Abstract
Context
Achievement of hypocortisolemia following transsphenoidal surgery (TSS) for Cushing’s disease (CD) is associated with successful adenoma resection. However, up to one third of these patients recur.
Objective
We assessed whether delay in reaching post-operative cortisol nadir may delineate patients at risk of recurrence for CD following TSS.
Methods
A retrospective review of of 257 patients who received 291 TSS procedures for CD at NIH, between 2003 and 2016. Early biochemical remission (serum cortisol nadir < 5 µg/dL), was confirmed with endocrinological and clinical follow up. Recurrence was detected by laboratory testing, clinical stigmata or medication-dependence during a median follow-up of 11 months.
Results
Of the 268 unique admissions, remission was recorded in 241 instances. Recurrence was observed in 9% of these cases with cortisol nadir ≤ 5 µg/dL and 6% of cases with cortisol nadir ≤ 2 µg/dL. The timing of hypocortisolemia was critical in detecting late recurrences. Morning POD-1 cortisol < 3.3µg/dL was 100% sensitive in predicting durable remission and morning POD-3 cortisol ≥ 18.5µg/dL was 98.6% specific in predicting remote recurrence. AUROC analysis revealed that hypocortisolemia ≤ 5 µg/dL before 15 hours (post-operative) had 95% sensitivity and an NPV of 0.98 for durable remission. Serum cortisol level ≤ 2 µg/dL, when achieved before 21 hours, improved sensitivity to 100%.
Conclusions
In our cohort, early, profound hypocortisolemia could be used as a clinical prediction tool for durable remission. Achievement of hypocortisolemia ≤ 2 µg/dL before 21 post-operative hours appeared accurately predict durable remission in the intermediate term.
Keywords: Pituitary adenoma, Hypercortisolism, Cushing’s disease, Transsphenoidal surgery, Remission, Post-operative testing
Introduction
Left untreated, chronically elevated endogenous hypercortisolemia resulting from Cushing’s disease (CD) is associated with significant morbidity and mortality.(1, 2) Transsphenoidal surgery (TSS) for resection of corticotropinoma is the first line treatment, offering the potential for remission in 77% to 98% of cases.(2, 3, 4, 5, 6, 7) Successful corticotropinoma resection leads to transient post-operative adrenocorticotropin (ACTH) and cortisol deficiency, due to suppression of the remaining normal pituitary corticotrophs by long-standing hypercortisolemia.(8, 9) Given the short serum half lives of ACTH and cortisol, a rapid drop in levels would be expected during the immediate post-operative period.(10, 11) As demonstrated by the faster rate of decline in serum levels achieved by total hypophysectomy, when compared to subcapsular resection, there is evidence to suggest that the residual corticotrophs do remain functional following complete removal of the ACTH-secreting adenoma.(12)
In the post-operative setting, failure to achieve hypocortisolemia is thought to reflect the presence of residual tumor cells.(13, 14) Therefore, serum cortisol has become a well-established biochemical marker of early surgical remission, with rates reported to range from 65–98% at experienced centers.(15, 16, 17, 18, 19) However, despite early remission, recurrence has been observed in 2–35% of these cases, occurring up to thirty years post-operatively.(12, 15, 16, 18, 20, 21, 22) Because pituitary adenomas are monoclonal in origin, disease recurrence is thought to result from microscopic residual tumor corticotrophs left at the adenoma margin or unrecognised dural invasion along the wall of the cavernous sinus.(1, 13, 23, 24) Early re-operation has been shown to achieve remission in the majority of these patients requiring further treatment.(13, 15, 25, 26) This highlights the possibility that post-operative cortisol values may reflect the extent of microscopic resection.
In attempt to define the subset of patients at high likelihood of recurrence, early identification of surgical success has been the focus of several recent studies.(17, 19, 20, 21, 27, 28, 29, 30) The technique of pseudocapsular resection, hypothesized to achieve complete tumour resection, has been associated with a rapid rate of post-operative cortisol decline.(12) More recently, normalised early post-operative values (NEPV), capitalizing on the potential for endoscopic TSS to act as an endogenous corticotrophin releasing hormone (CRH) stimulation test, have been shown to improve accuracy in predicting early non-remission.(30)
We hypothesized that the slope of serum cortisol decline in the immediate post-operative period may be used as a predictive biomarker for the completeness of tumor resection in ACTH-secreting pituitary adenoma. This may aid in the identification of patients who could benefit from enhanced biochemical follow-up to prevent disease relapse.
Subjects and Methods
Data from 328 patients who received 364 consecutive sublabial transsphenoidal surgeries for adrenocorticotropic (ACTH)–secreting pituitary adenomas by a team of surgeons (EHO, RRL and PC) from December 2003 until July 2015 was retrospectively reviewed. All operations took place at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD under research protocol NIH ID 03-N-0164 (clinicaltrials.gov identifier NCT00060541). Written informed consent was obtained from each patient for study participation, and the study was performed within the clinical and ethical guidelines of the Combined Neurosciences Institutional Review Board of the NIH.
Diagnosis of Cushing’s disease
The work-up for Cushing’s disease has previously been described and is summarized below.(31, 32, 33, 34, 35) Cushing’s syndrome was confirmed on the basis of elevations of at least two of the following laboratory tests: late night salivary cortisol, 24-hour urine free cortisol or low dose dexamethasone suppression testing (1mg overnight or 2mg over 48 hours, normal cortisol <1.8µg/L).(1, 35) Supplemental diagnostic testing included midnight serum cortisol and morning cortisol following 8mg overnight dexamethasone suppression testing (HDDST).(36, 37)
A diagnosis of Cushing’s disease was made in patients with ACTH-dependent hypercortisolism with at least one of the following: response of serum cortisol or ACTH to ovine corticotropin-releasing hormone (oCRH) stimulation testing, identification of adenoma on magnetic resonance imaging (MRI) of the pituitary or a central-to-peripheral ACTH gradient during inferior petrosal sinus sampling (IPSS).(33, 34, 38) Pituitary microadenomas were detected as hypoattenuated regions within the sella on early, post gadolinium contrast 3D gradient recalled echo imaging.(39, 40, 41) IPSS was performed in patients where HDDST, oCRH and pre-operative MRI were not concordant. (26)
The presence, size and location of lesions were recorded on the pre-operative MRI report. Surgical findings, denoting the size and location of the adenoma, were recorded on a graphical post-operative record of the pituitary gland. Presence and location of ACTH-positive pituitary adenoma on histopathology was noted. Concordance was referenced to imaging and/or surgical findings.
The biochemical assays and imaging modalities used in this study have previously been described in the literature.(7, 42, 43)
Post-operative outcomes
Serum cortisol levels were routinely obtained at 6-hour intervals from post-operative day 0 (POD-0) through day 3 (POD-3). The initial post-operative serum cortisol was obtained at 1200hr POD-0 or 1800hr depending upon the time of completion of surgery. After POD-3, daily morning cortisol levels were obtained until post-operative day 10 (POD-10) during their inpatient stay or until patient discharge.(27, 42)
Early biochemical remission was assigned by detecting nadir serum cortisol level of < 5µg/dL or < 2 µg/dL by POD-10, prior to administration of exogenous glucocorticoids, and sustained hypocortisolemia neccesiating glucocorticoid replacement.(33) All patients in whom this was not achieved (persistent CD) were excluded from further analysis. For patients undergoing early repeat TSS for persistent CD, cortisol values obtained after the final, successul TSS procedure were included for analysis.
Clinical stigmata of Cushing’s syndrome (recurrence of height arrest in children, weight gain, hypertension, plethora), biochemical evidence of hypercortisolemia (24 hour urinary free cortisol, diurnal serum cortisol and ACTH, or more recently, salivary cortisol), need medical therapy targeting Cushing’s syndrome (ketoconazole initiation to suppress serum cortisol), or requirement for further surgical management formed the criteria for assigning recurrence.
Statistical analysis
Patients were divided into remission and recurrence groups for statistical analysis. Baseline clinical characteristics were presented as percentages, or as means with standard deviations. Binomial variables were compared using two-sample tests of proportions or two sample T-tests with unequal variance (44) while categorical variables were compared using chi-square and Fisher’s exact tests, where appropriate. One-way Anova and Tukey post-hoc tests were used for the comparison of proportions of means, reported as mean difference (MD). Kaplan Meier analysis, using cortisol nadir as the survival event, was conducted to compare the time to reach post-operative cortisol nadir (≤5µg/dL or ≤2 µg/dL) between remission and recurrence groups. Statistical significance was assessed using the Log Rank test.(39) Additionally, CD recurrence was used as a survival event to distinguish the ability of cutoffs to predict durable remission. Predictors of recurrence were identified with logistic regression, using recurrence as the dependent variable, and evaluated for quality of fit using areas under the receiver operating characteristic curve (AUROC). Score cutoffs were chosen for sensitivity and specificity analysis after AUROC, confirmed using 2×2 tables. Standard errors were calculated by the DeLong method.(40) P values < 0.05 two-tailed were considered statistically significant. GraphPad Prism 6.0 software was used for statistical figures (GraphPad Software, La Jolla, California USA). Statistical analyses were performed using SPSS version 24.0 (IBM Corporation, Armonk, New York, USA).
Results
Two hundred and fifty seven patients receiving 291 TSS procedures were included in this study. The rest were excluded due to lack of adequate post-operative laboratory values or due to inadequate follow-up. Twenty three procedures were early repeat TSS surgeries for persistent CD; resulting in 268 unique admissions. Of these, remission was achieved in 241 (90%) patients, with post-operative cortisol nadir ≤ 5 µg/dL within 10 post operative days. Meanwhile, in 222 (82.8%) hypocortisolemia of ≤ 2 µg/dL was detected within 10 post operative days. Only the 241 instances with remission and hypocortisolemia ≤ 5µg/dL were included for further analysis. Patients were followed up for a mean of 14 months (median 7 months, interquartile range 1 – 17 months, range 1 – 150 months).
Recurrence
At the last follow up, recurrence was observed in 8.7% (n = 21/241) of cases achieving cortisol nadir ≤ 5 µg/dL and in 5.9% (n = 13/222) of cases achieving cortisol nadir ≤ 2 µg/dL. Of the 19 cases achieving cortisol nadir ≤5 µg/dL but not ≤ 2 µg/dL, 42% (n = 8) recurred. Recurrence occurred at a median of 11.86+/−20.52 months. There was a statistically significant difference in the rate of recurrence between the three groups (ANOVA F statistic (2, 479) = 1551, p<0.001). Upon post-hoc analysis, the difference remained significant [p =(0.05/3) = 0.0167, for exploratory analysis] between each of the three nadir cutoff values of ≤5µg/dL (A), ≤ 2 µg/dL (B) and 2.1 - 5 µg/dL (C) (A vs. B MD=0.03, p<0.001; A vs. C MD=−0.33 p<0.001; B vs. C MD =−0.36 P<0.001).
Patients did not differ significantly in BMI, gender age or race between the durable remission and recurrence groups. As expected (41), the proportion of MRI-positive (n=239); intra-operatively visualised macroscopic adenomas (n=233; pathology-confirmed adenomas (n=232); and location-concordance between imaging and histopathological findings (n=239) did not differ significantly between the two groups. The rate of location-concordance between imaging and surgical findings (n=153) was lower in the recurrence group, (57 vs 42%, p=0.026), suggesting that adenomas that were detected well both with imaging, during surgery tended to recur less frequently. (Table 1).
Table 1.
Univariate logistic regression analysis identifying predictors of recurrence in patients achieving early biochemical remission. Remission was defined as cortisol nadir ≤5µg/mL by POD-10. Measures of association are represented as odds ratio with corresponding p-values. Accounting for potential confounding variables of age, gender, BMI and ethnicity, adjusted odds ratio with corresponding p-values are presented.
| Variables | OR | 95% CI | Std Error | P value | Adj OR | P value | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | 1.023 | 0.99, 1.06 | 0.016 | 0.150 | |||
| BMI | 1.013 | 0.95, 1.08 | 0.031 | 0.663 | |||
| Gender | 0.737 | 0.26, 2.10 | 0.534 | 0.568 | |||
| White | 1.519 | 0.60, 3.84 | 0.474 | 0.378 | |||
| Black | 0.585 | 0.18, 1.87 | 0.594 | 0.366 | |||
| Asian | 0.320 | 0.06, 1.65 | 0.837 | 0.173 | |||
|
| |||||||
| Perioperative outcomes | |||||||
| MRI-positive | 1.030 | 0.38, 2.78 | 0.506 | 0.954 | 0.877 | 0.812 | |
| Visualised intra-operatively | 0.664 | 0.15, 3.00 | 0.770 | 0.595 | 0.683 | 0.637 | |
| Imaging/resection concordance | 3.758 | 1.17, 12.11 | 0.597 | 0.027* | 7.010 | 0.006** | |
| Pathology positive | 1.958 | 0.61, 6.34 | 0.600 | 0.262 | 2.196 | 0.238 | |
| Imaging/pathology concordance | 2.097 | 0.85, 5.16 | 0.460 | 0.107 | 1.039 | 0.074 | |
|
| |||||||
| Cortisol | |||||||
| 0000hr POD-0 | 0.971 | 0.94, 1.01 | 0.018 | 0.109 | 0.962 | 0.068 | |
| 1800hr POD-0 | 0.974 | 0.94, 1.01 | 0.017 | 0.116 | 0.970 | 0.135 | |
| 0000hr POD-1 | 0.970 | 0.94, 1.01 | 0.017 | 0.075 | 0.959 | 0.061 | |
| 0600hr POD-1 | 0.933 | 0.89, 0.98 | 0.026 | 0.007** | 0.904 | 0.002** | |
| 1200hr POD-1 | 0.907 | 0.82, 1.00 | 0.049 | 0.043* | 0.873 | 0.040* | |
| 1800hr POD-1 | 0.889 | 0.81, 0.98 | 0.048 | 0.014* | 0.887 | 0.028* | |
| 1800hr POD-2 | 0.894 | 0.78, 1.03 | 0.072 | 0.119 | 0.847 | 0.072 | |
| 0000hr POD-3 | 1.023 | 0.89 1.18 | 0.073 | 0.752 | 1.030 | 0.748 | |
| 0600hr POD-3 | 0.959 | 0.92, 1.00 | 0.022 | 0.062 | 0.959 | 0.062 | |
| 1200hr POD-3 | 0.900 | 0.82, 1.00 | 0.050* | 0.036* | 0.882 | 0.130 | |
| 1800hr POD-3 | 0.981 | 0.93, 1.04 | 0.028 | 0.487 | 1.010 | 0.842 | |
| NEPV cortisol | 0.931 | 0.87, 0.99 | 0.033* | 0.031* | 0.902 | 0.039* | |
Those of statistical signficance are marked with asterisks.
POD = post-operative day, BMI = body mass index, Adj = adjusted, OR = odds ratio, Std error = standard error, P value = probability value.
Early post-operative serum cortisol analysis
Survival analysis of time to reach nadir serum cortisol revealed a statistically significant difference between the durable remission and recurrence groups in patients achieving nadir serum cortisol ≤5µg/dL (p=0.002) (Figure 1A). Despite a statistically significant difference observed on Log Rank test, the median time to reach nadir cortisol ≤ 5 µg/dL was 42 hours in both the remission and recurrence groups. No difference in time to reach nadir cortisol was found in patients within subgroups nadir < 2 µg/dL (Figure 1B) or nadir 2 – 5 µg/dL (Figure 1C).
Figure 1.
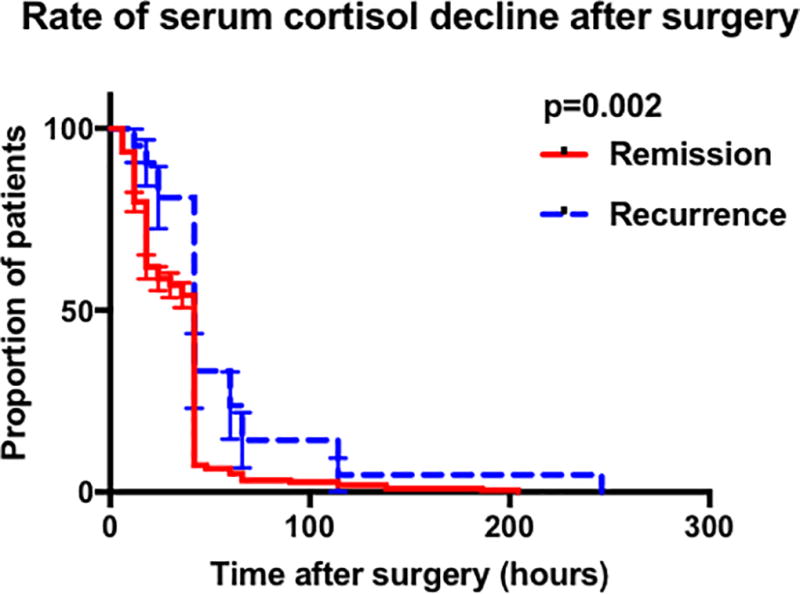
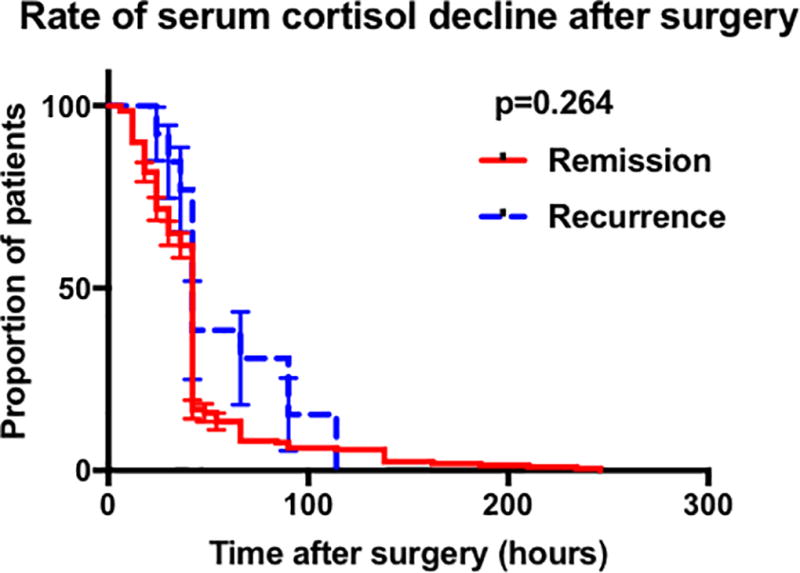
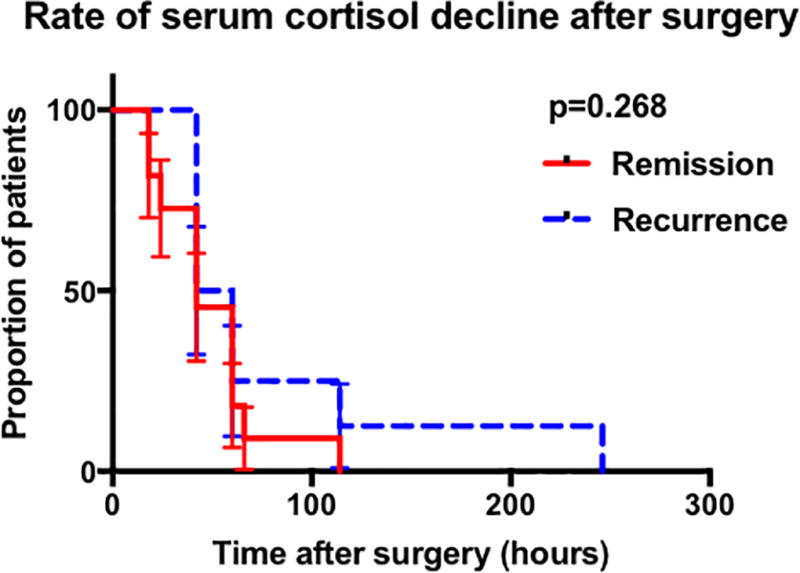
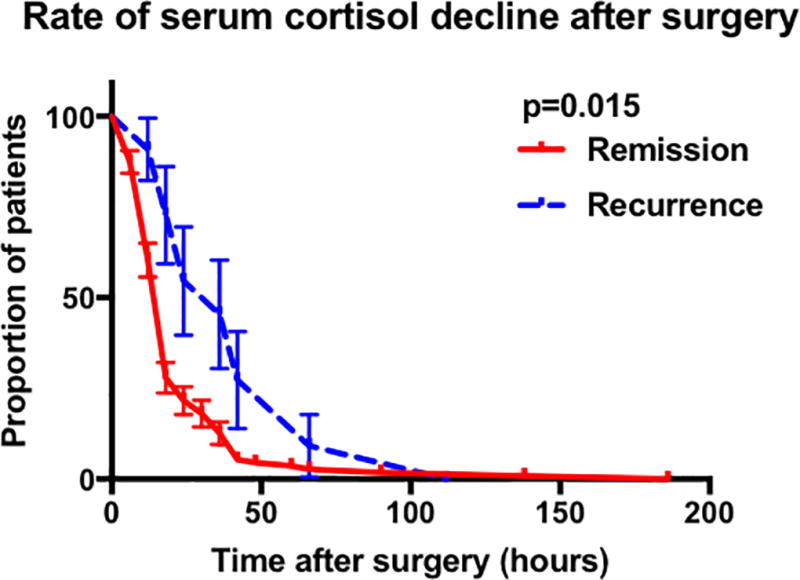
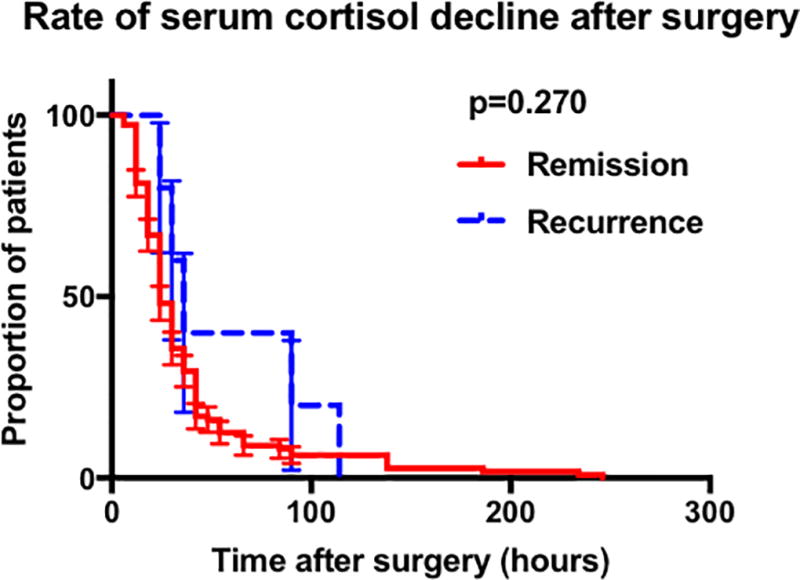
Graphs showing the results of the Kaplan meier analysis comparing remission and recurrence groups. A Comparison in all patients achieving cortisol nadir 5µg/dL by POD-10. B Comparison of in all patients achieving cortisol nadir 2µg/dL by POD-10. C Comparison in patients achieving cortisol nadir 2.1 – 5µg/dL by POD-10. D Comparison in patients who had the initial serum cortisol drawn within the first 42 post-operative hours and who achieved cortisol nadir 5µg/dL by POD-10. E Comparison in patients who had the initial serum cortisol drawn within the first 42 post-operative hours and who achieved cortisol nadir 2µg/dL by POD-10. (Abbreviations : POD, post-operative day).
To assess the effect of immediate cortisol decline on rates of recurrence, we performed a subgroup analysis on patients with available early (<42 hours) post-operative laboratory values (n = 128). This analysis enabled the exclusion of patients who had their first serum cortisol recorded at ≥ 42 hours but who may have achieved nadir cortisol prior to his time. The median time to reach cortisol nadir ≤ 5µg/dL was significantly different between the two groups (18 hours vs. 42 hours p=0.004) (Figure 1 D, 1E). These findings suggest that patients that have eventual recurrence may have a slight delay in serum cortisol nadir following TSS. The findings also suggest that early serum cortisol (< 42 hours) is critical to detect this slight delay.
Biochemical predictors of recurrence
To identify predictors of recurrence, we performed logistic regression analysis. After adjusting for age, BMI, gender and ethnicity. Morning (0600hr) POD-1 (p = 0.002), noon (1200hr) POD-1 (p = 0.040) and evening (1800hr) POD-1 (p = 0.028) cortisol were significantly associated with recurrence (Table 1). Normalized early post operative values, accurate predictors of early non-remission(30), were analysed for their potential to predict long-term recurrence. Logistic regression analysis revealed a significant difference between the durable remission and recurrence groups (p=0.026) (Table 1).
Area under the receiver operating characteristic curve (AUROC) was used to assess accuracy of association between post-operative cortisol levels and recurrence. Serum cortisol at 0600hr POD-1 and 1800hr POD-3 yielded the highest ROC 0.84, p < 0.001; 0.90 p < 0.023, respectively. NEPV and serum cortisol levels throughout POD-1 and POD-3 remained significant predictors of recurrence (Table 2). Sensitivity and specificity analysis identified a cutoff of < 3.3 µg/dL for serum cortisol at 0600hr POD-1. This achieved a sensitivity of 100%, specificity of 59% and NPV of 1.0 in predicting durable remission. Applying the cutoff of < 3.3 µg/dL to 0600hr POD-1, revealed a significant difference in recurrence rates. There were no observed recurrences in patients whose serum cortisol values reached < 3.3µg/dL by 0600 on POD1 (n=55). Paients with values above 3.3µg/dL (n=64) recurred on an average of 12 months after TSS (p = 0.02)(Figure 2). Furthermore, a cutoff of >18.5µg/dL for serum cortisol at 0600hr POD-3 achieved a specificity of 98.6%, sensitivity of 14% and PPV of 0.92 in predicting recurrence (despite nadir ≤ 5 µg/dL at other time points).
Table 2.
Comparison of post-operative serum cortisol in patients achieving early remission by POD-10. Results of AUROC analyses with recurrence as the reference variable. Remission defined as cortisol nadir ≤5µg/dL. Recurrence defined by biochemical hormone levels, clinical stigmata or medication requirement.
| Cortisol | AUROC | 95% CI | Std dev | n | P value |
|---|---|---|---|---|---|
| 0000hr POD-1 | 0.775 | 0.64, 0.91 | 0.067 | 94 | 0.010** |
| 0600hr POD-1 | 0.839 | 0.74, 0.94 | 0.052 | 119 | <0.001*** |
| 1200hr POD-1 | 0.761 | 063, 0.89 | 0.068 | 103 | 0.015* |
| 1800hr POD-1 | 0.724 | 0.52, 0.93 | 0.105 | 98 | 0.039* |
| 0600hr POD-3 | 0.742 | 0.63, 0.85 | 0.056 | 228 | <0.001*** |
| 1200hr POD-3 | 0.753 | 0.53, 0.97 | 0.111 | 52 | 0.044* |
| 1800hr POD-3 | 0.897 | 0.77, 1.03 | 0.055 | 42 | 0.023* |
| NEPV cortisol | 0.832 | 0.72, 0.94 | 0.055 | 65 | 0.007** |
| Nadir ≤5µg/dL | 0.714 | 0.60, 0.83 | 0.058 | 241 | 0.001*** |
| Nadir ≤2µg/dL | 0.661 | 0.51, 0.80 | 0.073 | 222 | 0.050* |
(Abbreviations: POD, post-operative day; NEPV, normalised early post-operative value, AUROC, area under the receiver operating curve; CI, confidence interval; Std dev, standard deviation; n, number; p value, probability value).
Figure 2.
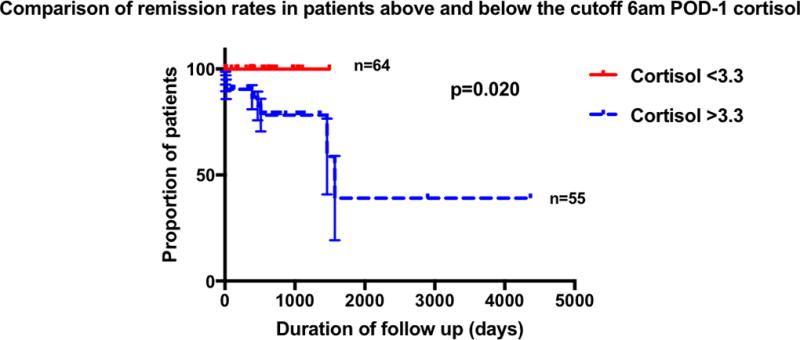
Kaplan-meier analysis comparing recurrence rates in patients above and below the cortisol cutoffs of 0600hr POD-1 <3.3µg/dL, (POD = post-operative day).
Further AUROC analyses performed to assess accuracy of assocation between time to reach post-operative cortisol nadir ≤ 5 µg/dL and remission yielded a ROC of 0.71, p = 0.001 (Table 2). Applying a cutoff of 15 hours to reach cortisol nadir ≤ 5 µg/dL achieved a sensitivity of 95%, specificity of 21% NPV 0.98 and PPV 0.10 in predicting durable remission. Association between time to reach post-operative cortisol nadir ≤ 2 µg/dL and durable remission yielded a ROC of 0.66, p = 0.05 (Table 2). Applying a cutoff of 21 hours to reach cortisol nadir ≤ 2 µg/dL achieved a sensitivity of 100%, specificity of 18% NPV 1.0 and PPV 0.7 in predicting durable remission. These results suggest that early, profound hypocortisolemia following TSS may predict durability of remission from CD.
Discussion
Identifying predictors of recurrence after resection of ACTH-secreting pituitary adenomas presents a significant clinical challenge. Transsphenoidal surgery, which has the ability to provide rapid symptom relief, effectively moderates the risk of permanent organ damage resulting from hormonal excess.(1, 6, 29) Post-operative biochemical markers, therefore, define immediate surgical outcomes.(4, 6, 9, 10, 22, 25, 27, 33, 38, 44, 45, 46) The reported remission rates vary widely, ranging from 65 – 97%, and may depend on surgical expertise and case volume.(2–7, 12, 14–27) In recent times, the need for clear criteria defining early biochemical remission has been recognized, in order to better standardise treatment effects in this patient group.(5, 47) Furthermore, in a subset of patients who do achieve early post-operative biochemical remission, subsequent recurrence of hypercortisolemia on long-term follow up has been documented.(4, 5, 6, 12, 15, 16, 18, 19, 20, 21, 29, 48)
In our retrospective review of 291 patients treated at a single centre, 87% and 79% of patients achieved early hypocortisolemia, ≤ 5 µg/dL and ≤2 µg/dL, respectively. Lindsay et al, in a review of 331 patients, have previously reported concordant rates of hypocortisolemia at 98% and 87% for serum cortisol ≤ 5µg/dL and ≤ 2µg/dL, respectively.(27) However, while their group found no significant difference in recurrence rates between the respective nadir cortisol levels, we were able to demonstrate a significantly higher recurrence rate in patients who did not achieve cortisol nadir < 2µg/dL. We suspect that we were able to detect a difference due to the availability of early (< 42 hours) post-operative serum cortisol values. As hypothesised by Monteith et al., this finding suggests that the rate of cortisol decline, and nadir cortisol level, may be associated with completeness of adenoma resection and thereby predict long-term outcomes in patients with CD.(12) This hypothesis is also supported by occurrence of same-site recurrence in patients with prior remission from CD.(49)
Post-operative hypocortisolemia following ACTH-secreting adenoma resection is thought to be due to chronic suppression of non-adenomatous pituitary corticotrophs in the setting of endogeonous hypercortisolemia.(9, 50, 51) However, observed differences in serum cortisol and ACTH levels following total hypophysectomy versus adenomectomy suggests some preserved corticotroph secretory function in the normal gland.(12) This preserved response was utilized by Asuzu et al., to detect a stress-response induced in the immediate post-operative period.(30, 52) Therefore, identification of optimal time points for recording serum cortisol are necessary to differentiate the contributions of normal gland and residual tumor. We found that normalised early post-operative cortisol, an effective marker of early remission (30), did not significantly predict long-term recurrence in our patient cohort. Therefore, same-day post-operative serum cortisol values may not be useful for prediction of eventual recurrence.
We then analysed post-operative serum cortisol levels to formulate a predictive model for risk of recurrence based on the magnitude and slope of hypocortisolemia. Achievement of nadir cortisol < 2 µg/dL or < 5 µg/dL from between 12 hours to five days post-operatively has previously been well documented to be a reliable marker of early remission.(25, 31, 53, 54) However, profound post-operative hypocortisolemia has been reported to have very poor accuracy in precting durable remission.(27, 55, 56, 57) In our cohort however, 0600hr POD-1 cortisol < 3.3 µg/dL was able to predict durable remission with a sensitivity of 100%. Additionally, 0600hr POD-3 cortisol > 18.6µg/dL demonstrated 99% specificity as a predictor of recurrence. Prior studies that tested the predictive ability of profound hypocortisolemia or undetectable serum cortisols had serum cortisols drawn more than 3 post-operative days.(27, 55, 56, 57) We found that unlike Lindsay et al. who obtained cortisol levels on post -operative days 3 – 5 (27), many patients (n = 127) in the current cohort had earlier (starting approximately 3 - 6 hours post-operatively) laboratory values available. We suspected that the slope of cortisol decline in the post-operative period is critical to determining the likelihood of the presence of microspopic residual tumor remnant. Therefore, we believe that inclusion of early post-operative (<42 hours) serum cortisols were critical to improve the predictive accuracy for durable remission. In patients with available early serum cortisol values, and eventual durable remission, hypocortisolemia ≤ 5 µg/dL was achieved within 15 hours (Figure 1D).
Based on the findings from descriptive analyses, we performed AUROC analyses to generate clinically useful predictive tools. In our model, we assessed whether the slope of fall in serum cortisol could predict durable remission. In our model, hypocortisolemia ≤ 5 µg/dL by 15 hours had 97% sensitivity and an NPV of 0.98 for durable remission. Similarly, in our model, a lower serum cortisol level of ≤ 2 µg/dL could be achieved by a later time-point of 21 hours to improve sensitivity to 100% and NPV to 1.0. Our findings suggest that early post-operative serum cortisol testing may have a role in predicting long-term outcomes following TSS for CD.
Although the data in this study was drawn from a large cohort of patients with Cushing’s disease, its quality is limited by the retrospective study design. Cortisol laboratory values in the first 42 post-operative hours were missing from a number of patients, who were excluded from further analysis. Semi-autonomous cortisol production due to adrenal nodule formation can severely inhibit correct application of these findings.(58) Furthermore, our mean 11-month follow-up period is unlikely to reflect the biochemical characteristics of all patients who experienced long-term recurrence, known to occur up to decades post-operatively. A median time to recurrence of 12 months in the subset of patients with 0600hr POD-1 cortisol >3.3µg/dL, highlights this. While surgical technique, surgeon expertise and volume of cases can affect outcomes(1); all included patients underwent surgery at a single center.
Conclusions
In our cohort of patients with CD, the slope in serum cortisol decline in the immediate post-operative period acted as a biochemical marker of recurrence. We found that achievement of hypocortisolemia ≤ 5 µg/dL by 15 hours or ≤ 2 µg/dL by 21 hours appeared to be an accurate predictor of durable remission in the intermediate term. Such a clinical prediction tool may have significant utility in the management of CD. Further validation of its accuracy in a multi-centre prospective study with longer-term follow up is, warranted.
Acknowledgments
The authors express gratitude to, and recognize the the foundational insights for this work from Dr. Edward H. Oldfield. Dr. Oldfield expired earlier this year, and was not able to critically review the final version of this manuscript.
Funding
This work was supported by National Institutes of Health Intramural Grant ZIA NS003150-01 awarded to Prashant Chittiboina. This work was supported by the Intramural Research Programs of the National Institute of Neurological Diseases and Stroke, and Eunice Kennedy Shriver National Institute for Child Health and Human Development, Bethesda, MD.
Abbreviations
- TSS
transsphenoidal surgery
- ACTH
adrenocorticotrophic hormone
- POD
post-operative day
- AUROC
area under the receiver operating characteristic curve
- NPV
negative predictive value
- PPV
positive predictive value.
Footnotes
Disclosure statement:
The authors have nothing to disclose.
Clinical Trial Information: The transsphenoidal surgeries and post-operative care was performed under the clinical trial NIH ID 03-N-0164 (clinicaltrials.gov identifier NCT00060541).
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author Contributions
Data collection: SB, ML, SS, LN, CAS, RR, PC; Data analysis: NI, GC, DA, PC; Manuscript drafting: NI, GC, PC; Critical review of manuscript: all authors; Study supervision: PC.
References
- 1.Lonser RR, Nieman L, Oldfield EH. Cushing’s disease: pathobiology, diagnosis, and management. Journal of neurosurgery. 2017;126:404–417. doi: 10.3171/2016.1.JNS152119. [DOI] [PubMed] [Google Scholar]
- 2.Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 Years in Stoke-on-Trent, UK: Audit and meta-analysis of literature. Journal of Clinical Endocrinology and Metabolism. 2011;96:632–642. doi: 10.1210/jc.2010-1942. [DOI] [PubMed] [Google Scholar]
- 3.Rees Da, Hanna FWF, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clinical Endocrinology. 2002;56:541–551. doi: 10.1046/j.1365-2265.2002.01511.x. [DOI] [PubMed] [Google Scholar]
- 4.Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012;15:71–83. doi: 10.1007/s11102-011-0347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czepielewski MA, Rollin GAFS, Casagrande A, Ferreira NP. Criteria of cure and remission in Cushing’s disease: an update. Arquivos Brasileiros de Endocrinologia & Metabologia. 2007;51:1362–1372. doi: 10.1590/S0004-27302007000800023. [DOI] [PubMed] [Google Scholar]
- 6.Hammer GD, Tyrrell JB, Lamborn KR, Applebury CB, Hannegan ET, Bell S, Rahl R, Lu A, Wilson CB. Transsphenoidal microsurgery for Cushing’s disease: Initial outcome and long-term results. Journal of Clinical Endocrinology and Metabolism. 2004;89:6348–6357. doi: 10.1210/jc.2003-032180. [DOI] [PubMed] [Google Scholar]
- 7.Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH. Outcome of surgical treatment of 200 children with cushing’s disease. Journal of Clinical Endocrinology and Metabolism. 2013;98:892–901. doi: 10.1210/jc.2012-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grino M, Boudouresque F, Conte-Devolx B, Gunz G, Grisoli F, Oliver C, Jaquet P. In vitro corticotropin-releasing hormone (CRH) stimulation of adrenocorticotropin release from corticotroph adenoma cells: effect of prolonged exposure to CRH and its interaction with cortisol. The Journal of clinical endocrinology and metabolism. 1988;66:770–775. doi: 10.1210/jcem-66-4-770. [DOI] [PubMed] [Google Scholar]
- 9.Hauger RL, Millan MA, Catt KJ, Aguilera G. Differential regulation of brain and pituitary corticotropin-releasing factor receptors by corticosterone. Endocrinology. 1987;120:1527–1533. doi: 10.1210/endo-120-4-1527. [DOI] [PubMed] [Google Scholar]
- 10.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Twenty-four-hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. Journal of Clinical Endocrinology and Metabolism. 1990;71:1616–1623. doi: 10.1210/jcem-71-6-1616. [DOI] [PubMed] [Google Scholar]
- 11.Iranmanesh A, Lizarralde G, Short D, Veldhuis JD. Intensive venous sampling paradigms disclose high frequency adrenocorticotropin release episodes in normal men. Journal of Clinical Endocrinology and Metabolism. 1990;71:1276–1283. doi: 10.1210/jcem-71-5-1276. [DOI] [PubMed] [Google Scholar]
- 12.Monteith SJ, Starke RM, Jane Ja, Oldfield EH. Use of the histological pseudocapsule in surgery for Cushing disease: rapid postoperative cortisol decline predicting complete tumor resection. Journal of Neurosurgery. 2012;116:721–727. doi: 10.3171/2011.12.JNS11886. [DOI] [PubMed] [Google Scholar]
- 13.Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. Journal of Neurosurgery. 2002;97:1343–1349. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]
- 14.Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM. Transsphenoidal resection in Cushing’s disease: undetectable serum cortisol as the definition of successful treatment. Clinical endocrinology. 1993;38:73–78. doi: 10.1111/j.1365-2265.1993.tb00975.x. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulou C, Schopohl J, Rachinger W, Buchfelder M, Honegger J, Reincke M, Stalla GK. Long-term remission and recurrence rates after first and second transsphenoidal surgery for Cushing’s disease: care reality in the Munich Metropolitan Region. European journal of endocrinology. 2014;170:283–292. doi: 10.1530/EJE-13-0634. [DOI] [PubMed] [Google Scholar]
- 16.Dallapiazza RF, Oldfield EH, Jane JA. Surgical management of Cushing’s disease. Pituitary. 2015;18:211–216. doi: 10.1007/s11102-015-0646-5. [DOI] [PubMed] [Google Scholar]
- 17.Costenaro F, Rodrigues TC, Rollin GAF, Ferreira NP, Czepielewski MA. Evaluation of Cushing’s disease remission after transsphenoidal surgery based on early serum cortisol dynamics. Clinical endocrinology. 2014;80:411–418. doi: 10.1111/cen.12300. [DOI] [PubMed] [Google Scholar]
- 18.Aranda G, Enseñat J, Mora M, Puig-Domingo M, Martínez de Osaba MJ, Casals G, Verger E, Ribalta MT, Hanzu FA, Halperin I. Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary. 2015;18:142–149. doi: 10.1007/s11102-014-0567-8. [DOI] [PubMed] [Google Scholar]
- 19.Alexandraki KI, Kaltsas GA, Isidori AM, Storr HL, Afshar F, Sabin I, Akker SA, Chew SL, Drake WM, Monson JP, Besser GM, Grossman AB. Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. European journal of endocrinology. 2013;168:639–648. doi: 10.1530/EJE-12-0921. [DOI] [PubMed] [Google Scholar]
- 20.Ramm-Pettersen J, Halvorsen H, Evang JA, Rønning P, Hol PK, Bollerslev J, Berg-Johnsen J, Helseth E. Low immediate postoperative serum-cortisol nadir predicts the short-term, but not long-term, remission after pituitary surgery for Cushing’s disease. BMC Endocrine Disorders. 2015;15:62. doi: 10.1186/s12902-015-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hameed N, Yedinak CG, Brzana J, Gultekin SH, Coppa ND, Dogan A, Delashaw JB, Fleseriu M. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary. 2013;16:452–458. doi: 10.1007/s11102-012-0455-z. [DOI] [PubMed] [Google Scholar]
- 22.Nakane T, Kuwayama A, Watanabe M, Takahashi T, Kato T, Ichihara K, Kageyama N. Long term results of transsphenoidal adenomectomy in patients with Cushing’s disease. Neurosurgery. 1987;21:218–222. doi: 10.1227/00006123-198708000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Trousseau H, Endocriniennes M. Monoclonality of Corticotroph Cushing’s Disease*. Journal of Clinical Endocrinology & Metabolism. 1992:472–475. doi: 10.1210/jcem.75.2.1322426. [DOI] [PubMed] [Google Scholar]
- 24.Schulte HM, Oldfield EH, Allolio B, Katz DA, Berkman RA, Ali IU. Clonal Composition of Pituitary Adenomas in Patients with Cushing’s Disease: Determination by X-Chromosome Inactivation Analysis*. The Journal of Clinical Endocrinology & Metabolism. 1991;73:1302–1308. doi: 10.1210/jcem-73-6-1302. [DOI] [PubMed] [Google Scholar]
- 25.Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB, Loriaux DL. Repeat transsphenoidal surgery for Cushing’s disease. Journal of Neurosurgery. 1989;71:520–527. doi: 10.3171/jns.1989.71.4.0520. [DOI] [PubMed] [Google Scholar]
- 26.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Loriaux DL. Petrosal Sinus Sampling with and without Corticotropin-Releasing Hormone for the Differential Diagnosis of Cushing’s Syndrome. New England Journal of Medicine. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 27.Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The Postoperative Basal Cortisol and CRH Tests for Prediction of Long-Term Remission from Cushing’s Disease after Transsphenoidal Surgery. The Journal of Clinical Endocrinology & Metabolism. 2011;96:2057–2064. doi: 10.1210/jc.2011-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu Dabrh AMA, Singh Ospina NM, Nofal AAl, Farah WH, Barrionuevo P, Sarigianni M, Mohabbat AB, Benkhadra K, Carranza Leon BG, Gionfriddo MR, Wang Z, Mohammed K, Ahmed AT, Elraiyah TA, Haydour Q, Alahdab F, Prokop LJ, Murad MH. Predictors of biochemical remissino and recurrence after surgical and radiation treatments of cushing disease: A systematic review and meta-analysis. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016;22:466–475. doi: 10.4158/EP15922.RA. [DOI] [PubMed] [Google Scholar]
- 29.Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB. Predictors of mortality and long-term outcomes in treated Cushing’s disease: a study of 346 patients. The Journal of clinical endocrinology and metabolism. 2013;98:1022–1030. doi: 10.1210/jc.2012-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asuzu D, Chatain GP, Hayes C, Benzo S, McGlotten R, Keil M, Beri A, Sharma ST, Nieman L, Lodish M, Stratakis C, Lonser RR, Oldfield EH, Chittiboina P. Normalized early post-operative cortisol and ACTH values predict nonremission after surgery for Cushing’s disease. The Journal of clinical endocrinology and metabolism. 2017 doi: 10.1210/jc.2016-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK. A Single Midnight Serum Cortisol Measurement Distinguishes Cushing’s Syndrome from Pseudo-Cushing States. The Journal of Clinical Endocrinology & Metabolism. 1998;83:1163–1167. doi: 10.1210/jcem.83.4.4733. [DOI] [PubMed] [Google Scholar]
- 32.Newell-Price J, Trainer P, Perry L, Wass J, Grossman A, Besser M. A single sleeping midnight cortisol has 100% sensitivity for the diagnosis of Cushing’s syndrome. Clinical Endocrinology. 1995;43:545–550. doi: 10.1111/j.1365-2265.1995.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 33.Dichek HL, Nieman LK, Oldfield EH, Pass HI, Malley JD, Cutler GB. A comparison of the standard high dose dexamethasone suppression test and the overnight 8-mg dexamethasone suppression test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. The Journal of clinical endocrinology and metabolism. 1994;78:418–422. doi: 10.1210/jcem.78.2.8106630. [DOI] [PubMed] [Google Scholar]
- 34.Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. The Journal of clinical endocrinology and metabolism. 1993;77:1308–1312. doi: 10.1210/jcem.77.5.8077325. [DOI] [PubMed] [Google Scholar]
- 35.Guignat L, Bertherat J. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline: commentary from a European perspective. European Journal of Endocrinology. 2010;163:9–13. doi: 10.1530/EJE-09-0627. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. Journal of Clinical Endocrinology and Metabolism. 2009;94:2930–2937. doi: 10.1210/jc.2009-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM, Edwards H. The diagnosis of Cushing’s syndrome: An endocrine society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Günes M, Celik O, Kadioglu P. Reliability of the diagnostic tests for Cushing’s syndrome performed in a tertiary referral center. Pituitary. 2013;16:139–145. doi: 10.1007/s11102-012-0387-7. [DOI] [PubMed] [Google Scholar]
- 39.Dwyer AJ, Frank JA, Doppman JL, Oldfield EH, Hickey AM, Cutler GB, Loriaux DL, Schiable TF. Pituitary adenomas in patients with Cushing disease: initial experience with Gd-DTPA-enhanced MR imaging. Radiology. 1987;163:421–426. doi: 10.1148/radiology.163.2.3562821. [DOI] [PubMed] [Google Scholar]
- 40.Doppman JL, Frank JA, Dwyer AJ, Oldfield EH, Miller DL, Nieman LK, Chrousos GP, Cutler GB, Loriaux DL. Gadolinium DTPA enhanced MR imaging of ACTH-secreting microadenomas of the pituitary gland. Journal of computer assisted tomography. 1988;12:728–735. doi: 10.1097/00004728-198809010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. The Journal of clinical endocrinology and metabolism. 2003;88:1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 42.Asuzu D, Chatain GP, Hayes C, Benzo S, McGlotten R, Keil M, Beri A, Sharma ST, Nieman L, Lodish M, Stratakis C, Lonser RR, Oldfield EH, Chittiboina P. Normalized early post-operative cortisol and ACTH values predict nonremission after surgery for Cushing’s disease. The Journal of clinical endocrinology and metabolism. 2017 doi: 10.1210/jc.2016-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK, Grant W, Clinical M, Branch DE, Institutes N. Spoiled Gradient Recalled Acquisition in the Steady State Technique Is Superior to Conventional Postcontrast Spin Echo Technique for Magnetic Resonance Imaging Detection of Adrenocorticotropin- Secreting Pituitary Tumors. Journal of Clinical Endocrinology and Metabolism. 2003;88:1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 44.Welch BL. The generalisation of student’s problems when several different population variances are involved. Biometrika. 1947;34:28–35. doi: 10.1093/biomet/34.1-2.28. [DOI] [PubMed] [Google Scholar]
- 45.Meij BP, Lopes MBS, Ellegala DB, Alden TD, Laws ER. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. Journal of Neurosurgery. 2002;96:195–208. doi: 10.3171/jns.2002.96.2.0195. [DOI] [PubMed] [Google Scholar]
- 46.Hassan-Smith ZK, Sherlock M, Reulen RC, Arlt W, Ayuk J, Toogood Aa, Cooper MS, Johnson AP, Stewart PM. Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. The Journal of clinical endocrinology and metabolism. 2012;97:1194–1201. doi: 10.1210/jc.2011-2957. [DOI] [PubMed] [Google Scholar]
- 47.Rutkowski MJ, Breshears JD, Kunwar S, Aghi MK, Blevins LS. Approach to the postoperative patient with Cushing’s disease. Pituitary. 2015;18:232–237. doi: 10.1007/s11102-015-0644-7. [DOI] [PubMed] [Google Scholar]
- 48.Alwani RA, Herder WWde, Aken MOvan, Berge JHvanden, Delwel EJ, Dallenga AHG, Jong FHDe, Lamberts SWJ, Lely AJvander, Feelders RA. Biochemical Predictors of Outcome of Pituitary Surgery for Cushing’s Disease. Neuroendocrinology. 2010;91:169–178. doi: 10.1159/000258677. [DOI] [PubMed] [Google Scholar]
- 49.Dickerman RD, Oldfield EH. Basis of persistent and recurrent Cushing disease: an analysis of findings at repeated pituitary surgery. Journal of neurosurgery. 2002;97:1343–1349. doi: 10.3171/jns.2002.97.6.1343. [DOI] [PubMed] [Google Scholar]
- 50.Ochedalski T, Rabadan-Diehl C, Aguilera G. Interaction between glucocorticoids and corticotropin releasing hormone (CRH) in the regulation of the pituitary CRH receptor in vivo in the rat. Journal of neuroendocrinology. 1998;10:363–369. doi: 10.1046/j.1365-2826.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 51.Anderson SM, Kant GJ, Souza EBDe. Effects of chronic stress on anterior pituitary and brain corticotropin-releasing factor receptors. Pharmacology, biochemistry, and behavior. 1993;44:755–761. doi: 10.1016/0091-3057(93)90002-b. [DOI] [PubMed] [Google Scholar]
- 52.Udelsman R, Norton JA, Jelenich SE, Goldstein DS, Linehan WM, Loriaux DL, Chrousos GP. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. Journal of Clinical Endocrinology and Metabolism. 1987;64:986–994. doi: 10.1210/jcem-64-5-986. [DOI] [PubMed] [Google Scholar]
- 53.Ram Z, Nieman LK, Cutler GB, Chrousos GP, Doppman JL, Oldfield EH. Early repeat surgery for persistent Cushing’s disease. Journal of Neurosurgery. 1994;80:37–45. doi: 10.3171/jns.1994.80.1.0037. [DOI] [PubMed] [Google Scholar]
- 54.Salassa RM, Laws ER, Carpenter PC, Northcutt RC. Transsphenoidal removal of pituitary microadenoma in Cushing’s disease. Mayo Clinic proceedings. 1978;53:24–28. [PubMed] [Google Scholar]
- 55.Imaki T, Tsushima T, Hizuka N, Odagiri E, Murata Y, Suda T, Takano K. Postoperative plasma cortisol levels predict long-term outcome in patients with Cushing’s disease and determine which patients should be treated with pituitary irradiation after surgery. Endocrine journal. 2001;48:53–62. doi: 10.1507/endocrj.48.53. [DOI] [PubMed] [Google Scholar]
- 56.Yap LB, Turner HE, Adams CBT, Wass JAH. Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: a single centre audit. Clinical endocrinology. 2002;56:25–31. doi: 10.1046/j.0300-0664.2001.01444.x. [DOI] [PubMed] [Google Scholar]
- 57.Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clinical endocrinology. 2005;63:549–559. doi: 10.1111/j.1365-2265.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 58.Acebes JJ, Martino J, Masuet C, Montanya E, Soler J. Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochirurgica. 2007;149:471–477. doi: 10.1007/s00701-007-1133-1. [DOI] [PubMed] [Google Scholar]


