogenesis of amyotrophic lateral sclerosis (ALS) is not well understood and remains a challenge to ALS therapy development. Proposed mechanisms of disease in ALS include motor neuron degeneration and astrocyte dysfunction secondary to damage from oxidative stress, as supported by both autopsy and laboratory studies1. A number of potential antioxidant therapies were tested in ALS clinical trials (e.g. acetylcysteine2, selegiline3, vitamin E4, and coenzyme Q105), but no clinically meaningful benefit was seen. More recently, urate has been proposed as an endogenous antioxidant6, 7 and neuroprotectant8–13.
Urate is present intracellularly and in bodily fluids as the anionic form of uric acid (2,6,8-trioxy-purine)14. The concentration of urate in the blood is dependent on dietary intake (e.g. purines), urate biosynthesis (i.e. oxidation via the enzyme xanthine oxidoreductase), and urate excretion14. In rodents, urate is converted to allantoin by urate oxidase (Uox). However, during primate evolution multiple independent mutations in the Uox gene arose, leading to the absence of functional urate oxidase. Thus, urate is the main end product of human purine metabolism and circulates in blood at high concentrations near the limits of its solubility (accounting for our susceptibility to gout) 14. The physiologic range of urate levels in blood is 3.6–8.5 mg/dL, with typically higher levels in men than women14. Peripherally-generated urate may not readily cross the blood-brain barrier (BBB), as suggested by the 10-fold gradient from the blood to cerebrospinal fluid (CSF), with CSF concentrations ranging from 0.3–0.5 mg/dL15. It has been speculated that mutations in the Uox gene during evolution, with resulting higher urate blood and CNS levels, conferred a selective advantage to primates related to urate’s antioxidant properties central to cancer and aging8, 14.
If urate were protective against neurodegeneration, one might expect high urate levels to correlate with either disease risk and/or prognosis in several neurodegenerative diseases. While genetically-determined hypouricemia is not associated with neurologic disease16–20, urate levels were found to be lower in people with ALS than healthy controls and disease mimics21, 22, while higher urate levels were found to be associated with lower risk of developing Parkinson’s disease (PD)23–25 and Alzheimer’s disease26. Further, urate levels correlated with prognosis in PD27–29 and urate elevation was neuroprotective in pre-clinical models of PD8, 30. Based on these data, clinical trials of urate elevation are being pursued in PD16 (NCT02642393). Urate levels have been more recently suggested to represent a prognostic factor for ALS survival31. However, previous studies in ALS cohorts ranging in size from 86 to 942 participants have reported conflicting results32–39.
The recent development of the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database has allowed the analysis of aggregate data from multiple independent ALS clinical trials40. Initial analyses suggested that urate, creatinine, and body mass index (BMI) are all strong predictors of ALS prognosis41. However, whether these three variables are independent or reflect shared pathophysiologic mechanisms is unclear. Here we leveraged the recent expansion of the PRO-ACT database to further evaluate the association between urate levels, creatinine, BMI and survival in the largest available cohort of ALS trial participants.
Methods
Database
Data used in the preparation of this article were obtained from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) Database. In 2011, Prize4Life, in collaboration with the Northeast ALS Consortium, and with funding from the ALS Therapy Alliance, formed the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) Consortium. The data available in the PRO-ACT Database has been volunteered by PRO-ACT Consortium members. The vision of the PRO-ACT project was to accelerate and enhance translational ALS research by designing and building a data set that would contain the merged data from as many completed ALS clinical trials as possible. Datasets from industry and academic clinical trials were collected, de-identified, cleaned, harmonized and organized according to a comprehensive common data structure, and imported according to the mappings. The initial release of PRO-ACT database has been described and included data from 8,635 people with ALS41. In December 2015, six trials were added to PRO-ACT and the database now contains data from 10,723 clinical trial participants. The complete PRO-ACT database is publically available online at www.ALSDatabase.org. It contains demographics data (10,723 records), longitudinal information on ALSFRS(-R) (68,041), vital capacity (55,753), vital signs (340,513), safety labs (2,485,021), concomitant medications (112,101) and adverse events (74,691).
Data Analysis
Mean and percentages of baseline characteristics were calculated for the study participants for age, gender, race, disease duration, time from symptom onset to diagnosis (diagnostic delay), site of onset (bulbar vs. limb), vital capacity, ALS Functional Rating Scale- Revised (ALSFRS-R), and riluzole use. Baseline was defined as the time of trial entry.
Survival was calculated as time until death. Cox proportional hazards regression models were used to estimate the adjusted effect of urate levels on survival. The following covariates were considered potential confounders and were included in the models: age, gender, time from symptom onset to diagnosis (diagnostic delay), baseline creatinine, and baseline body mass index (BMI). In order to calculate BMI for all individuals, height was imputed when missing by using the average height for the individual’s gender (N=582). We assessed the potential difference between imputed BMI and actual BMI in participants with both height and weight available. The mean difference was −0.02 (p=0.66, 95% CI: −0.11, 0.07). Correlations between uric acid, BMI, and creatinine were calculated by Pearson’s correlation coefficient.
Effects of uric acid on the change in ALSFRS-R from the study baseline were estimated from a random-slope linear mixed model with a fixed effect for time, uric acid, and time × uric acid and random participant specific intercepts and sloped with unstructured covariance. Sex, diagnosis delay, age, BMI, creatinine and their interaction with time were also entered as fixed effects to control for chance differences. The mean model was unstructured in time, whereas the covariance model assumed participant-specific linear deviations from the means.
Results
Study participants
At the time of this analysis, PRO-ACT included longitudinal data from 10,723 people with ALS from 23 separate clinical trials. Baseline characteristics are provided in Supplementary Table 1. Urate levels were available in some but not all trials included in PRO-ACT. For the present study, we included individuals for whom urate levels and potential confounders of urate levels (age, gender, diagnostic delay, creatinine, and BMI) were available (N=1,736). Clinical and demographic characteristics of study participants are summarized in Table 1. Baseline urate was significantly correlated with BMI (r= 0.30, p<0.001) and creatinine (r= 0.19, p=<0.001).
Table 1.
Characteristics of study participants (N=1,736)
| Characteristic | Percent or mean (±SD) |
|---|---|
| Age | 55.0 (±12.1) years |
| Gender | 36.7% Female |
| Diagnostic delay | 10.5 (±8.7) months |
| BMI | 25.3 (±4.5) kg/m2 |
| Creatinine | 0.9 (±0.2) mg/dL |
| Urate | 5.0 (±1.3) mg/dL |
BMI: Body Mass Index. N: number. SD: standard deviation.
Survival and functional decline by urate levels
In an adjusted Cox proportional hazards analysis, controlling for potential confounders, there was an 11% reduction in risk of death during the study (HR 0.89, 95% CI [0.82,0.97], p < 0.01) for each unit (mg/dL) increase in baseline urate (N=1,736) (Table 2). An initial Cox model was fit with an interaction between urate level and gender to see if the effect of urate on survival was different based on gender. The gender-urate interaction was removed for the final model when the interaction was found non-significant (p=0.76). Urate levels were not significantly different between individuals on riluzole and participants who were not on riluzole (p=0.24).
Table 2.
Hazard Ratios from Cox Proportional Hazards Model
| HR | se.HR | p | |
|---|---|---|---|
| Uric Acid (mg/dL) | 0.89 | 0.043 | 0.0098 |
| Gender (Male) | 1.017 | 0.11 | 0.88 |
| Diagnostic Delay (months) | 0.99 | 0.007 | 0.042 |
| Age (years) | 1.030 | 0.004 | < 0.001 |
| Creatinine (mg/dL) | 5.36 | 0.23 | < 0.001 |
| BMI (kg/m2) | 0.91 | 0.013 | < 0.001 |
BMI: Body Mass Index. HR: hazard ratio.
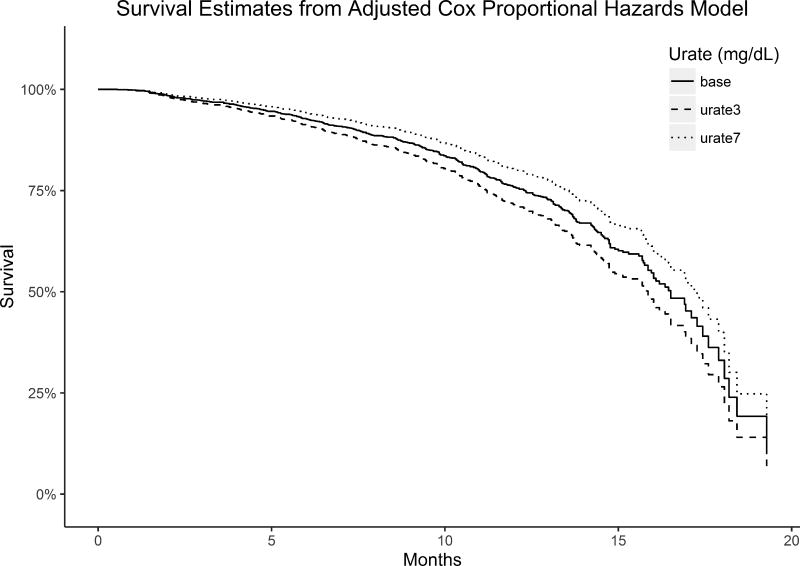
Figure 1 shows the predicted survival from the Cox proportional hazards model if all study participants had their urate levels set to 3 or 7mg/dL compared to the baseline survival. The cut-off of 7mg/dL was chosen because the current trials of urate elevation in PD and ALS are aimed at raising urate levels above 7mg/dL (NCT02642393; NCT02288091). The model predicts a 36% decrease in the risk of dying at any time point when urate levels are set to 7 mg/dL compared to when they are set to 3 mg/dL.
Figure 1. Predicted Survival from Cox Proportional Hazards Model.
The predicted survival if all study participants had their urate levels set to 3 mg/dL or 7mg/dL compared to baseline survival (solid line; participants had a mean urate level of 5.0 mg/dL).
A mixed model estimated a −1.5 point change in ALSFRS-R per month in our cohort (N=1,736; 95% CI: −1.8, −1.1; p < 0.001). The estimate for the rate of change in ALSFRS-R scores per month slowed by 0.041 (p=0.028) per unit (mg/dL) increase in baseline urate levels, with higher levels associated with improved outcomes. The same model estimated a −4.9% (95% CI: −6.1, −3.6; p < 0.001) change in vital capacity (VC) per month in the 1,285 individuals in whom longitudinal VC values were available. The estimate for the rate of change in VC scores per month slowed by 0.12% (p=0.06) per unit (mg/dL) increase in baseline urate levels.
Discussion
Our pooled analysis provides further support for urate as a predictor of ALS outcomes. Urate has been proposed as a prognostic factor for ALS by some, but not all authors32–37. Here we leveraged the expansion of PRO-ACT, the largest aggregation of ALS clinical trials, to examine the relationship between urate levels at study entry, survival, and functional decline. Our results showing a small, but significant association between urate and ALS outcomes are consistent with a growing literature supporting urate as a prognostic and potential neuroprotective agent in neurologic disease9,11–14,42–45. Of note, previous epidemiological studies on this topic ranged in size from 86 to 942 participants32–39 and it is possible that the reported lack of an association between urate and ALS survival in some series may have been due to lack of statistical power. Alternatively, differences in statistical methods and study populations or relatively small effect size may explain these discordant results.
Urate, creatinine, and BMI have all been identified as possible predictors of ALS prognosis40, 41, 46. Yet, whether these three variables are independent or reflect shared pathophysiologic mechanisms has been debated. Here we have shown that when controlling for both creatinine and BMI, urate was a significant predictor of ALS survival and functional decline as assessed by the ALSFRS-R. Another topic of debate in the literature is whether urate is a prognostic factor in both men and women, given the gender differences in physiologic urate concentration. In our study, the gender-urate interaction was found to be insignificant.
The PRO-ACT database has several limitations. There may be differences in participants of ALS clinical trials when compared to clinic-based populations (i.e. clinical trials tend to include younger ALS patients with more frequent limb onset and thus better prognosis)47 leading to selection bias. In addition, not every trial included the same variables thus limiting the number of individuals that could be included in the final analyses.
This study confirms the utility of the PRO-ACT database as a powerful epidemiological tool to analyze biomarkers of prognostic value in ALS40, 41 given the large amount of data that has been aggregated into a single database. PRO-ACT is a dynamic database; data from more clinical trials are being deposited and will serve to increase statistical power even further. It is expected that ALS researchers will continue to leverage PRO-ACT to further investigate ALS phenotypes and prognostic features when effect sizes are small. These analyses may in turn assist clinical trial design as identification of potential sub-populations of treatment responders upon stratification by biomarker, gene, or phenotype may also be possible.
As with any epidemiologic study, association does not imply causation and the possibility of unknown confounders cannot be excluded. However, the consideration of urate as a prognostic factor in ALS may have therapeutic implications and provides a foundation for relevant future avenues of research. Interestingly, pre-clinical studies of urate level modulation have already been performed in several models of neurodegeneration and supported urate as a neuroprotectant9,11–14,42–45. Pre-clinical studies on the role of urate in cellular and animal models of ALS are ongoing. These studies are of great translational potential. Indeed, urate modulation via its precursor inosine is rapidly advancing from the bench to the bedside in PD where a phase 2 clinical trial has demonstrated the safety and tolerability of urate-modulation15, 48. Clinical trials of urate elevation in ALS are ongoing (NCT03168711). Results from these studies will clarify whether urate modulation may be a valuable treatment for ALS. Ultimately, work on urate neurobiology may unravel novel therapeutic targets along urate-mediated pathways that may affect oxidative stress and damage and, in turn, ALS disease progression.
Supplementary Material
Acknowledgments
Funding: No targeted funding for this study.
Data used in the preparation of this article were obtained from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) Database. As such, the following organizations and individuals within the PRO-ACT Consortium contributed to the design and implementation of the PRO-ACT Database and/or provided data, but did not participate in the analysis of the data or the writing of this report: Neurological Clinical Research Institute, MGH; Northeast ALS Consortium; Novartis; Prize4Life Israel; Regeneron Pharmaceuticals, Inc.; Sanofi; Teva Pharmaceutical Industries, Ltd.
Sabrina Paganoni has received funding from the NIH (Career Development Award 2K12HD001097-16), Target ALS, the Salah Foundation, and the Spastic Paraplegia Foundation.
Katharine Nicholson has received funding from the Anne Young Fellowship, Brainstorm Cell Therapeutics, and ALS Finding a Cure Foundation.
Alexander Sherman receives funding from the AAN, MDA, ALS Association, and ALS Finding a Cure Foundation.
James Berry has consulted with Biogen-IDEC and Neuraltus Pharmaceuticals, and has received research support from Voyager Therapeutics, GSK, Cytokinetics, Brainstorm Cell Therapeutics, Novartis, ALS Therapy Development Institute, ALS Association, MDA and NIH.
Merit Cudkowicz has provided consulting for Cytokinetics, Astra Zeneca, Genentech, Biogen-IDEC, Voyager, Biohaven.
Nazem Atassi is funded by an NIH Career Development Award, receives fellowship grants from the American Academy of Neurology (AAN), Muscular Dystrophy Association (MDA), and the Anne Young Fellowship, has research grants from the Harvard NeuroDiscovery Center and ALS Therapy Alliance (ATA), and provided consulting for Biogen IDEC.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS functional rating scale - revised
- BBB
blood-brain barrier
- BMI
body mass index
- CI
Confidence Interval
- CNS
Central Nervous System
- CSF
cerebrospinal fluid
- HR
hazard ratio
- PD
Parkinson’s disease
- PRO-ACT
Pooled Resource Open-Access ALS Clinical Trials
- N
number
- SD
standard deviation
- VC
vital capacity
- Uox
urate oxidase
Footnotes
Statistical analysis was conducted by James Chan, Amy Shui, and David Schoenfeld; Harvard Medical School, Massachusetts General Hospital Biostatistics Center, Boston, MA.
Drs. Paganoni and Atassi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Individual authors’ contributions:
Sabrina Paganoni: study concept/design; analysis/interpretation of data; drafting/revising the manuscript.
Katharine Nicholson: study concept/design; analysis/interpretation of data; drafting/revising the manuscript.
James Chan: analysis/interpretation of data; drafting/revising the manuscript.
Amy Shui: analysis/interpretation of data; drafting/revising the manuscript.
David Schoenfeld: analysis/interpretation of data; drafting/revising the manuscript.
Alexander Sherman: analysis/interpretation of data; drafting/revising the manuscript.
James Berry: analysis/interpretation of data; drafting/revising the manuscript.
Merit Cudkowicz: analysis/interpretation of data; drafting/revising the manuscript.
Nazem Atassi: study concept/design; analysis/interpretation of data; drafting/revising the manuscript.
Disclosure of Interests:
James Chan reports no conflicts of interest.
Amy Shui reports no conflicts of interest.
David Schoenfeld reports no conflicts of interest.
References
- 1.D'Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–527. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louwerse ES, Weverling GJ, Bossuyt PM, Meyjes FE, de Jong JM. Randomized, double-blind, controlled trial of acetylcysteine in amyotrophic lateral sclerosis. Arch Neurol. 1995;52:559–564. doi: 10.1001/archneur.1995.00540300031009. [DOI] [PubMed] [Google Scholar]
- 3.Lange DJ, Murphy PL, Diamond B, et al. Selegiline is ineffective in a collaborative double-blind, placebo-controlled trial for treatment of amyotrophic lateral sclerosis. Arch Neurol. 1998;55:93–96. doi: 10.1001/archneur.55.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Graf M, Ecker D, Horowski R, et al. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm (Vienna) 2005;112:649–660. doi: 10.1007/s00702-004-0220-1. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann P, Thompson JL, Levy G, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol. 2009;66:235–244. doi: 10.1002/ana.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19:1331–1336. doi: 10.3233/JAD-2010-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Burdett TC, Desjardins CA, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:300–305. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinescu R, Zetterberg H. Urate as a marker of development and progression in Parkinson's disease. Drugs Today (Barc) 2011;47:369–380. doi: 10.1358/dot.2011.47.5.1591834. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N, Shu HY, Huang T, et al. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One. 2014;9:e100286. doi: 10.1371/journal.pone.0100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong L, Zhang QL, Zhang N, et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson's disease: linking to Akt/GSK3beta signaling pathway. J Neurochem. 2012;123:876–885. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama K, Matsumura N, Watabe M, Wang F, Kikuchi-Utsumi K, Nakaki T. Caffeine and uric acid mediate glutathione synthesis for neuroprotection. Neuroscience. 2011;181:206–215. doi: 10.1016/j.neuroscience.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55:463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 14.Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomark Med. 2010;4:701–712. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarzschild MA, Ascherio A, Beal MF, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71:141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiburkova B, Krijt J, Vyletal P, et al. Novel mutations in xanthine dehydrogenase/oxidase cause severe hypouricemia: biochemical and molecular genetic analysis in two Czech families with xanthinuria type I. Clin Chim Acta. 2012;413:93–99. doi: 10.1016/j.cca.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 17.Stiburkova B, Ichida K, Sebesta I. Novel homozygous insertion in SLC2A9 gene caused renal hypouricemia. Mol Genet Metab. 2011;102:430–435. doi: 10.1016/j.ymgme.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura Y, Matsuo H, Chiba T, et al. Pathogenic GLUT9 mutations causing renal hypouricemia type 2 (RHUC2) Nucleosides Nucleotides Nucleic Acids. 2011;30:1105–1111. doi: 10.1080/15257770.2011.623685. [DOI] [PubMed] [Google Scholar]
- 19.Gabrikova D, Bernasovska J, Sokolova J, Stiburkova B. High frequency of SLC22A12 variants causing renal hypouricemia 1 in the Czech and Slovak Roma population simple and rapid detection method by allele-specific polymerase chain reaction. Urolithiasis. 2015;43:441–445. doi: 10.1007/s00240-015-0790-4. [DOI] [PubMed] [Google Scholar]
- 20.Stiburkova B, Sebesta I, Ichida K, et al. Novel allelic variants and evidence for a prevalent mutation in URAT1 causing renal hypouricemia: biochemical, genetics and functional analysis. Eur J Hum Genet. 2013;21:1067–1073. doi: 10.1038/ejhg.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawton KA, Brown MV, Alexander D, et al. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics. Amyotroph Lateral Scler Frontotemporal Degener. 2014:1–9. doi: 10.3109/21678421.2014.908311. [DOI] [PubMed] [Google Scholar]
- 22.Keizman D, Ish-Shalom M, Berliner S, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–99. doi: 10.1016/j.jns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Aramburu I, Sanchez-Juan P, Jesus S, et al. Genetic variability related to serum uric acid concentration and risk of Parkinson's disease. Mov Disord. 2013;28:1737–1740. doi: 10.1002/mds.25507. [DOI] [PubMed] [Google Scholar]
- 25.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu N, Dubreuil M, Zhang Y, et al. Gout and the risk of Alzheimer's disease: a population-based, BMI-matched cohort study. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66:1460–1468. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65:716–723. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon KC, Eberly S, Gao X, et al. Mendelian randomization of serum urate and parkinson disease progression. Ann Neurol. 2014;76:862–868. doi: 10.1002/ana.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakshi R, Zhang H, Logan R, et al. Neuroprotective effects of urate are mediated by augmenting astrocytic glutathione synthesis and release. Neurobiol Dis. 2015;82:574–579. doi: 10.1016/j.nbd.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham A, Drory VE. Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: a meta-analysis. J Neurol. 2014 doi: 10.1007/s00415-014-7331-x. [DOI] [PubMed] [Google Scholar]
- 32.Paganoni S, Zhang M, Quiroz Zárate A, et al. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J Neurol. 2012;259:1923–1928. doi: 10.1007/s00415-012-6440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh SI, Baek S, Park JS, Piao L, Oh KW, Kim SH. Prognostic Role of Serum Levels of Uric Acid in Amyotrophic Lateral Sclerosis. J Clin Neurol. 2015;11:376–382. doi: 10.3988/jcn.2015.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chio A, Calvo A, Bovio G, et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71:1134–1142. doi: 10.1001/jamaneurol.2014.1129. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Z, Guo X, Wei Q, et al. Serum uric acid level is associated with the prevalence but not with survival of amyotrophic lateral sclerosis in a Chinese population. Metab Brain Dis. 2014;29:771–775. doi: 10.1007/s11011-014-9510-y. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda K, Hirayama T, Takazawa T, Kawabe K, Iwasaki Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51:1501–1508. doi: 10.2169/internalmedicine.51.7465. [DOI] [PubMed] [Google Scholar]
- 37.Keizman D, Ish-Shalom M, Berliner S, et al. Low uric acid levels in serum of patients with ALS: further evidence for oxidative stress? J Neurol Sci. 2009;285:95–99. doi: 10.1016/j.jns.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Abraham A, Drory VE. Influence of serum uric acid levels on prognosis and survival in amyotrophic lateral sclerosis: a meta-analysis. J Neurol. 2014;261:1133–1138. doi: 10.1007/s00415-014-7331-x. [DOI] [PubMed] [Google Scholar]
- 39.O'Reilly EJ, Liu D, Johns DR, et al. Serum urate at trial entry and ALS progression in EMPOWER. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:120–125. doi: 10.1080/21678421.2016.1214733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuffner R, Zach N, Norel R, et al. Crowdsourced analysis of clinical trial data to predict amyotrophic lateral sclerosis progression. Nat Biotechnol. 2015;33:51–57. doi: 10.1038/nbt.3051. [DOI] [PubMed] [Google Scholar]
- 41.Atassi N, Berry J, Shui A, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83:1719–1725. doi: 10.1212/WNL.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci U S A. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhu TG, Wang XX, Luo WF, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett. 2012;506:175–179. doi: 10.1016/j.neulet.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 45.Cipriani S, Desjardins CA, Burdett TC, Xu Y, Xu K, Schwarzschild MA. Protection of dopaminergic cells by urate requires its accumulation in astrocytes. J Neurochem. 2012;123:172–181. doi: 10.1111/j.1471-4159.2012.07820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chio A, Canosa A, Gallo S, et al. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology. 2011;77:1432–1437. doi: 10.1212/WNL.0b013e318232ab9b. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya S, Bakshi R, Logan R, Ascherio A, Macklin EA, Schwarzschild MA. Oral Inosine Persistently Elevates Plasma antioxidant capacity in Parkinson's disease. Mov Disord. 2016 doi: 10.1002/mds.26483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.