The population is aging rapidly worldwide, which will lead to an increased societal and economic burden of age-associated chronic disease, including cardiovascular diseases (CVD).1, 2 CVD remain the leading cause of morbidity and mortality in developed nations, and chronological age is the primary risk factor for CVD.3 Arterial stiffness and blood pressure (BP) both increase with advancing age4–7 and are independent predictors of CV events and mortality.8, 9 As such, there is strong, ongoing demand for evidence-based strategies that prevent, delay, or reverse age-associated increases in BP and arterial stiffness.10, 11 Indeed, the need for new approaches is expected to grow as the burden of age- and accelerated aging-associated cardiovascular dysfunction and disease continues to rise. In this review, we discuss the concept of healthy vascular aging (HVA) with regard to definition and contributing mechanisms, existing and promising HVA-enhancing lifestyle- and pharmacological-based strategies, and future directions. The focus will be primarily on data from observational and intervention studies in humans.
Components of HVA and Related Implications
Arterial stiffening and increases in BP occur with advancing age,4–7 although population-based studies indicate that this is not an inevitable consequence of aging, but rather results from an industrialized lifestyle.12, 13 The prevalence of hypertension dramatically increases with advancing age, affecting approximately two-thirds of Americans 60 years of age and older.3 Hypertension is also highly prevalent in populations with chronic disease, including chronic kidney disease (CKD) and type 2 diabetes.14, 15 The most recent Joint National Commission (JNC) 8 guidelines increased the BP treatment goal for individuals greater than 60 years of age to <150/90 mmHg, with a goal of <140/90 mmHg in adults 30–59 years of age, including individuals with diabetes and non-diabetic CKD16. However, the recently completed multi-center randomized controlled trial (RCT), the Systolic Blood Pressure Intervention Trial (SPRINT), conducted nationwide in over 9,000 adults17 challenged these guidelines. SPRINT was terminated early as a consequence of a 25% lower risk of the composite endpoint of CV events and death in individuals randomized to intensive BP lowering (systolic BP [SBP] <120 mmHg) compared to standard treatment (SBP <140 mm Hg). Notably, this finding was persistent across sub-groups including CKD and older adults (≥75 years of age).18 Although the technique used for BP measurement in SPRINT has been discussed19, the results of the trial have been influential, as a new report from the American College of Cardiology and American Heart Association Task Force of Clinical Practice Guidelines now defines high blood pressure as ≥130/80 mmHg for all ages.20
Large elastic artery stiffening (i.e., aorta and carotid arteries) also occurs with advancing age and is greater at any age in patients with chronic disease including CKD,21 diabetes,22 and hypertension.23 As a result, these and other clinical disorders featuring such CV changes can be viewed as states of accelerated vascular aging. Multiple techniques exist to assess arterial stiffness, including local distensibility (e.g., ultrasound and tonometry-measured carotid artery compliance), the carotid or aortic augmentation index, aortic distensibility by magnetic resonance imaging, and pulse-wave velocity (assessed between 2 arterial segments), as reviewed elsewhere.24–26 Of note, augmentation index is generally not considered an accurate marker of arterial stiffness as it is strongly influenced by heart rate, height, and contractility, and decreases in older age24, 25. As a result, augmentation index has not been included in the present assessment of the literature. Carotid-femoral pulse-wave velocity (CFPWV) is considered the gold-standard technique, measuring stiffness of the aorta,27 and can be measured by applanation tonometry or Doppler flow recordings. Unlike arterial BP, no formal medical guidelines or targets exist for CFPWV, nor is CFPWV routinely measured clinically; however, both 12 m/sec and 10 m/sec have been suggested as cut-offs for increased risk of CV events.27, 28
Arterial stiffness and BP/hypertension are dynamically interconnected, with each factor influencing the other in a bidirectional manner (Figure 1). Although arterial stiffness was long considered to be a complication of hypertension, there is growing evidence that arterial stiffening can precede the increase in SBP, and that an elevation of SBP further augments arterial stiffness.29–31
Figure 1. Components of healthy vascular aging.
Arterial stiffness and blood pressure/hypertension are dynamically interconnected, with each factor influencing the other in a bidirectional manner. With a shifting profile towards healthy vascular aging, blood pressure is lowered to a non-hypertensive range, and arterial stiffness is also reduced.
Arterial stiffness increases in the aorta and carotid arteries with aging, with a lack of stiffening in the large peripheral muscular arteries, thus reducing peripheral impedance to the forward component of the arterial pulse-wave and increasing pulsatile energy transmission to the microcirculation.32 This increased blood flow and pressure pulsatility can lead to damage of high flow, low impedance organs, including the kidneys and brain.32 Indeed, increases in arterial stiffness are associated with declines in renal function21, 33 and are considered a hallmark of end-stage renal disease.34 CFPWV is also independently associated with cognitive decline,35, 36 consistent with the concept of increased pulsatile energy transmission damaging the brain microcirculation and parenchymal tissues. Additionally, aortic stiffening-associated increases in pressure pulsatility and systolic load promote left ventricular remodeling featuring hypertrophy and dysfunction.37, 38
Recently in this journal, Niiranen et al. demonstrated in a community-dwelling cohort of middle-aged and older (MA/O) adults from the Framingham Heart Study, that HVA was independently associated with lower risk of incident CV events.39 HVA was defined as CFPWV <7.6 m/sec (mean±2 S.D. of a reference group of individuals less than 30 years of age) in combination with absence of hypertension (using the previous guideline SBP/DBP cutoff of 140/90 mmHg). These findings are consistent with evidence that increased CFPWV is an independent predictor of incident CV events and mortality8, 9 and improves prediction over traditional risk factors alone, including blood pressure.8, 40
Building upon the concept of HVA, this review will discuss mechanisms influencing HVA, as well as preventive strategies and therapeutic approaches for preserving/attaining HVA. Of note, very few interventions have achieved HVA in individuals or groups that lack HVA status at baseline when applying the definition employed in the Framingham Heart Study.39 As such, we will include studies that achieved significant CFPWV lowering, with or without changes in BP, even if full restoration of HVA status was not attained. Lastly, although the Framingham Heart Study definition of HVA used SBP and DBP to define BP component of this index, it should be emphasized that mean arterial pressure exerts an important physiologic influence on arterial stiffness41, and must be considered when assessing changes in CFPWV in response to the preventive and treatment strategies discussed below.
Mechanisms Influencing HVA (Figure 2)
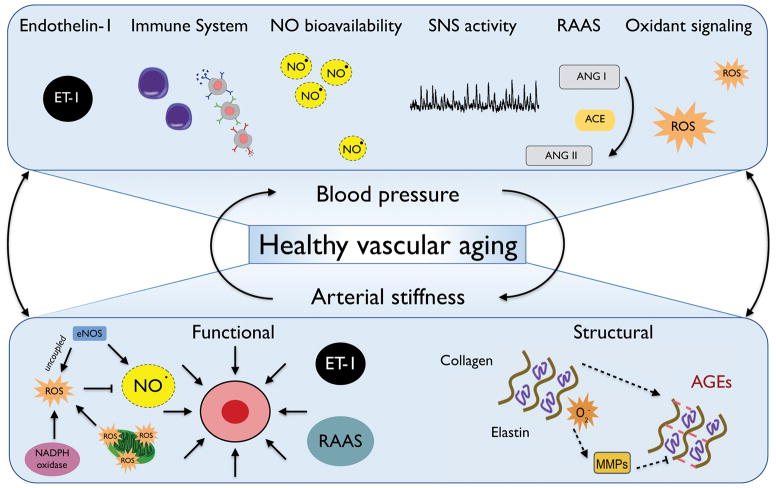
Figure 2. Mechanisms influencing healthy vascular aging.
Mechanisms influencing modulation of blood pressure with aging include vasodilation and vasoconstriction (e.g., nitric oxide [NO] and endothelin-1 [ET-1] bioavailability), immune activation and inflammation, sympathetic nervous system (SNS) activity, renin-angiotensin system (RAAS) activation, and oxidant signaling. Arterial stiffness is modulated by both functional (vascular smooth muscle cell tone) and/or structural components (extracellular matrix remodeling, including elastin degradation by matrix metalloproteinases [MMPs] and the formation of advanced glycation end products [AGEs]).
Modulation of BP with Aging
As the large elastic arteries become stiffer with aging, SBP increases, whereas diastolic BP decreases due to lessening of elastic recoil of the aorta;29, 42 as a result, pulse pressure widens with advancing age.43 Isolated systolic hypertension is the most common form of hypertension in individuals 50 years of age and older.44 Increases in large elastic artery stiffness are a major contributor to these changes in BP with aging, ultimately promoting the development of systolic hypertension.29–31 Age-associated endothelial dysfunction featuring decreased nitric oxide (NO) bioavailability and increased endothelin-1 production, as well as dysregulated vascular tone, further contribute to increased SBP.45, 46 These events are mediated in part by increased oxidative stress associated with excessive superoxide production.47 An interaction between the immune system and hypertension also may be involved, as immune activation and inflammation promoted by oxidative stress are implicated in the development of hypertension.48 Additionally, with advancing age sympathetic nervous system activity increases, and the association between sympathetic nervous system activity and BP becomes stronger, particularly in women.49 Furthermore, chronic activation of the renin angiotensin system promotes target organ damage, including the kidney and heart, as angiotensin II promotes both increased blood pressure as well as reactive oxygen species production.50
Modulation of Arterial Stiffness with Aging
Both functional and structural influences modulate arterial stiffness with aging. Functionally, arterial stiffness is modulated in part by the vasoconstrictor tone produced by the contractile state of vascular smooth muscle cells.42 Age-associated vascular endothelial dysfunction interacts closely with arterial stiffness,51 as endothelial NO synthase (eNOS) uncoupling can promote vascular remodeling and increased arterial stiffness via decreased NO bioavailability,52, 53 which may be exacerbated by oxidative stress.54, 55 Age-associated neurohumoral dysfunction, resulting from decreased sympathetic baroreflex sensitivity and increased sympathetic activation, also promotes arterial stiffness.56 Systemic inflammation, which also increases with aging, may contribute to arterial stiffness via immune activation and the development of hypertension.57
Structurally, extracellular matrix remodeling alters the composition of elastin and collagen in the large elastic arteries with advancing age. The medial layer undergoes elastin fragmentation and degradation,43, 58 which is mediated in part by up-regulation of matrix metalloproteinases (MMPs).59 Collagen deposition occurs, replacing the loss of elastin molecules,43, 58 and accelerated formation of advanced glycation end products (AGEs) occurs, which promote cross-linking of structural proteins and exacerbate increases in arterial stiffness.60 Oxidative stress and inflammation drive these structural changes via vascular damage, smooth muscle cell proliferation, collagen deposition, and arterial remodeling.61, 62 Angiotensin II may also modulate structural contributions to arterial stiffness by stimulating collagen formation, reducing elastin synthesis, and promoting matrix remodeling, in addition to influencing NO-signaling and reactive oxygen species production.63
Not only do changes in the extracellular matrix contribute to arterial stiffness, but intrinsic stiffening of the vascular smooth muscle cells, as measured by atomic force microscopy, also occurs with aging as well as hypertension.64, 65 Of note, intimal-medial thickening occurs with aging even in the absence of atherosclerotic plaques, mediated primarily by thickening of the intima,10 and is positively correlated with CFPWV in older adults.66, 67 Age-associated disease processes including diabetes (via impaired glucose tolerance)68 and CKD (via vascular calcification)69 can further exacerbate arterial stiffness.
It is difficult to separate hypertension and arterial stiffness due to their bidirectional interaction, common mechanisms, and overlapping presence in aging and age-associated disease. Although hypertension can promote aortic stiffening, large elastic artery stiffening may precede and promote an increase in SBP.29, 38 Large elastic artery stiffness is an independent predictor of incident hypertension in multiple longitudinal cohorts.30, 70, 71 Additionally, in rodents fed a high-fat, high sucrose-diet, increased aortic pulse-wave velocity is evident prior to an elevation in SBP.31 Notably, there are some interventions that have reduced arterial stiffness in a manner deemed at least partially BP-independent.72–75 Although, in general, interventions with the most profound influence on CFPWV typically also demonstrate a large SBP-lowering effect, there are examples in which arterial stiffness is reduced without lowering SBP. Of note, most of these latter examples have tended to be in populations without hypertension. Arterial stiffness and BP may be even more tightly intertwined when BP is already elevated.
Lifestyle-Based Strategies to Maintain or Restore HVA
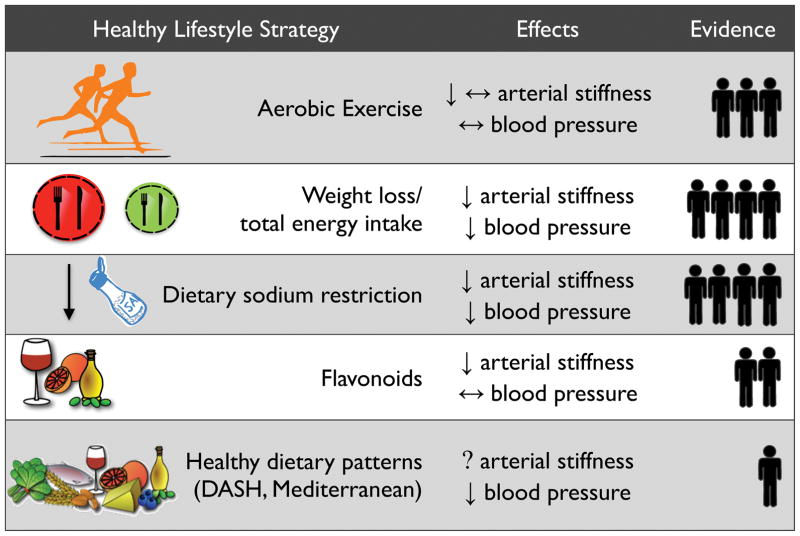
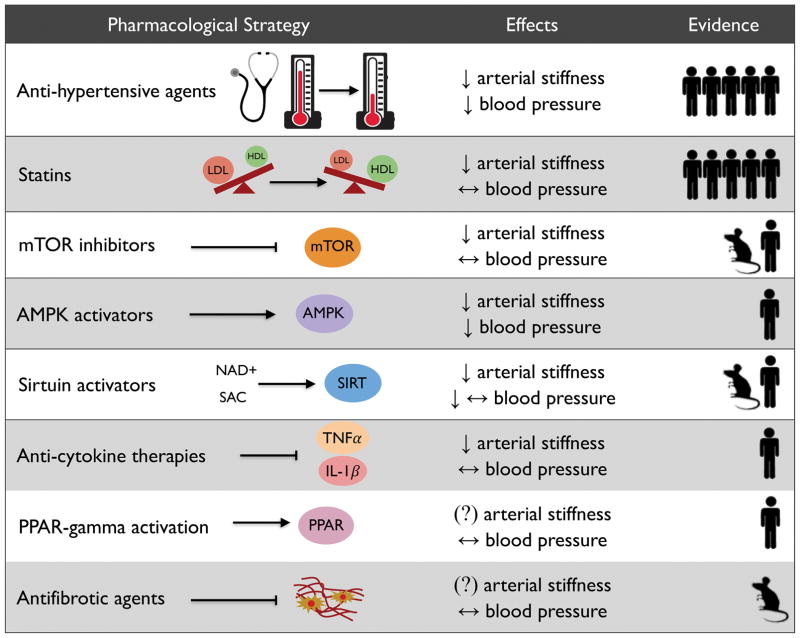
In this section, we will focus on lifestyle-based strategies (aerobic exercise, caloric restriction-based weight loss, and changes in diet composition) with evidence from RCTs demonstrating a reduction in CFPWV, with or without changes in SBP. Using an approach employed previously76, in Figure 3 we summarize current knowledge on the lifestyle-based strategies described below, including a semi-quantitative assessment of the weight of the available evidence for efficacy based on our review of the relevant literature.
Figure 3. Summary of healthy lifestyle-based strategies to maintain or restore healthy vascular aging.
Note: under “Effects”, ↓ represents a reduction, ↔ represents weak or conflicting evidence, and (?) represents a lack of available data for the indicated outcome (for arterial stiffness, this refers specifically to data on carotid-femoral pulse-wave velocity). Under “Evidence”, the human symbol represents clinical evidence and the number of symbols reflects the approximate semi-quantitative weight of evidence available for each strategy based on the authors’ review of the literature. For details, see references/discussion in the text.
Aerobic Exercise
The original observation associating aerobic exercise with HVA is from 1993 in rigorously screened healthy adults (primarily men) who participated in the Baltimore Longitudinal Study of Aging.77 In this cohort, CFPWV was inversely related to maximal oxygen consumption, suggesting that aerobic exercise may attenuate the age-associated increase in arterial stiffness. Subsequently, a similar observation was made in postmenopausal women, even in the presence of normal BP.78
Consistent with these cross-sectional findings, intervention studies conducted in healthy MA/O adults have demonstrated a significant reduction in arterial stiffness with aerobic exercise training. This was first demonstrated as an improvement in carotid artery compliance following a 3-month walking program administered to men,79 and later to postmenopausal women,80 consistent with earlier evidence of reduced arterial stiffness with 4 weeks of exercise training in healthy young sedentary men.81 Although a moderate intensity aerobic exercise intervention of similar duration was later shown to reduce CFPWV in healthy MA/O men82 and women83, the reductions in CFPWV were small and not clearly independent of small decreases in BP. Moreover, no improvement in CFPWV with exercise was observed in a year-long study conducted in healthy older adults84, and similar findings were reported in a group of overweight MA/O adults.85 Overall, the results of these trials suggest that aerobic exercise does not consistently lower SBP in healthy (non-hypertensive) MA/O adults.
The available evidence indicates a lack of efficacy of moderate intensity aerobic exercise for reducing CFPWV in MA/O adults with hypertension,86, 87 although exercise has been reported to reduce CFPWV in young to middle-aged pre-hypertensive and hypertensive adults88. A recent meta-analysis of 14 aerobic exercise trials conducted in pre-hypertensive and hypertensive adults concluded that aerobic exercise does not reduce arterial stiffness, although various indices of arterial stiffness were combined in this analysis.89
The efficacy of an aerobic exercise intervention to reduce arterial stiffness in the setting of age-associated disease is mixed. Although reductions in CFPWV and SBP have been observed with exercise training in adults with metabolic syndrome,90 aerobic exercise has been reported to both lower and have no effect on CFPWV and SBP in MA/O adults with type 2 diabetes.91, 92 Similarly, aerobic exercise does not appear to reduce CFPWV or SBP in patients with moderate to severe CKD,93, 94 although intradialytic exercise (i.e., during a dialysis session) may be efficacious in chronic dialysis patients.95
Overall, aerobic exercise appears to be an evidenced-based public health strategy for maintaining or restoring HVA in the setting of healthy (non-hypertensive) aging and in some diseases associated with accelerated vascular aging, although there are some inconsistencies across studies. The improvements in CFPWV appear at times to be independent of any change in BP, particularly in healthy MA/O adults who are free from hypertension. Of note, in contrast to aerobic exercise, resistance exercise training does not appear to reduce arterial stiffness,96 and intensive resistance exercise training performed without complementary aerobic exercise activities may actually increase CFPWV in young healthy individuals,97 consistent with earlier cross-sectional observations.98 Of note to public health translation, however, are data indicating limited adherence to aerobic exercise in long-term trials99 and in accordance with federal activity guidelines.100
Weight Loss and Total Energy Intake
Short-term (i.e., 3 months or less) caloric restriction-based weight loss administered in MA/O healthy overweight and obese adults significantly reduces CFPWV.101–103 Similar improvements are observed with one year of caloric-restriction based weight loss.104 The SBP-lowering effect in these trials was also notable (between 6–15 mm Hg in individuals free from hypertension at baseline). Caloric restriction-based weight loss is also efficacious for reducing CFPWV when administered in conjunction with other lifestyle interventions. Weight loss from an energy restricted diet plus exercise reduces CFPWV and slightly decreases SBP in young overweight and obese adults.105 In overweight and obese adults with moderately elevated SBP, caloric restriction-based weight loss in conjunction with the Dietary Approaches to Stop Hypertension (DASH) diet reduces both CFPWV and SBP.106 Of note, these improvements may have been mediated, at least in part, by the 30% reduction in sodium intake associated with the diet rather than by weight loss alone. The combination of reduction in total energy intake, exercise, and sodium restriction also has a significant CFPWV- and SBP-lowering effect in young to middle-aged, normotensive, overweight and obese adults.105, 107 Similarly, in adults with type 2 diabetes, the combination of weight loss via energy restriction, exercise, and the weight loss medication Orlistat promotes a profound lowering of CFPWV.108
In contrast to a shorter-term caloric restriction-based weight loss intervention, lifelong caloric restriction is challenging in humans due to adherence and has risk of negative side effects (such as loss of bone density and lean muscle mass observed in the recent 2 year Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy [CALERIE] trial of 25% caloric restriction in non-obese, healthy, younger adults).109 Data in rodents support that lifelong caloric restriction (40% reduction) reduces aortic PWV and SBP.110 Additionally, in a case-control study in MA/O humans, those self-practicing caloric restriction (n=18) for an average of 6 years had substantially lower SBP than age-matched healthy controls consuming a typical American diet, 111 and preliminary data indicate lower CFPWV as well in those practicing dietary restriction (Luigi Fontana, personal communication, 2017).
In summary, caloric restriction-based weight loss interventions have a consistent effect of reducing CFPWV as well as SBP and should be considered an important lifestyle-based strategy to restore or maintain HVA in overweight and obese adults. However, adherence to caloric restriction-based weight loss interventions in longer-term trials112 as well as maintenance of weight loss113 are large challenges, perhaps limiting public health translation. Improvements in HVA status may be mediated in part through modification of dietary components such as dietary sodium, which will be discussed more the subsequent section, or via administration through a combination lifestyle program, such as with exercise. Further evidence is needed regarding the efficacy of this strategy in diseases of accelerated CV aging such as CKD.
Dietary Components and Dietary Patterns
Dietary Sodium Restriction
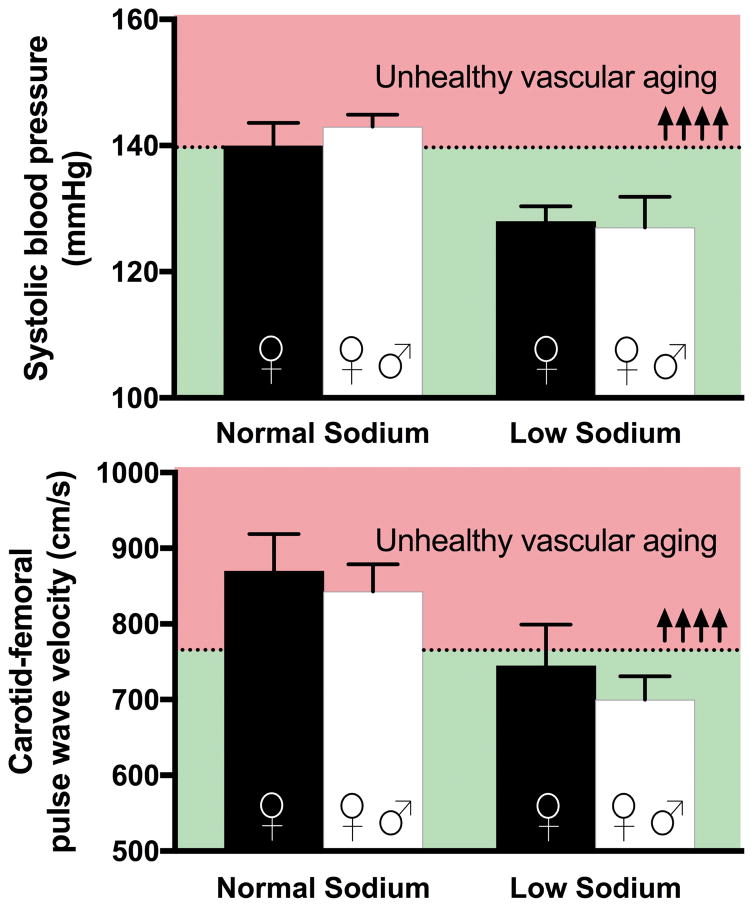
The first observation linking dietary sodium intake to arterial stiffness is a case-control study from 1986, which compared CFPWV in normotensive adults who voluntarily followed a low sodium diet (mean intake 44 mmol/d) for an average of two years to controls with the same mean arterial pressure. CFPWV was substantially lower in MA/O adults who practiced dietary sodium restriction.114 Subsequently, five trials of dietary sodium restriction have been conducted with CFPWV as an endpoint in MA/O, healthy adults of varying SBP (normotensive to hypertensive).87, 115–118 CFPWV was significantly reduced in four of these trials,87, 116–118 and SBP was lowered in all five. Of note, in two of these trials, individuals lacking HVA by the Framingham definition at baseline were restored to HVA-status by dietary sodium restriction (Figure 4).87, 118 The efficacy of this intervention for restoring HVA is further supported by evidence that dietary sodium restriction rapidly improves carotid artery compliance, another index of arterial stiffness, in MA/O adults with moderately elevated SBP.119
Figure 4. Dietary sodium restriction restores healthy vascular aging (HVA).
Changes in systolic blood pressure (SBP) (top panel) and carotid-femoral pulse-wave velocity (CFPWV) (bottom panel) in post-menopausal women (black bars) and post-menopausal women and middle-aged and older men (white bars) with elevated blood pressure in response to a low sodium diet (<90 mmol/d) compared to normal sodium intake (>120 mmol/d). Individuals lacking HVA by the Framingham definition at baseline were restored to healthy vascular aging status by dietary sodium restriction in both studies, as indicated by the reductions in SBP and CFPWV from the red- to the green-shaded zone (above and below the dashed line). Reproduced from87, 183 with permission.
Trials of dietary sodium restriction in populations of accelerated-aging diseases are lacking. One crossover trial of dietary sodium restriction has been conducted in hypertensive patients with stage 3–4 CKD, which demonstrated a non-significant reduction of CFPWV with a strong SBP-lowering effect.120 It also merits mention that sodium intake interacts closely with dietary potassium intake to influence CV risk.121 Evidence regarding the effect of potassium supplementation on CFPWV in healthy adults is mixed,72, 122 and the interactions of dietary sodium and potassium intake on CFPWV warrant additional research. Overall, dietary sodium restriction has a consistent SBP-lowering effect and significantly reduces CFPWV in healthy MA/O adults. Thus, dietary sodium restriction represents an important public health strategy to maintain or restore HVA, although further research is needed in populations with clinical disorders. Despite challenges in adhering to a low sodium diet, policy changes implemented at a national level in Finland support that population-level reductions in dietary sodium intake are possible.123
Flavonoids
Flavonoids are low molecular weight compounds composed of a three-ring structure with various substitutions and are found in abundance in citrus fruits, seeds, olive oil, tea, and red wine.124 Isoflavones are one class of flavonoids, found most often in legumes, including soybeans.125 Administration of isoflavones or an isoflavone metabolite reduces CFPWV in healthy MA/O men and postmenopausal women, with or without altering SBP.74, 126 Flavanones, flavanols, and anthocyanins are other classes of flavonoids124 with evidence of reducing CFPWV.73, 127–129 Grapefruit juice with high flavanones reduces CFPWV without lowering SBP in postmenopausal women with a large abdominal circumference.73 Similarly, cocoa flavanols reduce CFPWV in healthy MA/O men,127 as well as young healthy adults,128 and postmenopausal women with type 2 diabetes,129 along with possible reductions in SBP127, 128. Finally, cranberry juice with anthocyanins and polyphenols reduces CFPWV without changing SBP in MA/O adults with coronary artery disease.75 Thus, there is evidence that flavonoids may reduce CFPWV, with or without changes in SBP. Notably, adverse reactions are rare and flavonoids appear to have an exceptional safety record.124
Dietary Patterns
Specific patterns of dietary intake may modulate HVA. In a longitudinal cohort followed for 27 years, vegetable intake in childhood, as well as persistently high consumption of fruits and vegetable intake across the study period, were independently associated with lower CFPWV in adulthood.130 However, specific evidence on the effect of other dietary patterns such as the Mediterranean or vegetarian diet on CFPWV is currently lacking, although alternate measurements of arterial stiffness suggest that such patterns may lead to improvements.76 In trials implementing dietary patterns including DASH, the Mediterranean diet, and high fruit and vegetable intake, BP is also significantly reduced.131 This topic clearly represents an important and presently understudied area of future research.
Pharmacological-Based Strategies to Maintain or Restore HVA
Numerous pharmacological agents, both those routinely prescribed as well as novel agents, represent potential strategies for maintaining or restoring HVA. Agents that will be discussed in the upcoming sections include antihypertensive agents, statins, mammalian target of rapamycin (mTOR) inhibitors, AMP-activated protein kinase (AMPK) activators, sirtuin activators, anti-cytokine therapies, peroxisome proliferator-activated receptor-γ (PPAR-γ) activators, and antifibrotic agents. In Figure 5, we summarize current knowledge on the pharmacological strategies described below, including a semi-quantitative assessment of the weight of the available evidence for efficacy based on our review of the relevant literature.
Figure 5. Summary of pharmacological-based strategies to maintain or restore healthy vascular aging.
Note: under “Effects”, ↓ represents a reduction, ↔ represents weak or conflicting evidence, and (?) represents a lack of available data for the indicated outcome (for arterial stiffness, this refers specifically to data on carotid-femoral pulse-wave velocity). Under “Evidence”, human and mouse symbol represent clinical and preclinical evidence, respectively, and the number of symbols reflects the approximate semi-quantitative weight of evidence available for each strategy based on the authors’ review of the literature. For details, see references/discussion in the text. mTOR, mammalian target of rapamycin; AMPK, AMP-activated protein kinase; SAC, sirtuin activating compound; TNFα, tumor-necrosis factor-α; IL-1β, interleukin-1 β; PPAR-gamma, peroxisome proliferator-activated receptor-gamma
Antihypertensive Agents and BP Lowering
Trials evaluating the effect of antihypertensive agents on CFPWV have primarily been conducted in individuals with hypertension, although additional evidence is provided from a few studies conducted in healthy volunteers.132 Overall, most antihypertensive agents, including vasodilators133, β-blockers134, 135, calcium channel blockers,136, 137, diuretics,138 and angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB)138–141, appear to have some effect on CFPWV, with the best long-term evidence existing for ACEi/ARB agents. Of note, β-blockers may be less useful, as the slowing of HR can increase pulse pressure and central pressure augmentation.142 Spironolactone also significantly lowers CFPWV in patients with stage 2–3 CKD already on ACEi/ARB with good BP control.143
It may be the degree of SBP-lowering induced that is more important than the medication class regarding the effect on CFPWV. In SPRINT,17 CFPWV was measured in a sub-group of participants in an ancillary study, including a large number of patients with CKD and adults ≥75 years of age. The data are pending, but will provide important evidence regarding the influence of longer-term BP control (regardless of medication class) on arterial stiffness. A small study conducted in non-diabetic, hypertensive older adults suggests that intensive BP control does more effectively reduce CFPWV than standard BP management.144 However, despite well-known benefits of antihypertensive therapies, adherence is often suboptimal, particularly among older adults with multiple co-morbid conditions, and both drug-drug and drug-disease interactions increase the risk of adverse events with advancing age.145
Statins
Numerous trials have assessed the effect of statins (HMG-CoA reductase inhibitors) in CFPWV in MA/O adults with hypercholesterolemia, isolated systolic hypertension, or who are overweight/obese.146–152 With the exception of one trial,151 these studies have consistently reported significant reductions in CFPWV, generally without changing SBP.146, 148–150 The combination of a statin and an ARB also lowers CFPWV in healthy middle-aged men.153 Overall, statins appear quite effective at lowering CFPWV without changing SBP in MA/O adults. Statins have a well-established safety profile, although similar to antihypertensive agents, adherence can be sub-optimal, particularly with advancing age.145 As both antihypertensive agents and statins are commonly prescribed medications with advancing age, they should be considered effective strategies to maintain or restore HVA. This conclusion also emphasizes the importance of considering these effects when studying the efficacy of other interventions in populations taking these agents at baseline.
mTOR Inhibitors, AMPK Activators, and Sirtuin Activators
With advancing age, nutrient sensing pathways including mTOR, AMPK, and sirtuins become dysregulated.154 These pathways are among those modulated by chronic caloric restriction and, therefore, pharmacological manipulation might produce similar CV effects.76, 155 As such, interventions targeting these pathways may help maintain or restore HVA.
In a clinical trial that converted kidney transplant recipients from immunosuppression with cyclosporine A to the mTOR inhibitor sirolimus (both in addition to mycophenolate mofetil), conversion significantly reduced CFPWV, suggesting that mTOR inhibition reduces arterial stiffness.156 BP was also reduced, but may have been mediated by improved renal function and medication adjustments. The reduction in arterial stiffness is consistent with evidence that mTOR inhibition with rapamycin reduces aortic PWV in old mice (although without changing BP).157 However, rapamycin has notable side effects, including the potential for metabolic dysregulation, which may limit its translation as an anti-aging therapy.158 Consequently, safer analogs of rapamycin (rapalogs) are being developed as alternate anti-aging therapies.159
The AMPK activator metformin is another potential novel therapy to maintain or restore HVA. As proof of concept, metformin reduces CFPWV and BP in young women with polycystic ovary syndrome and is also well tolerated,160 thus may also reduce arterial stiffness in other states of impaired AMPK activation, including aging. Finally, sirtuin activators, including resveratrol and NAD+ precursors such as nicotinamide mononucleotide and nicotinamide riboside, are other potential strategies to reduce age-associated arterial stiffness. Resveratrol is a polyphenol found in red wine, grapes and other berries, and activates SIRT1.155 In non-human primates, resveratrol ameliorates high-fat and high-sucrose diet- induced increases in aortic PWV, without changing BP.161 Resveratrol also inhibits the mTOR/S6 kinase pathway.162 Of note, resveratrol may have off-target effects when administered in combination with other healthy lifestyle practices.155 Another potential strategy to augment the age-associated decline in SIRT1 activity is to increase bioavailability of the co-substrate NAD+.163 For example, supplementation with nicotinamide mononucleotide reduces aPWV without changing BP in old mice,164 and supplementation with nicotinamide riboside reduces BP and CFPWV in MA/O adults, particularly those with pre-hypertensive levels of SBP (Martens et al., in revision). However, additional research regarding the efficacy of NAD+ boosting compounds for reducing arterial stiffness in humans is needed, including data on clinical disorders of accelerating CV aging.
Anti-Cytokine Therapies
Anti-cytokine therapies are a potential novel therapeutic to restore HVA. Tumor-necrosis factor-α (TNF-α) antagonism reduces CFPWV without changing BP in chronic inflammatory diseases associated with increased aortic stiffness such as rheumatoid arthritis,165–167 but the potential side effects of anti-cytokine therapies may limit use in healthy aging populations. Of note, in the very recently completed Canakimumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), which enrolled over 10,000 patients with stable coronary artery disease and elevated C-reactive protein levels, the interleukin-1β inhibitor canakinumab significantly reduced risk of major CV events by 15%.168 These results provide initial support for the efficacy of anti-cytokine therapies for treating (and potentially preventing) CV diseases. However, the higher incidence of fatal infection observed with canakinumab may limit translation to a healthy again population.
PPAR- γ Activation
PPAR-γ is a regulator of fatty acid storage and glucose metabolism, and is activated by the thiazolidinedione pioglitazone. Short-term treatment with pioglitazone reduces brachial-ankle PWV in patients with type 2 diabetes169 and carotid-radial PWV in obese men with impaired glucose tolerance,170 without changing BP. However, the effects of these compounds on CFPWV and in the settings of age- and disease-associated arterial stiffening are currently unknown, and potential side effects of weight gain, edema, shortness of breath, and bone fracture need to be considered.171
Antifibrotic Agents
Pirfenidone is an antifibrotic agent that inhibits transforming growth factor-β, TNF-α, and other growth factors, and interferes with matrix formation.172 It is prescribed clinically to treat idiopathic pulmonary fibrosis, and is generally safe with an acceptable side effect profile.173 In a rodent model of diabetes, pirfenidone reverses cardiac fibrosis, attenuates cardiac stiffness, and also reduces renal fibrosis (without changing BP), and thus may hold promise in attenuating age-associated aortic stiffening.174
Overall, it is likely that novel pharmacological agents will have a future role in the treatment of diseases of accelerated vascular aging. Their use in the setting of healthy aging, to maintain or restore HVA, will require a more discerning consideration weighing potential side effects against potential benefits.
Mechanisms of Action
As discussed previously, arterial stiffness and elevated blood pressure share common mechanisms and bidirectional interactions. In general, shorter duration studies are more likely to modulate functional components of arterial stiffness (vascular smooth muscle tone) and to lower blood pressure than to change arterial structure (e.g., collagen or elastin composition), because the latter changes may require a longer-term treatment period (e.g., years) to induce.79 Structural changes may be even more difficult to reverse in disease states such as CKD, which is additionally characterized by medial calcification.175
Lifestyle-Based Strategies
We will focus this section on mechanisms by which lifestyle-based strategies may modulate arterial stiffness rather than blood pressure, and the reader is referred elsewhere for a discussion of the latter.176, 177 Lifestyle-based strategies to maintain or restore HVA appear more likely to influence functional components of arterial stiffness, although it is challenging to discern any structural changes that may occur if such interventions were maintained for a longer duration than typically evaluated in a RCT.
Aerobic exercise likely influences functional components of arterial stiffness, such as increased NO production,85 although long-term aerobic exercise may also influence arterial wall structure, including AGE cross-linking of proteins.178, 179 Indeed, results from preclinical work in mice supports the possibility that aerobic exercise may induce structural changes in the large elastic arteries of older animals, including reductions in collagen I and III, transforming growth factor-β1, and reduced smooth muscle α-actin180, 181.
Collectively, regression analyses in trials of caloric-restriction based weight loss suggest that reductions in arterial stiffness are independent of BP changes. Improvements in stiffness in these studies over a relatively short time period (e.g., 12 weeks) suggests that regulation of smooth muscle tone likely plays a larger role than structural changes. Functional influences on arterial stiffness, including NO production, may be mediated in part by reductions in circulating insulin or changes in other hormones, such as leptin.182
Reductions in arterial stiffness with caloric-restriction based weight loss may also be influenced by changes in diet composition, including dietary sodium restriction. Dietary sodium restriction rapidly improves carotid artery compliance, again suggesting a larger contribution of functional versus structural changes.119 Indeed, dietary sodium restriction both reduces vascular oxidative stress and increases NO bioavailability in humans,183 and rising sodium concentrations increase endothelial cell stiffness measured by atomic force microscopy, while downregulating NO production.184 Reductions in the endogenous Na+/K+ ATPase inhibitor marinobufagenin may also modulate the reductions in CFPWV with dietary sodium restriction.118
At least with shorter-term administration, flavonoids appear to also modulate functional components of arterial stiffness. Isoflavones are vasodilatory, reducing endothelin-1, increasing NO bioavailability, and improving vascular endothelial function.185 Flavanones may also increase NO bioavailability.186 Finally, intake of fruits and vegetables may modulate arterial stiffness via the effects of individual bioactive nutrients and phytochemicals, as well as via reductions in oxidative stress, inflammation, and insulin resistance.187, 188
Pharmacological-Based Strategies
Pharmacological-based strategies to maintain or restore HVA may modulate functional or structural components of arterial stiffness. Antihypertensive agents primarily target the functional (vasoconstrictive) component of arterial stiffness, through a direct modulation of BP.142 However ACEi/ARB may be particularly effective at reducing arterial stiffness, and indeed are more efficacious in the long-term than other antihypertensive agents because they also have antifibrotic effects.189 Statins also modulate smooth muscle tone via increased nitric oxide bioavailability,190 as well as reduced sympathetic neural activity,191 and oxidative stress.192 Metformin promotes eNOS activation by activating AMPK in the endothelium193, and additionally inhibits nuclear factor κ B signaling and decreases inflammation.149 Metformin may also modify arterial stiffness as well as lower BP by promoting weight loss.160
Additional agents modulating functional regulation of arterial stiffness are rapamycin, which activates arterial AMPK and decreases oxidative stress,157 and resveratrol, which increases eNOS activity, reduces superoxide generation by NAD(P)H oxidases, and reduces nuclear factor κ B -mediated inflammation and oxidative stress.161, 194, 195 Little is known regarding underlying mechanisms by which NAD+ precursor may reduce BP and aortic stiffness, but SIRT-1 activation may be involved.164 Anti-cytokine therapies likely lower arterial stiffness via anti-inflammatory effects,166, 167 and PPAR-γ activation also reduces circulating markers of inflammation.169, 170 Pharmacological agents may also target structural components of arterial stiffness, in particular antifibrotic agents.142 Rapamycin also decreases collagen and AGEs in the aorta, suggesting reduced cross-linking of collagens by AGEs with treatment.157
Conclusions and Future Directions
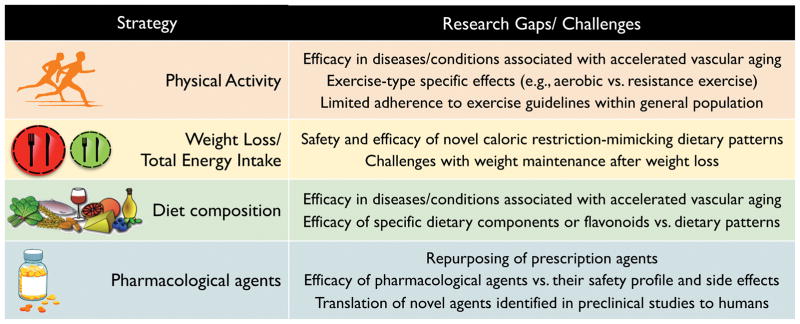
In this review, we have discussed the concept of HVA and contributing mechanisms, while also summarizing lifestyle- and pharmacological-based strategies to maintain or restore HVA in both healthy adults and patients with accelerated CV aging-related clinical disorders. There are notable gaps in the currently available research literature on this topic and practical challenges to implementing these interventions (Figure 6). In particular, there remains an unmet need to translate effective strategies to maintain or restore HVA in the clinic and at the public health level. An example this is the ongoing effort to reduce sodium intake at a population-level through policy statements,196 including government-industry partnerships to reduce sodium intake in several countries including Japan, Finland, and the United Kingdom.197 At the same time, preclinical models should continue to be utilized to discern the mechanisms modulating HVA in both healthy aging and diseased populations (reverse translation).198 Indeed, the combination of forward and reverse translational physiological approaches has been utilized effectively to better understand the mechanisms by which prevention and treatment strategies such as dietary sodium restriction modulate BP and vascular health.198
Figure 6. Current gaps in knowledge related to strategies to maintain or restore healthy vascular aging.
Notable gaps in the currently available literature and challenges to implementing discussed interventions to maintain or restore healthy vascular aging (HVA).
Novel strategies to maintain or restore HVA continue to be developed and tested. Examples of promising lifestyle interventions include inspiratory muscle strength training (breathing against a resistive load), which lowers SBP in both normotensive adults and patients with sleep apnea,199, 200 passive heat therapy, which lowers mean arterial BP and CFPWV even in young healthy adults201, and novel dietary patterns that may mimic the beneficial effects of long-term caloric restriction, including different forms of intermittent fasting.155 New pharmacological agents also continue to be developed, including anti-cytokine therapeutics and anti-senescence drugs. Additionally, a selective sodium-glucose cotransporter inhibitor (empaglifozin) was recently demonstrated to influence properties related to arterial stiffness, while lowering SBP in individuals with type 2 diabetes and established cardiovascular disease, thus may hold promise to maintain or restore HVA.202
Notably, in the Framingham Heart study, only about 1% of individuals over 70 years of age met the criteria for HVA.39 This observation highlights that it is difficult to maintain HVA into older age and that trials testing the efficacy of novel strategies are particularly needed for older adults. The recent SPRINT trial results indicate that this age group can indeed be very responsive to an intervention, contrary to what may have been believed previously.17 This was also the case for populations at high CV risk, including individuals with CKD. Thus, testing of novel interventions to restore HVA are also critically needed in diseases of accelerated CV aging, such as CKD and diabetes. An increased number of cardiovascular risk factors is also associated with greater annual increase in CFPWV, thus likely contributing to the progressive reduction in the prevalence of HVA with advancing age.203 Ultimately, shifting the distribution to a higher number of individuals with HVA status will reduce the burden of CV events and mortality in the population.
Acknowledgments
The authors thank Erzsebet Nagy for her contributions to the figures.
Sources of Funding
This work was supported by the National Heart, Lung and Blood Institute (NHLBI), R01HL134887, National Institutes on Aging (NIA), R01AG013038 and F32AG053009, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K01DK103678 and R01DK094796.
Footnotes
Disclosures
None.
References
- 1.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591. doi: 10.1126/science.1254405. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: Noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEniery CM, Yasmin, Maki-Petaja KM, McDonnell BJ, Munnery M, Hickson SS, Franklin SS, Cockcroft JR, Wilkinson IB. The impact of cardiovascular risk factors on aortic stiffness and wave reflections depends on age: The Anglo-Cardiff Collaborative Rrial (ACCT III) Hypertension. 2010;56:591–597. doi: 10.1161/HYPERTENSIONAHA.110.156950. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 11.Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999;47:613–625. doi: 10.1111/j.1532-5415.1999.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 12.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O’Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 13.Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo indians, a “no-salt” culture. Circulation. 1975;52:146–151. doi: 10.1161/01.cir.52.1.146. [DOI] [PubMed] [Google Scholar]
- 14.Kidney disease: Improving global outcomes (KDIGO) blood pressure work group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 15.Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 17.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. New Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: A randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers MG, Campbell NR. Unfounded concerns about the use of automated office blood pressure measurement in SPRINT. J Am Soc Hypertens. 2016;10:903–905. doi: 10.1016/j.jash.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension. 2017 doi.org/10.1161/HYP.0000000000000065. [Epub ahead of print]
- 21.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45:494–501. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 22.De Angelis L, Millasseau SC, Smith A, Viberti G, Jones RH, Ritter JM, Chowienczyk PJ. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension. 2004;44:67–71. doi: 10.1161/01.HYP.0000130482.81883.fd. [DOI] [PubMed] [Google Scholar]
- 23.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 26.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, task force III: Recommendations for user procedures. Am J Hypertens. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF. Arterial stiffness and hypertension: Chicken or egg? Hypertension. 2014;64:210–214. doi: 10.1161/HYPERTENSIONAHA.114.03449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J Appl Physiol (1985) 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda N, Takei T, Fujiu A, Ogawa T, Nitta K. Arterial stiffness in patients with non-diabetic chronic kidney disease (CKD) J Atheroscler Thromb. 2009;16:57–62. doi: 10.5551/jat.e602. [DOI] [PubMed] [Google Scholar]
- 34.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163–168. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 35.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 36.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–1040. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 37.Tan J, Pei Y, Hua Q, Xing X, Wen J. Aortic pulse wave velocity is associated with measures of subclinical target organ damage in patients with mild hypertension. Cell Biochem Biophys. 2014;70:167–171. doi: 10.1007/s12013-014-9876-9. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13–18. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, correlates, and prognosis of healthy vascular aging in a Western community-dwelling cohort: The Framingham Heart Study. Hypertension. 2017;70:267–274. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 41.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 43.Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: Changes in structure and function. J Am Coll Cardiol. 1987;10:42A–47A. doi: 10.1016/s0735-1097(87)80447-3. [DOI] [PubMed] [Google Scholar]
- 44.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: Analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. doi: 10.1161/01.hyp.37.3.869. [DOI] [PubMed] [Google Scholar]
- 45.Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol. 2008;23:350–355. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35:1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 47.Zalba G, San Jose G, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, Diez J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Saleh MA, Kirabo A, et al. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J Clin Invest. 2016;126:50–67. doi: 10.1172/JCI80761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- 50.Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension. 2012;60:878–883. doi: 10.1161/HYPERTENSIONAHA.110.155895. [DOI] [PubMed] [Google Scholar]
- 51.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 52.Oelze M, Kroller-Schon S, Steven S, et al. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 53.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased nos phosphorylation contributes to age-related vascular stiffness. J Appl Physiol (1985) 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 54.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 55.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 56.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, Vongpatanasin W, Levine BD, Fu Q. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59:98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension. 2013;62:226–230. doi: 10.1161/HYPERTENSIONAHA.113.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Z. Aging, arterial stiffness, and hypertension. Hypertension. 2015;65:252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gertontol A Biol Sc Med Sci. 2010;65:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep. 2000;2:98–105. doi: 10.1007/s11906-000-0066-3. [DOI] [PubMed] [Google Scholar]
- 62.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: Roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 63.Lyle AN, Raaz U. Killing me unsoftly: Causes and mechanisms of arterial stiffness. Aterioscler Thromb Vasc Biol. 2017;37:e1–e11. doi: 10.1161/ATVBAHA.116.308563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sehgel NL, Sun Z, Hong Z, Hunter WC, Hill MA, Vatner DE, Vatner SF, Meininger GA. Augmented vascular smooth muscle cell stiffness and adhesion when hypertension is superimposed on aging. Hypertension. 2015;65:370–377. doi: 10.1161/HYPERTENSIONAHA.114.04456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 67.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: A subgroup of the Cardiovascular Health Study. Am J Hypertens. 2002;15:16–23. doi: 10.1016/s0895-7061(01)02228-2. [DOI] [PubMed] [Google Scholar]
- 68.Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non-insulin-dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes: The ARIC study. Circulation. 1995;91:1432–1443. doi: 10.1161/01.cir.91.5.1432. [DOI] [PubMed] [Google Scholar]
- 69.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008;23:586–593. doi: 10.1093/ndt/gfm660. [DOI] [PubMed] [Google Scholar]
- 70.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45:426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 71.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55:681–688. doi: 10.1161/HYPERTENSIONAHA.109.147488. [DOI] [PubMed] [Google Scholar]
- 73.Habauzit V, Verny MA, Milenkovic D, Barber-Chamoux N, Mazur A, Dubray C, Morand C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: A randomized, controlled, crossover trial. Am J Clin Nutr. 2015;102:66–74. doi: 10.3945/ajcn.114.104646. [DOI] [PubMed] [Google Scholar]
- 74.Teede HJ, McGrath BP, DeSilva L, Cehun M, Fassoulakis A, Nestel PJ. Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arterioscler Thromb Vasc Biol. 2003;23:1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- 75.Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93:934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaRocca TJ, Martens CR, Seals DR. Nutrition and other lifestyle influences on arterial aging. Ageing Res Rev. 2017;39:106–119. doi: 10.1016/j.arr.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 78.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 80.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 81.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 82.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol. 2005;55:235–239. doi: 10.2170/jjphysiol.S2116. [DOI] [PubMed] [Google Scholar]
- 83.Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32–59 years. Br J Sports Med. 2009;43:615–618. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- 84.Oudegeest-Sander MH, Olde Rikkert MG, Smits P, Thijssen DH, van Dijk AP, Levine BD, Hopman MT. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: A randomized factorial design trial. Exp Gerontol. 2013;48:1509–1517. doi: 10.1016/j.exger.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kearney TM, Murphy MH, Davison GW, O’Kane MJ, Gallagher AM. Accumulated brisk walking reduces arterial stiffness in overweight adults: Evidence from a randomized control trial. J Am Soc Hypertens. 2014;8:117–126. doi: 10.1016/j.jash.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Ferrier KE, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38:222–226. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 87.Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: Role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 88.Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens. 2008;22:678–686. doi: 10.1038/jhh.2008.36. [DOI] [PubMed] [Google Scholar]
- 89.Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: A systematic review and meta-analysis. Int J Cardiol. 2014;173:361–368. doi: 10.1016/j.ijcard.2014.03.072. [DOI] [PubMed] [Google Scholar]
- 90.Donley DA, Fournier SB, Reger BL, DeVallance E, Bonner DE, Olfert IM, Frisbee JC, Chantler PD. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 2014;116:1396–1404. doi: 10.1152/japplphysiol.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Aerobic training-induced improvements in arterial stiffness are not sustained in older adults with multiple cardiovascular risk factors. J Hum Hypertens. 2013;27:335–339. doi: 10.1038/jhh.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32:1531–1535. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Headley S, Germain M, Wood R, Joubert J, Milch C, Evans E, Poindexter A, Cornelius A, Brewer B, Pescatello LS, Parker B. Short-term aerobic exercise and vascular function in CKD stage 3: A randomized controlled trial. Am J Kidney Dis. 2014;64:222–229. doi: 10.1053/j.ajkd.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol. 2013;8:1494–1501. doi: 10.2215/CJN.10141012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toussaint ND, Polkinghorne KR, Kerr PG. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int. 2008;12:254–263. doi: 10.1111/j.1542-4758.2008.00262.x. [DOI] [PubMed] [Google Scholar]
- 96.Miyachi M. Effects of resistance training on arterial stiffness: A meta-analysis. Br J Sports Med. 2013;47:393–396. doi: 10.1136/bjsports-2012-090488. [DOI] [PubMed] [Google Scholar]
- 97.Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP, Beckwith KA, Todd JS, Tanaka H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am J Hypertens. 2005;18:930–934. doi: 10.1016/j.amjhyper.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 98.Bertovic DA, Waddell TK, Gatzka CD, Cameron JD, Dart AM, Kingwell BA. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension. 1999;33:1385–1391. doi: 10.1161/01.hyp.33.6.1385. [DOI] [PubMed] [Google Scholar]
- 99.Saida T, Juul Sorensen T, Langberg H. Long-term exercise adherence after public health training in at-risk adults. Ann Phys Rehabil Med. 2017;60:237–243. doi: 10.1016/j.rehab.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 100.Ward BW, Clarke TC, Nugent NC, Schiller JS. Early release of selected estimates based on data from the 2015 National Health Interview Survey. National Center for Health Statistics. 2016 May; Available from: http://www.cdc.gov/nchs/nhis.htm.
- 101.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55:855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keogh JB, Brinkworth GD, Noakes M, Belobrajdic DP, Buckley JD, Clifton PM. Effects of weight loss from a very-low-carbohydrate diet on endothelial function and markers of cardiovascular disease risk in subjects with abdominal obesity. Am J Clin Nutr. 2008;87:567–576. doi: 10.1093/ajcn/87.3.567. [DOI] [PubMed] [Google Scholar]
- 103.Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and fmd using two low-fat diets. Int J Obes (Lond) 2005;29:1445–1451. doi: 10.1038/sj.ijo.0803039. [DOI] [PubMed] [Google Scholar]
- 104.Wycherley TP, Brinkworth GD, Keogh JB, Noakes M, Buckley JD, Clifton PM. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010;267:452–461. doi: 10.1111/j.1365-2796.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 105.Cooper JN, Buchanich JM, Youk A, Brooks MM, Barinas-Mitchell E, Conroy MB, Sutton-Tyrrell K. Reductions in arterial stiffness with weight loss in overweight and obese young adults: Potential mechanisms. Atherosclerosis. 2012;223:485–490. doi: 10.1016/j.atherosclerosis.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Arch Intern Med. 2010;170:126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol. 2012;11:114. doi: 10.1186/1475-2840-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barinas-Mitchell E, Kuller LH, Sutton-Tyrrell K, Hegazi R, Harper P, Mancino J, Kelley DE. Effect of weight loss and nutritional intervention on arterial stiffness in type 2 diabetes. Diabetes Care. 2006;29:2218–2222. doi: 10.2337/dc06-0665. [DOI] [PubMed] [Google Scholar]
- 109.Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV, Group CS. Effect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: A randomized clinical trial. J Bone MIner Res. 2016;31:40–51. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 113.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 114.Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O’Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166–169. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 115.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009;89:485–490. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 116.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54:482–488. doi: 10.1161/HYPERTENSIONAHA.109.133223. [DOI] [PubMed] [Google Scholar]
- 117.Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010;91:557–564. doi: 10.3945/ajcn.2009.28645. [DOI] [PubMed] [Google Scholar]
- 118.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DR. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;8:1952–1959. doi: 10.2215/CJN.00900113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 120.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–2103. doi: 10.1681/ASN.2013030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McDonough AA, Veiras LC, Guevara CA, Ralph DL. Cardiovascular benefits associated with higher dietary K+ vs.lower dietary Na+: Evidence from population and mechanistic studies. Am J Physiol Endocrinol Metab. 2017;312:E348–E356. doi: 10.1152/ajpendo.00453.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matthesen SK, Larsen T, Vase H, Lauridsen TG, Pedersen EB. Effect of potassium supplementation on renal tubular function, ambulatory blood pressure and pulse wave velocity in healthy humans. Scand J Clin Lab Invest. 2012;72:78–86. doi: 10.3109/00365513.2011.635216. [DOI] [PubMed] [Google Scholar]
- 123.Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 124.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 125.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 126.Nestel P, Fujii A, Zhang L. An isoflavone metabolite reduces arterial stiffness and blood pressure in overweight men and postmenopausal women. Atherosclerosis. 2007;192:184–189. doi: 10.1016/j.atherosclerosis.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 127.Heiss C, Sansone R, Karimi H, Krabbe M, Schuler D, Rodriguez-Mateos A, Kraemer T, Cortese-Krott MM, Kuhnle GG, Spencer JP, Schroeter H, Merx MW, Kelm M. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age (Dordr) 2015;37:9794. doi: 10.1007/s11357-015-9794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grassi D, Desideri G, Necozione S, di Giosia P, Barnabei R, Allegaert L, Bernaert H, Ferri C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J Hypertens. 2015;33:294–303. doi: 10.1097/HJH.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 129.Curtis PJ, Potter J, Kroon PA, Wilson P, Dhatariya K, Sampson M, Cassidy A. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: A double-blind randomized controlled trial. Am J Clin Nutr. 2013;97:936–942. doi: 10.3945/ajcn.112.043745. [DOI] [PubMed] [Google Scholar]
- 130.Aatola H, Koivistoinen T, Hutri-Kahonen N, Juonala M, Mikkila V, Lehtimaki T, Viikari JS, Raitakari OT, Kahonen M. Lifetime fruit and vegetable consumption and arterial pulse wave velocity in adulthood: The Cardiovascular Risk in Young Finns Study. Circulation. 2010;122:2521–2528. doi: 10.1161/CIRCULATIONAHA.110.969279. [DOI] [PubMed] [Google Scholar]
- 131.Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2016;7:76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asmar R. Effect of antihypertensive agents on arterial stiffness as evaluated by pulse wave velocity: Clinical implications. Am J Cardiovasc Drugs. 2001;1:387–397. doi: 10.2165/00129784-200101050-00008. [DOI] [PubMed] [Google Scholar]
- 133.Kahonen M, Ylitalo R, Koobi T, Turjanmaa V, Ylitalo P. Influence of captopril, propranolol, and verapamil on arterial pulse wave velocity and other cardiovascular parameters in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:483–489. [PubMed] [Google Scholar]
- 134.Kelly R, Daley J, Avolio A, O’Rourke M. Arterial dilation and reduced wave reflection. Benefit of dilevalol in hypertension. Hypertension. 1989;14:14–21. doi: 10.1161/01.hyp.14.1.14. [DOI] [PubMed] [Google Scholar]
- 135.Asmar RG, Kerihuel JC, Girerd XJ, Safar ME. Effect of bisoprolol on blood pressure and arterial hemodynamics in systemic hypertension. Am J Cardiol. 1991;68:61–64. doi: 10.1016/0002-9149(91)90711-s. [DOI] [PubMed] [Google Scholar]
- 136.Tedeschi C, Guarini P, Giordano G, Messina V, Cicatiello AM, Iovino L, Tagliamonte MR. Effects of nicardipine on intimal-medial thickness and arterial distensibility in hypertensive patients. Preliminary results after 6 months. Int Angiol. 1993;12:344–347. [PubMed] [Google Scholar]
- 137.Asmar RG, Benetos A, Chaouche-Teyara K, Raveau-Landon CM, Safar ME. Comparison of effects of felodipine versus hydrochlorothiazide on arterial diameter and pulse-wave velocity in essential hypertension. Am J Cardiol. 1993;72:794–798. doi: 10.1016/0002-9149(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 138.Benetos A, Cambien F, Gautier S, Ricard S, Safar M, Laurent S, Lacolley P, Poirier O, Topouchian J, Asmar R. Influence of the angiotensin ii type 1 receptor gene polymorphism on the effects of perindopril and nitrendipine on arterial stiffness in hypertensive individuals. Hypertension. 1996;28:1081–1084. doi: 10.1161/01.hyp.28.6.1081. [DOI] [PubMed] [Google Scholar]
- 139.Benetos A, Asmar R, Vasmant D, Thiery P, Safar M. Long lasting arterial effects of the ace inhibitor ramipril. J Hum Hypertens. 1991;5:363–368. [PubMed] [Google Scholar]
- 140.Heesen WF, Beltman FW, Smit AJ, May JF, de Graeff PA, Muntinga JH, Havinga TK, Schuurman FH, van der Veur E, Meyboom-de Jong B, Lie KI. Reversal of pathophysiologic changes with long-term lisinopril treatment in isolated systolic hypertension. J Cardiovasc Pharmacol. 2001;37:512–521. doi: 10.1097/00005344-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell GF, Dunlap ME, Warnica W, Ducharme A, Arnold JM, Tardif JC, Solomon SD, Domanski MJ, Jablonski KA, Rice MM, Pfeffer MA. Long-term trandolapril treatment is associated with reduced aortic stiffness: The prevention of events with angiotensin-converting enzyme inhibition hemodynamic substudy. Hypertension. 2007;49:1271–1277. doi: 10.1161/HYPERTENSIONAHA.106.085738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 143.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: A randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 144.Ichihara A, Hayashi M, Koura Y, Tada Y, Hirota N, Saruta T. Long-term effects of intensive blood-pressure lowering on arterial wall stiffness in hypertensive patients. Am J Hypertens. 2003;16:959–965. doi: 10.1016/s0895-7061(03)01004-5. [DOI] [PubMed] [Google Scholar]
- 145.Fleg JL, Aronow WS, Frishman WH. Cardiovascular drug therapy in the elderly: Benefits and challenges. Nat Rev Cardiol. 2011;8:13–28. doi: 10.1038/nrcardio.2010.162. [DOI] [PubMed] [Google Scholar]
- 146.Muramatsu J, Kobayashi A, Hasegawa N, Yokouchi S. Hemodynamic changes associated with reduction in total cholesterol by treatment with the HMG-CoA reductase inhibitor pravastatin. Atherosclerosis. 1997;130:179–182. doi: 10.1016/s0021-9150(96)06024-8. [DOI] [PubMed] [Google Scholar]
- 147.Orr JS, Dengo AL, Rivero JM, Davy KP. Arterial destiffening with atorvastatin in overweight and obese middle-aged and older adults. Hypertension. 2009;54:763–768. doi: 10.1161/HYPERTENSIONAHA.109.138248. [DOI] [PubMed] [Google Scholar]
- 148.Pirro M, Schillaci G, Mannarino MR, Savarese G, Vaudo G, Siepi D, Paltriccia R, Mannarino E. Effects of rosuvastatin on 3-nitrotyrosine and aortic stiffness in hypercholesterolemia. Nutr Metab Cardiovasc Dis. 2007;17:436–441. doi: 10.1016/j.numecd.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 149.Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN. Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens. 2013;26:608–616. doi: 10.1093/ajh/hps098. [DOI] [PubMed] [Google Scholar]
- 150.Ichihara A, Hayashi M, Koura Y, Tada Y, Kaneshiro Y, Saruta T. Long-term effects of statins on arterial pressure and stiffness of hypertensives. J Hum Hypertens. 2005;19:103–109. doi: 10.1038/sj.jhh.1001786. [DOI] [PubMed] [Google Scholar]
- 151.Raison J, Rudnichi A, Safar ME. Effects of atorvastatin on aortic pulse wave velocity in patients with hypertension and hypercholesterolaemia: A preliminary study. J Hum Hypertens. 2002;16:705–710. doi: 10.1038/sj.jhh.1001470. [DOI] [PubMed] [Google Scholar]
- 152.Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 153.Lunder M, Janic M, Jug B, Sabovic M. The effects of low-dose fluvastatin and valsartan combination on arterial function: A randomized clinical trial. Eur J Intern Med. 2012;23:261–266. doi: 10.1016/j.ejim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 154.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594:7177–7195. doi: 10.1113/JP272348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Joannides R, Monteil C, de Ligny BH, Westeel PF, Iacob M, Thervet E, Barbier S, Bellien J, Lebranchu Y, Seguin SG, Thuillez C, Godin M, Etienne I. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant. 2011;11:2414–2422. doi: 10.1111/j.1600-6143.2011.03697.x. [DOI] [PubMed] [Google Scholar]
- 157.Lesniewski LA, Seals DR, Walker AE, Henson GD, Blimline MW, Trott DW, Bosshardt GC, LaRocca TJ, Lawson BR, Zigler MC, Donato AJ. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16:17–26. doi: 10.1111/acel.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 2011;6:125–129. doi: 10.1007/s11523-011-0174-9. [DOI] [PubMed] [Google Scholar]
- 159.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: A randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95:722–730. doi: 10.1210/jc.2009-1985. [DOI] [PubMed] [Google Scholar]
- 161.Mattison JA, Wang M, Bernier M, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metabolism. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and ageing. Diabetes Obes Metab. 2013;15(Suppl 3):26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wong M, Oakley SP, Young L, Jiang BY, Wierzbicki A, Panayi G, Chowienczyk P, Kirkham B. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1277–1284. doi: 10.1136/ard.2007.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: A controlled study. Hypertension. 2010;55:333–338. doi: 10.1161/HYPERTENSIONAHA.109.143982. [DOI] [PubMed] [Google Scholar]
- 167.Maki-Petaja KM, Hall FC, Booth AD, Wallace SM, Yasmin, Bearcroft PW, Harish S, Furlong A, McEniery CM, Brown J, Wilkinson IB. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 168.Ridke PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med. 2017 doi: 10.1056/NEJMoa1707914. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 169.Satoh N, Ogawa Y, Usui T, Tagami T, Kono S, Uesugi H, Sugiyama H, Sugawara A, Yamada K, Shimatsu A, Kuzuya H, Nakao K. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care. 2003;26:2493–2499. doi: 10.2337/diacare.26.9.2493. [DOI] [PubMed] [Google Scholar]
- 170.Ryan KE, McCance DR, Powell L, McMahon R, Trimble ER. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–130. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 171.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. New Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kania DS, Smith CT, Nash CL, Gonzalvo JD, Bittner A, Shepler BM. Potential new treatments for diabetic kidney disease. Med Clin North Am. 2013;97:115–134. doi: 10.1016/j.mcna.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 173.King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. New Eng J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 174.Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jablonski KL, Chonchol M. Vascular calcification in end-stage renal disease. Hemodial Int. 2013;17(Suppl 1):S17–21. doi: 10.1111/hdi.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, American Heart A. Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 177.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, Fuchs FD, Hughes JW, Lackland DT, Staffileno BA, Townsend RR, Rajagopalan S. Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the American Heart Association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 178.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol (1985) 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: Reversal by aerobic exercise. J Physiol. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: Mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- 182.Rider OJ, Tayal U, Francis JM, Ali MK, Robinson MR, Byrne JP, Clarke K, Neubauer S. The effect of obesity and weight loss on aortic pulse wave velocity as assessed by magnetic resonance imaging. Obesity (Silver Spring) 2010;18:2311–2316. doi: 10.1038/oby.2010.64. [DOI] [PubMed] [Google Scholar]
- 183.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 186.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5:492–499. doi: 10.1007/s11883-003-0040-z. [DOI] [PubMed] [Google Scholar]
- 188.Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, Hong CP, Sinaiko AR. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Boutouyrie P, Lacolley P, Briet M, Regnault V, Stanton A, Laurent S, Mahmud A. Pharmacological modulation of arterial stiffness. Drugs. 2011;71:1689–1701. doi: 10.2165/11593790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 190.John S, Schlaich M, Langenfeld M, Weihprecht H, Schmitz G, Weidinger G, Schmieder RE. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: A randomized, placebo-controlled, double-blind study. Circulation. 1998;98:211–216. doi: 10.1161/01.cir.98.3.211. [DOI] [PubMed] [Google Scholar]
- 191.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Simvastatin therapy normalizes sympathetic neural control in experimental heart failure: Roles of angiotensin II type 1 receptors and NAD(P)H oxidase. Circulation. 2005;112:1763–1770. doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 192.Wang J, Xu J, Zhou C, Zhang Y, Xu D, Guo Y, Yang Z. Improvement of arterial stiffness by reducing oxidative stress damage in elderly hypertensive patients after 6 months of atorvastatin therapy. J Clin Hypertens (Greenwich) 2012;14:245–249. doi: 10.1111/j.1751-7176.2012.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- 194.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 195.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation. 2011;123:1138–1143. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 197.Mohan S, Campbell NR, Willis K. Effective population-wide public health interventions to promote sodium reduction. CMAJ. 2009;181:605–609. doi: 10.1503/cmaj.090361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Seals DR. Translational physiology: From molecules to public health. J Physiol. 2013;591:3457–3469. doi: 10.1113/jphysiol.2013.253195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vranish JR, Bailey EF. Daily respiratory training with large intrathoracic pressures, but not large lung volumes, lowers blood pressure in normotensive adults. Respir Physiol Neurobiol. 2015;216:63–69. doi: 10.1016/j.resp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 200.Vranish JR, Bailey EF. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep. 2016;39:1179–1185. doi: 10.5665/sleep.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594:5329–5342. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, Schmieder RE. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–1169. doi: 10.1161/CIRCULATIONAHA.117.029529. [DOI] [PubMed] [Google Scholar]
- 203.Terentes-Printzios D, Vlachopoulos C, Xaplanteris P, Ioakeimidis N, Aznaouridis K, Baou K, Kardara D, Georgiopoulos G, Georgakopoulos C, Tousoulis D. Cardiovascular risk factors accelerate progression of vascular aging in the general population results from the CRAVE study (Cardiovascular Risk Factors Affecting Vascular Age) Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.117.09633. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]