Abstract
Ionizing radiation (IR) is commonly used to treat central nervous system (CNS) cancers and metastases. While IR promotes remission, frequent side effects including impaired cognition and white matter loss occur following treatment. Fractionation is used to minimize these CNS late side effects, as it reduces IR effects in differentiated normal tissue, but not rapidly proliferating normal or tumor tissue. However, side effects occur even with the use of fractionated paradigms. Oligodendrocyte progenitor cells (OPCs) are a proliferative population within the CNS affected by radiation. We hypothesized that fractionated radiation would lead to OPC loss, which could contribute to the delayed white matter loss seen after radiation exposure. We found that fractionated IR induced a greater early loss of OPCs than an equivalent single dose exposure. Furthermore, OPC recovery was impaired following fractionated IR. Finally, reduced OPC differentiation and mature oligodendrocyte numbers occurred in single dose and fractionated IR paradigms. This work demonstrates that fractionation does not spare normal brain tissue and, importantly, highlights the sensitivity of OPCs to fractionated IR, suggesting that fractionated schedules may promote white matter dysfunction, a point that should be considered in radiotherapy.
Keywords: ionizing radiation, white matter, myelin, proliferation, apoptosis
Introduction
Radiation therapy is considered the primary non-surgical treatment for cancer; however the use of ionizing radiation (IR) is often limited by adverse effects in surrounding normal tissue (Barnett et al. 2009). With over 14.5 million U.S. citizens living with cancer diagnoses, either current or in remission (www.cancer.gov/about-cancer/what-is-cancer/statistics), there is ongoing interest in enhancing the therapeutic ratio, which is the ratio of cancer control to associated toxicity. To reduce side effects and increase radiosensitivity of cancer cells, therapeutic radiation is often given using small doses administered daily over the course of several weeks rather than as a large bolus dose (Nambiar et al. 2011). This fractionated radiation is considered beneficial to late responding tissues, which are comprised primarily of differentiated cells. Because radiation induced cell death occurs during proliferation, differentiated cells are largely spared.
The central nervous system (CNS) is considered a late responding tissue due to its constitutive populations of post-mitotic cells. In agreement with this classification, there is often a delay of months to years before progressive and irreversible side effects consisting of IR-induced tissue necrosis, demyelination, and cognitive impairment occur (Armstrong et al. 2004; Kureshi et al. 1994). In humans, impaired attention and memory, changes in personality, and white matter loss are common despite the use of fractionation (Correa et al. 2004; Harder et al. 2004). Although the brain has historically been treated as a late responding tissue in radiotherapy paradigms, this may not be entirely appropriate. In addition to post-mitotic cells, there are a number of CNS stem and progenitor populations overlooked in this assumption. Neurogenesis occurs in specific regions through adulthood in humans and mice (Rivera et al. 2013; Spalding et al. 2013). Microglia proliferate in cases of disease and injury (Gomez-Nicola et al. 2013; Li et al. 2013). Relevant to this study, oligodendrocyte progenitor cells (OPCs) proliferate, differentiate into oligodendrocytes, and myelinate throughout life (Dimou and Gallo 2015; Psachoulia et al. 2009; Rivers et al. 2008), making them susceptible to radiation insult. Acute OPC loss occurs in the murine CNS after radiation exposure (Chari et al. 2006; Irvine and Blakemore 2007). Importantly, reduced OPCs have also been noted in patients who received IR as part of their cancer treatment (Panagiotakos et al. 2007). While the OPC cell cycle is relatively long in adult mice, between 10 and 37 days, insult reduces OPC cell cycle length (Simon et al. 2011). Because fractionated radiation is known to modify cell cycle and promote synchrony, OPC dynamics may be altered with fractionated radiation (Pajonk et al. 2010). Additionally, while only half of adult OPCs may be cycling at any given time (Psachoulia et al. 2009), they all have the ability to divide (Kang et al. 2010); therefore, fractionation may also recruit non-cycling cells into the cell cycle to enhance OPC loss (Pajonk et al. 2010).
In addition to OPC depletion, maturation of OPCs and subsequent myelination may be affected by radiation exposure. An IR-induced DNA damage stress response can lead to cellular senescence (Campisi and d'Adda di Fagagna 2007; Suzuki and Boothman 2008); this may result in OPCs incapable of proliferation or maturation. The number of myelinated axons increases into adulthood in rats (Nunez et al. 2000; Yates and Juraska 2007); in addition to this de novo myelination, new myelin production is necessary to replace deteriorated myelin. Because oligodendrocyte and myelin turnover is slow, impairments in maturation may become obvious only after a prolonged period of time, perhaps similar to the latent period between radiation exposure and white matter late effects.
Because fractionated paradigms do not prevent white matter loss and OPCs are radiosensitive, we have investigated the effects of fractionated versus single dose irradiation on the oligodendrocyte lineage. To determine whether fractionation alters the acute response and fate of OPCs to radiation exposure, we induced yellow fluorescent protein (YFP) expression in Pdgfrα-CreERT2:Rosa26R-YFP mice, exposed them to single dose and fractionated radiation, and examined responses at time points ranging from 8 hours to 18 months from exposure. By directly comparing single dose and fractionated radiation paradigms, we were able to determine whether fractionation provides a benefit to normal OPCs and their progeny.
Methods
Animals
All animal procedures were approved by the University of Rochester Medical Center Committee on Animal Resources prior to experimentation. Pdgfrα-CreERT2:Rosa26R-YFP mice, developed by William D. Richardson, were used (Rivers et al. 2008). Mice were housed in temperature (23 ± 3°C) and light (12:12 light:dark) controlled rooms with free access to chow and water.
Tamoxifen Injections
Animals were injected intraperitoneally (i.p.) daily with tamoxifen (Sigma-Aldrich #T5648, St. Louis, MO) dissolved in 10% EtOH/90% sunflower seed oil (Sigma-Aldrich #S5007, St. Louis, MO) for 5 days at 6–8 weeks of age (Lagace et al. 2007; Wu et al. 2013) to induce yellow fluorescent protein (YFP) in PDGFRα+ OPCs (Fig. 1A). Injections of 180 mg/kg/d tamoxifen were used for 18 month and 8 hour time points. Due to animal loss, the dose was reduced to 120 mg/kg/d for some 8 hour, and all 3 day, 2, 3, and 4 week, 3 and 6 month time points.
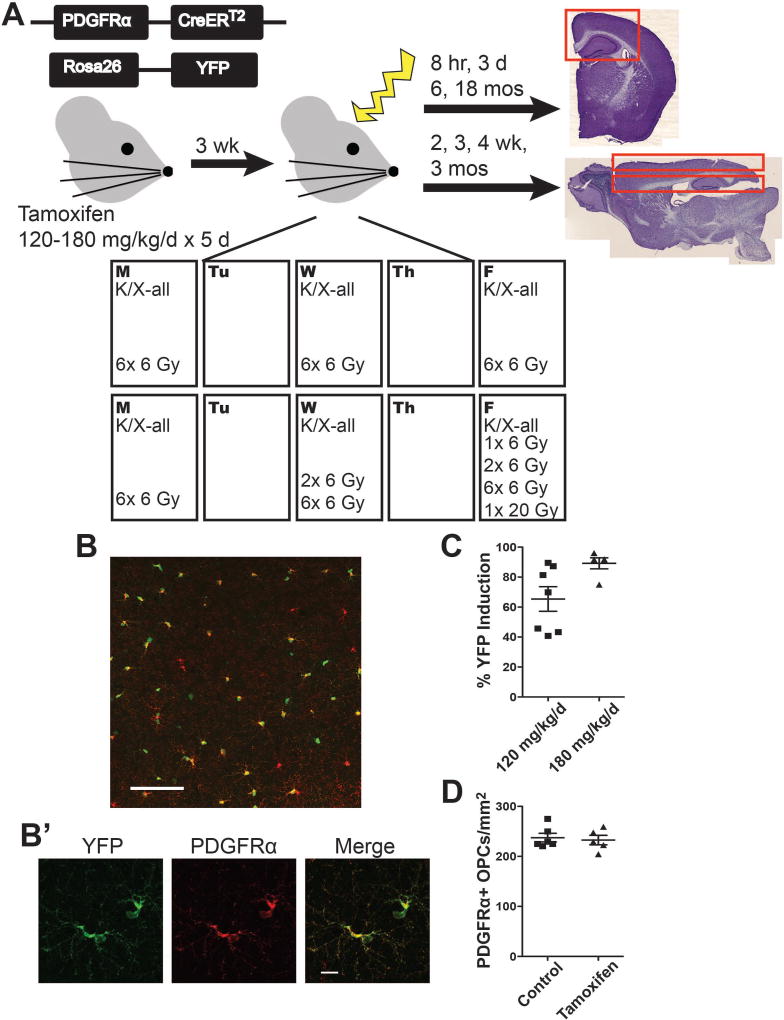
Figure 1. Experimental design.
A. Mice received intraperitoneal injections of 120–180 mg/kg/d tamoxifen for 5 consecutive days at 6–8 weeks of age. Three weeks was allowed for clearance of tamoxifen. Mice were anesthetized and exposed to 0 Gy, 1× 6 Gy, 2× 6 Gy, 6× 6 Gy, or 1× 20 Gy cranial irradiation, with exposures occurring Monday, Wednesday, and Friday for two consecutive weeks or on the final exposure days in the case of animals not exposed to the 6× 6 Gy paradigm. Animals were sacrificed at time points ranging from 8 hours to 18 months after exposure. Tissue was sectioned coronally for 8 hour, 3 day, 6 month, and 18 month time points and sagittally for 2, 3, 4 week and 3 month time points. B. Representative image shows YFP and PDGFRα expression in Pdgfrα-CreERT2:Rosa26R-YFP mice 3 weeks after intraperitoneal (i.p.) injection of 120 mg/kg/d tamoxifen for 5 days. Image scale bar = 100 µm. B’. Inset scale bar = 10 µm. C. YFP induction varied between 65.4% and 89.2% in the following conditions, respectively: 5 weeks following 5 days of 120 mg/kg/d tamoxifen and 5 weeks following 5 days of 180 mg/kg/d tamoxifen. n = 5–7 animals/group. D. Tamoxifen treatment did not alter the number of PDGFRα+ oligodendrocyte progenitor cells (OPCs) at 3 weeks post-injection of 120 mg/kg/d tamoxifen. n = 5–6 animals/group, Student’s t-test (p = 0.7189). Graphs represent mean ± SEM.
Irradiations
Three weeks were allowed for tamoxifen clearance prior to irradiations. Animals were anesthetized with 100 mg/kg ketamine/10 mg/kg xylazine i.p. on Monday, Wednesday, and Friday for 2 weeks, either with or without radiation. Irradiations were done using a 137Cs source with a 5 mm × 12.2 cm collimator. Mice were positioned supine with radiation directed between the eyes and ears, at a dose rate of 119–121 cGy/min to the surface of the skull (Moravan et al. 2011). The biologically effective dose (BED) equation, which applies the linear-quadratic model to take into account dose size per fraction and total dose administered when comparing different IR schedules (Fowler 1989), was used to design a fractionation paradigm comparable to 20 Gy single dose IR. The BED is a mathematical construct that provides clinicians the opportunity to compare dosing schedules by assigning arbitrary numbers in units of Gray (Gy). The equation is as follows: BED = D(1+d/(α/β)), where the total dose, D, is equal to (n × d), the number and size of dose fractions; α is a measure of susceptibility to direct cell death, and β is a measure of accumulated sub-lethal damage (Fowler 1989; Fowler 2010). Generally, late responding tissues are assigned an α/β ratio of 2–3 while early responding and tumor tissues are assigned an α/β ratio of 10, reflecting the importance of acute cell death in proliferative tissues versus the increased contribution of accumulated sub-lethal damage to cell survival in tissues with relatively few proliferating cells (Fowler 1989; Fowler 2010). While the CNS is typically considered a late responding tissue, OPCs are proliferative, albeit relatively slowly, and therefore have the potential to display an early response phenotype to radiation. Therefore, we based our fractionation schedule on an α/β ratio of 10:
Fractionated paradigm exposures were administered on Monday, Wednesday, and Friday over two weeks (Fig. 1A). Paradigms of 1 or two 6 Gy exposures (BED equivalents of 9.6 and 19.2 Gy10, respectively) were included at early time points to assess changes through the fractionation paradigm. Animals were sacrificed at 8 hours; 3 days; 2, 3, and 4 weeks; 3, 6, and 18 months following final radiation exposure.
BrdU Injections
Animals sacrificed at 8 hours and 3 days received 150 mg/kg 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich #B5002) i.p. 2 hours before sacrifice (Mandyam et al. 2007). Animals sacrificed at 2, 3, and 4 weeks received 150 mg/kg BrdU i.p. 3 days post-radiation (Mandyam et al. 2007).
Tissue Processing
For 8 hour, 3 day, and 6 and 18 month tissue collection, animals were anesthetized with ketamine and xylazine (100 mg ketamine/10mg xylazine) i.p. before transcardial perfusion with 0.15 M phosphate buffer (PB) containing 0.5% w/v sodium nitrite and 2 IU/ml heparin. Brains were removed and bisected along the midline. The right hemisphere was immersed in 4% paraformaldehyde (PFA) at 4°C for 24 hours, equilibrated in 30% sucrose overnight, frozen in isopentane, and stored at −80°C. Fixed brains were sectioned coronally at 30 µm on a sliding microtome and stored at −20°C in cryoprotectant (Fig. 1A). Animals sacrificed at 2, 3, 4 weeks or 3 months were similarly perfused with PB, followed by 4% PFA. Brains were immersion post-fixed at 4°C for 2 hours, equilibrated in 30% sucrose overnight, frozen in isopentane, and stored at −80°C (Hein et al. 2010; Moravan et al. 2011). Brains were sectioned sagittally at 30 µm on a sliding microtome and stored at −20°C in cryoprotectant (Fig. 1A).
Immunohistochemistry
Sections were washed in 0.15 M PB, blocked with 3–10% normal serum (Vector), and incubated in primary antibody, including: Goat anti-PDGFRα (R&D Systems #AF1062) 1:1000; Rabbit anti-GFP (Invitrogen #A6455) 1:2000 with Liberate Antigen Binding (L.A.B.) Solution pretreatment (Polysciences, Inc. # 24310-500); goat anti-GFP - Dylight 488 conjugated (Rockland #600-141-215) 1:2000; Rat anti-BrdU (Abcam #AB6326) 1:300 with 4N hydrochloric acid pretreatment for antigen retrieval; rabbit anti-Caspase 3 – activated (BD Pharmingen #559565) 1:2000; biotin anti-PCNA (Biolegend #307904) 1:300 with L.A.B. pretreatment; goat anti-GSTP1 diluted 1:1500 (Lifespan Biosciences #LS-B2376/31655) with L.A.B. pretreatment. Invitrogen Alexa Fluor secondary antibodies diluted 1:800 were used. Sections were mounted with Prolong Gold Antifade Mountant (Invitrogen #P36930). Images were captured using a Zeiss Axioplan IIi light microscope (Carl Zeiss), Sensicam QE camera (Cooke), and Slidebook software version 6 on Windows XP (Intelligent Imaging) or using an Olympus FV1000 laser scanning confocal microscope.
Quantification
ImageJ (NIH) was used for quantification. For quantification of YFP induction, 3 sections of the cortex, 720 µm apart, were imaged at 20× magnification. For quantification of YFP+ cells, apoptotic, and proliferative markers at 8 hours and 3 days post-radiation, 2 sections 720 µm apart were imaged at 10× and montaged to include corpus callosum, cortex, and hippocampus. These regions of interest were selected and the area and number of cells determined. The number of YFP+ cells normalized to area is reported. YFP cells positive for activated Caspase 3, BrdU, or PCNA were normalized to the number of YFP cells in the area analyzed. Two-way ANOVA with Bonferroni’s multiple comparisons test comparing all groups was used for statistical analysis at 8 hour and 3 day time points.
Images at 2, 3, and 4 weeks and 3 months were captured as a strip of rostral to caudal fields at 10× magnification. One strip of 10× images was taken across the hippocampus and another was taken across the cortex. Strips were analyzed in 445 µm segments rostral to caudal to compare across the unirradiated poles and irradiated medial field of the brain, with cell counts from corresponding hippocampal and cortical images averaged. To assess survival and proliferation, YFP cells positive for BrdU or PCNA were normalized to the number of YFP cells in the area analyzed. Two-way ANOVA with repeated measures across rostral-caudal location with Dunnett’s multiple comparisons test was used for statistical analysis. Logit transformation for variance stabilization of small proportions was used for statistical analysis of PCNA and BrdU positive proportions of YFP+ cells.
Images of YFP and GSTP1 expressing cells at 6 and 18 month time points were captured at 20× magnification, and quantified using five sections 720 µm apart, 3 cortical images per section, for each animal. Sections analyzed included the first two sections with dentate gyrus and the 3 preceding sections, which were expected to capture the irradiated area of the brain. The number of cells normalized to area quantified is reported using two-way ANOVA with Bonferroni’s multiple comparisons test for statistical analysis.
Prism 6 (GraphPad) was used for statistical analyses and graphs. Adobe Photoshop CS6 and Adobe Illustrator CS6 (Adobe Systems Incorporated) were used to create figures.
Results
YFP induction occurs in PDGFRα+ OPCs after tamoxifen exposure
YFP expression was induced in PDGFRα+ OPCs by 5 days of i.p. tamoxifen injection (Fig. 1B). Five weeks after 120 mg/kg/d tamoxifen the percent YFP induction of PDGFRα+ OPCs was 65.4%±8.2, Fig. 1C). With a tamoxifen dose of 180 mg/kg/d, 89.2%±3.7 of PDGFRα+ OPCs expressed YFP after 5 weeks (Fig. 1C). YFP induction did not affect the PDGFRα+ OPCs/mm2 numbers 3 weeks after injection by Student’s t-test (p=0.7189) (Fig. 1D), suggesting no OPC toxicity from tamoxifen exposure.
Fractionation enhances IR-induced OPC loss
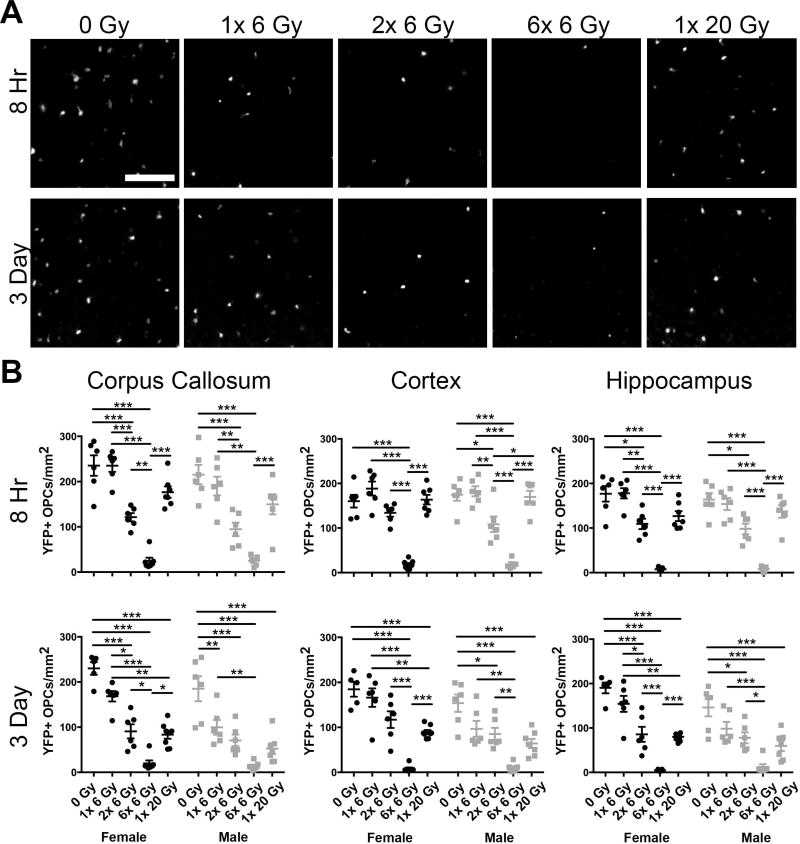
To assess the acute effects of single dose versus fractionated cranial IR on OPC numbers, we quantified YFP+ cells in the corpus callosum, cortex, and hippocampus of male and female mice at 8 hours and 3 days post exposure (Fig. 2). In addition to comparing the 6× 6 Gy fractionated dose to a single 20 Gy dose, mice treated with 1 or 2 exposures of 6 Gy IR were included to assess changes occurring through the fractionation schedule. At 8 hours, the number of YFP+ OPCs was reduced by radiation in the corpus callosum (F(4,51)=50.73, p<0.0001), cortex (F(4,51)=60.72, p<0.0001), and hippocampus (F(4,51)=59.4, p<0.0001) (Fig. 2B). Sex affected the number of YFP+ OPCs in the corpus callosum (F(1,51)=5.337, p=0.0250), but not the cortex (F(1,51)=0.07527, p=0.7849) or the hippocampus (F(1,51)=0.9844, p=0.3258). No interaction was observed in any region. The number of YFP+ OPCs was similarly reduced at 3 days in the corpus callosum (F(4,54)=53.99, p<0.0001), cortex (F(4,54)=40.78, p<0.0001), and hippocampus (F(4,54)=46.88, p<0.0001) (Fig. 2B). At 3 days, an effect of sex was also observed in the corpus callosum (F(1,54)=15.55, p=0.0002), cortex (F(1,54)=13.42, p=0.0006), and hippocampus (F(1,54)=2.18, p=0.0032), but there was no interaction between sex and dose. Specifically, we found that 2× 6 Gy, 6× 6 Gy, and 1× 20 Gy IR reduced YFP+ OPCs at 3 days after the final exposure in both sexes. While statistically significant differences were observed in the number of OPCs in males and females after a single dose of 6 Gy at 3 days, the overall response of OPCs to radiation was similar and statistically significant differences were not observed after 2 or 6 doses of 6 Gy or after a single 20 Gy radiation exposure. In both sexes, 6× 6 Gy caused a significantly greater loss of OPCs than a single 20 Gy IR exposure, indicating that fractionation enhances IR-induced OPC loss. This may be, in part, due to cumulative insult beginning earlier in the fractionated paradigm.
Figure 2. Fractionation enhances radiation-induced loss of oligodendrocyte progenitor cells.
A. Representative images of cortical yellow fluorescent protein (YFP) expressing cells 8 hours and 3 days after the final exposure to 0, 1× 6, 2× 6, 6× 6 or 1× 20 Gy irradiation. Scale bar = 200 µm. B. At both 8 hours and 3 days, the number of YFP+ OPCs was reduced by radiation in the corpus callosum, cortex, and hippocampus (p < 0.0001 for all). At 8 hours, an effect of sex was observed in the corpus callosum (p = 0.0250). At 3 days, an effect of sex was observed in the corpus callosum (p = 0.0002), cortex (p = 0.0006), and hippocampus (p = 0.0032). n = 5–8 animals/group, 2-way ANOVA with Bonferroni’s multiple comparisons test comparing all conditions. Graphs represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Graphs show statistically significant differences within sexes only. Significance was also found in the 1× 6 Gy IR female versus male groups at 3 days in the corpus callosum (p < 0.05) and cortex (p < 0.05) with Bonferroni’s post-test.
OPC apoptosis occurs at 8 hours following cranial IR
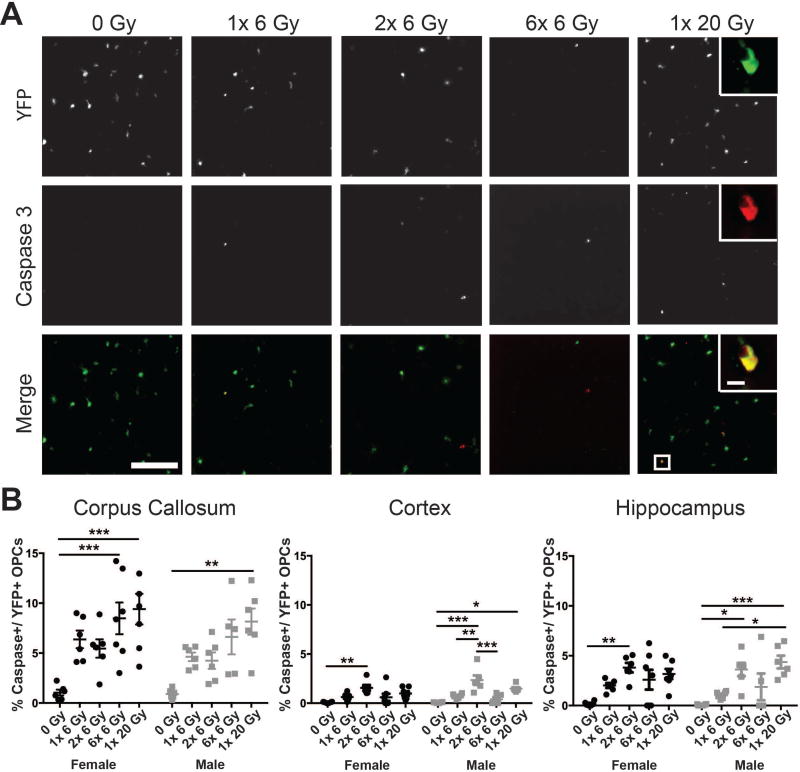
To determine the role of radiation-induced apoptosis in OPC loss after cranial IR, we assessed the proportion of YFP+ cells also positive for activated Caspase 3 (Fig. 3). We found an increase in apoptotic OPCs in the corpus callosum (F(4,51)=14.11, p<0.0001), cortex (F(4,51)=16.55, p<0.0001), and hippocampus (F(4,51)=12.00, p<0.0001) at 8 hours after the final IR exposure. No effect of sex was observed in the corpus callosum (F(1,51)=2.992, p=0.0897), cortex (F(1,51)=2.063, p=0.1570), or hippocampus (F(1,51)=0.1571, p=0.6935). Importantly, we observed a qualitatively increased proportion of YFP+ OPCs positive for activated Caspase 3 after 2 exposures of 6 Gy IR compared to a single dose in the cortex and hippocampus, suggesting that an increased proportion of OPCs are sensitive to IR-induced apoptosis with consecutive doses of radiation (Fig. 3B). The apoptotic fraction of OPCs also trended higher in 1× 20 Gy than 6× 6 Gy irradiated mice; however, very few YFP+ OPCs remained by the end of the 6× 6 Gy paradigm, pointing to the necessity of looking through the fractionation schedule to interpret OPC responses to fractionated versus single dose IR. The mean number of apoptotic OPCs (rather than proportion) at 8 hours after radiation exposure is shown in Supp. Info. Table 1.
Figure 3. Radiation exposure leads to oligodendrocyte progenitor cell apoptosis.
A. Representative images show apoptosis of cortical yellow fluorescent protein (YFP)-positive OPCs at 8 hours after radiation. Scale bar = 200 µm. Inset scale bar = 10 µm B. Radiation dose significantly affected the apoptotic fraction of OPCs in the irradiated corpus callosum, cortex, and hippocampus (p < 0.0001 for all). n = 5–7 animals/group, 2-way ANOVA with Bonferroni’s multiple comparisons test comparing all conditions. Graphs represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Proliferation of OPCs occurs at 3 days following IR exposure
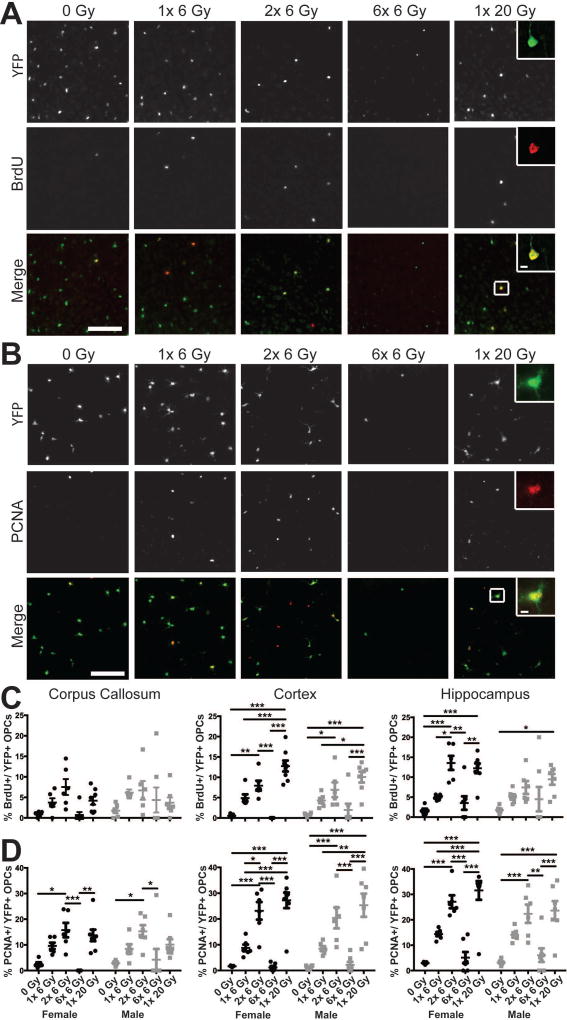
Radiosensitivity is linked to proliferation; if fractionation enhances radiation-induced cell killing, an IR-induced increase in proliferation or movement into radiosensitive phases of the cell cycle likely contributes to the phenomenon. Therefore, we assessed the proportion of YFP+ OPCs that were also positive for BrdU, a marker of S-phase cells and PCNA, a pan-proliferating cell marker (Fig. 4). Radiation dose significantly influenced the proportion of YFP+ OPCs in S-phase of the cell cycle in the corpus callosum (F(4,54)=3.697, p=0.0099), cortex (F(4,54)=33.1, p<0.0001), and hippocampus (F(4,54)=13.09, p<0.0001) at 3 days after the final exposure (Fig. 4A,C). No effect of sex or interaction was observed in the corpus callosum, cortex, or hippocampus. Radiation also increased the PCNA-positive fraction of YFP+ OPCs in the corpus callosum (F4,54)=12.91, p<0.0001), cortex (F(4,54)=40.37, p<0.0001), and hippocampus (F(4,54)=34.00, p<0.0001) (Fig. 4B,D). Sex did not affect the percent proliferating OPCs in the corpus callosum (F(1,54=0.008344, p=0.9276), cortex (F(1,54)=0.2803, p=0.5987), or hippocampus (F(1,54)=1.881, p=0.1759) and there was no interaction. The increased expression of proliferative markers in YFP+ cells seen with single doses of IR exposure indicates IR-induced OPC proliferation. Because 2 doses of 6 Gy qualitatively appeared to enhance the proportion of proliferating YFP+ OPCs over a single 6 Gy exposure, it is likely an increased proportion of OPCs enter the cell cycle with consecutive doses of radiation. A large increase in proliferation was also observed in the 1× 20 Gy condition, suggesting dose-related proliferation due to dose-related cell death. Because of the degree of depletion in the 6× 6 Gy dose, this is not an adequate representation of IR-related proliferation. The mean number of BrdU+ or PCNA+ OPCs at 3 days after radiation exposure is shown in Supp. Info. Table 1.
Figure 4. Proliferation of oligodendrocyte progenitor cells is enhanced by radiation exposure.
Three days after the final dose of radiation, proliferating OPCs were labeled by intraperitoneal injection of 5-bromo-2-deoxyuridine (BrdU) 2 hours prior to sacrifice. Representative images show enhanced BrdU (A) and PCNA (B) expression in cortical yellow fluorescent protein (YFP)-positive OPCs after radiation exposure. Scale bars = 200 µm. Inset scale bars = 10 µm. C. Radiation significantly increased the proportion of OPCs in S-phase of the cell cycle in the corpus callosum (p = 0.0099), cortex (p < 0.0001), and hippocampus (p < 0.0001). D. The PCNA labeled fraction of OPCs was affected by radiation dose in all areas examined (p < 0.0001 for each). n = 5–8/group, 2-way ANOVA with Bonferroni’s multiple comparisons test comparing all conditions. Graphs represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
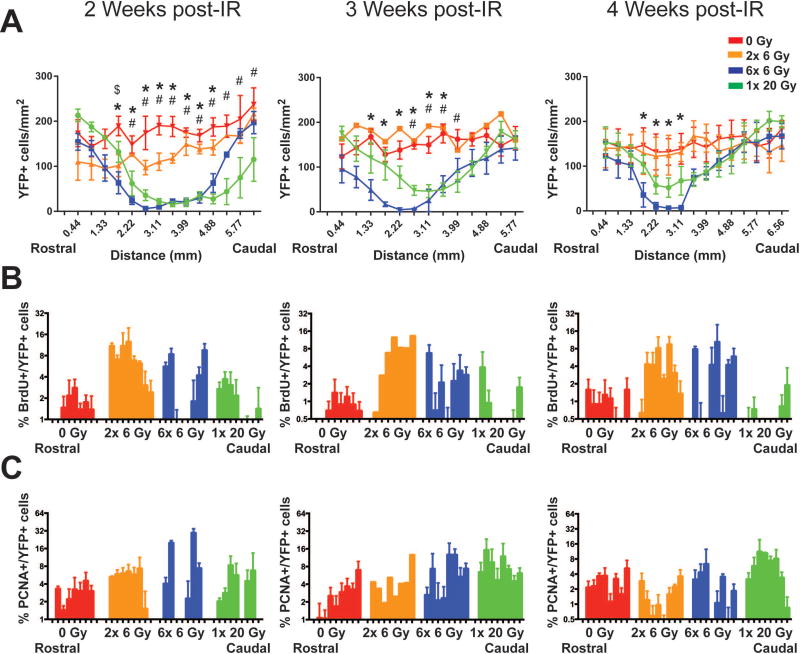
Recovery of oligodendrocyte progenitor cells is incomplete through 4 weeks post-radiation exposure
Given the substantial increase in OPC loss seen at 3 days after exposure to fractionated versus single dose IR, we assessed the ability of these cells to recover from radiation-induced depletion. To visualize potential cell migration from unirradiated poles into the irradiated area, brains were cut into sagittal sections and OPC numbers across the cortex and hippocampus were examined. Because a single 6 Gy dose did not cause a large OPC loss, this group was excluded from further studies. Moreover, although sex was balanced across these groups, we did not have enough animals in each group to test for specific sex differences. At 2, 3, and 4 weeks after IR, there was persistent depletion of YFP+ cells in 6× 6 Gy irradiated animals, while 1× 20 Gy irradiated mice showed some recovery by 4 weeks post-exposure and 2× 6 Gy irradiated animals showed recovery by 3 weeks post-exposure (Fig. 5A). Two-way ANOVA with repeated measures across rostral-caudal location indicated a significant effect of radiation exposure (p=0.0021), rostral-caudal location (p<0.0001), interaction (p<0.0001), and subjects (p<0.0001) at 2 weeks; a significant effect of exposure (p=0.0033), rostral-caudal location (p<0.0001), interaction (p=0.0009), and subjects (p<0.0001) at 3 weeks; and a significant effect of rostral-caudal location (p<0.0001), interaction (p<0.0001), and subjects (p<0.0001) at 4 weeks post-exposure (Fig. 5A, Supp. Info. Table 2). Dunnett’s multiple comparisons test comparing irradiation groups to controls was performed to establish specific differences in YFP+ cell numbers as a function of rostral-caudal location, as shown in Fig. 5A and Supp. Info. Table 3. Comparing recovery in 6× 6 Gy versus 1× 20 Gy groups demonstrates that OPC loss is greater at 3 and 4 weeks after completion of a fractionated paradigm. Additionally, the shrinking area of YFP cell depletion over time with areas proximal to the unirradiated poles being the first to recover suggests that YFP cell recovery as a function of time is due to migration of unirradiated YFP+ OPCs at the rostral and caudal poles into the OPC depleted brain region.
Figure 5. OPC recovery 2, 3, and 4 weeks after cranial irradiation.
A. YFP+ OPCs at 2, 3, and 4 weeks after the final exposure as a function of rostral-caudal location. There is an overall effect of radiation exposure at 2 weeks (p = 0.0021) and 3 weeks (p = 0.0033); rostral-caudal location at 2, 3, and 4 weeks (p < 0.0001); and interaction at 2 weeks (p < 0.0001), 3 weeks (p = 0.0009), and 4 weeks (p < 0.0001). B. Survival of OPCs at 2, 3, and 4 weeks after radiation. 5-bromo-2-deoxyuridine (BrdU) was administered intraperitoneally at 3 days after the final radiation exposure. Analysis of logit-transformed data shows a significant effect of radiation treatment at 2 weeks (p = 0.0002) and 4 weeks (p = 0.0036), of rostral-caudal location at 2 weeks (p = 0.0027), and of the interaction at 2 weeks (p < 0.0001) and 4 weeks (p = 0.0051). C. PCNA+ fraction of OPCs at 2, 3, and 4 weeks after radiation. Analysis of Logit-transformed data reveals an overall effect of rostral-caudal location at 2 weeks (p = 0.0007) and 4 weeks (p = 0.0125) and an interaction between dose and rostral-caudal position at 2 weeks (p < 0.0001). Quantitation was performed across the cortex and hippocampus. n = 1–4 animals/group, 2-way ANOVA with repeated measures, Dunnett’s multiple comparisons test comparing radiation groups to control. Graphs represent mean ± SEM. $: p<0.05 for 0 Gy versus 2× 6 Gy; *: p<0.05 for 0 Gy versus 6× 6 Gy; #: p<0.05 for 0 Gy versus 1× 20 Gy.
To investigate how surviving YFP+ OPCs may contribute to recovery post-IR, animals received i.p. injections of BrdU at 3 days post-IR, a time point with increased proliferation (see previous section), to assess the survival and contribution to recovery of the OPCs that are mitotically active at that time. Low numbers of BrdU+ YFP+ cells were observed in the irradiated area of the brain, suggesting that surviving OPCs in the irradiated field contribute little to recovery after 6× 6 Gy or 1× 20 Gy IR (Fig. 5B). BrdU levels at the rostral and caudal poles appear higher in 6× 6 Gy than 1× 20 Gy irradiated animals; because cell depletion occurred earlier through the fractionated paradigm, a proliferative response from the unirradiated poles likely began at an earlier time post-IR, resulting in greater cell labeling. At 2 weeks post-exposure, there was a significant effect of radiation exposure (p=0.0002), rostral-caudal location (p=0.0273), and interaction (p<0.0001) by 2-way ANOVA with repeated measures of logit-transformed data (Supp. Info. Table 2). At 3 weeks there was a significant effect for subjects (p<0.0001) and at 4 weeks a significant effect of exposure (p=0.0036) and interaction (p=0.0051) (Supp. Info. Table 2). Specific examples of alterations in the proportion of YFP+ cells that were BrdU+ are shown in Supp. Info. Table 4.
In addition to assessing differences in the survival of YFP+ OPCs proliferating at 3 days post-exposure, we analyzed proliferation at the time of sacrifice by assessing the proportion of YFP+ OPCs that expressed PCNA (Fig. 5C). Two-way ANOVA of the logit-transformed data suggested an effect for rostral-caudal location (p=0.0007) and interaction (p<0.0001) at 2 weeks post-exposure and for rostral-caudal location (p=0.0125) and subjects (p=0.0002) at 4 weeks post-exposure (Supp. Info. Table 2). While there appeared to be a change in the pattern of YFP+ OPC proliferation over time, suggesting the bulk of proliferating YFP+ cells in 6× 6 Gy and 1× 20 Gy irradiated animals moved from the rostral and caudal poles toward the radiation-depleted center of the brain (Fig. 5C), few specific examples of significant changes in proliferation were found using Dunnett’s multiple comparisons test comparing irradiated groups to control (Supp. Info. Table 5).
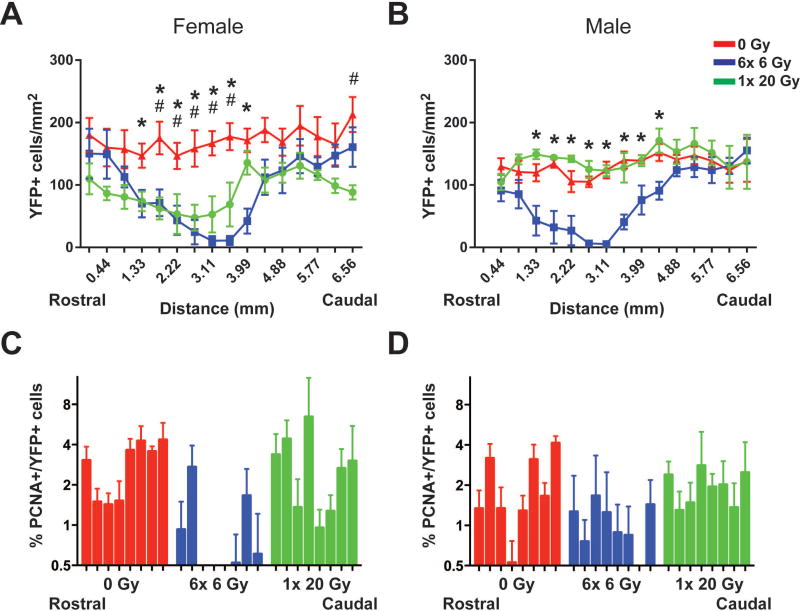
Recovery of YFP+ cells is sex- and dose-dependent at 3 months post-IR
At 3 months following the final exposure to IR, persistent depletion of YFP+ cells was apparent in the cortex and hippocampus of female mice exposed to 6× 6 Gy and 1× 20 Gy IR (Fig. 6A). A significant effect of IR exposure (p=0.0029), rostral-caudal location (p<0.0001), interaction (p<0.0001), and subjects (p<0.0001) was indicated by 2-way ANOVA with repeated measures (Supp. Info. Table 6). Conversely, while 6× 6 Gy irradiated males did not show recovery, YFP+ cell numbers in male mice exposed to 1× 20 Gy IR were indistinguishable from unirradiated controls (Fig. 6B). Two-way ANOVA indicated a significant effect of exposure (p=0.0119), rostral-caudal location (p<0.0001), interaction (p=0.0001), and subjects (p<0.0001) (Supp. Info. Table 6). A reduction in the PCNA+ fraction of YFP+ cells in 6× 6 Gy irradiated female mice (Fig. 6C, Supp. Info. Table 7) indicated a persistent effect of radiation on YFP+ OPC proliferation, which may contribute to the lack of recovery of YFP+ cells in these animals. An effect of exposure (p<0.0001) and rostral-caudal location (p=0.0009) was present by 2-way ANOVA with repeated measures on logit-transformed data (Supp. Info. Table 7). Two-way ANOVA with repeated measures suggested an overall effect of dose (p=0.0009) on YFP+ cell proliferation in male mice as well (Supp. Info. Table 6), though few specific examples were seen with Dunnett’s multiple comparisons test of the logit-transformed data (Supp. Info. Table 7).
Figure 6. Differential recovery of OPCs at 3 months post-radiation.
There is an overall effect of radiation exposure (p=0.0019), rostral-caudal location (p<0.0001), and interaction (p=0.0001) on OPCs in female mice (A), and an effect of exposure (p=0.0029), rostral-caudal location (p<0.0001), and the interaction (p<0.0001) in male mice (B). C. PCNA+ proliferative OPCs are reduced after fractionated IR in female mice, where there is a significant effect of exposure (p<0.0001) and rostral-caudal location (p=0.0009) in logit-transformed PCNA+ YFP+/ YFP+ cells. D. Radiation exposure affects the logit-transformed PCNA+ fraction of YFP+ cells in males (p=0.0009). Quantitation was performed across the cortex and hippocampus. n = 4–5 animals/group, 2-way ANOVA with repeated measures, Dunnett’s multiple comparisons test comparing radiation groups to control. Graphs represent mean ± SEM. *: p<0.05 for 0 Gy versus 6× 6 Gy; #: p<0.05 for 0 Gy versus 1× 20 Gy.
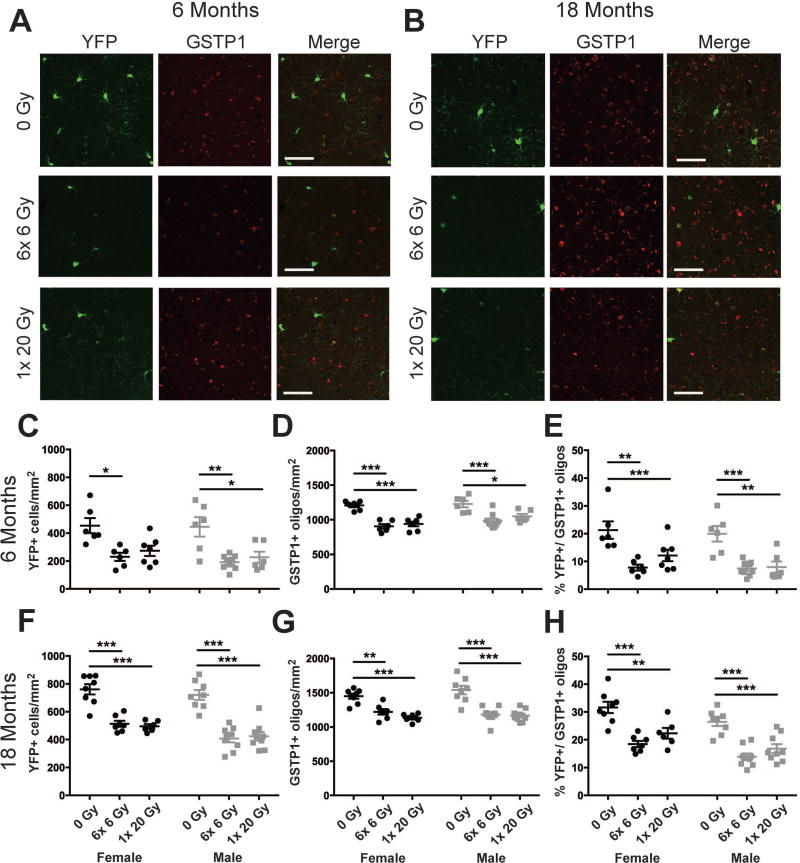
Reductions in GSTP1+ oligodendrocytes may be due to reduced YFP+ OPC maturation at 6 and 18 months from radiation exposure
To determine the long-term impact of early OPC loss we assessed the number of YFP+ cells and GSTP1+ oligodendrocytes, as well as the proportion of GSTP1+ oligodendrocytes expressing YFP, in the cortex at 6 and 18 months after radiation exposure (Fig. 7). Radiation reduced YFP+ cells at 6 months (F(2,34)=18.27, p<0.0001) (Fig. 7C). No effect of sex or interaction was observed. The number of GSTP1+ oligodendrocytes was affected by radiation dose (F(2,34)=32.52, p<0.0001) and sex (F(1,34)=5.701, p=0.0227) at 6 months, but no interaction was observed (Fig. 7D). The proportion of GSTP1+ oligodendrocytes that expressed YFP was reduced by radiation exposure at 6 months (F(2,32)=22.42, p<0.0001), while no effect of sex or interaction was observed (Fig. 7E). At 18 months, an effect of radiation exposure (F(2,41)=56.54, p<0.0001) and sex (F(1,41)=8.27, p=0.0064), without interaction, on YFP+ cells was observed (Fig. 7F). The number of GSTP1+ oligodendrocytes was reduced by radiation exposure (F(2,41)=40.7, p <0.0001) at 18 months (Fig. 7G), but there was no effect of sex or interaction. The proportion of GSTP1+ oligodendrocytes that expressed YFP at 18 months was reduced by radiation exposure (F(2,41)=35.58, p<0.0001) (Fig. 7H). An effect of sex (F(1,41)=15.22, p=0.0003), but not interaction, was also observed. These data show that YFP+ OPCs do not recover to control numbers through 18 months from single dose or fractionated radiation exposure in males or females. Moreover, at 6 months and 18 months from both radiation paradigms GSTP1+ mature oligodendrocytes are reduced in both sexes, with a reduced proportion of YFP+ cells differentiating into GSTP1+ oligodendrocytes in the same time frame. This suggests that radiation exposure reduces maturation of OPCs.
Figure 7. Reduced maturation of oligodendrocyte progenitor cells correlates with decreased numbers of mature oligodendrocytes at 6 and 18 months after radiation exposure.
A., B. Representative images of cortical YFP and GSTP1 expressing cells at 6 and 18 months from radiation. Scale bar = 50 µm. C. At 6 months, an effect of radiation dose (p < 0.0001) on YFP+ cells was observed. D. Both radiation dose (p < 0.0001) and sex (p = 0.0227) affected GSTP1 cell numbers at 6 months. E. The proportion of GSTP1+ oligodendrocytes that expressed YFP was reduced by radiation dose at 6 months (p < 0.0001). F. At 18 months, an effect of radiation dose (p < 0.0001) and sex (p = 0.0064) on YFP+ cells was observed. G. Radiation dose (p < 0.0001) affected the number of GSTP1 cells at 18 months. H. Radiation dose (p < 0.0001) and sex (p = 0.0003) affected the proportion of GSTP1+ oligodendrocytes that expressed YFP. n = 6–9/group. 2-way ANOVA with Bonferroni’s multiple comparisons test comparing all groups. Graphs represent mean ± SEM. *: p<0.05, **: p<0.01, ***: p<0.001.
Discussion
To compare the effects of single dose and fractionated IR paradigms on OPCs and their progeny, YFP expression was induced in PDGFRα+ OPCs. PDGFRα+ OPCs that did not express YFP at 5 weeks from tamoxifen injection in Fig. 1B may represent cells that were earlier lineage, not expressing PDGFRα at the time of tamoxifen injection, in addition to those attributable to inefficiency in cre-mediated recombination. Conversely, YFP+ cells that do not express PDGFRα at 5 weeks from tamoxifen injection are likely cells that were PDGFRα+ at the time of injection, but have since differentiated. While recombination has been observed in the piriform cortical neurons in this model, YFP expression in the areas we examined (corpus callosum, cortex, hippocampus) was described as overlapping with PDGFRα+ staining (Rivers et al. 2008). Moreover, the small fraction of neurons observed by Rivers et al. (2008) would not significantly affect interpretation of our results. Although we are not aware of any investigation into cell-fate of PDGFRα+ OPCs after radiation, we examined whether YFP overlapped with the astrocyte specific marker GFAP in irradiated tissues and found no evidence for radiation-associated changes in OPC cell fate (data not shown).
Animals were exposed to 6× 6 Gy or 1× 20 Gy IR and additional exposure groups of 1 or 2 doses of 6 Gy were used to assess OPC dynamics through the fractionated paradigm at acute time points. We report that the number of YFP+ OPCs in the mouse corpus callosum, cortex, and hippocampus was reduced 8 hours and 3 days after single dose or fractionated radiation exposure. The OPC reduction was significantly greater in mice exposed to 6× 6 Gy than 1× 20 Gy IR, indicating that fractionation may enhance acute IR-induced OPC loss. A caveat is the dose timing; loss of OPC populations began 2 weeks earlier in the fractionated paradigm. If delayed loss of OPCs occurs in response to 1× 20 Gy IR, loss may even out over time, as observed at 2 weeks and later time points. While fractionation can enhance IR-induced death in rapidly proliferating normal and tumor tissue, it is utilized in late responding normal tissue like the CNS to reduce side effects of radiotherapy (Panganiban et al. 2013). However, our data show that proliferating OPCs in the CNS are highly susceptible to fractionated IR, suggesting a possible role in CNS radiation side effects.
While mitotic catastrophe is the primary mechanism of radiation-induced cell death, its direct measure requires assessment of aberrant chromosomes, spindle formation, and multi- and micro-nucleated cells (Firat et al. 2011; Roninson et al. 2001). Because such assessments require in depth and time intensive analysis of many cells and because we were investigating the effects of radiation in large areas of the mouse brain as opposed to cell culture, we chose to look at apoptosis, which also contributes to IR-induced cell death (Panganiban et al. 2013). However, ongoing mitotic catastrophe likely contributed to IR-induced loss of OPCs, and may explain the increased loss that occurs between 8 hours and 3 days from 1× 6 Gy IR and between 3 days and 2 weeks from 1× 20 Gy IR, for example. In the cortex and hippocampus, 2 exposures of 6 Gy IR resulted in a greater proportion of apoptotic OPCs than a single dose at 8 hours from exposure, indicating that repeated exposure to radiation enhances the proportion of OPCs that are radiosensitive. In fact, the apoptotic fraction of OPCs was greater after 2× 6 Gy than 1× 20 Gy IR in the cortex. Conversely, in the corpus callosum the greatest increase in apoptosis was observed after 1× 20 Gy IR. This may be due to differences between white and gray matter. Because corpus callosum OPCs have a shorter cell cycle than cortical OPCs (Psachoulia et al. 2009), and insult is suggested to reduce cell cycle length (Simon et al. 2011), cell death may occur earlier after the 2nd exposure and we may have missed the peak of apoptosis after 2× 6 Gy in the corpus callosum, but not in the gray matter regions examined. The differences in proliferation rates may also affect which cell cycle phases OPCs are in 48 hours after exposure in white versus gray matter, and therefore related radiosensitivity. Additionally, the apoptotic fraction after a single dose of 6 Gy IR was higher in the corpus callosum than the gray matter areas investigated. If white matter cells are more sensitive to IR-induced death, as has been suggested (Li et al. 1996), the first exposure may kill a larger proportion, resulting in a smaller impact from the second fraction when compared to the first.
Our data support a role for proliferation in IR-induced apoptosis. Both fractionated and single dose radiation exposure increased proliferation as assessed by the BrdU+ and PCNA+ fractions of YFP+ cells at 3 days from the final exposure. Importantly, 2 doses of 6 Gy enhanced the proportion of proliferating YFP+ OPCs over a single 6 Gy exposure, suggesting an increased proportion of OPCs enter the cell cycle with consecutive doses. The increased proportion of cycling OPCs likely occurs due to cell loss; loss of OPCs from one radiation exposure recruits surviving OPCs into the cell cycle to repopulate the irradiated area, similar to observations using other OPC depletion strategies (Atkinson et al. 2005; Hughes et al. 2013). This may suggest IR-induced cell cycle synchrony, which is usually associated with early responding tissues and tumors and not traditionally associated with the late responding CNS.
Drawing conclusions about OPC behavior from the 6× 6 Gy irradiation group is problematic, since very few OPCs remain by the end of the fractionation paradigm. In rats, fractionation was shown to increase SVZ cell loss, which plateaued after a 4th daily exposure to 1.5 Gy cranial IR (Shinohara et al. 1997). Thus, with further 6 Gy exposures, we may have seen a plateau in cell loss and a reduced recruitment into the proliferative phase, which would explain the limited proliferation seen in our 6× 6 Gy irradiation group. Notably, the greatest increases in proliferation were often observed in the 1× 20 Gy irradiated group. However, after a single 20 Gy exposure, no additional radiation exposure occurred to promote further cell killing.
While there is agreement in the literature that OPC numbers are reduced following exposure to ionizing radiation (Atkinson et al. 2005; Chari and Blakemore 2002; Chari et al. 2006; Irvine and Blakemore 2007), there are contradictory reports regarding their recovery from IR-induced depletion (Irvine and Blakemore 2007; Panagiotakos et al. 2007; Piao et al. 2015). Irvine and Blakemore showed full recovery of the irradiated mouse cortex at 1 month following 40 Gy cranial IR. Conversely, Panagitotakos et al. and Piao et al. showed sustained loss of OPCs in the corpus callosum after a single dose of 25 Gy IR in 3 month old rats and a 50 Gy fractionated paradigm in 4 week old rats, respectively. In contrast to these experiments, we directly compared fractionated and single dose paradigms to assess the contribution of the dosing paradigm to recovery. We show sustained loss of YFP+ OPCs through 4 weeks after both fractionated and single dose IR. At 3 and 4 weeks from exposure, OPC loss appears qualitatively greater in animals exposed to the fractionated paradigm. Animal numbers were not large enough to separate by sex at these time points, but qualitatively the responses appeared similar. At 3 months, male mice exposed to a single dose of 20 Gy IR showed recovery of OPCs, while recovery was incomplete in males exposed to 6× 6 Gy IR in the same time frame. In females, we observed slight recovery in 1× 20 Gy compared to 6× 6 Gy exposed animals, but depletion was still significant. While these data indicate that both sex and the radiation paradigm may affect the ability of OPCs to recover into the irradiation-depleted zone, it does not explain what causes the sex or dosing paradigm dependent changes. Moreover, investigation of YFP+ cell numbers at 6 and 18 months from radiation revealed similar effects in male and female mice, indicating that the recovery seen in 1× 20 Gy irradiated male mice at 3 months is transient and raises question of its biological importance. A similar transient spike in immature oligodendrocytes, as assessed by the marker O4, was seen at 6 months following a single 25 Gy dose of cranial IR to female rats (Panagiotakos et al. 2007). It is unclear what causes this transient recovery, but the microenvironment or alterations intrinsic to the OPCs could be responsible for its impermanence. We can speculate that a unique microenvironment in males exposed to a single insult (Acaz-Fonseca et al. 2015; Lenz and McCarthy 2015) may contribute to the observed sex differences, but further investigation of the complex interaction of different cell types in the brain after radiation exposure is needed. In addition, sex differences have been observed in children exposed to cranial irradiation in terms of post-treatment cognition, but data is inconsistent (Mulhern et al. 2004; Tonning Olsson et al. 2014).
Qualitatively, recovery appeared to occur from the unirradiated poles and consist of unirradiated YFP+ cell proliferation and migration into the IR-depleted field after a single 20 Gy dose and the full 6× 6 Gy fractionated exposure. This is consistent with other reports suggesting OPC recovery is due to migration from unirradiated areas after IR exposure (Chari and Blakemore 2002; Irvine and Blakemore 2007) and with studies examining OPC recovery after other methods of depletion (Birey and Aguirre 2015; Robins et al. 2013). However, this migration did not yield full recovery of YFP+ OPCs and a low percentage of PCNA+ YFP+ OPCs at 3 months suggests that the proliferative response of surviving YFP+ OPCs was not sustained.
The lack of survival of BrdU+ YFP+ cells in the 1× 20 Gy IR groups suggests persistent loss of these cells over time, which, as noted above, may be due to delayed mitotic catastrophe (Firat et al. 2011). This would explain the relatively minor migration of these early proliferating YFP+ cells into the irradiated field. Alterations in the microenvironment specific to a single high dose exposure may also contribute to the lack of migration of peripheral BrdU+ YFP+ cells in this group. The pattern of PCNA+ YFP+ cells after 1× 20 Gy IR suggests later migration of YFP+ OPCs recruited from the unirradiated poles, which peaks at 3–4 weeks from exposure. While this migration may have contributed to the recovery of YFP+ OPCs in male mice exposed to 1× 20 Gy IR at 3 months, it did not appear to sufficiently promote female YFP+ OPC recovery. An ongoing proliferative response did not appear to be present in either sex at 3 months, based on the PCNA+ fraction of YFP+ OPCs at that time, which again may contribute to the low numbers of YFP cells seen at 6 and 18 months from IR exposure in both sexes.
In addition to the reduction in YFP+ cells, the number of GSTP1+ mature oligodendrocytes was reduced at both 6 and 18 months in 1× 20 Gy and 6× 6 Gy irradiation groups. While some depletion of mature oligodendrocytes could be attributed to direct injury, the fraction of GSTP1+ oligodendrocytes that was YFP+, a proxy for OPC maturation after the time of radiation exposure, was also significantly reduced in both paradigms and at both time points. This suggests that maturation of OPCs is inhibited long term in both males and females following radiation with or without fractionation. However, it is unclear from these experiments whether IR-induced impairment is dependent on a microenvironment that opposes proliferation and maturation of OPCs, as has been shown for neural progenitors (Monje et al. 2002), or on an OPC-intrinsic defect. Further studies will be required to elucidate the source of impaired OPC maturation and determine the role of the microenvironment in altering OPC behavior.
Because oligodendrocytes supply trophic factors to axons (Bradl and Lassmann 2010; Fruhbeis et al. 2013) and myelin plasticity is linked to learning and cognition (Bartzokis et al. 2010; McKenzie et al. 2014; Schlegel et al. 2012), dysfunction in this cell lineage could contribute to the side effects of radiation. Importantly, the susceptibility of OPCs to fractionated radiation may explain, in part, why cognitive side effects and demyelination still occur with fractionated radiotherapy paradigms. We have previously shown that the proportion of large, myelinated to small, unmyelinated axon fibers, but not velocity of signal transmission, in the corpus callosum is affected by both fractionated and single dose radiation (Begolly et al. 2016). Notably, in this same study we also showed decreased PDGFRα+ cell densities for both fractionated and single high dose irradiation at 3 weeks and 18 months (Begolly et al. 2016), consistent with the changes in YFP-labeled OPC populations described here.
While standard fractionated whole brain protocols are performed at doses varying from 1.8 to 2.5 Gy per fraction, suggesting the doses used in this study are high, stereotactic radiosurgery (SRS; single) doses commonly range from 15 to 20 Gy (Kohutek et al. 2015; Trifiletti et al. 2015). Moreover, the mouse brain does not exhibit the same degree of radiation sensitivity as does the human brain; preclinical investigations have shown an ED50 of over 32 Gy for radiation-induced brain death in mice (Chiang and McBride 1991), and no necrosis was observed following 25 Gy whole brain radiation at time points up to 9 months (Daigle et al. 2001). Although additional experiments with lower dose fractions may be required to fully characterize OPC responses in mouse brain, our data suggest the α/β ratio for OPCs falls somewhere between the currently assigned value of 2 and, perhaps, nearer the value of 10 often assigned to tumor tissue, indicating that traditional fractionation paradigms are unlikely to adequately spare the CNS from late effects. It is not clear what impact SRS versus whole brain exposure would have on recovery and long-term function of OPCs, but such questions could be addressed with a small animal research irradiator. While further work is also required to determine the contribution of OPC intrinsic deficits versus the contribution of the microenvironment to reduced OPC proliferation and maturation long term, if the early loss of OPCs contributes to late CNS IR side effects, our data suggest fractionation is insufficient to prevent late CNS side effects.
Supplementary Material
Main Points.
-
□
Fractionated radiation causes prolonged oligodendrocyte progenitor cell depletion
-
□
Fractionated radiation does not preferentially spare oligodendrocyte progenitor cell loss versus single high dose irradiation
-
□
Oligodendrocyte progenitor differentiation is reduced by radiation exposure
Acknowledgments
This work was supported by the University of Rochester Center for Medical Countermeasures Against Radiation (CMCR) Program, National Institute of Allergy and Infectious Diseases (NIAID) U19-AI091036, and University of Rochester Toxicology Training Grant (NIEHS) T32-ES07026. We thank Jack Walter, Lee Trojanzyck and Mallory Seaman for assistance with animals and tissue processing and the Confocal and Conventional Microscopy Core at the University of Rochester for assistance with image acquisition. Dr. O’Banion is a member of the Board of Directors for Clerisy Corporation.
References
- Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA. Sex differences in glia reactivity after cortical brain injury. Glia. 2015;63:1966–1981. doi: 10.1002/glia.22867. [DOI] [PubMed] [Google Scholar]
- Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev. 2004;14:65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- Atkinson SL, Li YQ, Wong CS. Apoptosis and proliferation of oligodendrocyte progenitor cells in the irradiated rodent spinal cord. Int J Radiat Oncol Biol Phys. 2005;62:535–44. doi: 10.1016/j.ijrobp.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, Burnet NG. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–42. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–62. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begolly S, Shrager PG, Olschowka JA, Williams JP, O'Banion MK. Fractionation Spares Mice From Radiation-Induced Reductions in Weight Gain But Does Not Prevent Late Oligodendrocyte Lineage Side Effects. Int J Radiat Oncol Biol Phys. 2016;96:449–57. doi: 10.1016/j.ijrobp.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Aguirre A. Age-Dependent Netrin-1 Signaling Regulates NG2+ Glial Cell Spatial Homeostasis in Normal Adult Gray Matter. J Neurosci. 2015;35:6946–51. doi: 10.1523/JNEUROSCI.0356-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chari DM, Blakemore WF. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–13. [PubMed] [Google Scholar]
- Chari DM, Gilson JM, Franklin RJ, Blakemore WF. Oligodendrocyte progenitor cell (OPC) transplantation is unlikely to offer a means of preventing X-irradiation induced damage in the CNS. Exp Neurol. 2006;198:145–53. doi: 10.1016/j.expneurol.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Chiang CS, McBride WH. Radiation enhances tumor necrosis factor alpha production by murine brain cells. Brain Res. 1991;566:265–9. doi: 10.1016/0006-8993(91)91707-8. [DOI] [PubMed] [Google Scholar]
- Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–55. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- Daigle JL, Hong JH, Chiang CS, McBride WH. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61:8859–65. [PubMed] [Google Scholar]
- Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–51. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat E, Gaedicke S, Tsurumi C, Esser N, Weyerbrock A, Niedermann G. Delayed cell death associated with mitotic catastrophe in gamma-irradiated stem-like glioma cells. Radiat Oncol. 2011;6:71. doi: 10.1186/1748-717X-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–68. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Fransen NL, Suzzi S, Perry VH. Regulation of microglial proliferation during chronic neurodegeneration. J Neurosci. 2013;33:2481–93. doi: 10.1523/JNEUROSCI.4440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder H, Holtel H, Bromberg JE, Poortmans P, Haaxma-Reiche H, Kluin-Nelemans HC, Menten J, van den Bent MJ. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62:544–7. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–53. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–76. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. A different regional response by mouse oligodendrocyte progenitor cells (OPCs) to high-dose X-irradiation has consequences for repopulating OPC-depleted normal tissue. Eur J Neurosci. 2007;25:417–24. doi: 10.1111/j.1460-9568.2007.05313.x. [DOI] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–81. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Yang TJ, Rosenblum MK, Ballangrud A, Young RJ, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol. 2015;125:149–56. doi: 10.1007/s11060-015-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureshi SA, Hofman FM, Schneider JH, Chin LS, Apuzzo ML, Hinton DR. Cytokine expression in radiation-induced delayed cerebral injury. Neurosurgery. 1994;35:822–9. doi: 10.1227/00006123-199411000-00004. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, Arguello AA, Donovan MH, Fischer SJ, Farnbauch LA, Beech RD, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–21. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Pang S, Yu Y, Wu X, Guo J, Zhang S. Proliferation of parenchymal microglia is the main source of microgliosis after ischaemic stroke. Brain. 2013;136:3578–88. doi: 10.1093/brain/awt287. [DOI] [PubMed] [Google Scholar]
- Li YQ, Jay V, Wong CS. Oligodendrocytes in the adult rat spinal cord undergo radiation-induced apoptosis. Cancer Res. 1996;56:5417–22. [PubMed] [Google Scholar]
- Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–22. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–22. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–62. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Moravan MJ, Olschowka JA, Williams JP, O'Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176:459–73. doi: 10.1667/rr2587.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern RK, White HA, Glass JO, Kun LE, Leigh L, Thompson SJ, Reddick WE. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10:180–9. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- Nambiar D, Rajamani P, Singh RP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011;728:139–57. doi: 10.1016/j.mrrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Nelson J, Pych JC, Kim JH, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Res Dev Brain Res. 2000;120:87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells. 2010;28:639–48. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotakos G, Alshamy G, Chan B, Abrams R, Greenberg E, Saxena A, Bradbury M, Edgar M, Gutin P, Tabar V. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban RA, Snow AL, Day RM. Mechanisms of radiation toxicity in transformed and non-transformed cells. Int J Mol Sci. 2013;14:15931–58. doi: 10.3390/ijms140815931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera PD, Shih HY, Leblanc JA, Cole MG, Amaral WZ, Mukherjee S, Zhang S, Lucero MJ, Decarolis NA, Chen BP, et al. Acute and fractionated exposure to high-LET (56)Fe HZE-particle radiation both result in similar long-term deficits in adult hippocampal neurogenesis. Radiat Res. 2013;180:658–67. doi: 10.1667/RR13480.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins SC, Villemain A, Liu X, Djogo T, Kryzskaya D, Storch KF, Kokoeva MV. Extensive regenerative plasticity among adult NG2-glia populations is exclusively based on self-renewal. Glia. 2013;61:1735–47. doi: 10.1002/glia.22554. [DOI] [PubMed] [Google Scholar]
- Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. 2001;4:303–13. doi: 10.1054/drup.2001.0213. [DOI] [PubMed] [Google Scholar]
- Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24:1664–70. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- Shinohara C, Gobbel GT, Lamborn KR, Tada E, Fike JR. Apoptosis in the subependyma of young adult rats after single and fractionated doses of X-rays. Cancer Res. 1997;57:2694–702. [PubMed] [Google Scholar]
- Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59:869–81. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–27. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS)--influence of SIPS on radiotherapy. J Radiat Res. 2008;49:105–12. doi: 10.1269/jrr.07081. [DOI] [PubMed] [Google Scholar]
- Tonning Olsson I, Perrin S, Lundgren J, Hjorth L, Johanson A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr Neurol. 2014;51:515–21. doi: 10.1016/j.pediatrneurol.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Trifiletti DM, Lee CC, Winardi W, Patel NV, Yen CP, Larner JM, Sheehan JP. Brainstem metastases treated with stereotactic radiosurgery: safety, efficacy, and dose response. J Neurooncol. 2015;125:385–92. doi: 10.1007/s11060-015-1927-6. [DOI] [PubMed] [Google Scholar]
- Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O'Banion MK. Sustained IL-1beta expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav Immun. 2013;32:9–18. doi: 10.1016/j.bbi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Increases in size and myelination of the rat corpus callosum during adulthood are maintained into old age. Brain Res. 2007;1142:13–8. doi: 10.1016/j.brainres.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.